Nutrigenomic Effect of Saturated and Unsaturated Long Chain Fatty Acids on Lipid-Related Genes in Goat Mammary Epithelial Cells: What Is the Role of PPARγ?
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture and Treatments
2.2. RNA Extraction, RNA Quality, Primer Design, and qPCR
2.3. Data Transformation, Relative mRNA Abundance, and Statistical Analysis
3. Results
3.1. Relative Abundance of Measured Transcripts
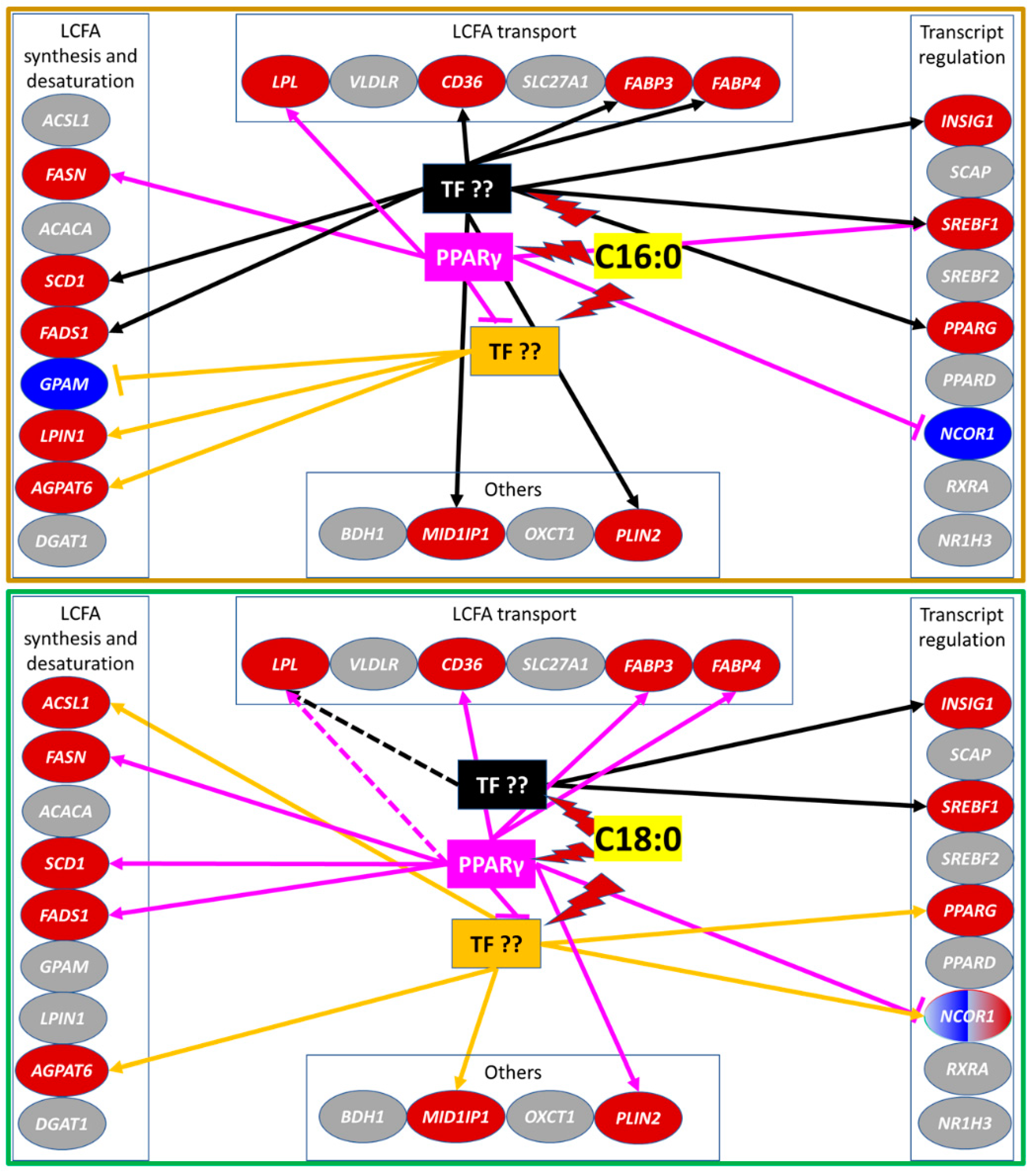
3.2. Genes Affected by Synthetic Agonist and Antagonist of PPARγ
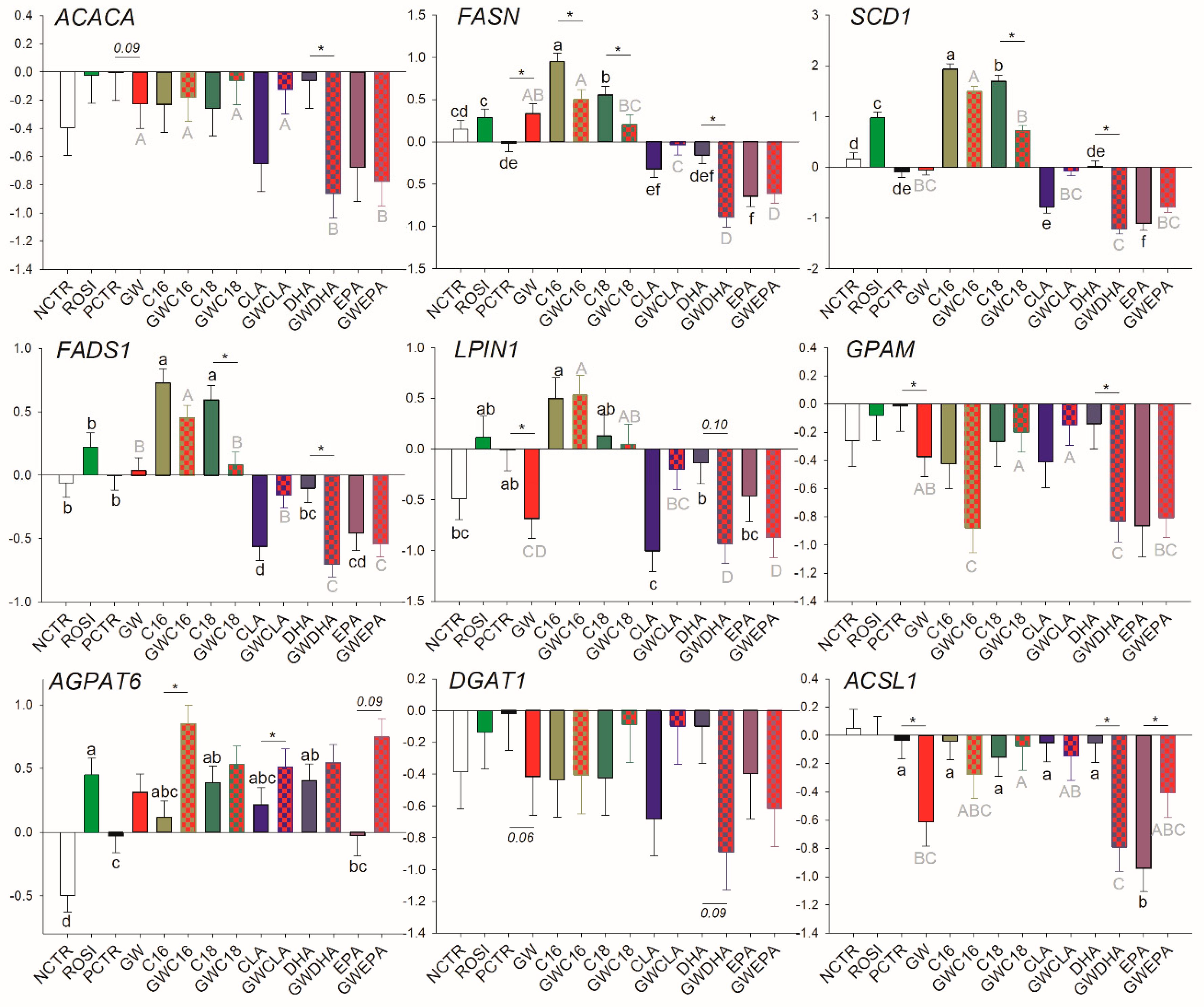
3.2.1. LCFA and Triacylglycerol Synthesis
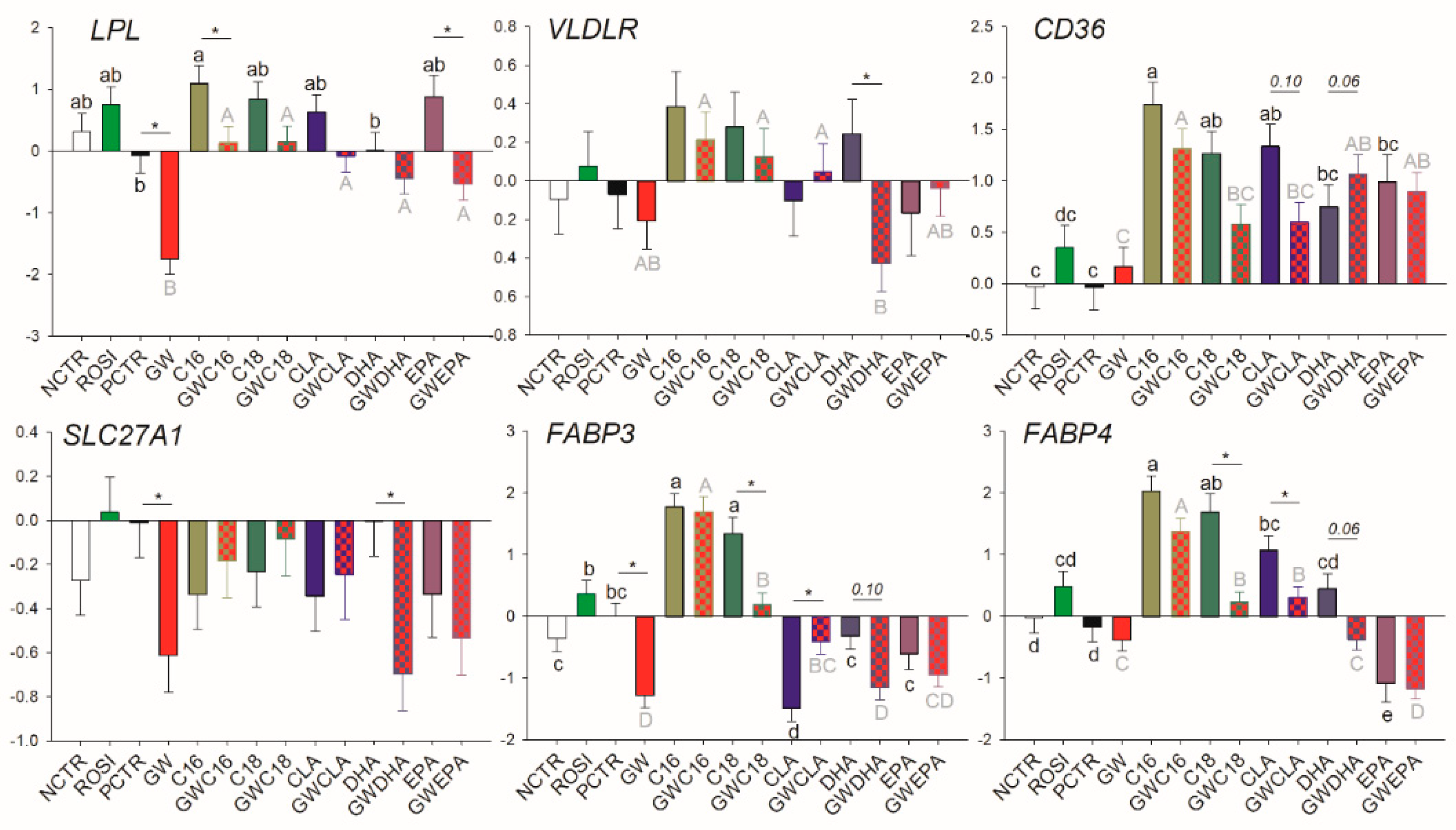
3.2.2. LCFA Transport
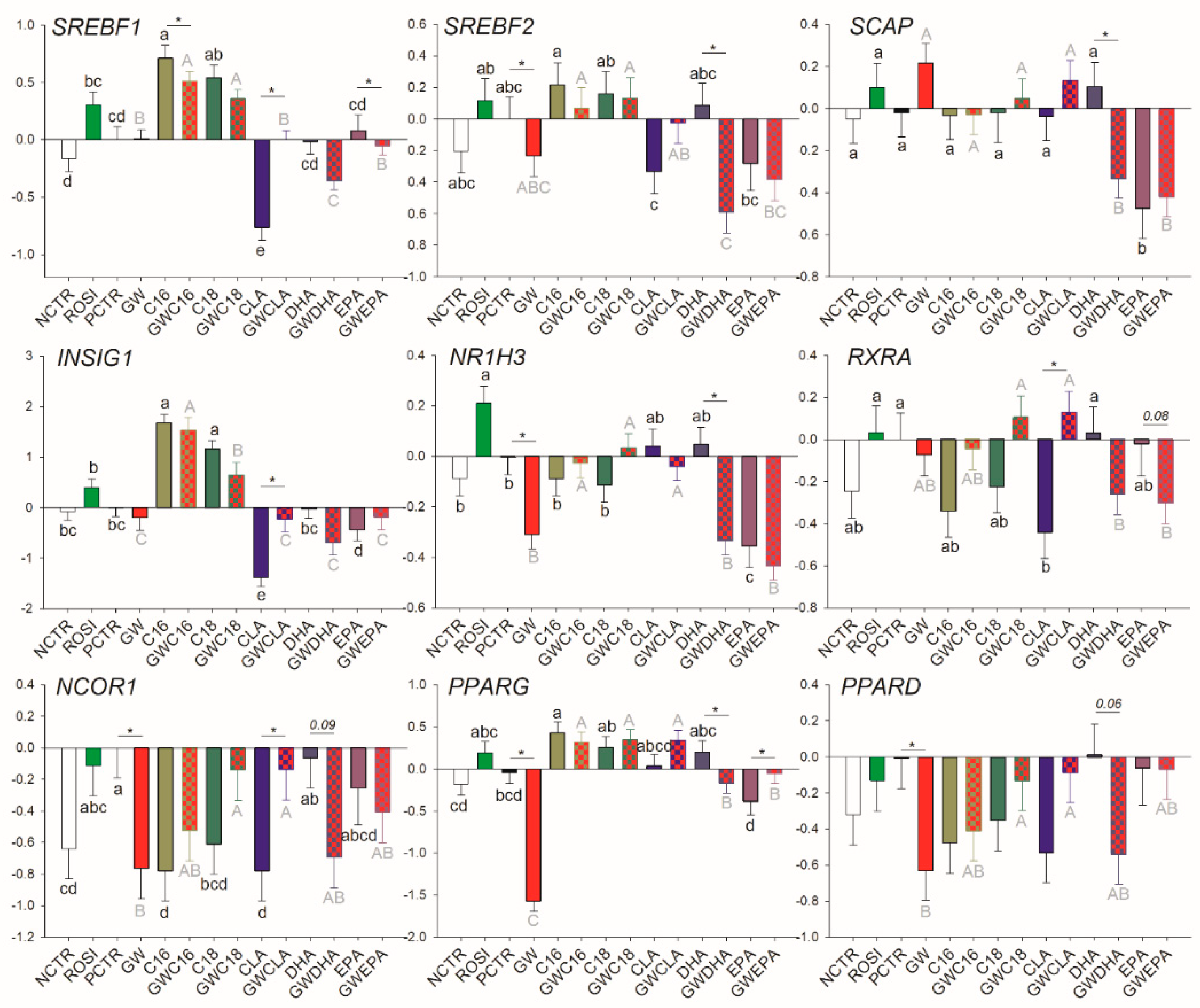
3.2.3. Transcription Regulation and other Functions
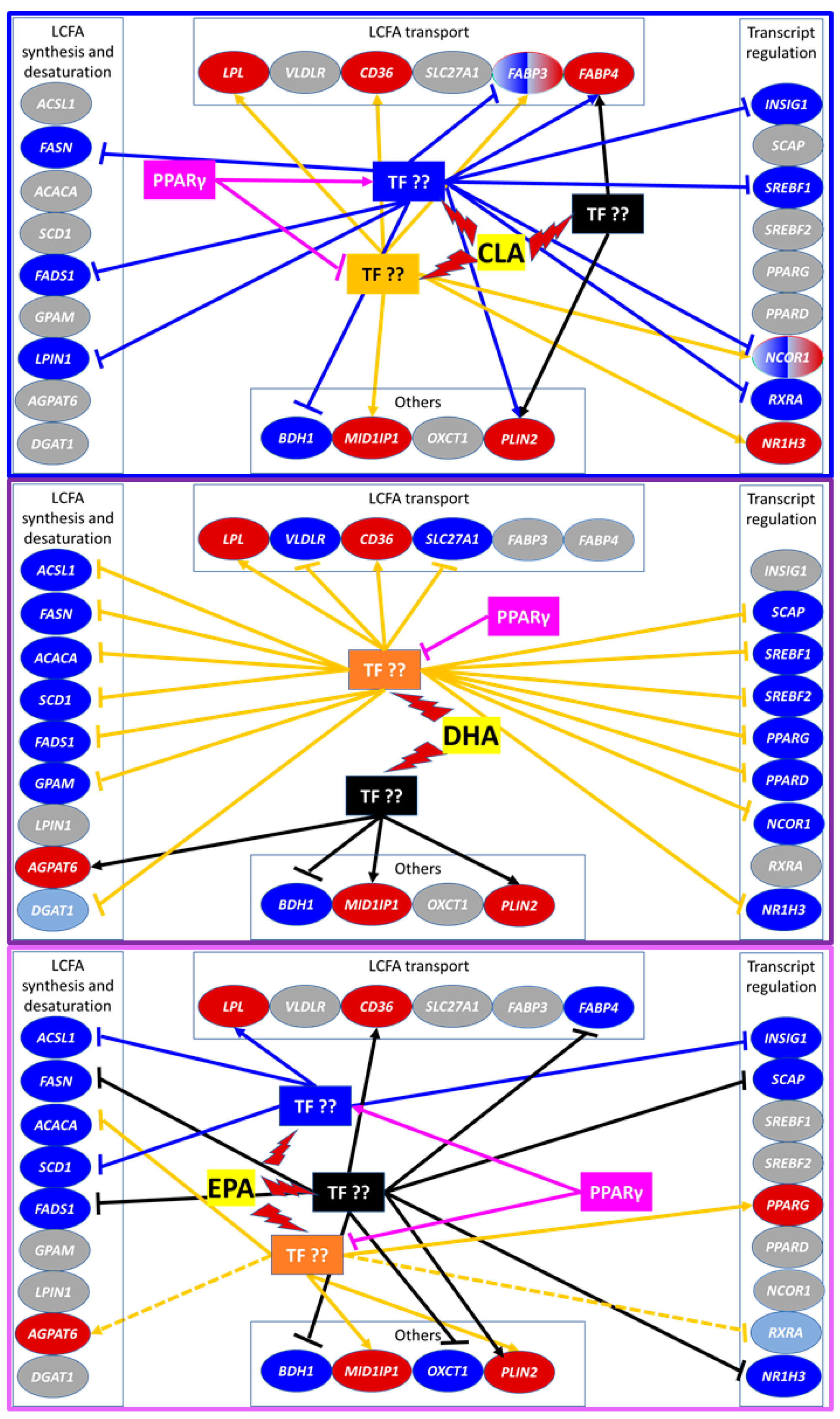
3.3. mRNA Abundance and Overall Effects of LCFA
3.3.1. Fatty Acid Synthesis and Desaturation
3.3.2. Fatty Acid Transport
3.3.3. Transcriptional Regulation of Lipogenesis
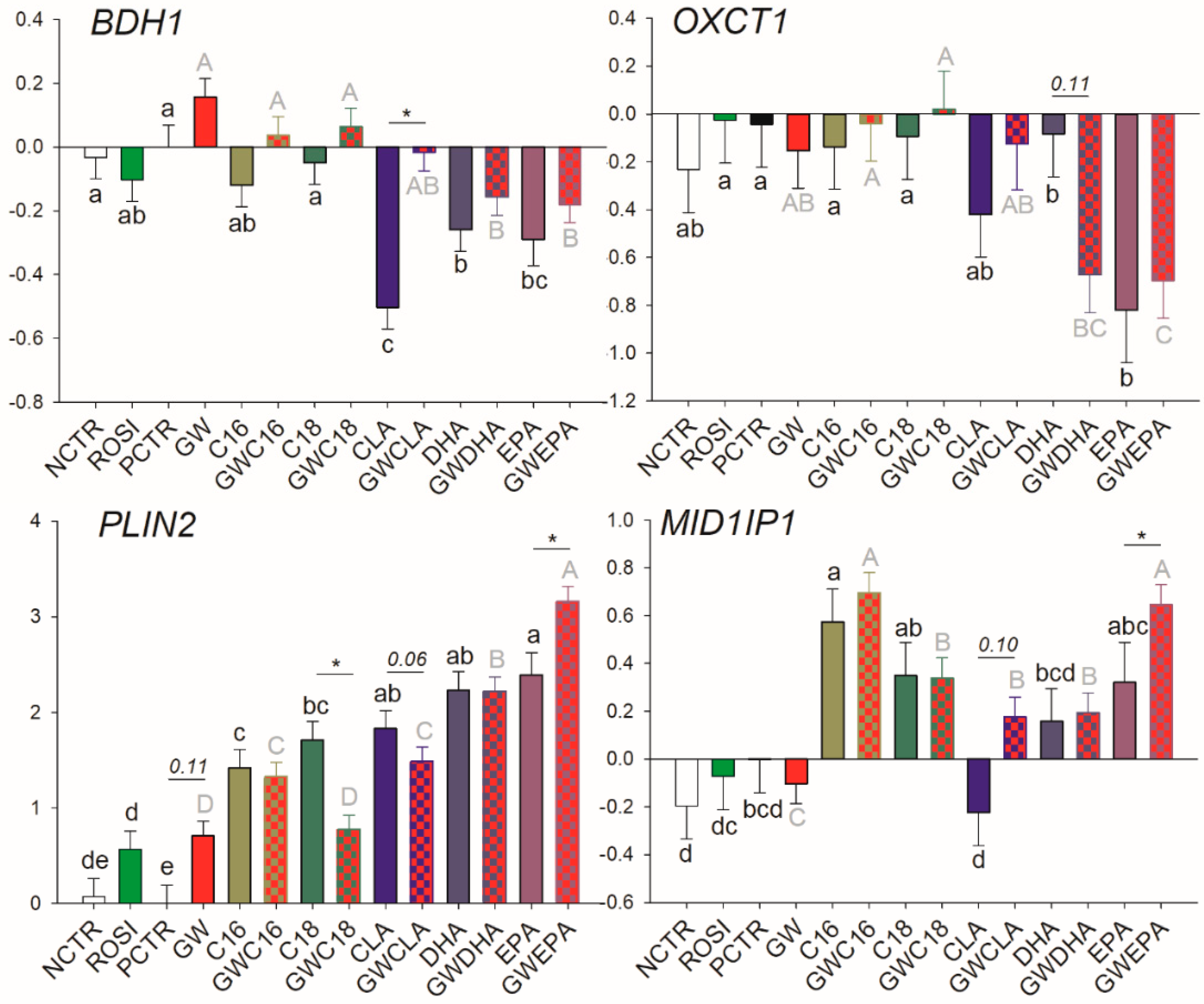
3.3.4. Lipid Droplet Formation and Ketone Body Utilization
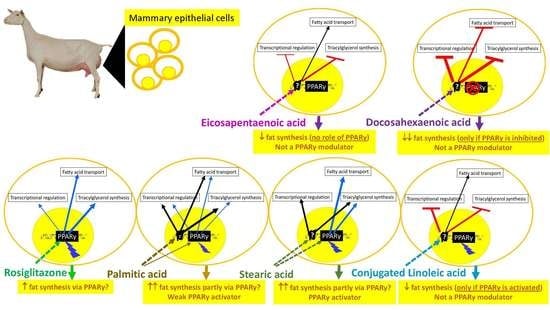
3.4. Transcriptomic Effect of LCFA via PPARγ
3.5. Comparison with MACT Cells
3.5.1. Proportion of mRNA Abundance of Lipogenic Genes between MACT and GMEC
3.5.2. C18:0 Affects Transcription of More Lipogenic Genes in GMEC than MACT
4. Discussion
4.1. GMEC Have a Weak Response to Rosiglitazone but Respond to PPARγ Antagonist
4.2. C16:0 Elicits a Strong Transcriptome Effect but C18:0 Is a More Specific PPARγ Agonist in GMEC
4.3. Basal Activation of PPAR Is Essential for Nutrigenomic Activity of CLA and DHA
4.4. Response of Mammary Cells from Goat vs. Bovine to LCFA: Similar but Different
4.5. Limitations of the Study
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Bauman, D.E.; Griinari, J.M. Regulation and nutritional manipulation of milk fat: Low-fat milk syndrome. Adv. Exp. Med. Biol. 2000, 480, 209–216. [Google Scholar] [CrossRef]
