The Chemical Composition, Fermentation End-Product of Silage, and Aerobic Stability of Cassava Pulp Fermented with Lactobacillus casei TH14 and Additives
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Treatments and Experiment Design
- (1)
- No LcTH14 + Urea 0% DM + Molasses 0% DM; CON
- (2)
- No LcTH14 + Urea 0% DM + Molasses 4% DM; M
- (3)
- No LcTH14 + Urea 4% DM + Molasses 0% DM; U
- (4)
- No LcTH14 + Urea 4% DM + Molasses 4% DM; UM
- (5)
- LcTH14 1 × 105 cfu/g FM + Urea 0% DM + Molasses 0% DM; L
- (6)
- LcTH14 1 × 105 cfu/g FM + Urea 0% DM + Molasses 4% DM; LM
- (7)
- LcTH14 1 × 105 cfu/g FM + Urea 4% DM + Molasses 0% DM; LU
- (8)
- LcTH14 1 × 105 cfu/g FM + Urea 4% DM + Molasses 4% DM; LUM
2.2. Material and Silage Preparation
2.3. Chemical Composition
2.4. Fermentation End-Product Analysis of Silage
2.5. Microbial Counting
2.6. Aerobic Stability Analysis
2.7. Statistical Analysis
3. Results
3.1. Microbiology and Chemical Composition of Cassava Pulp before Fermentation
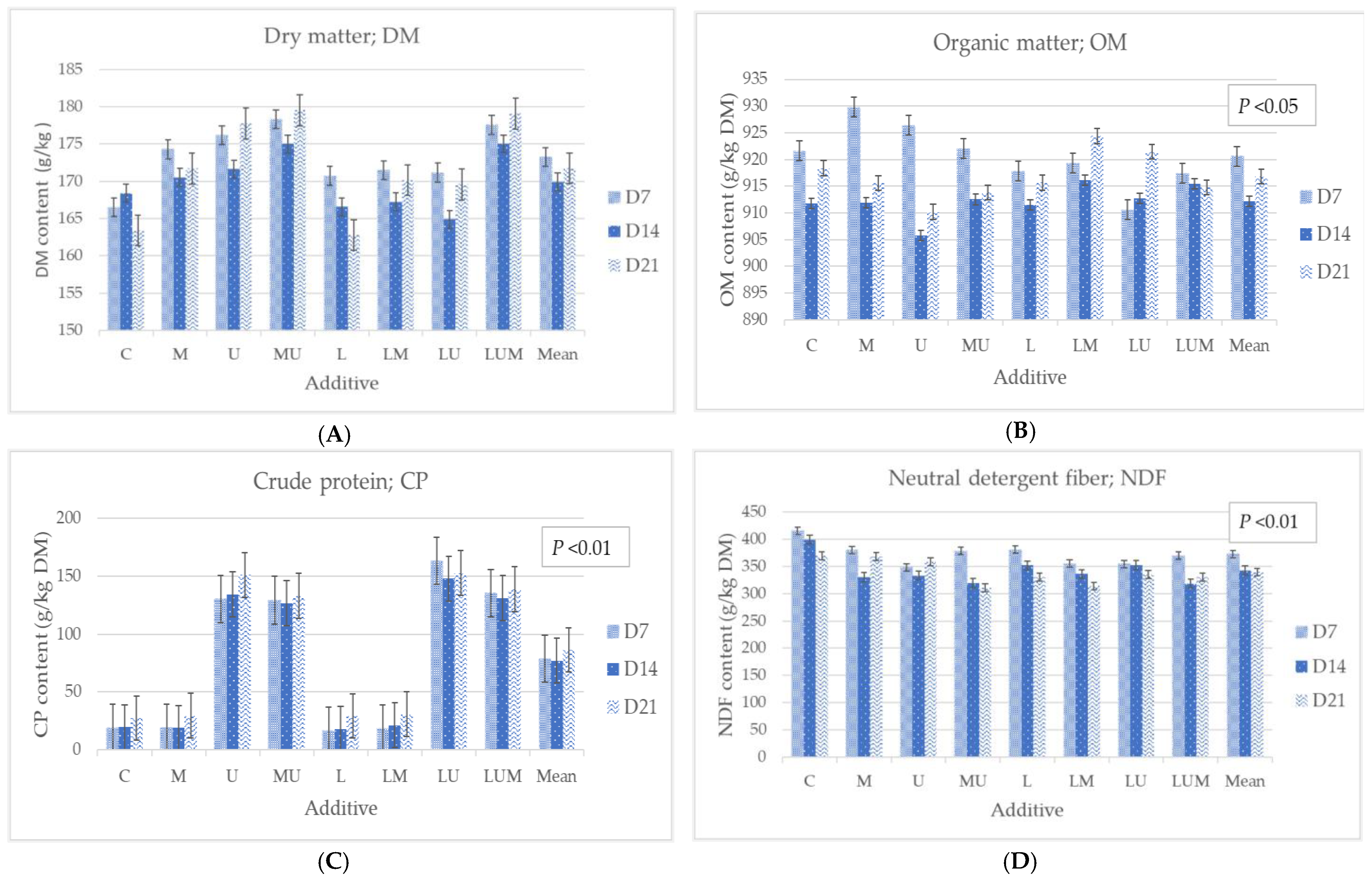
3.2. Chemical Composition of Cassava Pulp Treated with LcTH14, Urea, and Molasses after Fermentation
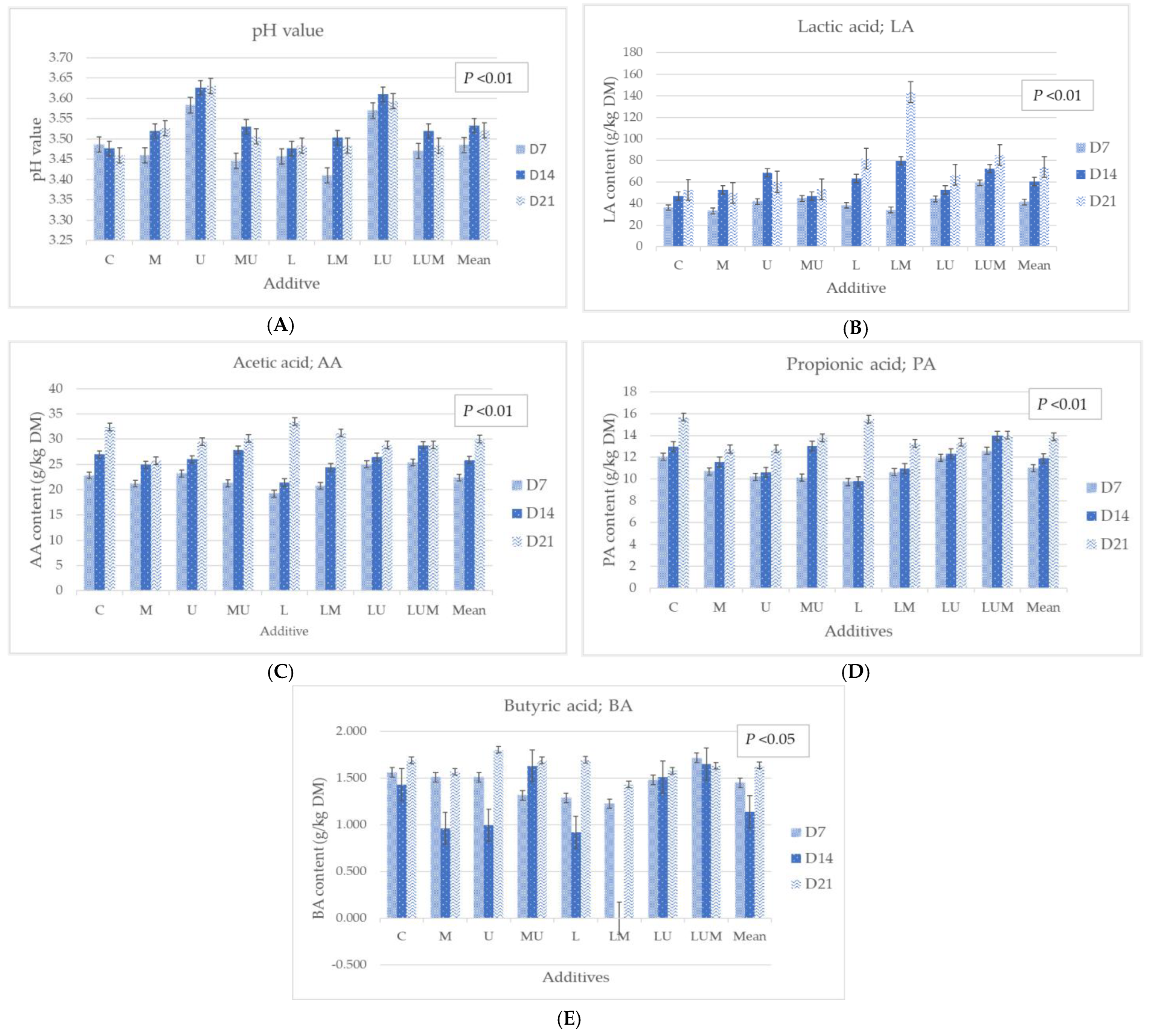
3.3. Effect of Cassava Pulp Treated with LcTH14, Urea, and Molasses on Characteristics after Fermentation
3.4. Microbiology and Aerobic Stability of Cassava Pulp Treated with LcTH14, Urea, and Molasses after 21 Days of Fermentation
4. Discussion
4.1. Microbial Populations and Chemical Composition of Cassava Pulp
4.2. Chemical Composition of Cassava Pulp Fermented with LcTH14, Urea, and Molasses after Fermentation
4.3. The Fermentation Characteristics of Cassava Pulp Fermented with LcTH14, Urea, and Molasses after Fermentation
4.4. Microbiology and Aerobic Stability of Cassava Pulp Treated with LcTH14, Urea, and Molasses after 21 Days of Fermentation
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
References
- Fathima, A.A.; Sanitha, M.; Tripathi, L.; Muiruri, S. Cassava (Manihot esculenta) dual use for food and bioenergy: A review. Food Energy Secur. 2022, e380. [Google Scholar] [CrossRef]
- FAO. Food Outlook: Biannual Report on Global Food Markets-November 2018. Mod. Sci.-Mod. Věda 2018, 15, 5–13. [Google Scholar]
- FAOSTAT. FAO Statistics, Food and Agriculture Organization of the United Nations. 2022. Available online: http://www.fao.org/faostat/en/ (accessed on 8 September 2022).
- Otekunrin, O.A.; Sawicka, B. Cassava, a 21st century staple crop: How can Nigeria harness its enormous trade potentials. Acta Sci. Agric. 2019, 3, 194–202. [Google Scholar] [CrossRef]
- Lerdlattaporn, R.; Phalakornkule, C.; Trakulvichean, S.; Songkasiri, W. Implementing circular economy concept by converting cassava pulp and wastewater to biogas for sustainable production in starch industry. Sustain. Environ. Res. 2021, 31, 20. [Google Scholar] [CrossRef]
