Tumor–Microenvironment Interaction: Analysis of Mast Cell Populations in Normal Tissue and Proliferative Disorders of the Canine Prostate
Abstract
1. Introduction
2. Materials and Methods
2.1. Case Selection
2.2. Histology and Immunohistochemistry
2.3. Double Immunofluorescence
2.4. Quantification of Mast Cell Density and Microvessel Density
2.5. Statistical Analysis
3. Result
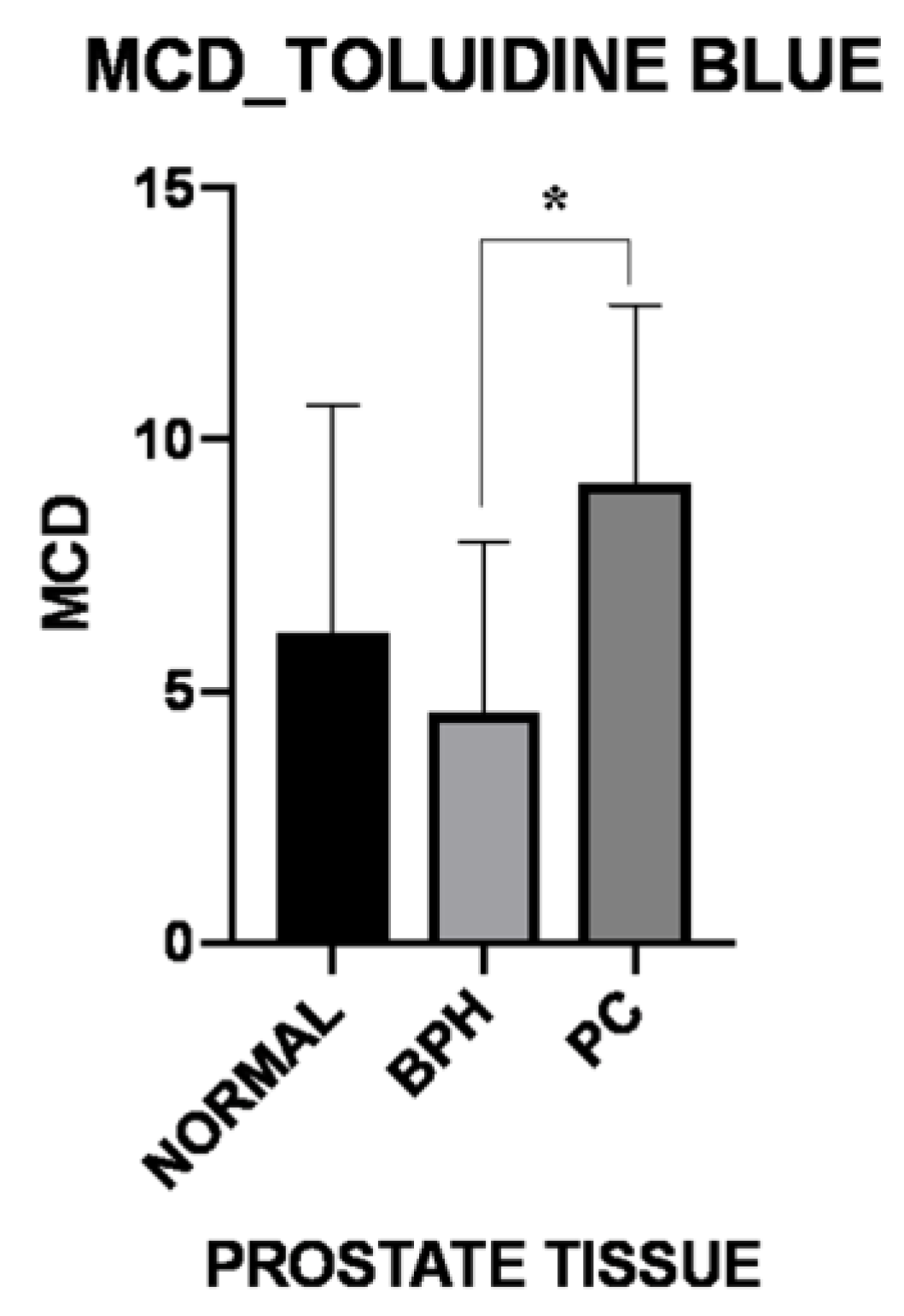
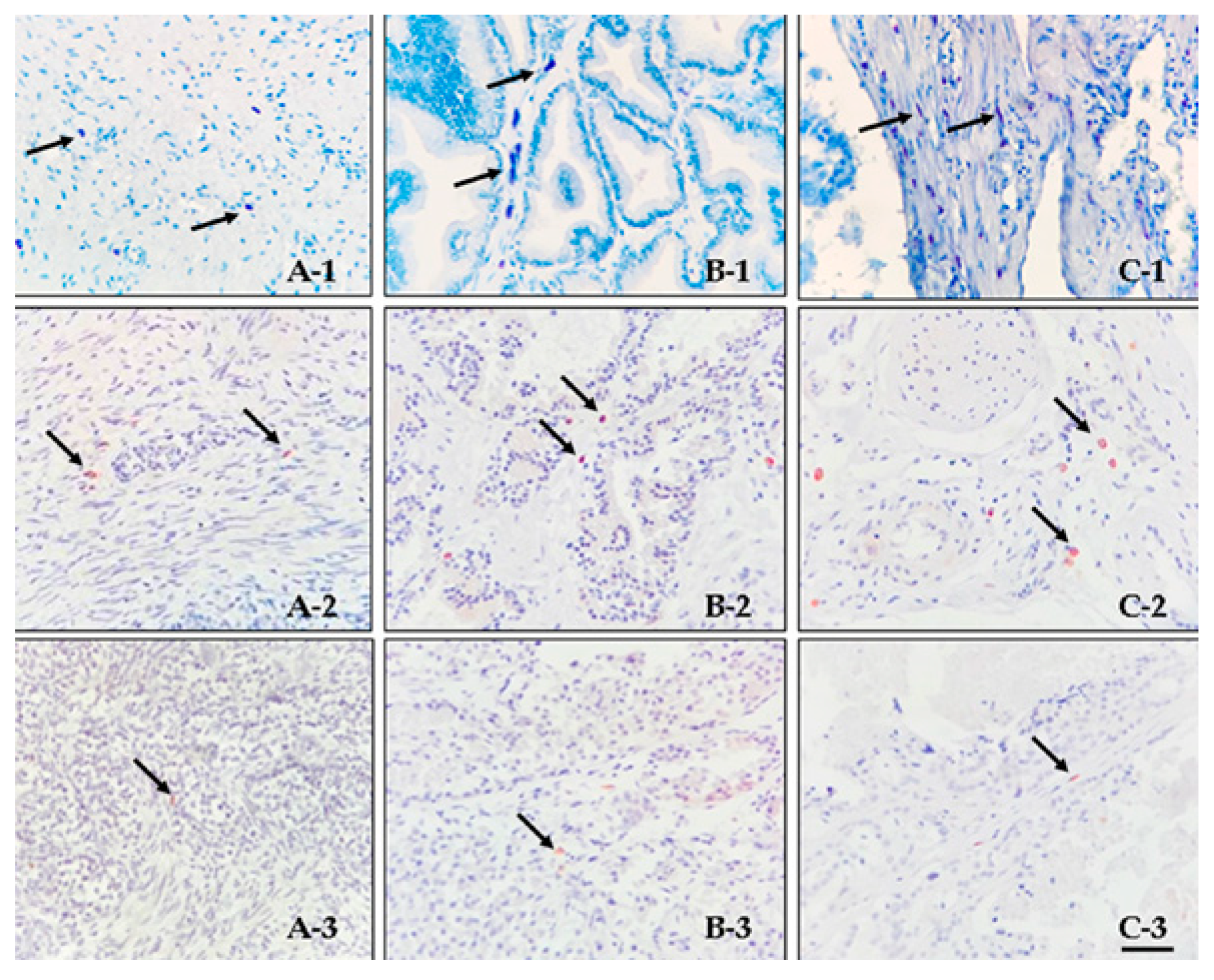
3.1. Histology and Immunohistochemistry
3.2. Double Immunofluorescence
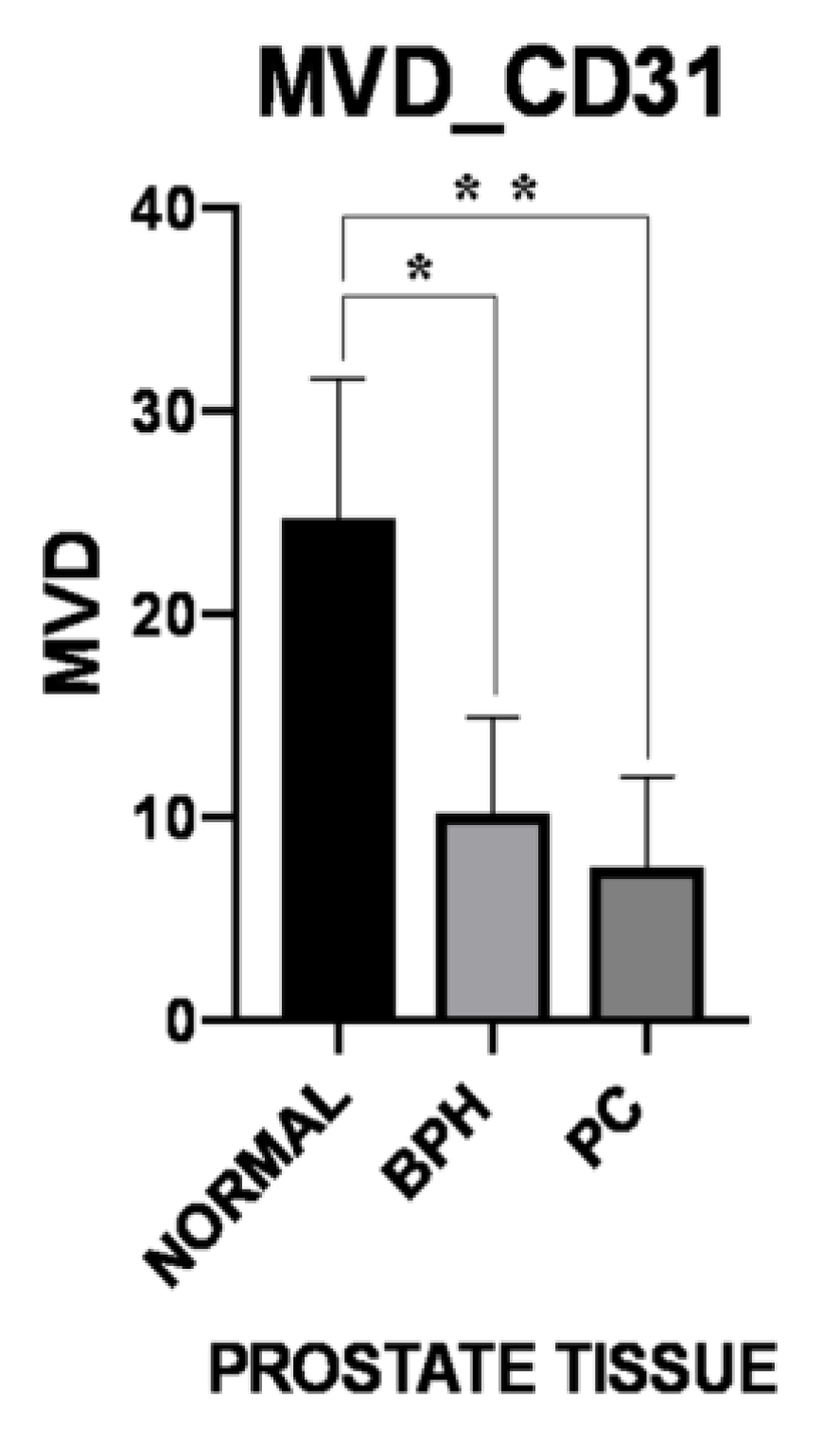
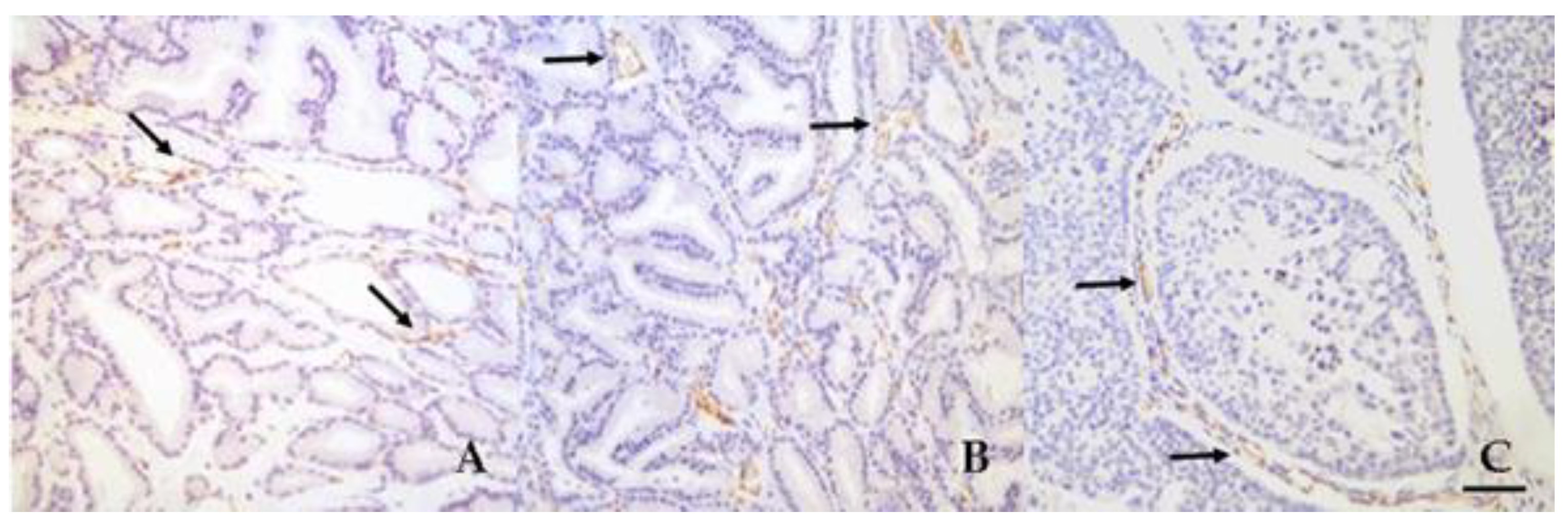
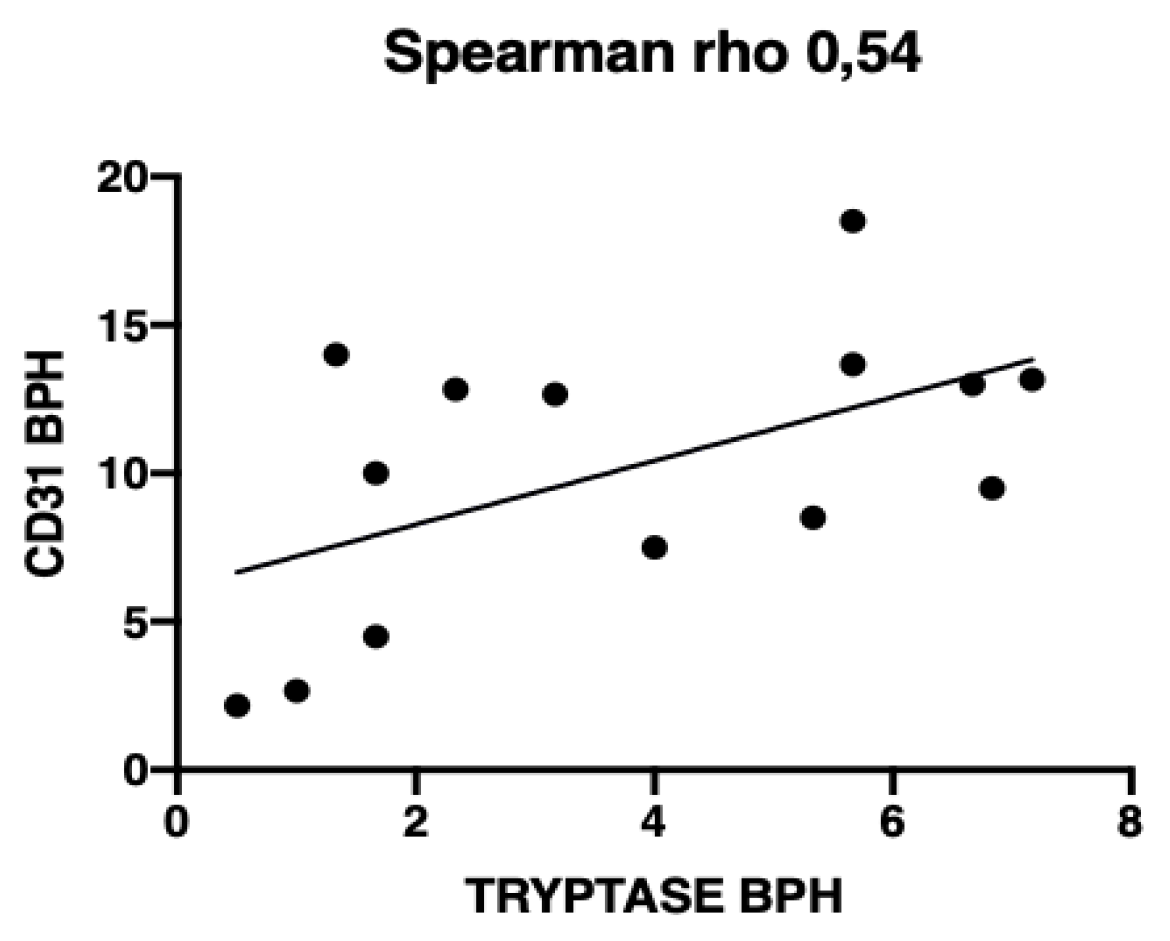
3.3. Microvessel Density
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Raposo, T.P.; Pires, I.; Prada, J.; Queiroga, F.L.; Argyle, D.J. Exploring new biomarkers in the tumour microenvironment of canine inflammatory mammary tumours. Vet. Comp. Oncol. 2017, 15, 655–666. