Epidemiology of Breed-Related Mast Cell Tumour Occurrence and Prognostic Significance of Clinical Features in a Defined Population of Dogs in West-Central Italy
Abstract
1. Introduction
2. Materials and Methods
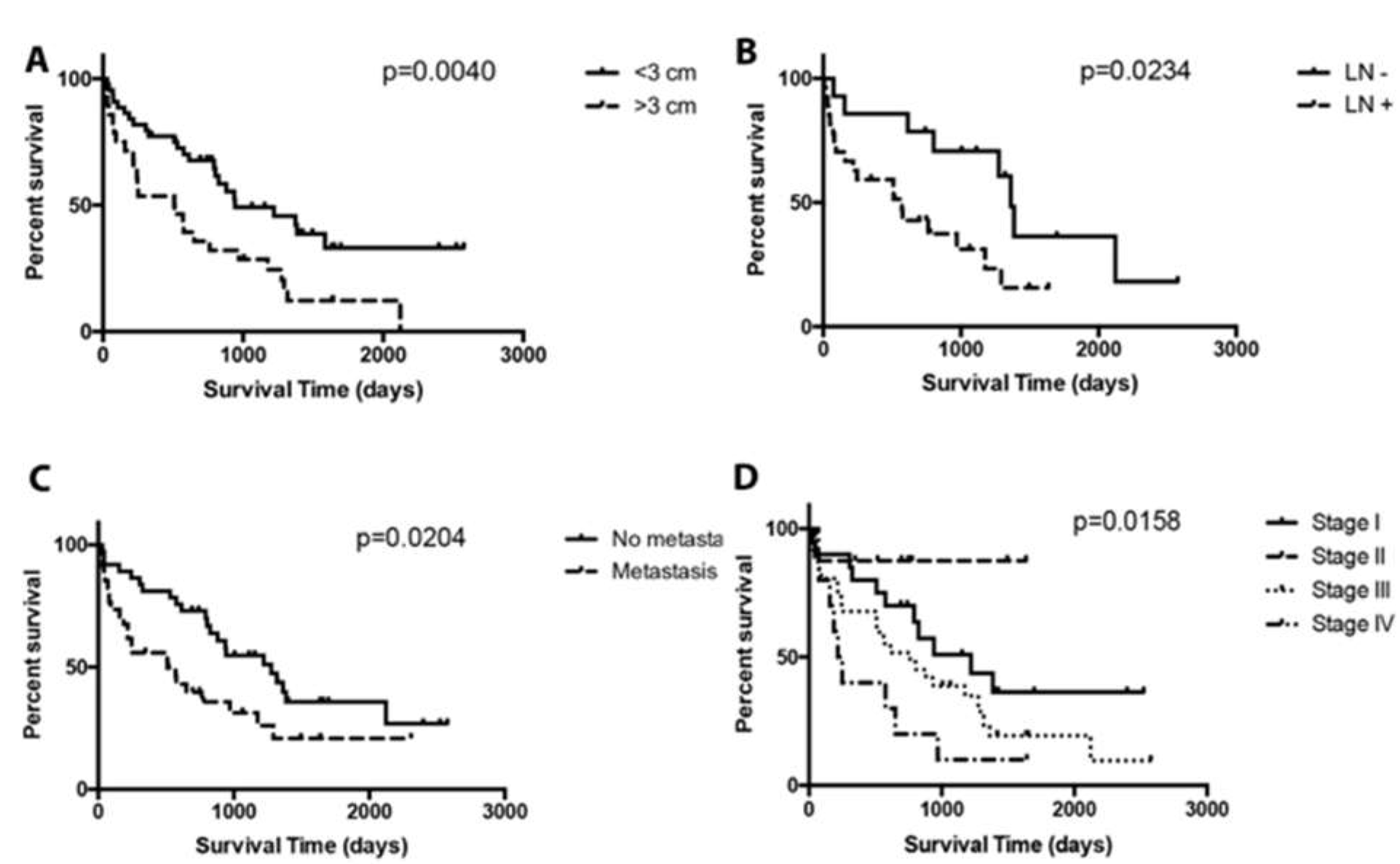
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bostock, D.E. Neoplasms of the Skin and Subcutaneous Tissues in Dogs and Cats. Br. Vet. J. 1986, 142, 1–19. [Google Scholar] [CrossRef]
- Rothwell, T.L.; Howlett, C.R.; Middleton, D.J.; Griffiths, D.A.; Duff, B.C. Skin Neoplasms of Dogs in Sydney. Aust. Vet. J. 1987, 64, 161–164. [Google Scholar] [CrossRef] [PubMed]
- Chaffin, K.; Thrall, D.E. Results of radiation therapy in 19 dogs with cutaneous mast cell tumor and regional lymph node metastasis. Vet. Radiol. Ultrasound 2002, 43, 392–395. [Google Scholar] [CrossRef]
- Rassnick, K.M.; Bailey, D.B.; Russell, D.S.; Flory, A.B.; Kiselow, M.A.; Intile, J.L.; Malone, E.K.; Balkman, C.E.; Barnard, S.M. A Phase II Study to Evaluate the Toxicity and Efficacy of Alternating CCNU and High-Dose Vinblastine and Prednisone (CVP) for Treatment of Dogs with High-Grade, Metastatic or Nonresectable Mast Cell Tumours. Vet. Comp. Oncol. 2010, 8, 138–152. [Google Scholar] [CrossRef] [PubMed]
- Kiupel, M.; Webster, J.D.; Bailey, K.L.; Best, S.; DeLay, J.; Detrisac, C.J.; Fitzgerald, S.D.; Gamble, D.; Ginn, P.E.; Goldschmidt, M.H.; et al. Proposal of a 2-Tier Histologic Grading System for Canine Cutaneous Mast Cell Tumors to More Accurately Predict Biological Behavior. Vet. Pathol. 2011, 48, 147–155. [Google Scholar] [CrossRef]
- Mochizuki, H.; Motsinger-Reif, A.; Bettini, C.; Moroff, S.; Breen, M. Association of Breed and Histopathological Grade in Canine Mast Cell Tumours. Vet. Comp. Oncol. 2017, 15, 829–839. [Google Scholar] [CrossRef]
- Horta, R.S.; Lavalle, G.E.; Monteiro, L.N.; Souza, M.C.C.; Cassali, G.D.; Araújo, R.B. Assessment of Canine Mast Cell Tumor Mortality Risk Based on Clinical, Histologic, Immunohistochemical, and Molecular Features. Vet. Pathol. 2018, 55, 212–223. [Google Scholar] [CrossRef] [PubMed]
- Baginski, H.; Davis, G.; Bastian, R.P. The Prognostic Value of Lymph Node Metastasis with Grade 2 MCTs in Dogs: 55 Cases (2001–2010). J. Am. Anim. Hosp. Assoc. 2014, 50, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Shoop, S.J.; Marlow, S.; Church, D.B.; English, K.; McGreevy, P.D.; Stell, A.J.; Thomson, P.C.; O’Neill, D.G.; Brodbelt, D.C. Prevalence and Risk Factors for Mast Cell Tumours in Dogs in England. Canine Genet. Epidemiol. 2015, 2, 1. [Google Scholar] [CrossRef] [PubMed]
