Revisiting Porcine Circovirus Disease Diagnostic Criteria in the Current Porcine Circovirus 2 Epidemiological Context
Abstract
1. Introduction
2. PCV-2 Infection Outcomes and Their Lesions
3. PCV-2 Infection Outcomes and Their Diagnoses
4. Diagnostic Tools for PCV-2 Infections
4.1. Diagnostic Criteria for PCVDs
4.2. Diagnostic Usefulness of PCR Methodologies
4.3. Diagnostic Usefulness of Antibody Detection Methodologies
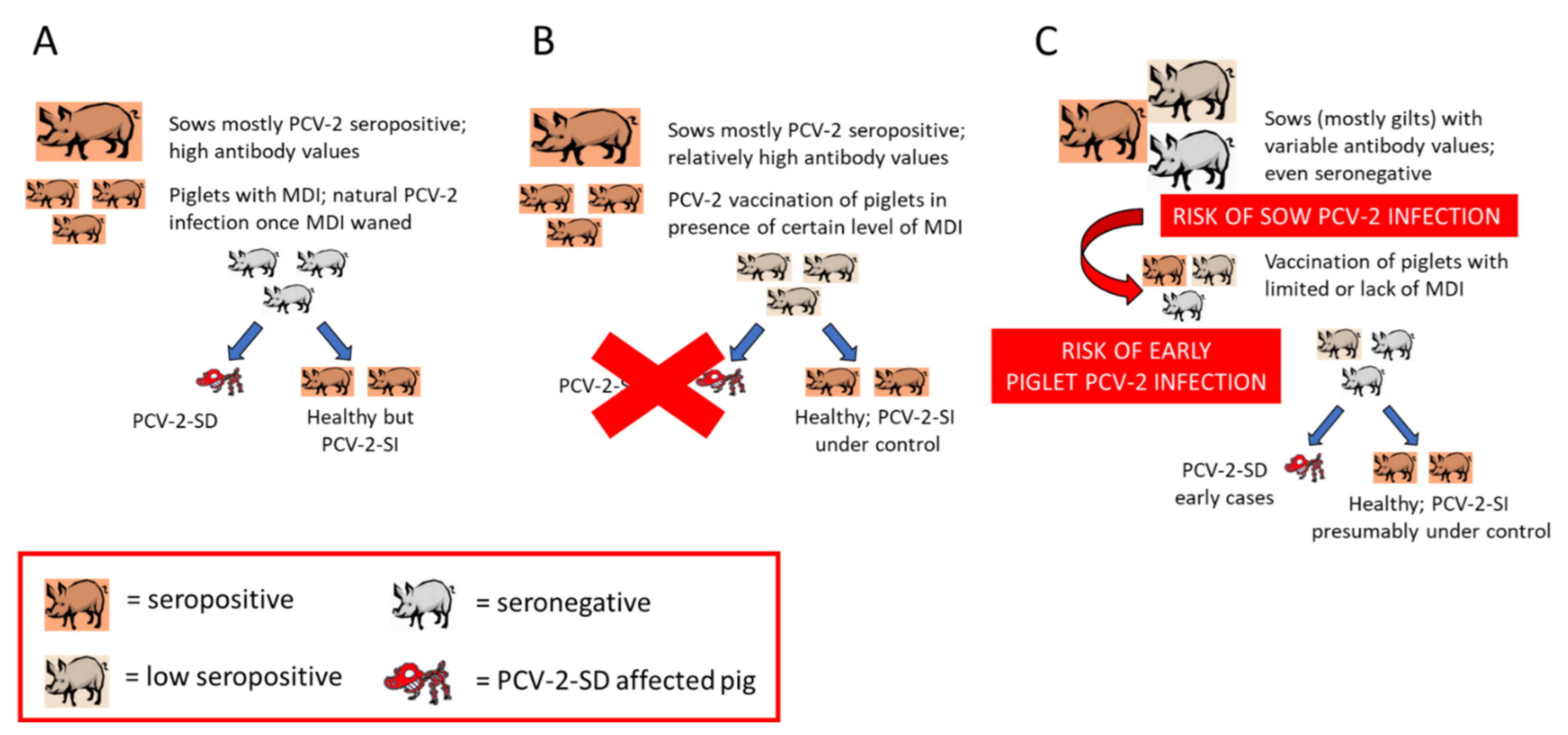
5. PCV-2 Epidemiology and Vaccination
5.1. Changing PCV-2 Epidemiology
5.2. PCVDs in Vaccinated Herds
6. Discussion
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Segalés, J. Porcine circovirus type 2 (PCV2) infections: Clinical signs, pathology and laboratory diagnosis. Virus Res. 2012, 164, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Segalés, J. Best practice and future challenges for vaccination against porcine circovirus type. Expert Rev. Vaccines 2015, 14, 473–487. [Google Scholar] [CrossRef]
- Alarcon, P.; Rushton, J.; Wieland, B. Cost of post-weaning multi-systemic wasting syndrome and porcine circovirus type-2 subclinical infection in England—An economic disease model. Prev. Vet. Med. 2013, 110, 88–102. [Google Scholar] [CrossRef] [PubMed]
- Franzo, G.; Segalés, J. Porcine Circovirus 2 Genotypes, Immunity and Vaccines: Multiple Genotypes but One Single Serotype. Pathogens 2020, 9, 1049. [Google Scholar] [CrossRef] [PubMed]
- Opriessnig, T.; Karuppannan, A.K.; Castro, A.M.; Xiao, C.-T. Porcine circoviruses: Current status, knowledge gaps and challenges. Virus Res. 2020, 286, 198044. [Google Scholar] [CrossRef] [PubMed]
- Kristensen, C.S.; Baadsgaard, N.P.; Toft, N. A meta-analysis comparing the effect of PCV2 vaccines on average daily weight gain and mortality rate in pigs from weaning to slaughter. Prev. Vet. Med. 2011, 98, 250–258. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, N.; Carriquiry, A.; O’Neill, K.; Opriessnig, T.; O’Connor, A. Mixed treatment comparison meta-analysis of porcine circovirus type 2 (PCV2) vaccines used in piglets. Prev. Vet. Med. 2014, 117, 413–424. [Google Scholar] [CrossRef]
- Allison, J.; Nitzel, G.P.; Taylor, L.P. Meta-analysis of porcine circovirus type 2 (PCV2) vaccines. Prev. Vet. Med. 2015, 119, 93. [Google Scholar] [CrossRef]
- Kristensen, C.S.; Hjulsager, C.K.; Larsen, L.E. A two-year follow-up study of the PCV2 status of a Danish pig herd that was initially assumed to be PCV2-free. Porc. Health Manag. 2015, 1, 1–5. [Google Scholar] [CrossRef][Green Version]
- Rosell, C.; Segalés, J.; Plana-Durán, J.; Balasch, M.; Rodríguez-Arrioja, G.; Kennedy, S.; Allan, G.; McNeilly, F.; Latimer, K.; Domingo, M. Pathological, Immunohistochemical, and In-situ Hybridization Studies of Natural Cases of Postweaning Multisystemic Wasting Syndrome (PMWS) in Pigs. J. Comp. Pathol. 1999, 120, 59–78. [Google Scholar] [CrossRef]
