Effects of Feeding Different Levels of Sprouted Barley on Fermentation Characteristics, Bacterial Quantification, and Rumen Morphology of Growing Lambs
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Welfare and Ethics Clearance
2.2. Diets and Management Practices
2.3. Animals and Management Practices
2.4. Feed Analyses
2.5. Growth Performance and Digestion Trial
2.6. Rumen Fermentation Characteristics
2.7. Rumen Tissue Color Measurements
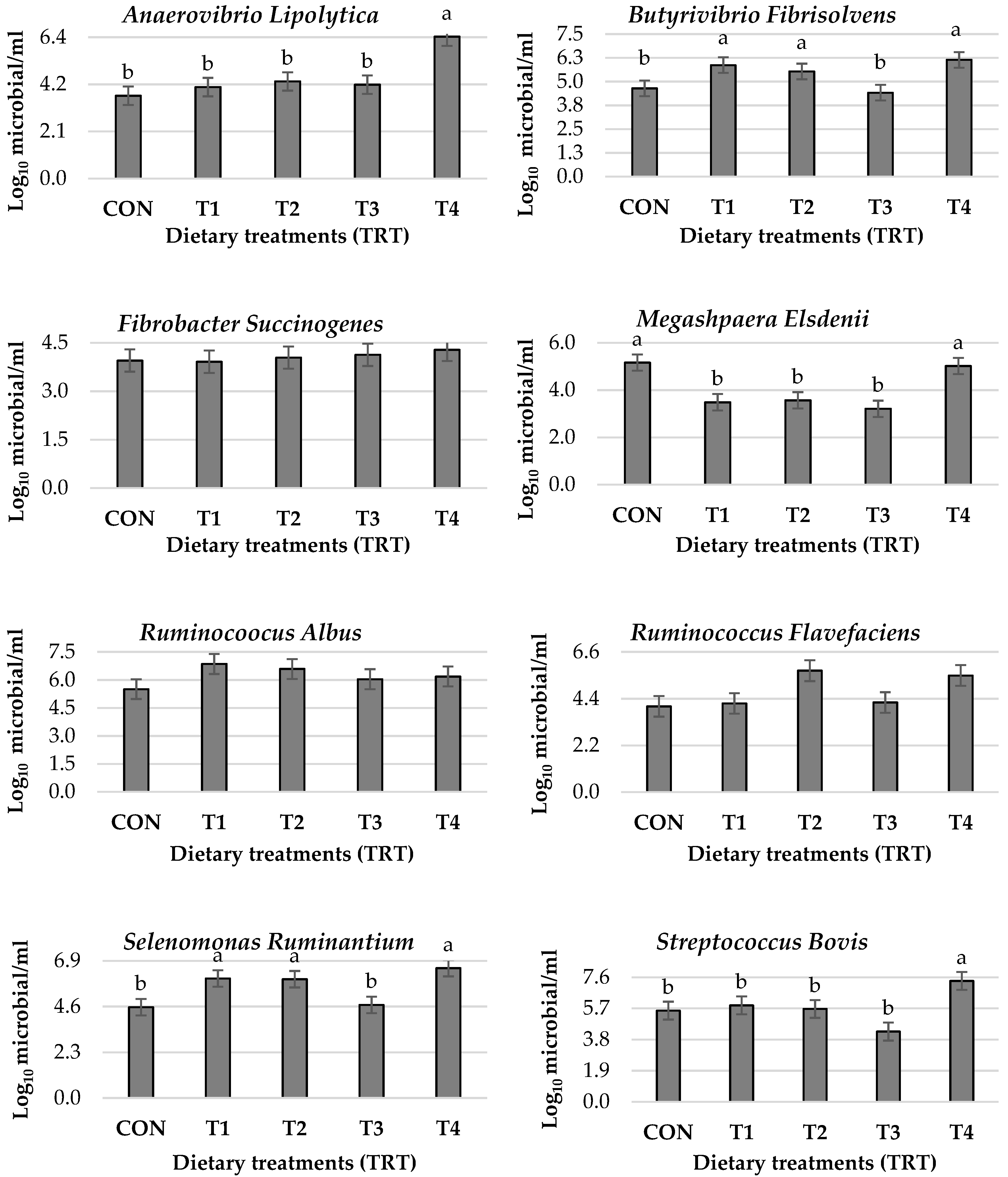
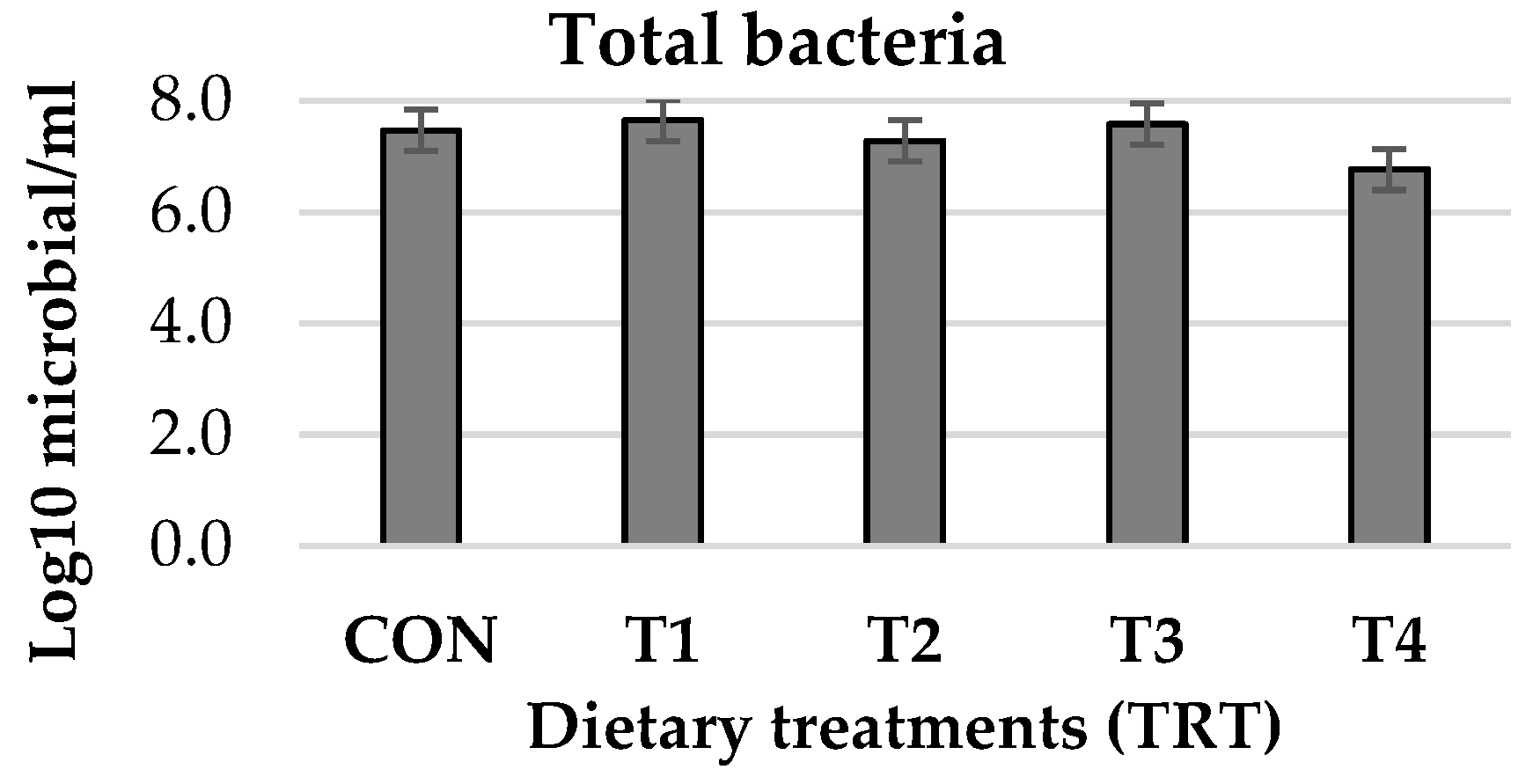
2.8. Ruminal Bacterial Quantification
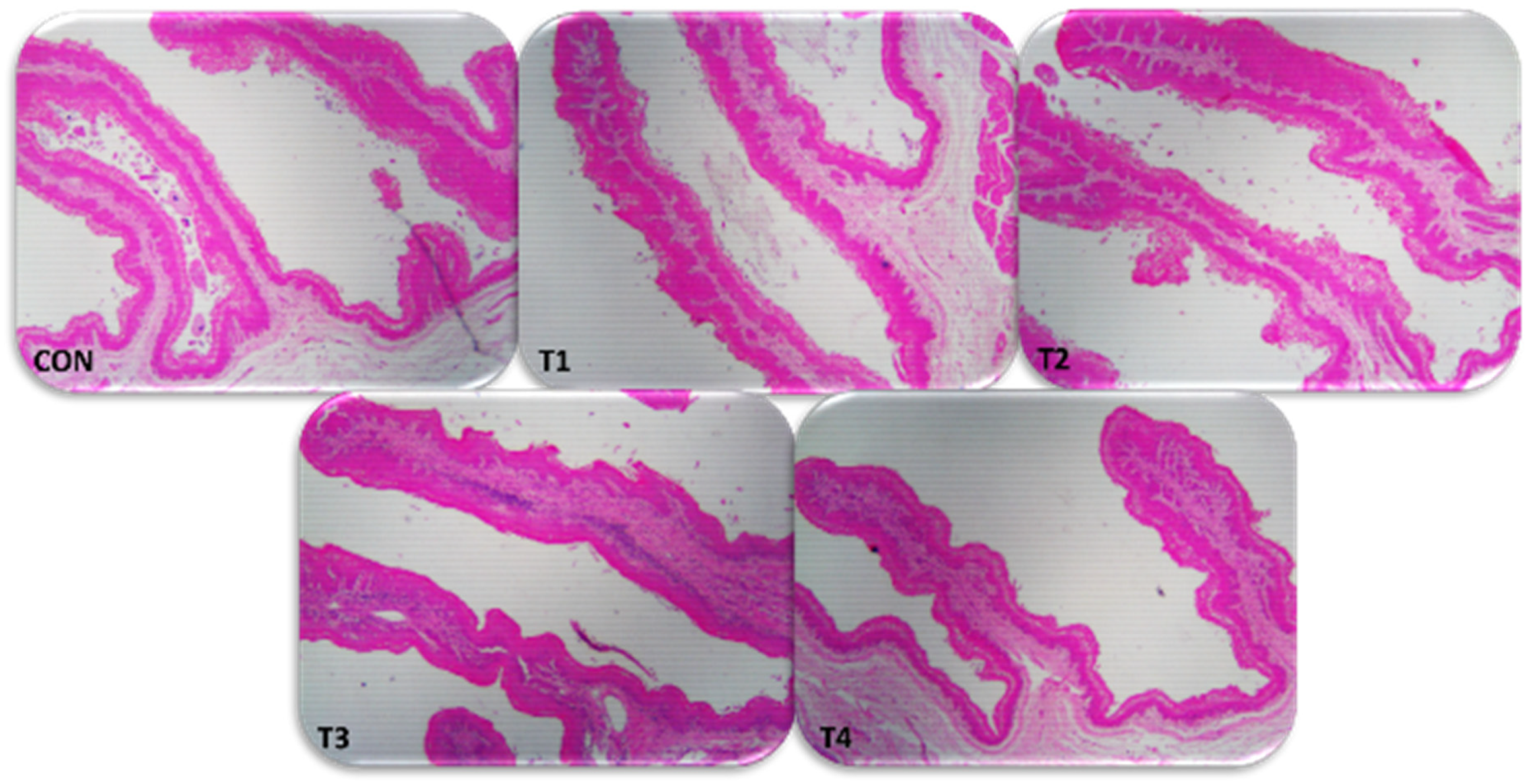
2.9. Rumen Histomorphometric
2.10. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Safwat, M.S.; Sarmiento-Franco, L.; Santos-Ricalde, R.H. Rabbit production using local resources as feedstuffs in the tropics. Trop. Subtrop. Agroecosyst 2014, 17, 161–171. [Google Scholar]
- Prakash, D.S. Effect of Replacement of Concentrate Mixture by Maize Hydroponic Fodder on Performance of Goat. Ph.D. Thesis, Maharashtra Animal and Fishery, Sciences University, Nagpur, India, April 2017. [Google Scholar]
- Al-Baadani, H.H.; Alowaimer, A.N.; Al-Badwi, M.A.; Abdelrahman, M.M.; Soufan, W.H.; Alhidary, I.A. Evaluation of the nutritive value and digestibility of sprouted barley as feed for growing lambs: In Vivo and In Vitro studies. Animals 2022, 12, 1206. [Google Scholar] [CrossRef] [PubMed]
- Hafla, A.N.; Soder, K.J.; Brito, A.F.; Rubano, M.D.; Dell, C.J. Effect of sprouted barley grain supplementation of an herbage-based or haylage-based diet on ruminal fermentation and methane output in continuous culture. J. Dairy Sci. 2014, 97, 7856–7869. [Google Scholar] [CrossRef] [PubMed]
- Tawfeeq, J.A.; Hassan, S.A.; Kadori, S.H.; Shaker, R.M.; Hamz, Z.R. Evaluation of feeding hydroponics barley on digestibility and rumen fermentations in Awassi lambs. Iraqi J. Agric. Sci. 2018, 49, 636–645. [Google Scholar]
- Huws, S.A.; Creevey, C.J.; Oyama, L.B.; Mizrahi, I.; Denman, S.E.; Popova, M.; Muñoz-Tamayo, R.; Forano, E.; Waters, S.M.; Hess, M. Addressing global ruminant agricultural challenges through understanding the rumen microbiome: Past, present and future. Front. Microbiol. 2018, 9, 2161–2193. [Google Scholar] [CrossRef]
- Mamuad, L.L.; Lee, S.S.; Lee, S.S. Recent insight and future techniques to enhance rumen fermentation in dairy goats. Asian Australas. J. Anim. Sci. 2019, 32, 1321–1330. [Google Scholar] [CrossRef]