- Bauman, D.E.; Griinari, J.M. Nutritional regulation of milk fat synthesis. Annu. Rev. Nutr. 2003, 23, 203–227. [Google Scholar] [CrossRef] [PubMed]
- Clegg, R.A.; Barber, M.C.; Pooley, L.; Ernens, I.; Larondelle, Y.; Travers, M.T. Milk fat synthesis and secretion: Molecular and cellular aspects. Livest. Prod. Sci. 2001, 70, 3–14. [Google Scholar] [CrossRef]
- Bionaz, M.; Loor, J.J. Gene networks driving bovine milk fat synthesis during the lactation cycle. BMC Genomics 2008, 9, 366. [Google Scholar] [CrossRef] [PubMed]
- Osorio, J.S.; Lohakare, J.; Bionaz, M. Biosynthesis of milk fat, protein, and lactose: Roles of transcriptional and posttranscriptional regulation. Physiol. Genomics 2016, 48, 231–256. [Google Scholar] [CrossRef] [PubMed]
- Dubois, V.; Eeckhoute, J.; Lefebvre, P.; Staels, B. Distinct but complementary contributions of PPAR isotypes to energy homeostasis. J. Clin. Investig. 2017, 127, 1202–1214. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, M.T.; Yudell, B.E.; Loor, J.J. Regulation of energy metabolism by long-chain fatty acids. Prog. Lipid Res. 2014, 53, 124–144. [Google Scholar] [CrossRef] [PubMed]
- Kadegowda, A.K.; Bionaz, M.; Piperova, L.S.; Erdman, R.A.; Loor, J.J. Peroxisome proliferator-activated receptor-gamma activation and long-chain fatty acids alter lipogenic gene networks in bovine mammary epithelial cells to various extents. J. Dairy Sci. 2009, 92, 4276–4289. [Google Scholar] [CrossRef]
- Shi, H.B.; Zhao, W.S.; Luo, J.; Yao, D.W.; Sun, Y.T.; Li, J.; Shi, H.P.; Loor, J.J. Peroxisome proliferator-activated receptor gamma1 and gamma2 isoforms alter lipogenic gene networks in goat mammary epithelial cells to different extents. J. Dairy Sci. 2014, 97, 5437–5447. [Google Scholar] [CrossRef]
- Bionaz, M.; Osorio, J.; Loor, J.J. TRIENNIAL LACTATION SYMPOSIUM: Nutrigenomics in dairy cows: Nutrients, transcription factors, and techniques. J. Anim. Sci. 2015, 93, 5531–5553. [Google Scholar] [CrossRef]
- Pegorier, J.P.; Le May, C.; Girard, J. Control of gene expression by fatty acids. J. Nutr. 2004, 134, 2444s–2449s. [Google Scholar] [CrossRef] [PubMed]