- West, K.H.; Bystrom, J.M.; Wojnarowicz, C.; Shantz, N.; Jacobson, M.; Allan, G.M.; Haines, D.M.; Clark, E.G.; Krakowka, S.; McNeilly, F.; et al. Myocarditis and abortion associated with intrauterine infection of sows with porcine circovirus 2. J. Vet. Diagn. Investig. 1999, 11, 530–532. [Google Scholar] [CrossRef]
- Brunborg, I.M.; Jonassen, C.M.; Moldal, T.; Bratberg, B.; Lium, B.; Koenen, F.; Schönheit, J. Association of Myocarditis with High Viral Load of Porcine Circovirus Type 2 in Several Tissues in Cases of Fetal Death and High Mortality in Piglets. A Case Study. J. Vet. Diagn. Investig. 2007, 19, 368–375. [Google Scholar] [CrossRef]
- Smith, W.J.; Thomson, J.; Done, S. Dermatitis/nephropathy syndrome of pigs. Vet. Rec. 1993, 132, 47. [Google Scholar] [CrossRef]
- McNeilly, F.; Kennedy, S.; Moffett, D.; Meehan, B.; Foster, J.; Clarke, E.; Ellis, J.; Haines, D.; Adair, B.; Allan, G. A comparison of in situ hybridization and immunohistochemistry for the detection of a new porcine circovirus in formalin-fixed tissues from pigs with post-weaning multisystemic wasting syndrome (PMWS). J. Virol. Methods 1999, 80, 123–128. [Google Scholar] [CrossRef]
- Olvera, A.; Sibila, M.; Calsamiglia, M.; Segalés, J.; Domingo, M. Comparison of porcine circovirus type 2 load in serum quantified by a real time PCR in postweaning multisystemic wasting syndrome and porcine dermatitis and nephropathy syndrome naturally affected pigs. J. Virol. Methods 2004, 117, 75–80. [Google Scholar] [CrossRef]
- Unterweger, C.; Brunthaler, R.; Auer, A.; Fux, R.; Weissenbacher-Lang, C.; Ladinig, A. Reconsideration of the diagnostic criteria required for PCV2 reproductive disease. Vet. J. 2021, 272, 105660. [Google Scholar] [CrossRef]
- Brunborg, I.M.; Moldal, T.; Jonassen, C.M. Quantitation of porcine circovirus type 2 isolated from serum/plasma and tissue samples of healthy pigs and pigs with postweaning multisystemic wasting syndrome using a TaqMan-based real-time PCR. J. Virol. Methods 2004, 122, 171–178. [Google Scholar] [CrossRef]
- Hansen, M.S.; Hjulsager, C.K.; Bille-Hansen, V.; Haugegaard, S.; Dupont, K.; Hogedal, P.; Kunstmann, L.; Larsen, L.E. Selection of method is crucial for the diagnosis of porcine circovirus type 2 associated reproductive failures. Vet. Microbiol. 2010, 144, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Oropeza-Moe, M.; Delgado, A.J.O.; Framstad, T. Porcine circovirus type 2 associated reproductive failure in a specific pathogen free (SPF) piglet producing herd in Norway: A case report. Porc. Health Manag. 2017, 3, 25. [Google Scholar] [CrossRef]
- Harding, J.C.S.; Clark, E.G.; Strokappe, J.H.; Willson, P.I.; Ellis, J.A. Postweaning multisystemic wasting syndrome: Epidemiology and clinical presentation. Swine Health Prod. 1998, 6, 249–254. [Google Scholar]