- Qiu, Q.; Gao, C.; Gao, Z.; Rahman, A.M.; He, Y.; Cao, B.; Su, H. Temporal dynamics in rumen bacterial community composition of finishing steers during an adaptation period of three months. Microorganisms 2019, 7, 410. [Google Scholar] [CrossRef]
- Belanche, A.; Kingston-Smith, A.H.; Griffith, G.W.; Newbold, C.J. A multi-kingdom study reveals the plasticity of the rumen microbiota in response to a shift from non-grazing to grazing diets in sheep. Front. Microbiol. 2019, 10, 122–138. [Google Scholar] [CrossRef]
- Salo, S. Effects of hydroponic fodder feeding on milk yield and composition of dairy cow: Review. J. Nat. Sci. Res. 2019, 9, 1–8. [Google Scholar]
- Raeisi, Z.; Tahmasbi, R.; Dayani, O.; Ayatollahi, M.A.; Tavassolian, I. Digestibility, microbial protein synthesis, rumen and blood parameters in sheep fed diets containing hydroponic barley fodder. J. Livest. Sci. Technol. 2018, 6, 9–17. [Google Scholar]
- Girma, F.; Gebremariam, B. Review on hydroponic feed value to livestock production. J. Sci. Innov. Res. 2018, 7, 106–109. [Google Scholar] [CrossRef]
- Ikram, A.; Saeed, F.; Afzaal, M.; Imran, A.; Niaz, B.; Tufail, T.; Anjum, F.M. Nutritional and end-use perspectives of sprouted grains: A comprehensive review. Food Sci. Nutr. 2021, 9, 4617–4628. [Google Scholar] [CrossRef] [PubMed]
- Al-Saadi, M.J.; Al-Zubiadi, I.A. Effects of substitution barley by 10%, 30% of sprouted barley on rumen characters, digestibility and feed efficiency in diet of Awassi male lambs. Int. J. Sci. Res. 2016, 5, 2228–2233. [Google Scholar]
- Dung, D.D.; Godwin, I.R.; Nolan, J.V. Digestive characteristics, rumen ammonia nitrogen and volatile fatty acids levels in sheep fed commercial pellets supplemented with grimmett barley grain or freeze-dried or fresh barley sprouts. JS Pac. Agric. 2012, 16, 1–10. [Google Scholar]
- Mpanza, T.D.E.; Dhlamini, T.C.; Pierneef, R.E.; Mbatha, K.R. Enteric methane emission, rumen fermentation and microbial profiles of meat-master lambs supplemented with barley fodder sprouts. Fermentation 2022, 8, 434. [Google Scholar] [CrossRef]
- Farghaly, M.M.; Abdullah, M.A.; Youssef, I.M.; Abdel-Rahim, I.R.; Abouelezz, K. Effect of feeding hydroponic barley sprouts to sheep on feed intake, nutrient digestibility, nitrogen retention, rumen fermentation and ruminal enzymes activity. Livest. Sci. 2019, 228, 31–37. [Google Scholar] [CrossRef]