- Trakulvichean, S.; Chaiprasert, P.; Otmakhova, J.; Songkasiri, W. Comparison of fermented animal feed and mushroom growth media as two value-added options for waste cassava pulp management. Waste Manag. Res. 2017, 35, 1210–1219. [Google Scholar] [CrossRef] [PubMed]
- Akaracharanya, A.; Kesornsit, J.; Leepipatpiboon, N.; Srinorakutara, T.; Kitpreechavanich, V.; Tolieng, V. Evaluation of the waste from cassava starch production as a substrate for ethanol fermentation by Saccharomyces cerevisiae. Ann. Microbiol. 2011, 61, 431–436. [Google Scholar] [CrossRef]
- Bizzuti, B.E.; de Abreu Faria, L.; da Costa, W.S.; Lima, P.d.M.T.; Ovani, V.S.; Krüger, A.M.; Louvandini, H.; Abdalla, A.L. Potential use of cassava by-product as ruminant feed. Trop. Anim. Health Prod. 2021, 53, 1–7. [Google Scholar] [CrossRef]
- Kongphitee, K.; Sommart, K.; Phonbumrung, T.; Gunha, T.; Suzuki, T. Feed intake, digestibility and energy partitioning in beef cattle fed diets with cassava pulp instead of rice straw. Asian-Australas. J. Anim. Sci. 2018, 31, 1431–1441. [Google Scholar] [CrossRef] [PubMed]
- Keaokliang, O.; Kawashima, T.; Angthong, W.; Suzuki, T.; Narmseelee, R. Chemical composition and nutritive values of cassava pulp for cattle. Anim. Sci. J. 2018, 89, 1120–1128. [Google Scholar] [CrossRef]
- Sharma, D.; Joshi, A.; Schiere, J.; Ibrahim, M. Treatment of crop residues: A review. In Handbook for Straw Feeding Systems in Livestock Production; Indo-Dutch Project on Bioconversion of Crop Residues: New Delhi, India; Wageningen, The Netherlands, 1995; pp. 263–270. [Google Scholar]
- Suntara, C.; Cherdthong, A.; Uriyapongson, S.; Wanapat, M.; Chanjula, P. Comparison effects of ruminal crabtree-negative yeasts and crabtree-positive yeasts for improving ensiled rice straw quality and ruminal digestion using in vitro gas production. J. Fungi 2020, 6, 109. [Google Scholar] [CrossRef]
- Dagaew, G.; Cherdthong, A.; Wongtangtintharn, S.; Wanapat, M.; Suntara, C. Manipulation of in vitro ruminal fermentation and feed digestibility as influenced by yeast waste-treated cassava pulp substitute soybean meal and different roughage to concentrate ratio. Fermentation 2021, 7, 196. [Google Scholar] [CrossRef]
- Kaewpila, C.; Thip-uten, S.; Cherdthong, A.; Khota, W. Impact of cellulase and lactic acid bacteria inoculant to modify ensiling characteristics and in vitro digestibility of sweet corn stover and cassava pulp silage. Agriculture 2021, 11, 66. [Google Scholar] [CrossRef]
- Phesatcha, K.; Phesatcha, B.; Chunwijitra, K.; Wanapat, M.; Cherdthong, A. Changed rumen fermentation, blood parameters, and microbial population in fattening steers receiving a high concentrate diet with saccharomyces cerevisiae improve growth performance. Vet. Sci. 2021, 8, 294. [Google Scholar] [CrossRef] [PubMed]