- Jacobsen, B.; Krueger, L.; Seeliger, F.; Bruegmann, M.; Segalés, J.; Baumgaertner, W. Retrospective study on the occurrence of porcine circovirus 2 infection and associated entities in Northern Germany. Vet. Microbiol. 2009, 138, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Segalés, J.; Allan, G.M.; Domingo, M. Porcine circovirus diseases. Anim. Health Res. Rev. 2005, 6, 119–142. [Google Scholar] [CrossRef] [PubMed]
- Pensaert, M.B.; Sanchez, R.E., Jr.; Ladekjaer-Mikkelsen, A.S.; Allan, G.M.; Nauwynck, H.J. Viremia and effect of fetal infection with porcine viruses with special reference to porcine circovirus 2 infection. Vet. Microbiol. 2004, 98, 175–183. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.; Wang, C.; Madson, D.M.; Opriessnig, T. High prevalence of porcine circovirus viremia in newborn piglets in five clinically normal swine breeding herds in North America. Prev. Vet. Med. 2010, 97, 228–236. [Google Scholar] [CrossRef]
- Oliver-Ferrando, S.; Segalés, J.; López-Soria, S.; Callén, A.; Merdy, O.; Joisel, F.; Sibila, M. Exploratory field study on the effect of Porcine circovirus 2 (PCV2) sow vaccination on serological, virological and reproductive parameters in a PCV2 subclinically infected sow herd. BMC Vet. Res. 2018, 14, 130. [Google Scholar] [CrossRef]
- Pleguezuelos, P.; Sibila, M.; Cuadrado, R.; López-Jiménez, R.; Pérez, D.; Huerta, E.; Llorens, A.M.; Núñez, J.I.; Segalés, J.; López-Soria, S. Exploratory field study on the effects of porcine circovirus 2 (PCV-2) sow vaccination at different physiological stages mimicking blanket vaccination. Porc. Health Manag. 2021, 7, 1–12. [Google Scholar] [CrossRef]
- Rosell, C.; Segalés, J.; Ramos-Vara, J.A.; Folch, J.M.; Rodriguez-Arrioja, G.M.; Duran, C.O.; Balasch, M.; Plana-Durán, J.; Domingo, M. Identification of porcine circovirus in tissues of pigs with porcine dermatitis and nephropathy syndrome. Vet. Rec. 2000, 146, 40–43. [Google Scholar] [CrossRef]
- Segalés, J.; Allan, G.M.; Domingo, M. Circoviruses. In Diseases of Swine, 11th ed.; Zimmerman, J.J., Karriker, L.A., Ramírez, A., Schwartz, K.J., Stevenson, G.W., Zhang, J.Q., Eds.; Wiley Blackwell: Hoboken, NJ, USA, 2019; pp. 473–487. [Google Scholar]
- Krakowka, S.; Hartunian, C.; Hamberg, A.; Shoup, D.; Rings, M.; Zhang, Y.; Allan, G.; Ellis, J.A. Evaluation of induction of porcine dermatitis and nephropathy syndrome in gnotobiotic pigs with negative results for porcine circovirus type. Am. J. Vet. Res. 2008, 69, 1615–1622. [Google Scholar] [CrossRef]
- Jiang, H.; Wang, D.; Wang, J.; Zhu, S.; She, R.; Ren, X.; Tian, J.; Quan, R.; Hou, L.; Li, Z.; et al. Induction of Porcine Dermatitis and Nephropathy Syndrome in Piglets by Infection with Porcine Circovirus Type. J. Virol. 2019, 93, e02045-18. [Google Scholar] [CrossRef]
- Tico, G.; Segalés, J.; Martinez, J. The blurred border between porcine circovirus type 2-systemic disease and porcine respir-atory disease complex. Vet. Microbiol. 2013, 163, 242–247. [Google Scholar] [CrossRef]
- Baro, J.; Segalés, J.; Martinez, J. Porcine circovirus type 2 (PCV2) enteric disease: An independent condition or part of the systemic disease? Vet. Microbiol. 2015, 176, 83–87. [Google Scholar] [CrossRef]