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-H.; Frantz, A.M.; Anderson, K.L.; Graef, A.J.; Scott, M.C.; Robinson, S.; Sharkey, L.C.; O’Brien, T.D.; Dickerson, E.B.; Modiano, J.F. Interleukin-8 Promotes Canine Hemangiosarcoma Growth by Regulating the Tumor Microenvironment. Exp. Cell Res. 2014, 323, 155–164. [Google Scholar] [CrossRef] [PubMed]
- de Souza, T.A.; de Campos, C.B.; De Biasi Bassani Gonçalves, A.; Nunes, F.C.; Monteiro, L.N.; de Oliveira Vasconcelos, R.; Cassali, G.D. Relationship between the inflammatory tumor microenvironment and different histologic types of canine mammary tumors. Res. Vet. Sci. 2018, 119, 209–214. [Google Scholar] [CrossRef] [PubMed]
- Weber, C.E.; Kuo, P.C. The tumor microenvironment. Surg. Oncol. 2012, 21, 172–177. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Fan, X.; Houghton, J. Tumor microenvironment: The role of the tumor stroma in cancer. J. Cell. Biochem. 2007, 101, 805–815. [Google Scholar] [CrossRef] [PubMed]
- Swartz, M.A.; Iida, N.; Roberts, E.W.; Sangaletti, S.; Wong, M.H.; Yull, F.E.; Coussens, L.M.; DeClerck, Y.A. Tumor Microenvironment Complexity: Emerging Roles in Cancer Therapy. Cancer Res. 2012. [Google Scholar] [CrossRef]