- Pholsen, S.; Khota, W.; Pang, H.; Higgs, D.; Cai, Y. Characterization and application of lactic acid bacteria for tropical silage preparation. Anim. Sci. J. 2016, 87, 1202–1211. [Google Scholar] [CrossRef] [PubMed]
- Cherdthong, A.; Suntara, S.; Khota, W.; Wanapat, M. Feed utilization and rumen fermentation characteristics of Thai-indigenous beef cattle fed ensiled rice straw with Lactobacillus casei TH14, molasses, and cellulase enzymes. Livest. Sci. 2021, 245, 104405. [Google Scholar] [CrossRef]
- So, S.; Cherdthong, A.; Wanapat, M. Effect of sugarcane bagasse as industrial by-products treated Lactobacillus casei TH14, cellulase, and molasses on feed utilization, ruminal ecology and milk production of mid-lactating Holstein Friesian cows. J. Sci. Food Agri. 2021, 101, 4481–4489. [Google Scholar] [CrossRef]
- Khota, W.; Pholsen, S.; Higgs, D.; Cai, Y. Fermentation quality and in vitro methane production of sorghum silage prepared with cellulase and lactic acid bacteria. Asian Australas. J. Anim. Sci. 2017, 30, 1568. [Google Scholar] [CrossRef]
- Norrapoke, T.; Wanapat, M.; Cherdthong, A.; Kang, S.; Phesatcha, K.; Pongjongmit, T. Improvement of nutritive value of cassava pulp and in vitro fermentation and microbial population by urea and molasses supplementation. J. Appl. Anim. Res. 2018, 46, 242–247. [Google Scholar] [CrossRef]
- So, S.; Cherdthong, A.; Wanapat, M.; Uriyapongson, S. Fermented sugarcane bagasse with Lactobacillus combined with cellulase and molasses promotes in vitro gas kinetics, degradability, and ruminal fermentation patterns compared to rice straw. Anim. Biotechnol. 2020, 33, 116–127. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis; Association of Official Analytical Chemists: Washington, DC, USA, 1990. [Google Scholar]
- Van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef]
- Cai, Y.; Benno, Y.; Ogawa, M.; Kumai, S. Effect of applying lactic acid bacteria isolated from forage crops on fermentation characteristics and aerobic deterioration of silage. J. Dairy Sci. 1999, 82, 520–526. [Google Scholar] [CrossRef]
- Porter, M.; Murray, R. The volatility of components of grass silage on oven drying and the inter-relationship between dry-matter content estimated by different analytical methods. Grass Forage Sci. 2001, 56, 405–411. [Google Scholar] [CrossRef]
- Fawcett, J.; Scott, J. A rapid and precise method for the determination of urea. J. Clin. Pathol. 1960, 13, 156–159. [Google Scholar] [CrossRef]
- Carvalho, B.; Avila, C.; Krempser, P.; Batista, L.; Pereira, M.; Schwan, R. Occurrence of mycotoxins and yeasts and moulds identification in corn silages in tropical climate. J. Appl. Microbiol. 2016, 120, 1181–1192. [Google Scholar] [CrossRef] [PubMed]