- Cino-Ozuna, A.G.; Henry, S.; Hesse, R.; Nietfeld, J.C.; Bai, J.; Scott, H.M.; Rowland, R.R.R. Characterization of a New Disease Syndrome Associated with Porcine Circovirus Type 2 in Previously Vaccinated Herds. J. Clin. Microbiol. 2011, 49, 2012–2016. [Google Scholar] [CrossRef]
- Roma, L.G.; Fraile, L.; Segalés, J. Recent advances in the epidemiology, diagnosis and control of diseases caused by porcine circovirus type. Vet. J. 2011, 187, 23–32. [Google Scholar] [CrossRef]
- Harms, P.A.; Sorden, S.D.; Halbur, P.G.; Bolin, S.R.; Lager, K.M.; Morozov, I.; Paul, P.S. Experimental reproduction of severe disease in CD/CD pigs concurrently infected with type 2 porcine circovirus and porcine reproductive and respiratory syn-drome virus. Vet. Pathol. 2001, 38, 528–539. [Google Scholar] [CrossRef]
- Pallarés, F.J.; Halbur, P.G.; Opriessnig, T.; Sorden, S.D.; Villar, D.; Janke, B.H.; Yaeger, M.J.; Larson, D.J.; Schwartz, K.J.; Yoon, K.J.; et al. Porcine Circovirus Type 2 (PCV-2) Coinfections in US Field Cases of Postweaning Multisystemic Wasting Syndrome (PMWS). J. Vet. Diagn. Investig. 2002, 14, 515–519. [Google Scholar] [CrossRef]
- Grau-Roma, L.; Stockmarr, A.; Kristensen, C.S.; Enoe, C.; Lopez-Soria, S.; Nofrarias, M.; Bille-Hansen, V.; Hjulsager, C.K.; Sibila, M.; Jorsal, S.E.; et al. Infectious risk factors for individual post-weaning multisystemic wasting syndrome (PMWS) development in pigs from affected farms in Spain and Denmark. Res. Vet. Sci. 2012, 93, 1231–1240. [Google Scholar] [CrossRef]
- Larochelle, R.; Bielanski, A.; Müller, P.; Magar, R. PCR detection and evidence of shedding of porcine circovirus type 2 in boar semen. J. Clin. Microbiol. 2000, 38, 4629–4632. [Google Scholar] [CrossRef]
- Shibata, I.; Okuda, Y.; Kitajima, K.; Asai, T. Shedding of Porcine Circovirus into Colostrum of Sows. J. Vet. Med. Ser. B 2006, 53, 278–280. [Google Scholar] [CrossRef]
- Ramirez, A.; Wang, C.; Prickett, J.R.; Pogranichniy, R.; Yoon, K.-J.; Main, R.; Johnson, J.K.; Rademacher, C.; Hoogland, M.; Hoffmann, P.; et al. Efficient surveillance of pig populations using oral fluids. Prev. Vet. Med. 2012, 104, 292–300. [Google Scholar] [CrossRef]
- Verreault, D.; Létourneau, V.; Gendron, L.; Massé, D.; Gagnon, C.A.; Duchaine, C. Airborne porcine circovirus in Canadian swine confinement buildings. Vet. Microbiol. 2010, 141, 224–230. [Google Scholar] [CrossRef]
- Viancelli, A.; Garcia, L.; Schiochet, M.; Kunz, A.; Steinmetz, R.; Ciacci-Zanella, J.; Esteves, P.; Barardi, C. Culturing and molecular methods to assess the infectivity of porcine circovirus from treated effluent of swine manure. Res. Vet. Sci. 2012, 93, 1520–1524. [Google Scholar] [CrossRef] [PubMed]
- Dvorak, C.M.; Lilla, M.P.; Baker, S.R.; Murtaugh, M.P. Multiple routes of porcine circovirus type 2 transmission to piglets in the presence of maternal immunity. Vet. Microbiol. 2013, 166, 365–374. [Google Scholar] [CrossRef] [PubMed]