- Mbeunkui, F.; Johann, D.J. Cancer and the tumor microenvironment: A review of an essential relationship. Cancer Chemother. Pharmacol. 2009, 63, 571–582. [Google Scholar] [CrossRef]
- van Kempen, L.C.; Ruiter, D.J.; van Muijen, G.N.; Coussens, L.M. The tumor microenvironment: A critical determinant of neoplastic evolution. Eur. J. Cell Biol. 2003, 82, 539–548. [Google Scholar] [CrossRef]
- Joyce, J.A. Therapeutic targeting of the tumor microenvironment. Cancer Cell 2005, 7, 513–520. [Google Scholar] [CrossRef]
- Fukumura, D.; Jain, R.K. Tumor microenvironment abnormalities: Causes, consequences, and strategies to normalize. J. Cell. Biochem. 2007, 101, 937–949. [Google Scholar] [CrossRef]
- Shiao, S.L.; Chu, G.C.-Y.; Chung, L.W.K. Regulation of prostate cancer progression by the tumor microenvironment. Cancer Lett. 2016, 380, 340–348. [Google Scholar] [CrossRef] [PubMed]
- Varricchi, G.; Galdiero, M.R.; Loffredo, S.; Marone, G.; Iannone, R.; Marone, G.; Granata, F. Are Mast Cells MASTers in Cancer? Front. Immunol. 2017, 8, 424. [Google Scholar] [CrossRef] [PubMed]