- M.E.W.A. Small Ruminant Vaccine Protocol and Programs; Veterinary Vaccine Centre: Riyadh, Saudi Arabia, 2015. [Google Scholar]
- A.O.A.C. Official Methods of Analysis, 19th ed.; Association of Official Analytical Chemists: Rockville, MD, USA, 2012. [Google Scholar]
- Van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for dietary fiber, neutral detergent fiber and non-starch polysaccharides in relation to animal nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef]
- Mekonnen, E.; Mekuriaw, Y.; Tegegne, F.; Asmare, B. Evaluation of fodder biomass yield of hydroponically-grown barley and oats and the effects on intake, digestibility and weight gain of Washera sheep when fed as a supplement to a basal diet of natural pasture hay in Ethiopia. Trop. Grassl. Forrajes Trop. 2019, 7, 519–526. [Google Scholar] [CrossRef]
- Szulc, P.; Mravčáková, D.; Szumacher-Strabel, M.; Varadyova, Z.; Várady, M.; Čobanová, K.; Cieslak, A. Ruminal fermentation, microbial population and lipid metabolism in gastrointestinal nematode-infected lambs fed a diet supplemented with herbal mixtures. PLoS ONE 2020, 15, e0231516. [Google Scholar] [CrossRef]
- Alhidary, I.; Abdelrahman, M.M.; Alyemni, A.H.; Khan, R.U.; Al-Mubarak, A.H.; Albaadani, H.H. Characteristics of rumen in Naemi lamb: Morphological changes in response to altered feeding regimen. Acta Histochem. 2016, 118, 331–337. [Google Scholar] [CrossRef]
- Li, C.; Zhang, Q.; Wang, G.; Niu, X.; Wang, W.; Li, F.; Zhang, Z. The functional development of the rumen is influenced by weaning and associated with ruminal microbiota in lambs. Anim. Biotechnol. 2022, 33, 612–628. [Google Scholar] [CrossRef] [PubMed]
- Izuddin, W.I.; Loh, T.C.; Samsudin, A.A.; Foo, H.L.; Humam, A.M.; Shazali, N. Effects of postbiotic supplementation on growth performance, ruminal fermentation and microbial profile, blood metabolite and GHR, IGF-1 and MCT-1 gene expression in post-weaning lambs. BMC Vet. Res. 2019, 15, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Alharthi, A.S.; Al-Baadani, H.H.; Al-Badwi, M.A.; Abdelrahman, M.M.; Alhidary, I.A.; Khan, R.U. Effects of sunflower hulls on productive performance, digestibility indices and rumen morphology of growing Awassi lambs fed with total mixed rations. Vet. Sci. 2021, 8, 174. [Google Scholar] [CrossRef] [PubMed]