- Lorincz, M.; Csagola, A.; Biksi, I.; Szeredi, L.; Dan, A.; Tuboly, T. Detection of porcine circovirus in rodents—Short commu-nication. Acta Vet. Hung. 2010, 58, 265–268. [Google Scholar] [CrossRef] [PubMed]
- Blunt, R.; McOrist, S.; McKillen, J.; McNair, I.; Jiang, T.; Mellits, K. House fly vector for porcine circovirus 2b on commercial pig farms. Vet. Microbiol. 2011, 149, 452–455. [Google Scholar] [CrossRef]
- Harding, J.C.; Baker, C.D.; Tumber, A.; McIntosh, K.A.; Parker, S.E.; Middleton, D.M.; Hill, J.E.; Ellis, J.A.; Krakowka, S. Porcine circovirus-2 DNA concentration distinguishes wasting from nonwasting pigs and is correlated with lesion distribu-tion, severity, and nucleocapsid staining intensity. J. Vet. Diagn. Investig. 2008, 20, 274–282. [Google Scholar] [CrossRef]
- Fort, M.; Olvera, A.; Sibila, M.; Segalés, J.; Mateu, E. Detection of neutralizing antibodies in postweaning multisystemic wasting syndrome (PMWS)-affected and non-PMWS-affected pigs. Vet. Microbiol. 2007, 125, 244–255. [Google Scholar] [CrossRef]
- Roma, L.G.; Hjulsager, C.K.; Sibila, M.; Kristensen, C.S.; López-Soria, S.; Enøe, C.; Casal, J.; Bøtner, A.; Nofrarías, M.; Bille-Hansen, V.; et al. Infection, excretion and seroconversion dynamics of porcine circovirus type 2 (PCV2) in pigs from post-weaning multisystemic wasting syndrome (PMWS) affected farms in Spain and Denmark. Vet. Microbiol. 2009, 135, 272–282. [Google Scholar] [CrossRef]
- Harding, J.C.S.; Baker, C.; Rhodes, C.; McIntosh, K.A.; Bonneau, M. Ring tests to evaluate the performance of Porcine circovirus-2 (PCV-2) polymerase chain reaction (PCR) assays used in North American diagnostic laboratories. Can. J. Vet. Res. 2009, 73, 7–14. [Google Scholar]
- Hjulsager, C.; Grau-Roma, L.; Sibila, M.; Enøe, C.; Larsen, L.; Segalés, J. Inter-laboratory and inter-assay comparison on two real-time PCR techniques for quantification of PCV2 nucleic acid extracted from field samples. Vet. Microbiol. 2009, 133, 172–178. [Google Scholar] [CrossRef]
- Aramouni, M.; Segalés, J.; Sibila, M.; Martin-Valls, G.; Nieto, D.; Kekarainen, T. Torque teno sus virus 1 and 2 viral loads in postweaning multisystemic wasting syndrome (PMWS) and porcine dermatitis and nephropathy syndrome (PDNS) affected pigs. Vet. Microbiol. 2011, 153, 377–381. [Google Scholar] [CrossRef]
- Prickett, J.R.; Johnson, J.; Murtaugh, M.P.; Puvanendiran, S.; Wang, C.; Zimmerman, J.J.; Opriessnig, T. Prolonged Detection of PCV2 and Anti-PCV2 Antibody in Oral Fluids Following Experimental Inoculation. Transbound. Emerg. Dis. 2011, 58, 121–127. [Google Scholar] [CrossRef]
- Turlewicz-Podbielska, H.; Włodarek, J.; Pomorska-Mól, M. Noninvasive strategies for surveillance of swine viral diseases: A review. J. Vet. Diagn. Investig. 2020, 32, 503–512. [Google Scholar] [CrossRef]