- Mantovani, A.; Allavena, P.; Sica, A.; Balkwill, F. Cancer-related inflammation. Nature 2008, 454, 436–444. [Google Scholar] [CrossRef] [PubMed]
- Barron, D.A.; Rowley, D.R. The Reactive Stroma Microenvironment and Prostate Cancer Progression. Endocr. Relat. Cancer 2012, 19, R187–R204. [Google Scholar] [CrossRef] [PubMed]
- Ribatti, D.; Crivellato, E. Mast cells, angiogenesis, and tumour growth. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 2012, 1822, 2–8. [Google Scholar] [CrossRef] [PubMed]
- Crivellato, E.; Nico, B.; Ribatti, D. Mast cells and tumour angiogenesis: New insight from experimental carcinogenesis. Cancer Lett. 2008, 269, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Ribatti, D.; Crivellato, E. Chapter 4 The Controversial Role of Mast Cells in Tumor Growth. In International Review of Cell and Molecular Biology; Academic Press: Cambridge, MA, USA, 2009; Volume 275, pp. 89–131. [Google Scholar]
- Maltby, S.; Khazaie, K.; McNagny, K.M. Mast cells in tumor growth: Angiogenesis, tissue remodelling and immune-modulation. Biochim. Biophys. Acta (BBA) Rev. Cancer 2009, 1796, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Mukai, K.; Tsai, M.; Saito, H.; Galli, S.J. Mast cells as sources of cytokines, chemokines, and growth factors. Immunol. Rev. 2018, 282, 121–150. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhang, Y.; Zhao, J.; Yang, Z.; Li, D.; Katirai, F.; Huang, B. Mast cell: Insight into remodeling a tumor microenvironment. Cancer Metast. Rev. 2011, 30, 177–184. [Google Scholar] [CrossRef]
- Globa, T.; Şaptefrţi, L.; Ceauşu, R.A.; Gaje, P.; Cimpean, A.M.; Raica, M. Mast cell phenotype in benign and malignant tumors of the prostate. Pol. J. Pathol. 2014, 65, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Nonomura, N.; Takayama, H.; Nishimura, K.; Oka, D.; Nakai, Y.; Shiba, M.; Tsujimura, A.; Nakayama, M.; Aozasa, K.; Okuyama, A. Decreased number of mast cells infiltrating into needle biopsy specimens leads to a better prognosis of prostate cancer. Br. J. Cancer 2007, 97, 952–956. [Google Scholar] [CrossRef] [PubMed]