- Abdelrahman, M.M.; Alhidary, I.; Albaadani, H.H.; Alobre, M.; Khan, R.U.; Aljumaah, R.S. Effect of palm kernel meal and malic acid on rumen characteristics of growing Naemi lambs fed total mixed ration. Animals 2019, 9, 408. [Google Scholar] [CrossRef]
- SAS Institute. SAS Users Guide: Statistics; SAS Institute Inc.: Cary, NC, USA, 2008. [Google Scholar]
- Belote, B.L.; Soares, I.; Tujimoto-Silva, A.; Sanches, A.W.; Kraieski, A.L.; Santin, E. Applying I see inside histological methodology to evaluate gut health in broilers challenged with Eimeria. Vet. Parasitol. 2019, 276, 1–7. [Google Scholar] [CrossRef]
- Morales, M.A.; Juárez, M.; Ávila, E. Effect of substituting hydroponic green barley forage for a commercial feed on performance of growing rabbits. World Rabbit Sci. 2009, 17, 35–38. [Google Scholar] [CrossRef][Green Version]
- Muhammad, S.; Afzal, H.; Mudassar, S. Use of sprouted grains in the diets of poultry and ruminants. Indian Res. J. 2013, 2, 20–27. [Google Scholar]
- Helal, H.G. Productive and reproductive performance of Barki ewes fed on sprouted barley grains on desert by-products during lactating period. Res. J. Anim. Vet. Sci. 2018, 10, 37–48. [Google Scholar]
- Dung, D.D.; Godwin, I.R.; Nolan, J.V. Nutrient content and in sacco digestibility of barley grain and sprouted barley. J. Anim. Vet. Adv. 2010, 9, 2485–2492. [Google Scholar] [CrossRef]
- Den Besten, G.; Van Eunen, K.; Groen, A.K.; Venema, K.; Reijngoud, D.J.; Bakker, B.M. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J. Lipid Res. 2013, 54, 2325–2340. [Google Scholar] [CrossRef]
- Ma, J.; Shah, A.M.; Shoa, Y.; Wang, Z.; Zou, H.; Hu, R.; Peng, Q.; Kang, K.; Wanapat, M. Effects of yeast cell wall on the growth performance, ruminal fermentation and microbial community of weaned calves. Livest. Sci. 2020, 239, 104170–104177. [Google Scholar] [CrossRef]
- Guo, J.; Li, P.; Liu, S.; Miao, B.; Zeng, B.; Jiang, Y.; Li, L.; Wang, L.; Chen, Y.; Zhang, H. Characterization of the rumen microbiota and volatile fatty acid profile of weaned goat kid under shrub-grassland grazing and indoor feeding. Animals 2020, 10, 176. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Fang, L.; Meng, Q.; Li, S.; Chai, S.; Liu, S.; Schonewille, J.T. Assessment of ruminal bacterial and archaeal community structure in yak (Bos grunniens). Front. Microbiol. 2017, 8, 179–188. [Google Scholar] [CrossRef] [PubMed]