- Bionaz, M.; Chen, S.; Khan, M.J.; Loor, J.J. Functional role of PPARs in ruminants: Potential targets for fine-tuning metabolism during growth and lactation. PPAR Res. 2013, 2013, 684159. [Google Scholar] [CrossRef] [PubMed]
- Varga, T.; Czimmerer, Z.; Nagy, L. PPARs are a unique set of fatty acid regulated transcription factors controlling both lipid metabolism and inflammation. Biochim. Biophys. Acta 2011, 1812, 1007–1022. [Google Scholar] [CrossRef] [PubMed]
- Bauman, D.E.; Mather, I.H.; Wall, R.J.; Lock, A.L. Major advances associated with the biosynthesis of milk. J. Dairy Sci. 2006, 89, 1235–1243. [Google Scholar] [CrossRef]
- Bionaz, M.; Loor, J.J. Identification of reference genes for quantitative real-time PCR in the bovine mammary gland during the lactation cycle. Physiol. Genomics 2007, 29, 312–319. [Google Scholar] [CrossRef] [PubMed]
- Fleige, S.; Pfaffl, M.W. RNA integrity and the effect on the real-time qRT-PCR performance. Mol. Asp. Med. 2006, 27, 126–139. [Google Scholar] [CrossRef] [PubMed]
- Vandesompele, J.; De Preter, K.; Pattyn, F.; Poppe, B.; Van Roy, N.; De Paepe, A.; Speleman, F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002, 3, RESEARCH0034. [Google Scholar] [CrossRef]
- Huang, X.Q.; Miller, W. A time-efficient, linear-space local similarity algorithm. Adv. Appl. Math. 1991, 12, 337–357. [Google Scholar] [CrossRef]
- Lehmann, J.M.; Moore, L.B.; Smitholiver, T.A.; Wilkison, W.O.; Willson, T.M.; Kliewer, S.A. An antidiabetic thiazolidinedione is a high-affinity ligand for peroxisome proliferator-activated receptor gamma (Ppar-Gamma). J. Biol. Chem. 1995, 270, 12953–12956. [Google Scholar] [CrossRef]
- Chen, Z.; Luo, J.; Ma, L.; Wang, H.; Cao, W.; Xu, H.; Zhu, J.; Sun, Y.; Li, J.; Yao, D.; et al. MiR130b-regulation of PPARgamma coactivator-1alpha suppresses fat metabolism in goat mammary epithelial cells. PLoS ONE 2015, 10, e0142809. [Google Scholar] [CrossRef]