- McNiel, E.A.; Prink, A.L.; O’Brien, T.D. Evaluation of Risk and Clinical Outcome of Mast Cell Tumours in Pug Dogs. Vet. Comp. Oncol. 2006, 4, 2–8. [Google Scholar] [CrossRef]
- Patnaik, A.K.; Ehler, W.J.; MacEwen, E.G. Canine Cutaneous Mast Cell Tumor: Morphologic Grading and Survival Time in 83 Dogs. Vet. Pathol. 1984, 21, 469–474. [Google Scholar] [CrossRef] [PubMed]
- Webster, J.D.; Yuzbasiyan-Gurkan, V.; Miller, R.A.; Kaneene, J.B.; Kiupel, M. Cellular Proliferation in Canine Cutaneous Mast Cell Tumors: Associations with c-KIT and Its Role in Prognostication. Vet. Pathol. 2007, 44, 298–308. [Google Scholar] [CrossRef] [PubMed]
- Thompson, J.J.; Pearl, D.L.; Yager, J.A.; Best, S.J.; Coomber, B.L.; Foster, R.A. Canine Subcutaneous Mast Cell Tumor: Characterization and Prognostic Indices. Vet. Pathol. 2011, 48, 156–168. [Google Scholar] [CrossRef] [PubMed]
- Stefanello, D.; Buracco, P.; Sabattini, S.; Finotello, R.; Giudice, C.; Grieco, V.; Iussich, S.; Tursi, M.; Scase, T.; Di Palma, S.; et al. Comparison of 2- and 3-Category Histologic Grading Systems for Predicting the Presence of Metastasis at the Time of Initial Evaluation in Dogs with Cutaneous Mast Cell Tumors: 386 Cases (2009–2014). J. Am. Vet. Med. Assoc. 2015, 246, 765–769. [Google Scholar] [CrossRef] [PubMed]
- vonHoldt, B.M.; Pollinger, J.P.; Lohmueller, K.E.; Han, E.; Parker, H.G.; Quignon, P.; Degenhardt, J.D.; Boyko, A.R.; Earl, D.A.; Auton, A.; et al. Genome-Wide SNP and Haplotype Analyses Reveal a Rich History Underlying Dog Domestication. Nature 2010, 464, 898–902. [Google Scholar] [CrossRef] [PubMed]
- Peters, J.A. Canine Mastocytoma: Excess Risk as Related to Ancestry. JNCI J. Natl. Cancer Inst. 1969, 42, 435–443. [Google Scholar] [CrossRef] [PubMed]
- MacFarlane, M.J.; MacFarlane, L.L.; Scase, T.; Parkin, T.; Morris, J.S. Use of Neutrophil to Lymphocyte Ratio for Predicting Histopathological Grade of Canine Mast Cell Tumours. Vet. Rec. 2016, 179, 491. [Google Scholar] [CrossRef] [PubMed]
- Torres de la Riva, G.; Hart, B.L.; Farver, T.B.; Oberbauer, A.M.; Messam, L.L.M.; Willits, N.; Hart, L.A. Neutering Dogs: Effects on Joint Disorders and Cancers in Golden Retrievers. PLoS ONE 2013, 8, e55937. [Google Scholar] [CrossRef]
- Hart, B.L.; Hart, L.A.; Thigpen, A.P.; Willits, N.H. Long-Term Health Effects of Neutering Dogs: Comparison of Labrador Retrievers with Golden Retrievers. PLoS ONE 2014, 9, e102241. [Google Scholar] [CrossRef] [PubMed]
- White, C.R.; Hohenhaus, A.E.; Kelsey, J.; Procter-Gray, E. Cutaneous MCTs: Associations with Spay/Neuter Status, Breed, Body Size, and Phylogenetic Cluster. J. Am. Anim. Hosp. Assoc. 2011, 47, 210–216. [Google Scholar] [CrossRef]
