Pathogens Detected in 205 German Farms with Porcine Neonatal Diarrhea in 2017
Abstract
1. Introduction
2. Materials and Methods
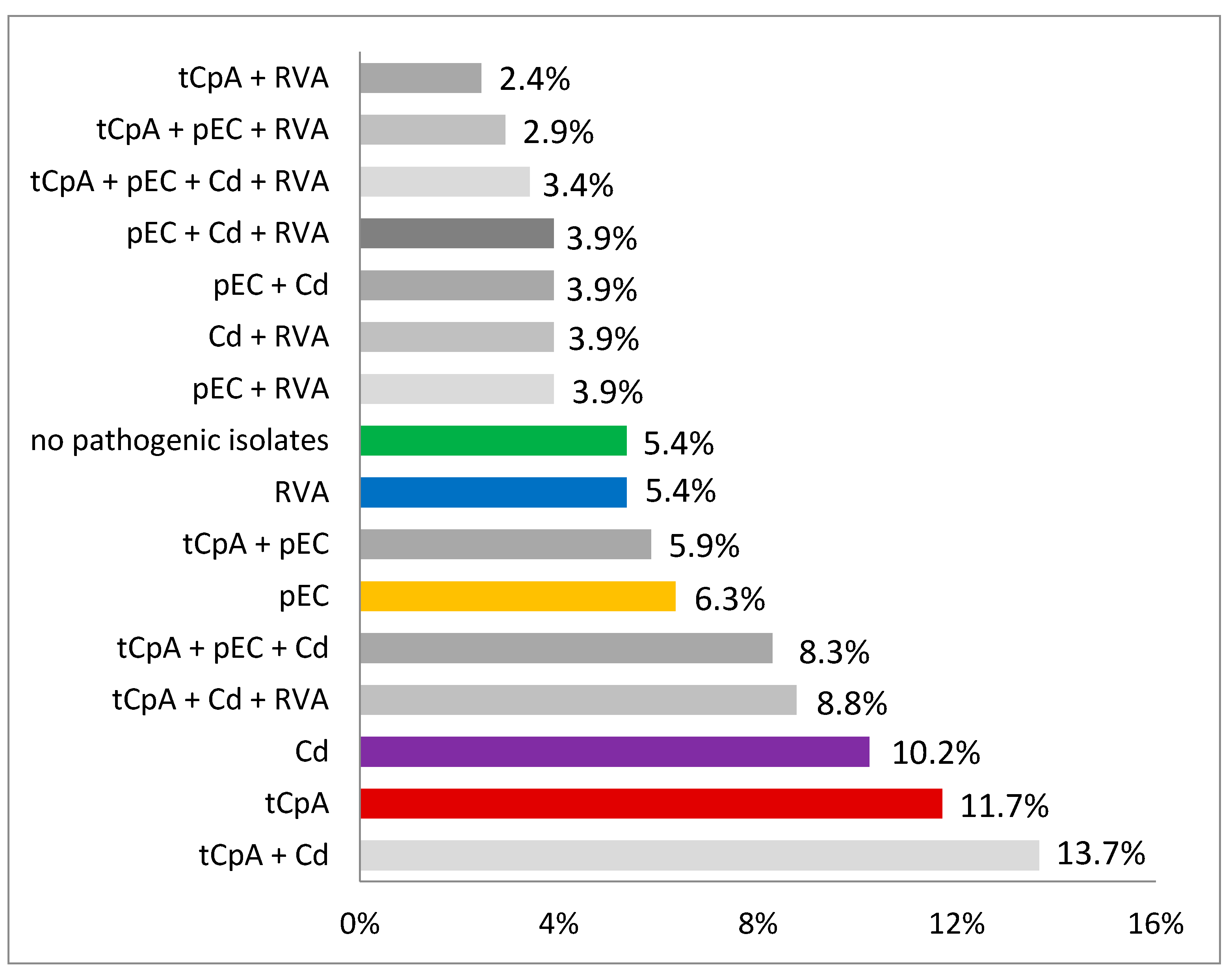
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kongsted, H.; Stege, H.; Toft, N.; Nielsen, J.P. The effect of New Neonatal Porcine Diarrhoea Syndrome (NNPDS) on average daily gain and mortality in 4 Danish pig herds. BMC Vet. Res. 2014, 10, 90. [Google Scholar] [CrossRef]
- Thomson, J.R.; Friendship, R.M. Digestive system. In Diseases of Swine, 11th ed.; Zimmerman, J.J., Karriker, L.A., Ramirez, A., Schwartz, K.J., Stevenson, G.W., Eds.; Wiley-Blackwell: Chichester, UK, 2019; pp. 234–263. [Google Scholar]
- Mertens, N.; Köchling, M.; von Berg, S.; Gotter, V.; Dohmann, K.; Strutzberg-Minder, K.; Lillie-Jaschniski, K. Vorkommen von bakteriellen und viralen Saugferkeldurchfallerregern auf landwirtschaftlichen Betrieben in Deutschland. Tierärztl. Umsch. 2018, 73, 199–206. [Google Scholar]
- Mesonero-Escuredo, S.; Strutzberg-Minder, K.; Casanovas, C.; Segalés, J. Viral and bacterial investigations on the aetiology of recurrent pig neonatal diarrhea cases in Spain. Porc. Health Manag. 2018, 4, 5. [Google Scholar] [CrossRef]
- Gould, L.H.; Limbago, B. Clostridium difficile in Food and Domestic Animals: A New Foodborne Pathogen? Clin. Infect. Dis. 2010, 51, 577–582. [Google Scholar] [CrossRef]
- Songer, J.G.; Uzal, F.A. Clostridial enteric infections in pigs. J. Vet. Diag. Investig. 2005, 17, 528–536. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.Y.; Cho, A.; Kim, J.W.; Kim, H.; Kim, B. High prevalence of Clostridium difficile PCR ribotype 078 in pigs in Korea. Anaerobe 2018, 51, 42–46. [Google Scholar] [CrossRef] [PubMed]
- De Rycke, J.; Milon, A.; Oswald, E. Nectrotoxic Escherichia coli (NTEC): Two emerging categories of human and animal pathogens. Vet. Res. 1999, 30, 221–233. [Google Scholar]
- Van Bost, S.; Babe, M.H.; Jacquemin, E.; Mainil, J. Characteristics of necrotoxigenic Escherichia coli isolated from septicemic and diarrheic calves between 1958 and 1970. Vet. Microbiol. 2001, 82, 311–320. [Google Scholar] [CrossRef]
- Marthaler, D.; Homwong, N.; Rossow, K.; Culhane, M.; Goyal, S.; Collins, J.; Matthijnssens, J.; Ciarlet, M. Rapid detection and high occurrence of porcine rotavirus A, B, and C by RT-qPCR in diagnostic samples. J. Virol. Methods 2014, 209, 30–34. [Google Scholar] [CrossRef]