- Fazaeli, H.; Golmohammadi, H.A.; Tabatabayee, S.N.; Asghari-Tabrizi, M. Productivity and nutritive value of barley green fodder yield in hydroponic system. World Appl. Sci. J. 2012, 16, 531–539. [Google Scholar]
- Cui, K.; Qi, M.; Wang, S.; Diao, Q.; Zhang, N. Dietary energy and protein levels influenced the growth performance, ruminal morphology and fermentation and microbial diversity of lambs. Sci. Rep. 2019, 9, 1–10. [Google Scholar] [CrossRef]
- Steele, M.A.; Garcia, F.; Lowerison, M.; Gordon, K.; Metcalf, J.A.; Hurtig, M. Three-dimensional imaging of rumen tissue for morphometric analysis using micro-computed tomography. J. Dairy Sci. 2014, 97, 7691–7696. [Google Scholar] [CrossRef]
- Suárez, B.J.; Van Reenen, C.G.; Gerrits, W.J.; Stockhofe, N.; Van Vuuren, A.M.; Dijkstra, J. Effects of supplementing concentrates differing in carbohydrate composition in veal calf diets: II. Rumen development. J. Dairy Sci. 2006, 89, 4376–4386. [Google Scholar] [CrossRef]
- Shen, Z.; Seyfert, H.M.; Löhrke, B.; Schneider, F.; Zitnan, R.; Chudy, A.; Voigt, J. An energy-rich diet causes rumen papillae proliferation associated with more IGF type 1 receptors and increased plasma IGF-1 concentrations in young goats. J. Nutr. 2004, 134, 11–17. [Google Scholar] [CrossRef]
- Álvarez-Rodríguez, J.; Monleón, E.; Sanz, A.; Badiola, J.J.; Joy, M. Rumen fermentation and histology in light lambs as affected by forage supply and lactation length. Res. Vet. Sci. 2012, 92, 247–253. [Google Scholar] [CrossRef]
- Van Ackeren, C.; Steingaß, H.; Hartung, K.; Funk, R.; Drochner, W. Effect of roughage level in a total mixed ration on feed intake, ruminal fermentation patterns and chewing activity of early-weaned calves with ad libitum access to grass hay. Anim. Feed Sci. Technol. 2009, 153, 48–59. [Google Scholar] [CrossRef]
- Wang, Y.H.; Xu, M.; Wang, F.N.; Yu, Z.P.; Yao, J.H.; Zan, L.S.; Yang, F.X. Effect of dietary starch on rumen and small intestine morphology and digesta pH in goats. Livest. Sci. 2009, 122, 48–52. [Google Scholar] [CrossRef]
| Ingredients, % | Dietary Treatments (TRT) | ||||
|---|---|---|---|---|---|
| CON | T1 | T2 | T3 | T4 | |
| Barley grain | 70.0 | 52.5 | 35.0 | 17.5 | 0.0 |
| Alfalfa hay | 30.0 | 22.5 | 15.0 | 7.5 | 0.0 |
| Sprouted barley | 0.0 | 25.0 | 50.0 | 75.0 | 100.0 |
| Total | 100 | 100 | 100 | 100 | 100 |