- Zink, M.C.; Farhoody, P.; Elser, S.E.; Ruffini, L.D.; Gibbons, T.A.; Rieger, R.H. Evaluation of the Risk and Age of Onset of Cancer and Behavioral Disorders in Gonadectomized Vizslas. J. Am. Vet. Med. Assoc. 2014, 244, 309–319. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, R.; Marconato, L.; Buracco, P.; Boracchi, P.; Giudice, C.; Iussich, S.; Grieco, V.; Chiti, L.E.; Favretto, E.; Stefanello, D. The Impact of Extirpation of Non-Palpable/Normal-Sized Regional Lymph Nodes on Staging of Canine Cutaneous Mast Cell Tumours: A Multicentric Retrospective Study. Vet. Comp. Oncol. 2018, 16, 505–510. [Google Scholar] [CrossRef] [PubMed]
- Thamm, D.H.; Turek, M.M.; Vail, D.M. Outcome and Prognostic Factors Following Adjuvant Prednisone/Vinblastine Chemotherapy for High-Risk Canine Mast Cell Tumour: 61 Cases. J. Vet. Med. Sci. 2006, 68, 581–587. [Google Scholar] [CrossRef] [PubMed]
- Worley, D.R. Incorporation of Sentinel Lymph Node Mapping in Dogs with Mast Cell Tumours: 20 Consecutive Procedures. Vet. Comp. Oncol. 2014, 12, 215–226. [Google Scholar] [CrossRef] [PubMed]
| Breed | MCT Dogs (%) | VTH Dogs (%) | p-Value | OR (95% CI) |
|---|---|---|---|---|
| Mixed † | 24 (24.5) | 3576 (27.1) | 0.64 | |
| Boxer † | 23 (23.5) | 537 (4.1) | <0.0001 | 7.2 (4.5–11.6) |
| Labrador Ret. † | 15 (15.3) | 866 (6.6) | 0.0011 | 2.6 (1.5–4.5) |
| Golden Ret. † | 7 (7.1) | 430 (3.3) | 0.06 | |
| French Bulldog ‡ | 4 (4.1) | 126 (1.0) | 0.0156 | 4.4 (1.6–12.2) |
| American Pit Bull Terrier ‡ | 3 (3.1) | 77 (0.6) | 0.0213 | 5.3 (1.7–17.3) |
| English Setter ‡ | 3 (3.1) | 240 (1.8) | 0.27 | |
| Bolognese ‡ | 2 (2.0) | 79 (0.6) | 0.12 | |
| Pug ‡ | 2 (2.0) | 135 (1.0) | 0.27 | |
| Miniature Poodle ‡ | 1 (1.0) | 450 (3.4) | 0.27 | |
| Dachshund ‡ | 1 (1.0) | 391 (3.0) | 0.37 | |
| Dobermann Pinscher ‡ | 1 (1.0) | 186 (1.4) | 1.00 | |
| German Shepherd ‡ | 1 (1.0) | 743 (5.6) | 0.045 | 0.2 (0.0–1.3) |
| Pointer ‡ | 1 (1.0) | 61 (0.5) | 0.37 | |
| Shih tzu ‡ | 1 (1.0) | 127 (1.0) | 0.61 | |
| Springer Spaniel ‡ | 1 (1.0) | 159 (1.2) | 1.00 | |
| Yorkshire Terrier ‡ | 1 (1.0) | 180 (1.4) | 1.00 |
| Sex and Spay/Neuter Status | MCT Dogs (%) | VTH Dogs (%) | p-Value | OR (95% CI) |
|---|---|---|---|---|
| Sex | ||||
| Male | 47 (48.0) | 6739 (51.5) | 0.48 † | |
| Female | 51 (52.0) | 6338 (48.5) | ||
| Neutered male | 4 (4.1) | 312 (2.4) | 0.28 ‡ | |
| Intact male | 43 (43.9) | 6427 (49.1) | ||
| Intact female | 25 (25.5) | 4372 (33.4) | 0.0022 † | 1 |
| Spayed female | 26 (26.5) | 1966 (15.1) | 2.31 (1.33–4.02) | |
| Sexual status | ||||
| Intact | 68 (69.4) | 10,799 (82.6) | 0.0006 † | 1 |