- Khota, W.; Pholsen, S.; Higgs, D.; Cai, Y. Natural lactic acid bacteria population of tropical grasses and their fermentation factor analysis of silage prepared with cellulase and inoculant. J. Dairy Sci. 2016, 99, 9768–9781. [Google Scholar] [CrossRef] [PubMed]
- Kozaki, M.; Uchimura, T.; Okada, S. Experimental Manual of Lactic Acid Bacteria; Asakurasyoten: Tokyo, Japan, 1992; pp. 34–37. [Google Scholar]
- Ranjit, N.K.; Kung, L., Jr. The effect of Lactobacillus buchneri, Lactobacillus plantarum, or a chemical preservative on the fermentation and aerobic stability of corn silage. J. Dairy Sci. 2000, 83, 526–535. [Google Scholar] [CrossRef]
- Acosta Aragón, Y.; Jatkauskas, J.; Vrotniakiene, V. The effect of a silage inoculant on silage quality, aerobic stability, and meat production on farm scale. Int. Sch. Res. Not. 2012, 2012, 6. [Google Scholar] [CrossRef]
- Steel, R.G.; Torrie, J.H. Principles and Procedures of Statistics: A Biometrical Approach; McGraw-Hill: New York, NY, USA, 1980; Volume 2, p. 633. [Google Scholar]
- Kaewpila, C.; Gunun, P.; Kesorn, P.; Subepang, S.; Thip-Uten, S.; Cai, Y.; Pholsen, S.; Cherdthong, A.; Khota, W. Improving ensiling characteristics by adding lactic acid bacteria modifies in vitro digestibility and methane production of forage-sorghum mixture silage. Sci. Rep. 2021, 11, 1968. [Google Scholar] [CrossRef]
- Napasirth, V.; Napasirth, P.; Sulinthone, T.; Phommachanh, K.; Cai, Y. Microbial population, chemical composition and silage fermentation of cassava residues. Anim. Sci. J. 2015, 86, 842–848. [Google Scholar] [CrossRef]
- Sriroth, K.; Chollakup, R.; Chotineeranat, S.; Piyachomkwan, K.; Oates, C.G. Processing of cassava waste for improved biomass utilization. Bioresour. Technol. 2000, 71, 63–69. [Google Scholar] [CrossRef]
- Huang, Y.; Liang, L.; Dai, S.; Wu, C.; Chen, C.; Hao, J. Effect of different regions and ensiling periods on fermentation quality and the bacterial community of whole-plant maize silage. Front. Microbiol. 2021, 12, 743695. [Google Scholar] [CrossRef] [PubMed]
- Grace, M. Cassava Processing; Publications Division, Food and Agriculture Organization of the United Nations: Rome, Italy, 1977. [Google Scholar]
- Yunus, M.; Ohba, N.; Shimojo, M.; Furuse, M.; Masuda, Y. Effects of adding urea and molasses on Napiergrass silage quality. Asian-Australas. J. Anim. Sci. 2000, 13, 1542–1547. [Google Scholar] [CrossRef]
- Alli, I.; Fairbairn, R.; Noroozi, E.; Baker, B.E. The effects of molasses on the fermentation of chopped whole-plant leucaena. J. Sci. Food Agric. 1984, 35, 285–289. [Google Scholar] [CrossRef]
- Kim, D.H.; Lee, K.D.; Choi, K.C. Role of LAB in silage fermentation: Effect on nutritional quality and organic acid production—An overview. AIMS Agric. Food 2021, 6, 216–234. [Google Scholar] [CrossRef]
