Pulse Pressure Variation Can Predict the Hemodynamic Response to Pneumoperitoneum in Dogs: A Retrospective Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Anesthetic Protocol
2.2. Study Protocol
2.3. Statistical Analysis
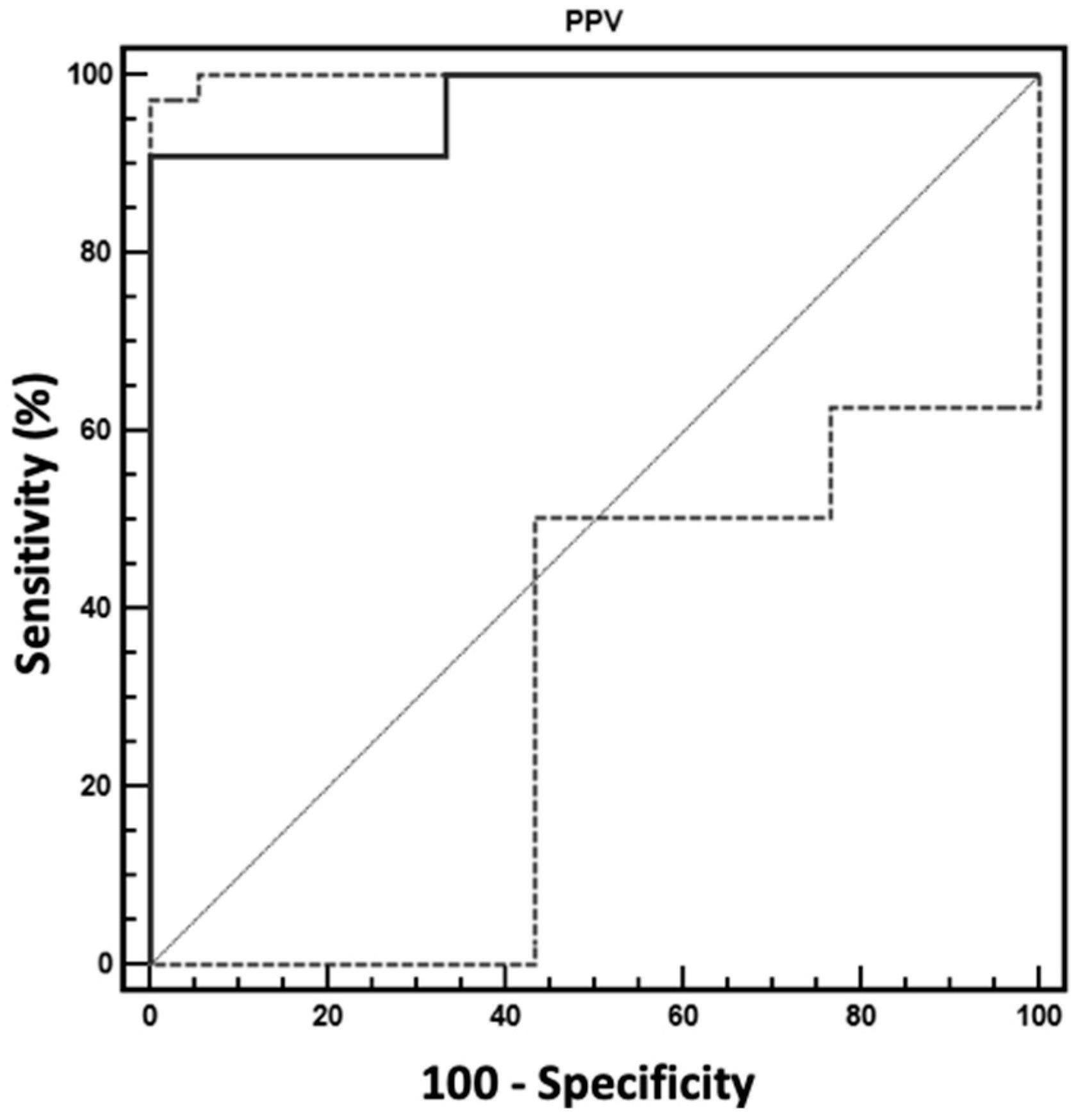
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Cheifetz, I.M. Cardiorespiratory interactions: The relationship between mechanical ventilation and hemodynamics. Respir. Care 2014, 59, 1937–1945. [Google Scholar] [CrossRef]
- Michard, F. Changes in arterial pressure during mechanical ventilation. Anesthesiology 2005, 103, 419–428. [Google Scholar] [CrossRef] [PubMed]
- Cannesson, M. Arterial pressure variation and goal-directed fluid therapy. J. Cardiothorac. Vasc. Anesth. 2010, 24, 487–497. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Du, B. Does pulse pressure variation predict fluid responsiveness in critically ill patients? A systematic review and meta-analysis. Crit. Care 2014, 18, 650. [Google Scholar] [CrossRef] [PubMed]
- Perel, A.; Pizov, R.; Cotev, S. Respiratory variations in the arterial pressure during mechanical ventilation reflect volume status and fluid responsiveness. Intensive Care Med. 2014, 40, 798–807. [Google Scholar] [CrossRef]
- Marik, P.E.; Cavallazzi, R.; Vasu, T.; Hirani, A. Dynamic changes in arterial waveform derived variables and fluid responsiveness in mechanically ventilated patients: A systematic review of the literature. Crit. Care Med. 2009, 37, 2642–2647. [Google Scholar] [CrossRef]
- Drozdzynska, M.J.; Chang, Y.M.; Stanzani, G.; Pelligand, L. Evaluation of the dynamic predictors of fluid responsiveness in dogs receiving goal-directed fluid therapy. Vet. Anaesth. Analg. 2018, 45, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Berkenstadt, H.; Friedman, Z.; Preisman, S.; Keidan, I.; Livingstone, D.; Perel, A. Pulse pressure and stroke volume variations during severe haemorrhage in ventilated dogs. Br. J. Anaesth. 2005, 94, 721–726. [Google Scholar] [CrossRef]
- Sano, H.; Seo, J.; Wightman, P.; Cave, N.J.; Gieseg, M.A.; Johnson, C.B.; Chambers, P. Evaluation of pulse pressure variation and pleth variability index to predict fluid responsiveness in mechanically ventilated isoflurane-anesthetized dogs. J. Vet. Emerg. Crit. Care 2018, 28, 301–309. [Google Scholar] [CrossRef]
- Fantoni, D.T.; Ida, K.K.; Gimenes, A.M.; Mantovani, M.M.; Castro, J.R.; Patricio, G.C.F.; Ambrosio, A.M.; Otsuki, D.A. Pulse pressure variation as a guide for volume expansion in dogs undergoing orthopedic surgery. Vet. Anaesth. Analg. 2017, 44, 710–718. [Google Scholar] [CrossRef]