| Nutrient composition, % | |||||
| Dry matter | 95.5 | 76.6 | 57.8 | 38.9 | 20.1 |
| Crude protein | 15.0 | 14.7 | 14.4 | 14.2 | 13.9 |
| Neutral detergent fiber | 34.2 | 34.9 | 35.5 | 36.2 | 36.9 |
| Acid detergent fiber | 19.8 | 19.1 | 18.3 | 17.5 | 16.8 |
| Lignin | 5.8 | 4.9 | 4.0 | 3.0 | 2.1 |
| Non-fibrous carbohydrates | 43.9 | 43.8 | 43.7 | 43.6 | 43.5 |
| Fat | 1.7 | 1.9 | 2.2 | 2.4 | 2.7 |
| Ash | 5.2 | 4.6 | 4.1 | 3.5 | 3.0 |
| Calcium | 0.6 | 0.5 | 0.4 | 0.3 | 0.2 |
| Phosphorus | 0.2 | 0.2 | 0.3 | 0.3 | 0.4 |
| Magnesium | 0.2 | 0.2 | 0.2 | 0.1 | 0.1 |
| Potassium | 1.5 | 1.2 | 1.0 | 0.7 | 0.5 |
| Sulfur | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 |
| Sodium | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 |
| Zinc, ppm | 33.0 | 42.2 | 51.5 | 60.7 | 70.0 |
| Copper, ppm | 5.0 | 5.2 | 5.5 | 5.7 | 6.0 |
| Net energy, Mcal/kg | 1.85 | 1.87 | 1.88 | 1.89 | 1.91 |
| Target Gene | Forward (F) and Reverse (R) Primer (5′ → 3′) | Product Length | GenBank Number 1 |
|---|---|---|---|
| Anaerovibrio Lipolytica | F: CACCAAGGCGACGATCAGTA R: CTGCCTCCCGTAGGAGTTTG | 86 | AB034191.1 |
| Butyrivibrio Fibrisolvens | F: AGTAACGCGTGGGTAACCTG R: AAATCATGCGATTCCGTGCG | 89 | U41167.1 |
| Fibrobacter Succinogenes | F: GTGCAAGCGTTGTTCGGAAT R: TCTACGCATTCCACCGCTAC | 163 | AB275514.1 |
| Megashpaera Elsdenii | F: CGGCTACATTTCCCCGTACA R: GCGGTCCGTAATGAGGATGT | 85 | AY608424.1 |
| Ruminocoocus Albus | F: AAAGAAGAAAGGCGGAGCGA R: CAGGCTTCGGCTCGATATGT | 143 | CP002407.1 |
| Ruminococcus Flavefaciens | F: CGTTACCGCCCTTTCCTGAT R: AGGACGGCAAGCAATGAGAA | 74 | NC_001758.1 |
| Selenomonas Ruminantium | F: GGTCTGAGAGGATGAACGGC R: CGAGCCGAAACCCTTCTTCA | 137 | M62703.1 |
| Streptococcus Bovis | F: GCCGGTCTGAGAGGATGAAC R: AGACTTTCGTCCATTGCGGA | 99 | NR_113306.1 |
| Total bacteria | F: GTGSTGCAYGGYTGTCGTCA R: ACGTCRTCCMCACCTTCCTC | 130 | NR_2828033 |
| Parameters 1 | Dietary Treatments (TRT) 2 | p-Value 4 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| CON | T1 | T2 | T3 | T4 | SEM 3 | TRT | L | Q | |
| LWG (kg) | 13.18 a | 10.60 a | 10.50 a | 10.35 a | 2.90 b | 1.46 | 0.009 | 0.010 | 0.18 |
| DMI (kg/day) | 1.46 a | 1.31 a | 0.95 b | 0.77 b | 0.23 c | 0.09 | <0.0001 | <0.0001 | 0.11 |
| OMI (kg/day) | 1.38 a | 1.25 a | 0.91 b | 0.74 b | 0.22 c | 0.09 | <0.0001 | <0.0001 | 0.10 |
| Apparent total tract digestibility (%) | |||||||||