- Hempel, H.A.; Cuka, N.S.; Kulac, I.; Barber, J.R.; Cornish, T.C.; Platz, E.A.; Marzo, A.M.D.; Sfanos, K.S. Low Intratumoral Mast Cells Are Associated with a Higher Risk of Prostate Cancer Recurrence. Prostate 2017, 77, 412–424. [Google Scholar] [CrossRef] [PubMed]
- Johansson, A.; Rudolfsson, S.; Hammarsten, P.; Halin, S.; Pietras, K.; Jones, J.; Stattin, P.; Egevad, L.; Granfors, T.; Wikström, P.; et al. Mast Cells Are Novel Independent Prognostic Markers in Prostate Cancer and Represent a Target for Therapy. Am. J. Pathol. 2010, 177, 1031–1041. [Google Scholar] [CrossRef] [PubMed]
- Fleischmann, A.; Schlomm, T.; Köllermann, J.; Sekulic, N.; Huland, H.; Mirlacher, M.; Sauter, G.; Simon, R.; Erbersdobler, A. Immunological microenvironment in prostate cancer: High mast cell densities are associated with favorable tumor characteristics and good prognosis. Prostate 2009, 69, 976–981. [Google Scholar] [CrossRef] [PubMed]
- LeRoy, B.E.; Northrup, N. Prostate cancer in dogs: Comparative and clinical aspects. Vet. J. 2009, 180, 149–162. [Google Scholar] [CrossRef] [PubMed]