- Santos, A.P.M.D.; Santos, E.M.; Oliveira, J.S.D.; Ribeiro, O.L.; Perazzo, A.F.; Martins Araújo Pinho, R.; Macêdo, A.J.D.S.; Pereira, G.A. Effects of urea addition on the fermentation of sorghum (Sorghum bicolor) silage. Afr. J. Range Forage Sci. 2018, 35, 55–62. [Google Scholar] [CrossRef]
- Bilal, M.Q. Effect of molasses and corn as silage additives on the characteristics of mott dwarf elephant grass silage at different fermentation periods. Pak. Vet. J. 2009, 29, 19–23. [Google Scholar]
- Hu, W.; Schmidt, R.; McDonell, E.; Klingerman, C.; Kung, L., Jr. The effect of Lactobacillus buchneri 40788 or Lactobacillus plantarum MTD-1 on the fermentation and aerobic stability of corn silages ensiled at two dry matter contents. J. Dairy Sci. 2009, 92, 3907–3914. [Google Scholar] [CrossRef]
- Mayne, C.; Gordon, F. Effect of harvesting system on nutrient losses during silage making. 2. In-silo losses. Grass Forage Sci. 1986, 41, 341–351. [Google Scholar] [CrossRef]
- Neumann, M.; Oliboni, R.; Oliveira, M.R.; Faria, M.V.; Ueno, R.K.; Reinerh, L.L.; Durman, T. Chemicals additive used in silages. Appl. Res. Agrotech. 2010, 3, 187–208. [Google Scholar]
- Thao, N.T.; Wanapat, M.; Cherdthong, A.; Kang, S. Effects of eucalyptus crude oils supplementation on rumen fermentation, microorganism and nutrient digestibility in swamp buffaloes. Asian-Australas. J. Anim. Sci. 2014, 27, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, F.; Garcia, R.; Pires, A.; Pereira, O.; Carvalho, G.; Olivindo, C. Forage sorghum silage with added urea in two storage periods. Rev. Bras. Zootec. 2009, 38, 2111–2115. [Google Scholar] [CrossRef]
- Wachirapakorn, C.; Pilachai, K.; Wanapat, M.; Pakdee, P.; Cherdthong, A. Effect of ground corn cobs as a fiber source in total mixed ration on feed intake, milk yield and milk composition in tropical lactating crossbred Holstein cows. Anim. Nutr. 2016, 2, 334–338. [Google Scholar] [CrossRef] [PubMed]
- Buettner, M.; Lechtenberg, V.; Hendrix, K.; Hertel, J. Composition and digestion of ammoniated tall fescue (Festuca arundinacea Schreb.) hay. Anim. Sci. J. 1982, 54, 173–178. [Google Scholar] [CrossRef]
- Wanapat, M.; Kang, S.; Khejornsart, P.; Pilajun, R. Improvement of whole crop rice silage nutritive value and rumen degradability by molasses and urea supplementation. Trop. Anim. Health Prod. 2013, 45, 1777–1781. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.; Wanapat, M.; Nunoi, A. Effect of urea and molasses supplementation on quality of cassava top silage. J. Anim. Feed Sci. 2018, 27, 74–80. [Google Scholar] [CrossRef]
- McDonald, P.; Henderson, N.; Heron, S. The Biochemistry of Silage; Chalcombe Publications: Southampton, UK, 1991; p. 340. [Google Scholar]