- Liu, L.; Lin, Y.; Liu, L.; Wang, L.; Bian, Y.; Gao, X.; Li, Q. Regulation of peroxisome proliferator-activated receptor gamma on milk fat synthesis in dairy cow mammary epithelial cells. In Vitro Cell Dev. Biol. Anim. 2016, 52, 1044–1059. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.B.; Zhao, W.S.; Zhang, C.H.; Shahzad, K.; Luo, J.; Loor, J.J. Transcriptome-wide analysis reveals the role of PPAR gamma controlling the lipid metabolism in goat mammary epithelial cells. PPAR Res. 2016, 2016. [Google Scholar] [CrossRef] [PubMed]
- Tang, K.Q.; Wang, Y.N.; Zan, L.S.; Yang, W.C. miR-27a controls triacylglycerol synthesis in bovine mammary epithelial cells by targeting peroxisome proliferator-activated receptor gamma. J. Dairy Sci. 2017, 100, 4102–4112. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.C.; Guo, W.L.; Zan, L.S.; Wang, Y.N.; Tang, K.Q. Bta-miR-130a regulates the biosynthesis of bovine milk fat by targeting peroxisome proliferator-activated receptor gamma. J. Anim. Sci. 2017, 95, 2898–2906. [Google Scholar] [CrossRef] [PubMed]
- Kurtz, A.; Vogel, F.; Funa, K.; Heldin, C.H.; Grosse, R. Developmental regulation of mammary-derived growth inhibitor expression in bovine mammary tissue. J. Cell Biol. 1990, 110, 1779–1789. [Google Scholar] [CrossRef]
- Bionaz, M.; Loor, J.J. ACSL1, AGPAT6, FABP3, LPIN1, and SLC27A6 are the most abundant isoforms in bovine mammary tissue and their expression is affected by stage of lactation. J. Nutr. 2008, 138, 1019–1024. [Google Scholar] [CrossRef]
- Leesnitzer, L.M.; Parks, D.J.; Bledsoe, R.K.; Cobb, J.E.; Collins, J.L.; Consler, T.G.; Davis, R.G.; Hull-Ryde, E.A.; Lenhard, J.M.; Patel, L.; et al. Functional consequences of cysteine modification in the ligand binding sites of peroxisome proliferator activated receptors by GW9662. Biochemistry 2002, 41, 6640–6650. [Google Scholar] [CrossRef]
- Hansen, H.O.; Knudsen, J. Effect of exogenous long-chain fatty acids on individual fatty acid synthesis by dispersed ruminant mammary gland cells. J. Dairy Sci. 1987, 70, 1350–1354. [Google Scholar] [CrossRef]