- Sibila, M.; Calsamiglia, M.; Segales, J.; Blanchard, P.; Badiella, L.; Le Dimna, M.; Jestin, A.; Domingo, M. Use of a polymerase chain reaction assay and an ELISA to monitor porcine circovirus type 2 infection in pigs from farms with and without postweaning multisystemic wasting syndrome. Am. J. Vet. Res. 2004, 65, 88–92. [Google Scholar] [CrossRef]
- Feng, H.; Blanco, G.; Segalés, J.; Sibila, M. Can Porcine circovirus type 2 (PCV2) infection be eradicated by mass vaccination? Vet. Microbiol. 2014, 172, 92–99. [Google Scholar] [CrossRef]
- Oliver-Ferrando, S.; Segalés, J.; Sibila, M.; Díaz, I. Comparison of cytokine profiles in peripheral blood mononuclear cells between piglets born from Porcine circovirus 2 vaccinated and non-vaccinated sows. Vet. Microbiol. 2018, 214, 148–153. [Google Scholar] [CrossRef]
- O’Connor, B.; Gauvreau, H.; West, K.; Bogdan, J.; Ayroud, M.; Clark, E.G.; Konoby, C.; Allan, G.; Ellis, J.A. Multiple porcine circovirus 2-associated abortions and reproductive failure in a multisite swine production unit. Can. Vet. J. 2001, 42, 551–553. [Google Scholar]
- Madson, D.M.; Patterson, A.R.; Ramamoorthy, S.; Pal, N.; Meng, X.J.; Opriessnig, T. Reproductive Failure Experimentally Induced in Sows via Artificial Insemination with Semen Spiked with Porcine Circovirus Type. Vet. Pathol. 2009, 46, 707–716. [Google Scholar] [CrossRef]
- Eddicks, M.; Koeppen, M.; Willi, S.; Fux, R.; Reese, S.; Sutter, G.; Stadler, J.; Ritzmann, M. Low prevalence of porcine circovirus type 2 infections in farrowing sows and corresponding pre-suckling piglets in southern German pig farms. Vet. Microbiol. 2016, 187, 70–74. [Google Scholar] [CrossRef]
- Opriessnig, T.; Xiao, C.; Gerber, P.F.; Halbur, P.G. Emergence of a novel mutant PCV2b variant associated with clinical PCVAD in two vaccinated pig farms in the U.S. concurrently infected with PPV. Vet. Microbiol. 2013, 163, 177–183. [Google Scholar] [CrossRef]
- Seo, H.W.; Park, C.; Kang, I.; Choi, K.; Jeong, J.; Park, S.-J.; Chae, C. Genetic and antigenic characterization of a newly emerging porcine circovirus type 2b mutant first isolated in cases of vaccine failure in Korea. Arch. Virol. 2014, 159, 3107–3111. [Google Scholar] [CrossRef]
- Sibila, M.; Rocco, C.; Franzo, G.; Huerta, E.; Domingo, M.; Núñez, J.I.; Segalés, J. Genotyping of Porcine Circovirus 2 (PCV-2) in Vaccinated Pigs Suffering from PCV-2-Systemic Disease between 2009 and 2020 in Spain. Pathogens 2021, 10, 1016. [Google Scholar] [CrossRef] [PubMed]
- Opriessnig, T.; Patterson, A.R.; Elsener, J.; Meng, X.J.; Halbur, P.G. Influence of Maternal Antibodies on Efficacy of Porcine Circovirus Type 2 (PCV2) Vaccination to Protect Pigs from Experimental Infection with PCV2. Clin. Vaccine Immunol. 2008, 15, 397–401. [Google Scholar] [CrossRef] [PubMed]
- Fort, M.; Sibila, M.; Perez-Martin, E.; Nofrarías, M.; Mateu, E.; Segalés, J. One dose of a porcine circovirus 2 (PCV2) sub-unit vaccine administered to 3-week-old conventional piglets elicits cell-mediated immunity and significantly reduces PCV2 viremia in an experimental model. Vaccine 2009, 27, 4031–4037. [Google Scholar] [CrossRef] [PubMed]