- Nathues, H.; Nienhoff, H.; Grosse Beilage, E.; Blaha, T.; Ritzmann, M.; Reiner, G.; Lahrmann, K.H.; Kaufhold, J.; Waberski, D.; Hennig-Pauka, I.; et al. Monitoring-Systeme in Zuchtschweinebeständen aus Sicht der Wissenschaft. Dtsch. Tierärztebl. 2010, 10, 1324–1334. [Google Scholar]
- Baums, C.G.; Schotte, U.; Amtsberg, G.; Goethe, R. Diagnostic multiplex PCR for toxin genotyping of Clostridium perfringens isolates. Vet. Microbiol. 2004, 100, 11–16. [Google Scholar] [CrossRef]
- Doosti, A.; Mokhtari-Farsani, A. Study of the frequency of Clostridium difficile tcdA, tcdB, cdtA and cdtB genes in feces of Calves in south west of Iran. Ann. Clin. Microbiol. Antimicrob. 2014, 13, 21. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.I.; Kang, S.G.; Kang, M.L.; Yoo, H.S. Development of multiplex polymerase chain reaction assays for detecting enterotoxigenic Escherichia coli and their application to field isolates from piglets with diarrhea. J. Vet. Diagn. Investig. 2008, 20, 492–496. [Google Scholar] [CrossRef] [PubMed]
- Fairbrother, J.M.; Nadeau, E. Colibacillosis. In Diseases of Swine, 11th ed.; Zimmerman, J.J., Karriker, L.A., Ramirez, A., Schwartz, K.J., Stevenson, G.W., Eds.; Wiley-Blackwell: Chichester, UK, 2019; pp. 807–834. [Google Scholar]
- Unterweger, C.; Kahler, A.; Gerlach, G.F.; Viehmann, M.; Henning-Pauka, I. Administration of non-pathogenic isolates of Escherichia coli and Clostridium perfringens type A to piglets in a herd affected with high incidence of neonatal diarrhea. Animal 2016, 30, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Pang, X.L.; Lee, B.; Boroumand, N.; Leblanc, B.; Preiksaitis, J.K.; Yu Ip, C.C. Increased detection of rotavirus using a real time reverse transcription-polymerase chain reaction (RT-PCR) assay in stool specimens from children with diarrhea. J. Med. Virol. 2004, 72, 496–501. [Google Scholar] [CrossRef] [PubMed]
- Pang, X.; Cao, M.; Zhang, M.; Lee, B. Increased sensitivity for various rotavirus genotypes in stool specimen by amending three mismatched nucleotides in the forward primer of a real-time RT-PCR assay. J. Virol. Methods 2011, 172, 85–87. [Google Scholar] [CrossRef]