- Piantoni, P.; Lock, A.L.; Allen, M.S. Milk production responses to dietary stearic acid vary by production level in dairy cattle. J. Dairy Sci. 2015, 98, 1938–1949. [Google Scholar] [CrossRef]
- Oswal, D.P.; Balanarasimha, M.; Loyer, J.K.; Bedi, S.; Soman, F.L.; Rider, S.D.; Hostetler, H.A. Divergence between human and murine peroxisome proliferator-activated receptor alpha ligand specificities. J. Lipid Res. 2013, 54, 2354–2365. [Google Scholar] [CrossRef]
- Khan, S.A.; Heuvel, J.P.V. Reviews: Current topics—Role of nuclear receptors in the regulation of gene expression by dietary fatty acids (Review). J. Nutr. Biochem. 2003, 14, 554–567. [Google Scholar] [CrossRef]
- Bauman, D.E.; Harvatine, K.J.; Lock, A.L. Nutrigenomics, rumen-derived bioactive fatty acids, and the regulation of milk fat synthesis. Annu. Rev. Nutr. 2011, 31, 299–319. [Google Scholar] [CrossRef] [PubMed]
- Ricote, M.; Glass, C.K. PPARs and molecular mechanisms of transrepression. Bba Mol. Cell Biol. Lipids 2007, 1771, 926–935. [Google Scholar] [CrossRef] [PubMed]
- Jump, D.B.; Tripathy, S.; Depner, C.M. Fatty acid-regulated transcription factors in the liver. Annu. Rev. Nutr. 2013, 33, 249–269. [Google Scholar] [CrossRef] [PubMed]
- Dentin, R.; Benhamed, F.; Pegorier, J.P.; Foufelle, F.; Viollet, B.; Vaulont, S.; Girard, J.; Postic, C. Polyunsaturated fatty acids suppress glycolytic and lipogenic genes through the inhibition of ChREBP nuclear protein translocation. J. Clin. Investig. 2005, 115, 2843–2854. [Google Scholar] [CrossRef]
- Iizuka, K.; Wu, W.; Horikawa, Y.; Saito, M.; Takeda, J. Feedback looping between ChREBP and PPAR alpha in the regulation of lipid metabolism in brown adipose tissues. Endocr. J. 2013, 60, 1145–1153. [Google Scholar] [CrossRef] [PubMed]
- Harvatine, K.J.; Boisclair, Y.R.; Bauman, D.E. Liver x receptors stimulate lipogenesis in bovine mammary epithelial cell culture but do not appear to be involved in diet-induced milk fat depression in cows. Physiol. Rep. 2014, 2, e00266. [Google Scholar] [CrossRef]