- Yuan, X.; Li, J.; Dong, Z.; Shao, T. The reconstitution mechanism of Napier grass microiota during the ensiling of alfalfa and their contributions to fermentation quality of silage. Bioresour. Technol. 2020, 297, 122391. [Google Scholar] [CrossRef] [PubMed]
- Yitbarek, M.B.; Tamir, B. Silage additives. Open J. Appl. Sci. 2014, 4, 17. [Google Scholar]
- Fang, J.; Matsuzaki, M.; Suzuki, H.; Cai, Y.; Horiguchi, K.I.; Takahashi, T. Effects of lactic acid bacteria and urea treatment on fermentation quality, digestibility and ruminal fermentation of roll bale rice straw silage in wethers. Grassl. Sci. 2012, 58, 73–78. [Google Scholar] [CrossRef]
- Muck, R.; Nadeau, E.; McAllister, T.; Contreras-Govea, F.; Santos, M.; Kung, L., Jr. Silage review: Recent advances and future uses of silage additives. J. Dairy Sci. 2018, 101, 3980–4000. [Google Scholar] [CrossRef]
- Kung, L., Jr.; Shaver, R.; Grant, R.; Schmidt, R. Silage review: Interpretation of chemical, microbial, and organoleptic components of silages. J. Dairy Sci. 2018, 101, 4020–4033. [Google Scholar] [CrossRef]
- Ely, L.O. The use of added feedstuffs in silage production. In Fermentation of Silage—A Review; McCullough, M.E., Ed.; National Feed Ingredients Association Publisher: West Des Moines, IA, USA, 1978. [Google Scholar]
- Shirley, J.; Brown, L.; Toman, F.; Stroube, W. Influence of varying amounts of urea on the fermentation pattern and nutritive value of corn silage. J. Dairy Sci. 1972, 55, 805–810. [Google Scholar] [CrossRef]
- Wang, F.; Nishino, N. Ensiling of soybean curd residue and wet brewers grains with or without other feeds as a total mixed ration. J. Dairy Sci. 2008, 91, 2380–2387. [Google Scholar] [CrossRef]
- Woolford, M. The significance of Propionibacterium species and Micrococcus lactilyticus to the ensiling process. J. Appl. Bacteriol. 1975, 39, 301–306. [Google Scholar] [CrossRef]
- Schlegel, H.G. General Microbiology; Cambridge University Press: Cambridge, UK, 1987. [Google Scholar]
- Muck, R.E. Silage microbiology and its control through additives. Rev. Bras. Zootec. 2010, 39, 183–191. [Google Scholar] [CrossRef]
- Kung, L., Jr.; Stokes, M.R.; Lin, C. Silage additives. Silage Sci. Technol. 2003, 42, 305–360. [Google Scholar]
- García, Á. Ammonia-N concentration in alfalfa silage and its effects on dairy cow performance: A meta-analysis. Rev. Colomb. Cienc. Pecu. 2017, 30, 175–184. [Google Scholar]
- Kung, L., Jr. Understanding the biology of silage preservation to maximize quality and protect the environment. In Proceedings of the 2010 California Alfalfa & Forage Symposium and Corn/Cereal Silage Conference, Visalia, CA, USA, 1–2 December 2010; pp. 1–2. [Google Scholar]
- Rooke, J.A.; Hatfield, R.D. Biochemistry of ensiling. Silage Sci. Technol. 2003, 42, 95–139. [Google Scholar]