- Kashtan, J.; Green, J.F.; Parsons, E.Q.; Holcroft, J.W. Hemodynamic effect of increased abdominal pressure. J. Surg. Res. 1981, 30, 249–255. [Google Scholar] [CrossRef]
- Zuckerman, R.S.; Heneghan, S. The duration of hemodynamic depression during laparoscopic cholecystectomy. Surg. Endosc. 2002, 16, 1233–1236. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Zhu, S.; Ji, Q.; Li, W.; Liu, J. The impact of intra-abdominal pressure on the stroke volume variation and plethysmographic variability index in patients undergoing laparoscopic cholecystectomy. Biosci. Trends 2015, 9, 129–133. [Google Scholar] [CrossRef] [PubMed]
- Branche, P.E.; Duperret, S.L.; Sagnard, P.E.; Boulez, J.L.; Petit, P.L.; Viale, J.P. Left ventricular loading modifications induced by pneumoperitoneum: A time course echocardiographic study. Anesth. Analg. 1998, 86, 482–487. [Google Scholar] [CrossRef] [PubMed]
- Takata, M.; Wise, R.A.; Robotham, J.L. Effects of abdominal pressure on venous return: Abdominal vascular zone conditions. J. Appl. Physiol. 1990, 69, 1961–1972. [Google Scholar] [CrossRef] [PubMed]
- Briganti, A.; Evangelista, F.; Centonze, P.; Rizzo, A.; Bentivegna, F.; Crovace, A.; Staffieri, F. A preliminary study evaluating cardiac output measurement using Pressure Recording Analytical Method (PRAM) in anaesthetized dogs. BMC Vet. Res. 2018, 14, 72. [Google Scholar] [CrossRef]
- Struthers, A.D.; Cuschieri, A. Cardiovascular consequences of laparoscopic surgery. Lancet 1998, 352, 568–570. [Google Scholar] [CrossRef]
- Koivusalo, A.M.; Lindgren, L. Effects of carbon dioxide pneumoperitoneum for laparoscopic cholecystectomy. Acta Anaesthesiol. Scand. 2000, 44, 834–841. [Google Scholar] [CrossRef]
- Atkinson, T.M.; Giraud, G.D.; Togioka, B.M.; Jones, D.B.; Cigarroa, J.E. Cardiovascular and Ventilatory Consequences of Laparoscopic Surgery. Circulation 2017, 135, 700–710. [Google Scholar] [CrossRef]
- Ivankovich, A.D.; Miletich, D.J.; Albrecht, R.F.; Heyman, H.J.; Bonnet, R.F. Cardiovascular effects of intraperitoneal insufflation with carbon dioxide and nitrous oxide in the dog. Anesthesiology 1975, 42, 281–287. [Google Scholar] [CrossRef]
- Duke, T.; Steinacher, S.L.; Remedios, A.M. Cardiopulmonary effects of using carbon dioxide for laparoscopic surgery in dogs. Vet. Surg. 1996, 25, 77–82. [Google Scholar] [CrossRef] [PubMed]
- Dexter, S.P.; Vucevic, M.; Gibson, J.; McMahon, M.J. Hemodynamic consequences of high- and low-pressure capnoperitoneum during laparoscopic cholecystectomy. Surg. Endosc. 1999, 13, 376–381. [Google Scholar] [CrossRef] [PubMed]