- Witte, N.; Muenzner, M.; Rietscher, J.; Knauer, M.; Heidenreich, S.; Nuotio-Antar, A.M.; Graef, F.A.; Fedders, R.; Tolkachov, A.; Goehring, I.; et al. The glucose sensor ChREBP links de novo lipogenesis to PPAR gamma activity and adipocyte differentiation. Endocrinology 2015, 156, 4008–4019. [Google Scholar] [CrossRef]
- Scirpo, R.; Fiorotto, R.; Villani, A.; Amenduni, M.; Spirli, C.; Strazzabosco, M. Stimulation of nuclear receptor peroxisome proliferator-activated receptor-gamma limits NF-kappaB-dependent inflammation in mouse cystic fibrosis biliary epithelium. Hepatology 2015, 62, 1551–1562. [Google Scholar] [CrossRef]
- He, X.X.; Liu, W.J.; Shi, M.Y.; Yang, Z.T.; Zhang, X.C.; Gong, P.T. Docosahexaenoic acid attenuates LPS-stimulated inflammatory response by regulating the PPAR gamma/NF-kappa B pathways in primary bovine mammary epithelial cells. Res. Vet. Sci. 2017, 112, 7–12. [Google Scholar] [CrossRef]
- Marion-Letellier, R.; Savoye, G.; Ghosh, S. Polyunsaturated fatty acids and inflammation. In Iubmb Life; John Wiley & Sons: Hoboken, NJ, USA, 2015; pp. 659–667. [Google Scholar] [CrossRef]
- Sessler, A.M.; Ntambi, J.M. Polyunsaturated fatty acid regulation of gene expression. J. Nutr. 1998, 128, 923–926. [Google Scholar] [CrossRef] [PubMed]
- Loor, J.J.; Doreau, M.; Chardigny, J.M.; Ollier, A.; Sebedio, J.L.; Chilliard, Y. Effects of ruminal or duodenal supply of fish oil on milk fat secretion and profiles of trans-fatty acids and conjugated linoleic acid isomers in dairy cows fed maize silage. Anim. Feed Sci. Tech. 2005, 119, 227–246. [Google Scholar] [CrossRef]
- Magee, P.; Pearson, S.; Whittingham-Dowd, J.; Allen, J. PPAR gamma as a molecular target of EPA anti-inflammatory activity during TNF-alpha-impaired skeletal muscle cell differentiation. J. Nutr. Biochem. 2012, 23, 1440–1448. [Google Scholar] [CrossRef] [PubMed]
- Grygiel-Gorniak, B. Peroxisome proliferator-activated receptors and their ligands: Nutritional and clinical implications—A review. Nutr. J. 2014, 13. [Google Scholar] [CrossRef] [PubMed]