- Katsuda, K.; Kohmoto, M.; Kawashima, K.; Tsunemitsu, H. Frequency of enteropathogen detection in suckling and weaned pigs with diarrhea in Japan. J. Vet. Diagn. Investig. 2006, 18, 350–354. [Google Scholar] [CrossRef]
- Vidal, A.; Martín-Valls, G.E.; Tello, M.; Mateu, E.; Martín, M.; Darwich, L. Prevalence of enteric pathogens in diarrheic and non-diarrheic samples from pig farms with neonatal diarrhea in the North EAST-1 of Spain. Vet. Microbiol. 2019, 237, 108419. [Google Scholar] [CrossRef]
- Dors, A.; Czyzewska-Dors, E.; Wasyl, D.; Pomorska-Mol, M. Prevalence and factors associated with occurrence of bacterial enteropathogens in suckling piglets in farrow-to-finish herds. Vet. Rec. 2016, 179, 598. [Google Scholar] [CrossRef]
- Springer, S.; Finzel, J.; Florian, V.; Schoepe, H.; Woitow, G.; Selbitz, H.J. Occurrence and control of the Clostridium perfringens type A associated diarrhea of the suckling pigs with special consideration of the immunoprophylaxis. Tierärztl. Prax. 2012, 40, 375–382. [Google Scholar]
- Johannsen, U.; Arnold, P.; Köhler, B.; Selbitz, H.J. Untersuchungen zur experimentellen Clostridium perfringens-Typ-A-Enterotoxämie der Saugferkel. MH Vet. Med. 1993, 48, 129–136. [Google Scholar]
- Waters, M.; Savoie, A.; Garmory, H.S.; Bueschel, D.; Popoff, M.R.; Songer, G.; Tiball, R.W.; McClane, B.A.; Sarker, M.R. Genotyping and Phenotyping of Beta2-Toxigenic Clostridium perfringens Fecal Isolates Associated with Gastrointestinal Diseases in Piglets. J. Clin. Microbiol. 2003, 41, 3584–3591. [Google Scholar] [CrossRef] [PubMed]
- Dinleyici, M.; Vandenplas, Y. Clostridium difficile Colitis Prevention and Treatment. In Probiotics and Child Gastrointestinal Health; Advances in Experimental Medicine and Biology; Springer: Berlin, Germany, 2019; pp. 139–146. [Google Scholar]
- Chan, G.; Farzan, A.; DeLay, J.; McEwen, B.; Prescott, J.F.; Friendship, R.M. A retrospective study on the etiological diagnoses of diarrhea in neonatal piglets in Ontario, Canada, between 2001 and 2010. Can. J. Vet. Res. 2013, 77, 254–260. [Google Scholar] [PubMed]
- Zajacova, Z.S.; Konstantinova, L.; Alexa, P. Detection of virulence factors of Escherichia coli focused on prevalence of EAST-11 toxin in stool of diarrheic and non-diarrheic piglets and presence of adhesion involving virulence factors in astA positive strains. Vet. Microbiol. 2012, 154, 369–375. [Google Scholar] [CrossRef] [PubMed]