- Safran, D.; Sgambati, S.; Orlando, R., 3rd. Laparoscopy in high-risk cardiac patients. Surg. Gynecol. Obstet. 1993, 176, 548–554. [Google Scholar] [CrossRef] [PubMed]
- Ho, H.S.; Saunders, C.J.; Corso, F.A.; Wolfe, B.M. The effects of CO2 pneumoperitoneum on hemodynamics in hemorrhaged animals. Surgery 1993, 114, 381–387, discussion 387–388. [Google Scholar] [PubMed]
| Dog | Phase | Breed | Age (Years) | HR (beats/min) | MAP (mmHg) | CI (L/min/m2) | PPV (%) | Crs (mL/cmH2O/kg) | Group |
|---|---|---|---|---|---|---|---|---|---|
| 1 | BASE | cocker spaniel | 2 | 87 | 98 | 2.9 | 21 | 0.9 | S |
| P10 | 86 | 117 | 1.1 | 11 | 0.8 | ||||
| 2 | BASE | mixed breed | 3 | 72 | 79 | 5.9 | 32 | 1.0 | S |
| P10 | 96 | 76 | 2.6 | 12 | 0.8 | ||||
| 3 | BASE | mixed breed | 3 | 75 | 79 | 2.2 | 18 | 1.6 | S |
| P10 | 84 | 128 | 1.4 | 7 | 0.9 | ||||
| 4 | BASE | bull terrier | 4 | 41 | 76 | 6.3 | 22 | 1.6 | S |
| P10 | 85 | 121 | 4.7 | 21 | 0.9 | ||||
| 5 | BASE | beagle | 4 | 108 | 64 | 6.5 | 13.6 | 2.1 | S |
| P10 | 109 | 89 | 4.5 | 18 | 0.6 | ||||
| 6 | BASE | beagle | 2 | 88 | 86 | 5.6 | 19 | 1 | S |
| P10 | 102 | 54 | 4.7 | 13 | 0.9 | ||||
| 7 | BASE | mixed breed | 4 | 116 | 76 | 2.7 | 20 | 1.1 | S |
| P10 | 131 | 59 | 1.6 | 7 | 1.1 | ||||
| 8 | BASE | boxer | 5 | 128 | 88 | 3.4 | 26 | 1.1 | S |
| P10 | 120 | 100 | 2.6 | 16 | 0.6 | ||||
| 9 | BASE | cocker spaniel | 2 | 72 | 75 | 3.6 | 33 | 1.8 | S |
| P10 | 83 | 82 | 2.3 | 12 | 0.9 | ||||
| 10 | BASE | mixed breed | 1 | 99 | 54 | 2.7 | 24 | 1.4 | S |
| P10 | 78 | 95 | 2.1 | 7.8 | 0.8 | ||||
| 11 | BASE | beagle | 4 | 71 | 81 | 5.3 | 18 | 1.9 | S |
| P10 | 71 | 91 | 4.6 | 12 | 0.7 | ||||
| 12 | BASE | mixed breed | 3 | 107 | 81 | 4.6 | 6.3 | 3.1 | NS |
| P10 | 101 | 87 | 4.4 | 5 | 1.1 | ||||
| 13 | BASE | cocker spaniel | 5 | 56 | 70 | 7.5 | 11 | 2.3 | NS |
| P10 | 80 | 99 | 7.6 | 10 | 1.3 | ||||
| 14 | BASE | mixed breed | 2 | 102 | 83 | 3.7 | 7.3 | 0.9 | NS |
| P10 | 73 | 74 | 4.1 | 10 | 0.5 | ||||
| 15 | BASE | mixed breed | 2 | 60 | 76 | 3.5 | 8.8 | 1.4 | NS |
| P10 | 91 | 96 | 4.6 | 7 | 1.2 | ||||
| 16 | BASE | beagle | 5 | 84 | 80 | 3.2 | 14 | 1.9 | NS |
| P10 | 90 | 101 | 3.7 | 9 | 1.2 | ||||
| 17 | BASE | bull terrier | 4 | 67 | 68 | 4.7 | 14 | 1.3 | NS |
| P10 | 89 | 100 | 5.7 | 9 | 1 | ||||
| 18 | BASE | boxer | 6 | 97 | 70 | 3.6 | 9 | 1.4 | NS |
| P10 | 89 | 95 | 3.3 | 14 | 1.4 | ||||
| 19 | BASE | mixed breed | 3 | 76 | 70 | 4.2 | 11 | 1.6 | NS |
| P10 | 102 | 76 | 4.4 | 9 | 1.3 | ||||
| 20 | BASE | mixed breed | 2 | 91 | 83 | 2.8 | 16 | 1.7 | NS |