- Nautrup, B.P.; Van Vlaenderen, I.; Mah, C.; Angulo, J. Do High Levels of Maternally Derived Antibodies Interfere with the Vaccination of Piglets against Porcine Circovirus Type 2? A Literature Review and Data Analysis. Vaccines 2021, 9, 923. [Google Scholar] [CrossRef]
- Feng, H.; Segalés, J.; Fraile, L.; Lopez-Soria, S.; Sibila, M. Effect of high and low levels of maternally derived antibodies on porcine circovirus type 2 (PCV2) infection dynamics and production parameters in PCV2 vaccinated pigs under field condi-tions. Vaccine 2016, 34, 3044–3050. [Google Scholar] [CrossRef]
- Haake, M.; Palzer, A.; Rist, B.; Weissenbacher-Lang, C.; Fachinger, V.; Eggen, A.; Ritzmann, M.; Eddicks, M. Influence of age on the effectiveness of PCV2 vaccination in piglets with high levels of maternally derived antibodies. Vet. Microbiol. 2014, 168, 272–280. [Google Scholar] [CrossRef]
- Mair, K.; Sedlak, C.; Käser, T.; Pasternak, J.A.; Levast, B.; Gerner, W.; Saalmüller, A.; Summerfield, A.; Gerdts, V.; Wilson, H.; et al. The porcine innate immune system: An update. Dev. Comp. Immunol. 2014, 45, 321–343. [Google Scholar] [CrossRef]
- Canelli, E.; Borghetti, P.; Ferrari, L.; De Angelis, E.; Ferrarini, G.; Catella, A.; Ogno, G.; Martelli, P. Immune response to PCV2 vaccination in PRRSV viraemic piglets. Vet. Rec. 2016, 178, 193. [Google Scholar] [CrossRef]
- Sinha, A.; Shen, H.G.; Schalk, S.; Beach, N.; Huang, Y.; Halbur, P.G.; Meng, X.J.; Opriessnig, T. Porcine Reproductive and Respiratory Syndrome Virus Infection at the Time of Porcine Circovirus Type 2 Vaccination Has No Impact on Vaccine Efficacy. Clin. Vaccine Immunol. 2010, 17, 1940–1945. [Google Scholar] [CrossRef]
- Segalés, J.; Domingo, M. Clinical and pathological findings of PMWS cases in Europe. In Leman Swine Conference; Allen, D., Ed.; University of Minnesota: Minneapolis, MN, USA, 1999; pp. 246–249. [Google Scholar]
- Sorden, S.D. Update on porcine circovirus and postweaning multisystemic wasting syndrome (PMWS). Swine Health Prod. 2000, 8, 133–136. [Google Scholar]
- Segalés, J.; Kekarainen, T.; Cortey, M. The natural history of porcine circovirus type 2: From an inoffensive virus to a dev-astating swine disease? Vet. Microbiol. 2013, 165, 13–20. [Google Scholar] [CrossRef]
- Kekarainen, T.; Segalés, J. Porcine circovirus 2 immunology and viral evolution. Porc. Health Manag. 2015, 1, 17. [Google Scholar] [CrossRef]
- Figueras-Gourgues, S.; Fraile, L.; Segalés, J.; Hernández-Caravaca, I.; López-Úbeda, R.; García-Vázquez, F.A.; Gomez-Duran, O.; Grosse-Liesner, B. Effect of Porcine circovirus 2 (PCV-2) maternally derived antibodies on performance and PCV-2 viremia in vaccinated piglets under field conditions. Porc. Health Manag. 2019, 5, 1–11. [Google Scholar] [CrossRef]