| DM | 81.8 b | 81.6 b | 87.3 a | 88.1 a | 90.9 a | 1.69 | 0.004 | 0.010 | 0.88 |
| OM | 82.7 b | 82.5 b | 88.3 a | 88.9 a | 91.5 a | 1.63 | 0.004 | 0.010 | 0.95 |
| Dietary Treatments (TRT) 2 | p-Value 4 | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Parameters 1 | CON | T1 | T2 | T3 | T4 | SEM 3 | TRT | L | Q |
| Day 45 | |||||||||
| pH0 | 7.0 | 7.3 | 7.1 | 7.3 | 6.9 | 0.22 | 0.61 | 0.72 | 0.22 |
| pH3 | 6.4 | 6.4 | 6.8 | 6.5 | 6.2 | 0.15 | 0.11 | 0.48 | 0.02 |
| pH different | 0.7 | 0.8 | 0.4 | 0.7 | 0.8 | 0.14 | 0.48 | 0.78 | 0.56 |
| Day 75 | |||||||||
| pH0 | 6.5 | 6.2 | 6.3 | 6.4 | 6.4 | 0.18 | 0.85 | 0.40 | 0.34 |
| pH3 | 6.2 | 6.1 | 6.1 | 5.9 | 6.3 | 0.18 | 0.57 | 0.81 | 0.24 |
| pH different | 0.4 | 0.2 | 0.4 | 0.5 | 0.2 | 0.12 | 0.40 | 0.56 | 0.57 |
| Rumen Color | |||||||||
| L* | 57.3 | 61.2 | 61.1 | 59.5 | 52.4 | 2.5 | 0.12 | 0.68 | 0.01 |
| a* | 1.5 | 2.0 | 0.8 | 1.5 | 0.4 | 0.4 | 0.15 | 0.56 | 0.42 |
| b* | 28.3 a | 29.2 a | 27.0 a | 23.2 ab | 18.1 b | 2.0 | 0.03 | 0.09 | 0.07 |
| Dietary Treatments (TRT) 2 | p-Value 4 | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Parameters | CON | T1 | T2 | T3 | T4 | SEM 3 | TRT | L | Q |
| Lactic acid (%) | 24.2 a | 11.7 c | 10.1 c | 16.1 b | 3.4 d | 1.3 | <0.0001 | <0.0001 | 0.450 |
| Formic acid (C1; %) | 0.6 c | 1.9 b | 2.5 b | 1.7 b | 6.9 a | 0.3 | <0.0001 | <0.0001 | 0.006 |
| Acetic acid (C2; %) | 29.5 c | 37.2 b | 47.3 a | 37.2 b | 44.8 a | 1.7 | <0.0001 | <0.0001 | 0.038 |
| Propionic acid (C3; %) | 35.8 a | 32.3 a | 23.0 b | 24.6 b | 34.1 a | 1.7 | <0.0001 | 0.001 | <0.0001 |
| Butyric Acid (C4; %) | 9.9 c | 16.7 b | 16.9 b | 20.2 a | 10.7 c | 0.9 | <0.0001 | <0.0001 | <0.0001 |
| C2/C3 ratio | 0.8 c | 1.1 bc | 2.1 a | 1.5 b | 1.4 b | 0.1 | <0.0001 | <0.0001 | 0.005 |
| TVFA (mmol/mL) 1 | 0.184 a | 0.141 b | 0.114 c | 0.141 b | 0.077 d | 0.01 | <0.0001 | <0.0001 | 0.763 |
| Dietary Treatments (TRT) 2 | p-Value 4 | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Parameters 1 | CON | T1 | T2 | T3 | T4 | SEM 3 | TRT | L | Q |
| Papilla height (mm) | 1.64 c | 1.73 bc | 1.90 ab | 1.94 a | 1.34 d | 0.06 | <0.0001 | 0.219 | <0.0001 |
| Papilla width (mm) | 0.28 c | 0.30 bc | 0.33 a | 0.32 ab | 0.28 c | 0.01 | <0.0001 | 0.002 | <0.0001 |
| SA (mm)2 | 1.45 c | 1.66 b | 2.01 a | 1.98 a | 1.18 d | 0.07 | <0.0001 | 0.001 | <0.0001 |
| Density (n/cm2) | 60.5 ab | 66.8 a | 61.6 ab | 55.0 b | 65.3 a | 2.51 | 0.014 | 0.574 | 0.510 |