| P10 | 76 | 96 | 2.6 | 7 | 1.1 |
| Parameter | BASE | P10 | p Value |
|---|---|---|---|
| HR (beats/min) | 84.8 ± 21.6 | 91.8 ± 15.4 | 0.111 |
| MV (L/min/kg) | 0.17 ± 0.03 | 0.19 ± 0.04 | 0.127 |
| MAP (mmHg) | 76.8 ± 9.4 | 91.8 ± 18.5 | 0.003 * |
| CI (L/min/m2) | 4.28 ± 1.4 | 3.67 ± 1.6 | 0.019 * |
| SVR (dyn*sec/cm5) | 3117 ± 1485 | 3003 ± 1341 | 0.775 |
| PPV (%) | 17.2 ± 7.6 | 11.1 ± 4.0 | 0.001* |
| EtCO2 (mmHg) | 45.9 ± 4.9 | 52.6 ± 7.4 | 0.067 |
| SpO2 (%) | 98.2 ± 1.1 | 97.6 ± 1.4 | 0.765 |
| Crs (mL/cmH2O/kg) | 1.6 ± 0.6 | 0.9 ± 0.2 | 0.001 * |
| Ppeak (cmH2O) | 8.9 ± 1.8 | 12.3 ± 2.9 | 0.001 * |
| Pplat (cmH2O) | 8.7 ± 1.8 | 11.6 ± 2.3 | 0.001 * |
| Parameter | Phase | S Group | NO-S Group | p Value |
|---|---|---|---|---|
| HR (beats/min) | BASE | 86.4 ± 25.1 | 81.1 ± 20.1 | 0.601 |
| P10 | 96.1 ± 26.8 | 94.1 ± 12.5 | 0.822 | |
| MAP (mmHg) | BASE | 77.9 ± 11.6 | 74.6 ± 6.8 | 0.471 |
| P10 | 89.1 ± 27.1 | 92.1 ± 7.4 | 0.742 | |
| CI (L/m2) | BASE | 4.32 ± 1.62 | 4.22 ± 1.38 | 0.88 |
| P10 | 2.97 ± 1.4 # | 4.51 ± 1.41 | 0.02 * | |
| SVR (dyn*sec/cm5) | BASE | 3081 ± 1007 | 3160 ± 1991 | 0.902 |
| P10 | 3573 ± 1245 | 2305 ±1138 | 0.032 * | |
| EtCO2 | BASE | 46.2 ± 5.24 | 43.2 ± 7.2 | 0.602 |
| P10 | 50.1 ± 7.32 | 48.2 ±5.6 | 0.732 | |
| PPV (%) | BASE | 22.4 ± 6.1 | 10.9 ± 3.3 | 0.000 * |
| P10 | 12.6 ± 4.3 # | 9.1 ± 2.5 | 0.05 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Bella, C.; Lacitignola, L.; Fracassi, L.; Skouropoulou, D.; Crovace, A.; Staffieri, F. Pulse Pressure Variation Can Predict the Hemodynamic Response to Pneumoperitoneum in Dogs: A Retrospective Study. Vet. Sci. 2019, 6, 17. https://doi.org/10.3390/vetsci6010017
Di Bella C, Lacitignola L, Fracassi L, Skouropoulou D, Crovace A, Staffieri F. Pulse Pressure Variation Can Predict the Hemodynamic Response to Pneumoperitoneum in Dogs: A Retrospective Study. Veterinary Sciences. 2019; 6(1):17. https://doi.org/10.3390/vetsci6010017
Chicago/Turabian StyleDi Bella, Caterina, Luca Lacitignola, Laura Fracassi, Despoina Skouropoulou, Antonio Crovace, and Francesco Staffieri. 2019. "Pulse Pressure Variation Can Predict the Hemodynamic Response to Pneumoperitoneum in Dogs: A Retrospective Study" Veterinary Sciences 6, no. 1: 17. https://doi.org/10.3390/vetsci6010017
APA StyleDi Bella, C., Lacitignola, L., Fracassi, L., Skouropoulou, D., Crovace, A., & Staffieri, F. (2019). Pulse Pressure Variation Can Predict the Hemodynamic Response to Pneumoperitoneum in Dogs: A Retrospective Study. Veterinary Sciences, 6(1), 17. https://doi.org/10.3390/vetsci6010017