| Spay/neuter | 30 (30.6) | 2278 (17.4) | 2.09 (1.36–3.22) |
| Variable | Dogs n (%) |
|---|---|
| Localisation † (n = 90) | |
| Extremities | 15 (15.3) |
| Trunk | 42 (42.8) |
| Head | 16 (16.3) |
| Miscellaneous | 17 (17.3) |
| Single/Multiple (n = 98) | |
| Single | 80 (81.6) |
| Multiple | 18 (18.4) |
| Size ‡ (n = 86) | |
| <3 cm | 53 (60.2) |
| >3 cm | 33 (39.8) |
| Ulceration (n = 89) | |
| Present | 15 (16.8) |
| Absent | 74 (83.1) |
| Presence of metastasis | |
| Lymph node (n = 48) | 28 (58.3) |
| Spleen and/or liver (n = 88) | 12 (13.6) |
| WHO clinical staging (n = 84) | |
| 0 | 1 (1.2) |
| I | 25 (29.8) |
| II | 8 (9.5) |
| III | 38 (45.2) |
| IV | 12 (14.3) |
| Histological grading § (n = 68) | |
| G1P | 8 (11.8) |
| G2P | 54 (79.4) |
| G3P | 2 (2.9) |
| G1P-LGK | 2 (6.0) |
| G2P-LGK | 27 (81.8) |
| G2P-HGK | 1 (3.0) |
| G3P-HGK | 3 (9.1) |
| Subcutaneous | 4 (5.9) |
| Histological margins (n = 53) | |
| Complete | 30 (56.7) |
| Narrow | 10 (18.9) |
| Incomplete | 13 (24.5) |
| Treatment (n = 82) | |
| Surgery | 40 (48.8) |
| Chemotherapy and/or TKI | 8 (9.8) |
| Surgery and radiotherapy | 2 (2.4) |
| Surgery and chemotherapy and/or TKI | 22 (26.8) |
| Radiotherapy and chemotherapy and/or TKI | 2 (2.4) |
| No therapy | 12 (14.6) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pierini, A.; Lubas, G.; Gori, E.; Binanti, D.; Millanta, F.; Marchetti, V. Epidemiology of Breed-Related Mast Cell Tumour Occurrence and Prognostic Significance of Clinical Features in a Defined Population of Dogs in West-Central Italy. Vet. Sci. 2019, 6, 53. https://doi.org/10.3390/vetsci6020053
Pierini A, Lubas G, Gori E, Binanti D, Millanta F, Marchetti V. Epidemiology of Breed-Related Mast Cell Tumour Occurrence and Prognostic Significance of Clinical Features in a Defined Population of Dogs in West-Central Italy. Veterinary Sciences. 2019; 6(2):53. https://doi.org/10.3390/vetsci6020053
Chicago/Turabian StylePierini, Alessio, George Lubas, Eleonora Gori, Diana Binanti, Francesca Millanta, and Veronica Marchetti. 2019. "Epidemiology of Breed-Related Mast Cell Tumour Occurrence and Prognostic Significance of Clinical Features in a Defined Population of Dogs in West-Central Italy" Veterinary Sciences 6, no. 2: 53. https://doi.org/10.3390/vetsci6020053
APA StylePierini, A., Lubas, G., Gori, E., Binanti, D., Millanta, F., & Marchetti, V. (2019). Epidemiology of Breed-Related Mast Cell Tumour Occurrence and Prognostic Significance of Clinical Features in a Defined Population of Dogs in West-Central Italy. Veterinary Sciences, 6(2), 53. https://doi.org/10.3390/vetsci6020053