| TSP (mm2/cm2) | 56.4 b | 70.9 a | 78.9 a | 69.6 a | 49.7 b | 3.91 | <0.0001 | 0.016 | <0.0001 |
| SC (mm) | 0.03 b | 0.03 b | 0.03 b | 0.03 b | 0.04 a | 0.001 | <0.0001 | 0.216 | 0.196 |
| WE (mm) | 0.08 c | 0.09 b | 0.11 a | 0.09 b | 0.10 b | 0.002 | <0.0001 | <0.0001 | <0.0001 |
| LP (mm) | 0.10 c | 0.12 bc | 0.13 b | 0.17 a | 0.11 c | 0.005 | <0.0001 | <0.0001 | <0.0001 |
| SM (mm) | 0.11 e | 0.14 d | 0.20 b | 0.23 a | 0.18 c | 0.006 | <0.0001 | <0.0001 | <0.0001 |
| Papilla Height | TSP | pH Values | ||||
|---|---|---|---|---|---|---|
| Ruminal Bacteria Metabolites | Correlation Coefficient | |||||
| rxy | p Value | rxy | p Value | rxy | p Value | |
| Lactic acid | 0.563 | 0.003 | 0.088 | 0.675 | −0.012 | 0.927 |
| Formic acid | −0.680 | 0.002 | −0.413 | 0.039 | 0.246 | 0.235 |
| Acetic acid | −0.179 | 0.391 | 0.242 | 0.242 | 0.104 | 0.618 |
| Propionic acid | −0.487 | 0.013 | −0.359 | 0.077 | 0.040 | 0.846 |
| Butyric Acid | 0.388 | 0.055 | 0.176 | 0.399 | −0.323 | 0.114 |
| TVFA | 0.589 | 0.001 | 0.343 | 0.092 | −0.184 | 0.378 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alharthi, A.S.; Al-Baadani, H.H.; Abdelrahman, M.M.; Alhidary, I.A. Effects of Feeding Different Levels of Sprouted Barley on Fermentation Characteristics, Bacterial Quantification, and Rumen Morphology of Growing Lambs. Vet. Sci. 2023, 10, 15. https://doi.org/10.3390/vetsci10010015
Alharthi AS, Al-Baadani HH, Abdelrahman MM, Alhidary IA. Effects of Feeding Different Levels of Sprouted Barley on Fermentation Characteristics, Bacterial Quantification, and Rumen Morphology of Growing Lambs. Veterinary Sciences. 2023; 10(1):15. https://doi.org/10.3390/vetsci10010015
Chicago/Turabian StyleAlharthi, Abdulrahman S., Hani H. Al-Baadani, Mutassim M. Abdelrahman, and Ibrahim A. Alhidary. 2023. "Effects of Feeding Different Levels of Sprouted Barley on Fermentation Characteristics, Bacterial Quantification, and Rumen Morphology of Growing Lambs" Veterinary Sciences 10, no. 1: 15. https://doi.org/10.3390/vetsci10010015
APA StyleAlharthi, A. S., Al-Baadani, H. H., Abdelrahman, M. M., & Alhidary, I. A. (2023). Effects of Feeding Different Levels of Sprouted Barley on Fermentation Characteristics, Bacterial Quantification, and Rumen Morphology of Growing Lambs. Veterinary Sciences, 10(1), 15. https://doi.org/10.3390/vetsci10010015