- Nagy, B.; Casey, T.A.; Whipp, S.C.; Moon, H.W. Susceptibility of porcine intestine to pilus-mediated adhesion by some isolates of piliated enterotoxigenic Escherichia coli increases with age. Infect. Immun. 1992, 60, 1285–1294. [Google Scholar] [CrossRef]
- Ménard, L.P.; Dubreuil, J.D. Enteroaggregative Escherichia coli heat-stable enterotoxin 1 (EAST-11): A new toxin with an old twist. Crit. Rev. Microbiol. 2002, 28, 43–60. [Google Scholar] [CrossRef]
- Renzhammer, R.; Loncaric, I.; Roch, F.F.; Pinior, B.; Käsbohrer, A.; Spergser, J.; Ladinig, A.; Unterweger, C. Prevalence of Virulence Genes and Antimicrobial Resistances in E. coli Associated with Neonatal Diarrhea, Postweaning Diarrhea, and Edema Disease in Pigs from Austria. Antibiotics 2020, 9, 208. [Google Scholar] [CrossRef]
- Kim, H.J.; Park, S.I.; Ha, T.P.; Jeong, Y.J.; Kim, H.H.; Kwon, H.J.; Kang, M.I.; Cho, K.O.; Park, S.J. Detection and genotyping of Korean porcine rotaviruses. Vet. Microbiol. 2010, 144, 274–286. [Google Scholar] [CrossRef]
- Park, J.G.; Alfajaro, M.M.; Cho, E.H.; Kim, J.Y.; Soliman, M.; Baek, Y.B.; Park, C.H.; Lee, J.H.; Son, K.Y.; Cho, K.O.; et al. Development of a live attenuated trivalent porcine rotavirus A vaccine against disease caused by recent strains most prevalent in South Korea. Vet. Res. 2019, 50, 2. [Google Scholar] [CrossRef]
- Pensaert, M.B.; Martelli, P. Porcine epidemic diarrhea: A retrospect from Europe and matters of debate. Virus Res. 2016, 226, 1–6. [Google Scholar] [CrossRef]
- Stadler, J.; Zoels, S.; Fux, R.; Hanke, D.; Pohlmann, A.; Blome, S.; Weissenböck, H.; Weissenbacher-Lang, C.; Ritzmann, M.; Ladinig, A. Emergence of porcine epidemic diarrhea virus in southern Germany. BMC Vet. Res. 2015, 11, 142. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, L.J.; Malmkvist, J.; Kammersgaard, T.; Jorgensen, E. Avoiding hypothermia in neonatal pigs: Effect of duration of floor heating at different room temperatures. J. Anim. Sci. 2013, 91, 425–432. [Google Scholar] [CrossRef] [PubMed]
- Le Dividich, J.; Noblet, J. Colostrum intake and thermoregulation in the neonatal pig in relation to environmental temperature. Neonatology 1981, 40, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Muns, R.; Nuntapaitoon, M.; Tummaruk, P. Non-infectious causes of pre-weaning mortality in piglets. Livest. Sci. Vol. 2016, 184, 46–57. [Google Scholar] [CrossRef]