- Toral, P.G.; Chilliard, Y.; Rouel, J.; Leskinen, H.; Shingfield, K.J.; Bernard, L. Comparison of the nutritional regulation of milk fat secretion and composition in cows and goats. J. Dairy Sci. 2015, 98, 7277–7297. [Google Scholar] [CrossRef]
| Transcript | PPARγ * | ||
|---|---|---|---|
| Activator | Inhibitor | Target Gene | |
| LCFA Synthesis and Desaturation and TAG Synthesis | |||
| ACACA | No | ↓ (p = 0.09) | Maybe |
| ACSL1 | No | ↓ | Likely |
| AGPAT6 | ↑ | No | Likely not # |
| GPAM | No | ↓ | Likely |
| DGAT1 | No | ↓ (p = 0.06) | Maybe |
| FADS1 | No | No | No |
| FASN | ↑ | ↑ | ?? |
| LPIN1 | No | ↓ | Likely |
| SCD1 | ↑ | No | Yes |
| LCFA Transport | |||
| CD36 | No | No | No |
| FABP3 | No | ↓ | Likely |
| FABP4 | No | No | No |
| LPL | No | ↓ | Likely |
| SLC27A1 | No | ↓ | Likely |
| VLDLR | No | No | No |
| Transcription Regulation | |||
| INSIG1 | No | No | No |
| NCOR1 | No | ↓ | Likely |
| NR1H3 | ↑ | ↓ | Yes |
| PPARD | No | ↓ | Likely |
| PPARG | No | ↓ | Likely |
| RXRA | No | No | No |
| SCAP | No | No | No |
| SREBF1 | No | No | No |
| SREBF2 | No | ↓ | Likely |
| Others | |||
| BDH1 | No | No | No |
| MID1lP1 | No | No | No |
| OXCT1 | No | No | No |
| PLIN2 | ↑ | ↑ (p = 0.11) | Maybe |
| Transcript | Effect of LCFA (without/with GW9662) * | ||||
|---|---|---|---|---|---|
| C16:0 | C18:0 | CLA | DHA | EPA | |
| LCFA Synthesis and Desaturation and TAG Synthesis | |||||
| ACACA | -/- | -/- | -/- | -/↓ | -/↓ |
| ACSL1 | -/- | -/↑ | -/- | -/↓ | ↓/- |
| AGPAT6 | -/↑ | ↑/↑ | -/↑ | ↑/↑ | -/↑ |
| DGAT1 | -/- | -/- | -/- | -/↓# | -/- |
| FADS1 | ↑/↑ | ↑/- | ↓/- | -/↓ | ↓/↓ |
| FASN | ↑/- | ↑/- | -/- | -/↓ | ↓/↓ |
| GPAM | -/↓ | -/- | -/- | -/↓ | ↓/↓ |
| LPIN1 | -/↑ | -/- | ↓/- | -/- | -/- |
| SCD1 | ↑/↑ | ↑/- | -/- | -/↓$ | ↓/- |
| LCFA Transport | |||||
| CD36 | ↑/↑ | ↑/- | ↑/- | -/↑ | -/↑ |
| FABP3 | ↑/↑ | ↑/- | ↓/↑ | -/- | -/- |
| FABP4 | ↑/↑ | ↑/- | ↑/↑ | -/- | ↓/- |