- Cherdthong, A.; Suntara, C.; Khota, W. Lactobacillus casei TH14 and additives could modulate the quality, gas kinetics and the in vitro digestibility of ensilaged rice straw. J. Anim. Physiol. Anim. Nutr. 2020, 104, 1690–1703. [Google Scholar] [CrossRef]
- So, S.; Cherdthong, A.; Wanapat, M. Improving sugarcane bagasse quality as ruminant feed with Lactobacillus, cellulase, and molasses. J. Anim. Sci. Technol. 2020, 62, 648. [Google Scholar] [CrossRef]
- Ganji Jamehshooran, E.; Jafari Khorshidi, K.; Bayat Kouhsar, J. Chemical composition, aerobic stability and fermentation pattern of tomato pomace and pumpkin waste silage using fibrolytic enzymes and lactic acid bacteria. Int. J. Recycl. Org. Waste Agric. 2022, 11, 47–59. [Google Scholar]
- Pahlow, G.; Rammer, C.; Slottner, D.; Tuori, M. Ensiling of legumes. In Legume silages for animal production—LEGSIL; Wilkins, R.J., Paul, C., Eds.; Landbauforschung Voelkenrode: Brunswick, Germany, 2002; Volume 234, pp. 27–31. [Google Scholar]
- Owen, T. The effects of a combination of a silage inoculant and a chemical preservative on the fermentation and aerobic stability of whole crop cereal and maize silage. In Proceedings of the 13th International Silage Conference, Auchincruive, Scotland, 9–28 September 2002; pp. 196–197. [Google Scholar]
| Item 1 | Cassava Pulp |
|---|---|
| Microbial counts (cfu/g FM) | |
| LAB | 8.8 × 104 |
| Coliform bacteria | ND |
| Aerobic bacteria | 1.91 × 108 |
| Yeasts | 1.98 × 107 |
| Molds | ND |
| Chemical composition (g/kg DM) | |
| DM (g/kg) | 165.50 |
| OM | 973.60 |
| CP | 25.94 |
| EE | 2.53 |
| NDF | 438.87 |
| ADF | 246.79 |
| pH | 3.96 |
| Additive 1 | DM (g/kg) | OM | CP | EE | NDF | ADF |
|---|---|---|---|---|---|---|
| (g/kg DM) | ||||||
| CON | 166.10 d | 917.25 | 22.10 | 4.52 | 394.37 | 222.03 |
| M | 172.19 bc | 919.09 | 22.55 | 4.81 | 359.56 | 207.80 |
| U | 175.19 ab | 914.12 | 138.55 | 3.72 | 346.32 | 222.94 |
| UM | 177.62 a | 915.80 | 129.64 | 3.32 | 335.67 | 220.54 |
| L | 166.69 d | 915.31 | 21.34 | 4.20 | 353.90 | 198.84 |
| LM | 169.62 cd | 919.94 | 23.46 | 4.09 | 334.71 | 216.77 |
| LU | 168.54 cd | 914.90 | 154.69 | 3.69 | 347.00 | 214.96 |
| LUM | 177.23 a | 915.87 | 132.91 | 2.93 | 339.29 | 200.53 |
| SEM | 1.35 | 2.91 | 2.86 | 0.57 | 9.60 | 8.25 |
| p-value Interaction | ||||||
| L × U × M | <0.05 | 0.68 | 0.09 | 0.99 | 0.65 | 0.08 |
| L×U | 0.21 | 0.82 | <0.05 | 0.71 | <0.05 | 0.57 |
| L × M | 0.43 | 0.81 | 0.19 | 0.63 | 0.51 | 0.40 |
| U × M | 0.59 | 0.65 | <0.01 | 0.42 | 0.21 | 0.40 |
| Main effect | ||||||
| L | <0.05 | 0.98 | 0.03 | 0.38 | 0.04 | 0.10 |
| U | <0.01 | 0.21 | <0.01 | <0.05 | <0.05 | 0.57 |
| M | <0.01 | 0.29 | <0.01 | 0.55 | <0.05 | 0.58 |
| Additive 1 | pH | LA | AA | PA | BA | Ammonia-N |
|---|---|---|---|---|---|---|
| (g/kg DM) | ||||||
| CON | 3.47 | 45.29 | 27.41 | 13.57 | 1.56 | 2.23 |