- Opriessnig, T.; Patterson, A.R.; Madson, D.M.; Pal, N.; Rothschild, M.; Kuhar, D.; Lunney, J.K.; Juhan, N.M.; Meng, X.J.; Halbur, P.G. Difference in severity of porcine circovirus type two-induced pathological lesions between Landrace and Pietrain pigs. J. Anim. Sci. 2009, 87, 1582–1590. [Google Scholar] [CrossRef]
- Rose, N.; Abhervé-Guéguen, A.; Le Diguerher, G.; Eveno, E.; Jolly, J.-P.; Blanchard, P.; Oger, A.; Jestin, A.; Madec, F. Effect of the Pietrain breed used as terminal boar on Post-weaning Multisystemic Wasting Syndrome (PMWS) in the offspring in four PMWS-affected farms. Livest. Prod. Sci. 2005, 95, 177–186. [Google Scholar] [CrossRef]
- López-Soria, S.; Nofrarías, M.; Calsamiglia, M.; Espinal, A.; Valero, O.; Ramírez-Mendoza, H.; Mínguez, A.; Serrano, J.M.; Marín, O.; Callén, A.; et al. Post-weaning multisystemic wasting syndrome (PMWS) clinical expression under field conditions is modulated by the pig genetic background. Vet. Microbiol. 2011, 149, 352–357. [Google Scholar] [CrossRef]
- López-Soria, S.; Sibila, M.; Nofrarías, M.; Calsamiglia, M.; Manzanilla, E.; Ramírez-Mendoza, H.; Mínguez, A.; Serrano, J.; Marín, O.; Joisel, F.; et al. Effect of porcine circovirus type 2 (PCV2) load in serum on average daily weight gain during the postweaning period. Vet. Microbiol. 2014, 174, 296–301. [Google Scholar] [CrossRef]
| PCVD (Acronym) | Major Clinical Signs | Individual Diagnostic Criteria |
|---|---|---|
| PCV-2 subclinical infection (PCV-2-SI) | Decreased average daily gain (approx. 10–40 g/day) without any evident clinical sign |
|
| PCV-2 systemic disease (PCV-2-SD) | Wasting, weight loss, decreased rate of weight gain clinically evident, ill thrift or poor-doing animals, sometimes with respiratory and/or digestive disorders |
|
| PCV2 reproductive disease (PCV-2-RD) | Abortions or mummifications |
|
| Regular return-to-estrus |
| |
| Porcine dermatitis and nephropathy syndrome (PDNS) ** | Dark red papules and macules on skin, mainly in hind limbs and perineal area |
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Segalés, J.; Sibila, M. Revisiting Porcine Circovirus Disease Diagnostic Criteria in the Current Porcine Circovirus 2 Epidemiological Context. Vet. Sci. 2022, 9, 110. https://doi.org/10.3390/vetsci9030110
Segalés J, Sibila M. Revisiting Porcine Circovirus Disease Diagnostic Criteria in the Current Porcine Circovirus 2 Epidemiological Context. Veterinary Sciences. 2022; 9(3):110. https://doi.org/10.3390/vetsci9030110
Chicago/Turabian StyleSegalés, Joaquim, and Marina Sibila. 2022. "Revisiting Porcine Circovirus Disease Diagnostic Criteria in the Current Porcine Circovirus 2 Epidemiological Context" Veterinary Sciences 9, no. 3: 110. https://doi.org/10.3390/vetsci9030110
APA StyleSegalés, J., & Sibila, M. (2022). Revisiting Porcine Circovirus Disease Diagnostic Criteria in the Current Porcine Circovirus 2 Epidemiological Context. Veterinary Sciences, 9(3), 110. https://doi.org/10.3390/vetsci9030110