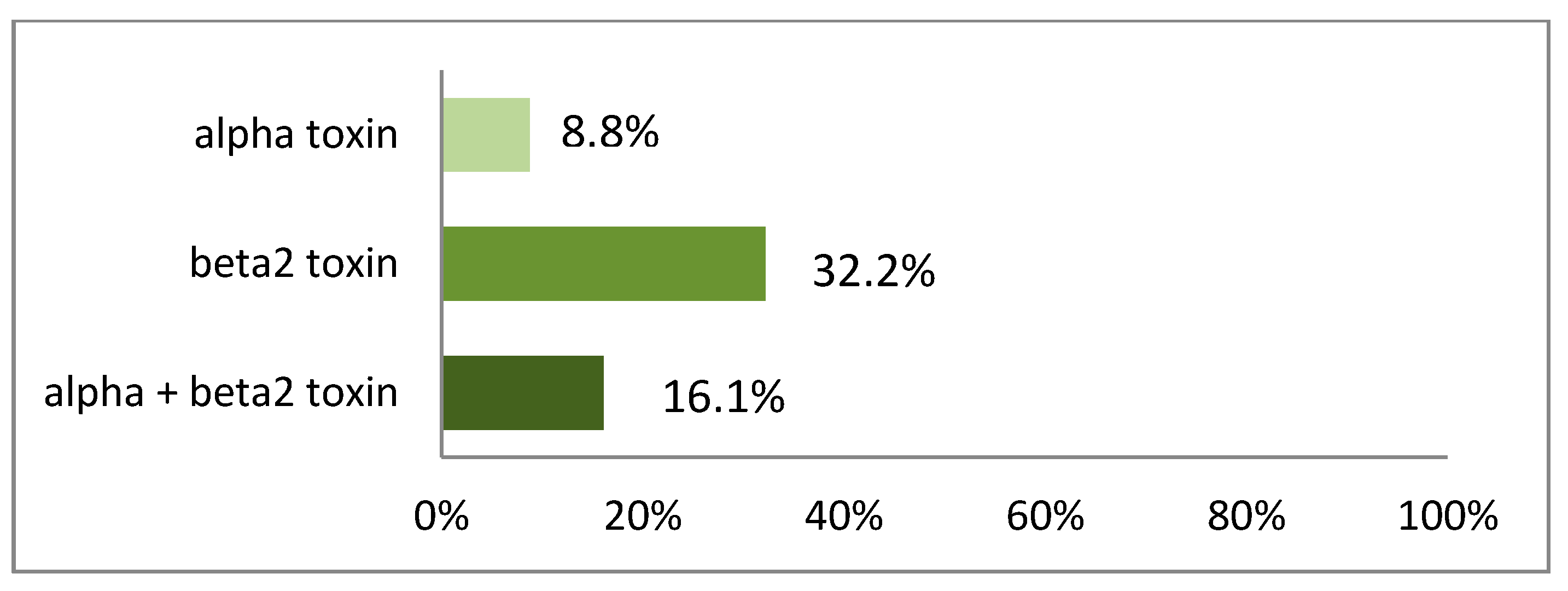
| Bacterial Mponents/Products. | Target Genes | Virulence Factors |
|---|---|---|
| Fimbriae | faeG (F4) | F4 fimbriae |
| faeC (F5) | F5 fimbriae | |
| fasA (F6) | F6 fimbriae | |
| fedA (F18) | F18 fimbriae | |
| fim41A (F41) | F41 fimbriae | |
| fimH (F1) | Type 1 fimbriae | |
| fimA (F1) | Type 1 fimbriae | |
| Adhesins | papC | P fimbriae |
| aidA (AIDA) | AIDA-I autotransporter adhesin | |
| paa | porcine attaching-effacing associated protein | |
| eaeA (intimin) | intimin | |
| Toxins | eltB (LTI) | heat-sensitive enterotoxin |
| estA (STI) | heat-resistant enterotoxin | |
| estB (STII) | heat-resistant enterotoxin | |
| astA (EAST-1) | heat-resistant enterotoxin | |
| stx2e | Shiga toxin-2e | |
| cdtB | cytolethal distending toxin | |
| Others | cnf1 | cytotoxic necrotizing factor type 1 |
| iucD | aerobactin siderophore | |
| escV | type III secretion system | |
| pic | serin protease autotransporter |
| Pathotype | Fimbriae/Adhesins | Toxins/Virulence Factors |
|---|---|---|
| ETEC * | F4, F5, F6, F18, F41, AIDA, (paa) | STI, STII, LTI, EAST-1 |
| EPEC * | Intimin, (paa) | escV, (pic) |
| EDEC * | F18, (AIDA) | stx2e |
| NTEC * | papC | cnf1, (cdtB) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mertens, N.; Theuß, T.; Köchling, M.; Dohmann, K.; Lillie-Jaschniski, K. Pathogens Detected in 205 German Farms with Porcine Neonatal Diarrhea in 2017. Vet. Sci. 2022, 9, 44. https://doi.org/10.3390/vetsci9020044
Mertens N, Theuß T, Köchling M, Dohmann K, Lillie-Jaschniski K. Pathogens Detected in 205 German Farms with Porcine Neonatal Diarrhea in 2017. Veterinary Sciences. 2022; 9(2):44. https://doi.org/10.3390/vetsci9020044
Chicago/Turabian StyleMertens, Nicolas, Tobias Theuß, Monika Köchling, Karen Dohmann, and Kathrin Lillie-Jaschniski. 2022. "Pathogens Detected in 205 German Farms with Porcine Neonatal Diarrhea in 2017" Veterinary Sciences 9, no. 2: 44. https://doi.org/10.3390/vetsci9020044
APA StyleMertens, N., Theuß, T., Köchling, M., Dohmann, K., & Lillie-Jaschniski, K. (2022). Pathogens Detected in 205 German Farms with Porcine Neonatal Diarrhea in 2017. Veterinary Sciences, 9(2), 44. https://doi.org/10.3390/vetsci9020044