| M | 3.50 | 45.29 | 23.93 | 11.66 | 1.35 | 1.02 |
| U | 3.61 | 50.97 | 26.24 | 11.18 | 1.43 | 51.66 |
| UM | 3.49 | 48.34 | 26.42 | 12.30 | 1.54 | 32.61 |
| L | 3.47 | 61.14 | 24.69 | 11.66 | 1.50 | 1.07 |
| LM | 3.47 | 85.77 | 25.49 | 11.61 | 0.88 | 1.54 |
| LU | 3.59 | 54.53 | 26.77 | 12.54 | 1.52 | 54.62 |
| LUM | 3.49 | 72.40 | 27.66 | 13.52 | 1.66 | 38.38 |
| SEM | 0.01 | 9.65 | 2.86 | 0.57 | 9.60 | 8.25 |
| p-value Interaction | ||||||
| L × U × M | 0.13 | 0.88 | 0.36 | 0.24 | 0.44 | 0.73 |
| L × U | 0.70 | 0.31 | 0.45 | 0.01 | 0.20 | <0.05 |
| L × M | 0.56 | 0.12 | 0.21 | 0.30 | 0.51 | 0.20 |
| U × M | <0.01 | 0.74 | 0.34 | <0.05 | 0.07 | <0.01 |
| Main effect | ||||||
| L | 0.05 | <0.01 | 0.87 | 0.70 | 0.58 | <0.05 |
| U | <0.01 | 0.69 | 0.16 | 0.53 | 0.13 | <0.01 |
| M | <0.01 | 0.17 | 0.68 | 0.93 | 0.31 | <0.01 |
| Additive 1 | Microbial Counts | ||
|---|---|---|---|
| LAB | Aerobic Bacteria | Yeasts | |
| 106 cfu/g FM | 105 cfu/g FM | 104 cfu/g FM | |
| CON | 0.22 d | 9.00 a | 1.00 |
| M | 0.74 d | 3.60 c | ND |
| U | 0.48 d | 1.77 d | 1.33 |
| UM | 0.62 d | 0.82 d | 0.33 |
| L | 2.14 c | 5.40 b | 0.50 |
| LM | 9.10 a | 4.60 cb | 2.50 |
| LU | 1.38 cd | 0.67 d | 0.50 |
| LUM | 3.87 b | 0.29 d | 1.00 |
| SEM | 0.37 | 0.48 | 0.74 |
| p-value Interaction | |||
| L × U × M | <0.01 | <0.05 | 0.65 |
| L × U | <0.01 | 0.55 | 0.22 |
| L × M | <0.01 | <0.01 | 0.08 |
| U × M | <0.01 | <0.01 | 0.67 |
| Main effect | |||
| L | <0.01 | <0.01 | 0.28 |
| U | <0.01 | <0.01 | 0.55 |
| M | <0.01 | <0.01 | 0.96 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pongsub, S.; Suntara, C.; Khota, W.; Boontiam, W.; Cherdthong, A. The Chemical Composition, Fermentation End-Product of Silage, and Aerobic Stability of Cassava Pulp Fermented with Lactobacillus casei TH14 and Additives. Vet. Sci. 2022, 9, 617. https://doi.org/10.3390/vetsci9110617
Pongsub S, Suntara C, Khota W, Boontiam W, Cherdthong A. The Chemical Composition, Fermentation End-Product of Silage, and Aerobic Stability of Cassava Pulp Fermented with Lactobacillus casei TH14 and Additives. Veterinary Sciences. 2022; 9(11):617. https://doi.org/10.3390/vetsci9110617
Chicago/Turabian StylePongsub, Sunisa, Chanon Suntara, Waroon Khota, Waewaree Boontiam, and Anusorn Cherdthong. 2022. "The Chemical Composition, Fermentation End-Product of Silage, and Aerobic Stability of Cassava Pulp Fermented with Lactobacillus casei TH14 and Additives" Veterinary Sciences 9, no. 11: 617. https://doi.org/10.3390/vetsci9110617
APA StylePongsub, S., Suntara, C., Khota, W., Boontiam, W., & Cherdthong, A. (2022). The Chemical Composition, Fermentation End-Product of Silage, and Aerobic Stability of Cassava Pulp Fermented with Lactobacillus casei TH14 and Additives. Veterinary Sciences, 9(11), 617. https://doi.org/10.3390/vetsci9110617