| LPL | ↑↑/↑ | -/↑ | -/↑ | -/↑ | -/↑ |
| SLC27A1 | -/- | -/- | -/- | -/↓$ | -/- |
| VLDLR | -/- | -/- | -/- | -/↓$ | -/- |
| Transcription Regulation | |||||
| INSIG1 | ↑/↑ | ↑/↑ | ↓/- | -/- | ↓/- |
| NCOR1 | ↓/- | ↓/↑ | ↓/↑ | -/↓$ | -/- |
| NR1H3 | -/- | -/- | -/- | -/↓ | ↓/↓ |
| PPARD | -/- | -/- | -/- | -/↓ | -/- |
| PPARG | ↑/↑ | -/↑ | -/- | -/↓ | -/↑ |
| RXRA | -/- | -/- | ↓/- | -/- | -/↓# |
| SCAP | -/- | -/- | -/- | -/↓ | ↓/↓ |
| SREBF1 | ↑↑/↑ | ↑/↑ | ↓/- | -/↓ | -/- |
| SREBF2 | -/- | -/- | -/- | -/↓$ | -/- |
| Others | |||||
| BDH1 | -/- | -/- | ↓/- | ↓/↓ | ↓/↓ |
| MID1lP1 | ↑/↑ | -/↑ | -/↑ | -/↑ | -/↑ |
| OXCT1 | -/- | -/- | -/- | -/- | ↓/↓ |
| PLIN2 | ↑/↑ | ↑/- | ↑/↑ | ↑/↑ | ↑/↑↑ |
| PPARγ agonist | Weak | Weak+ | No | No | No |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vargas-Bello-Pérez, E.; Zhao, W.; Bionaz, M.; Luo, J.; Loor, J.J. Nutrigenomic Effect of Saturated and Unsaturated Long Chain Fatty Acids on Lipid-Related Genes in Goat Mammary Epithelial Cells: What Is the Role of PPARγ? Vet. Sci. 2019, 6, 54. https://doi.org/10.3390/vetsci6020054
Vargas-Bello-Pérez E, Zhao W, Bionaz M, Luo J, Loor JJ. Nutrigenomic Effect of Saturated and Unsaturated Long Chain Fatty Acids on Lipid-Related Genes in Goat Mammary Epithelial Cells: What Is the Role of PPARγ? Veterinary Sciences. 2019; 6(2):54. https://doi.org/10.3390/vetsci6020054
Chicago/Turabian StyleVargas-Bello-Pérez, Einar, Wangsheng Zhao, Massimo Bionaz, Jun Luo, and Juan J. Loor. 2019. "Nutrigenomic Effect of Saturated and Unsaturated Long Chain Fatty Acids on Lipid-Related Genes in Goat Mammary Epithelial Cells: What Is the Role of PPARγ?" Veterinary Sciences 6, no. 2: 54. https://doi.org/10.3390/vetsci6020054
APA StyleVargas-Bello-Pérez, E., Zhao, W., Bionaz, M., Luo, J., & Loor, J. J. (2019). Nutrigenomic Effect of Saturated and Unsaturated Long Chain Fatty Acids on Lipid-Related Genes in Goat Mammary Epithelial Cells: What Is the Role of PPARγ? Veterinary Sciences, 6(2), 54. https://doi.org/10.3390/vetsci6020054