- Lai, C.-L.; van den Ham, R.; van Leenders, G.; van der Lugt, J.; Mol, J.A.; Teske, E. Histopathological and immunohistochemical characterization of canine prostate cancer. Prostate 2008, 68, 477–488. [Google Scholar] [CrossRef]
- Bigliardi, E.; Bresciani, C.; Maria Cantoni, A.; Di Ianni, F.; Morini, G.; Voccia, S.; Corradi, A.; Parmigiani, E. Canine Prostate Carcinoma: Four Clinical Cases in Sexually Intact and Neutered Dogs. Open J. Urol. 2012, 2, 232–236. [Google Scholar] [CrossRef]
- Fonseca-Al, C.E.; Tiagua Vic, I.S.; Calazans, S.G.; Laufer-Amo, R. Canine Prostate Cancer: Would the Dog be an Important Model for the Study of New Drugs? Am. J. Drug Discov. Dev. 2013, 3, 220–224. [Google Scholar] [CrossRef]
- Teske, E.; Naan, E.C.; van Dijk, E.M.; Van Garderen, E.; Schalken, J.A. Canine prostate carcinoma: Epidemiological evidence of an increased risk in castrated dogs. Mol. Cell. Endocrinol. 2002, 197, 251–255. [Google Scholar] [CrossRef]
- Pittoni, P.; Colombo, M.P. The Dark Side of Mast Cell–Targeted Therapy in Prostate Cancer. Cancer Res. 2012, 72, 831–835. [Google Scholar] [CrossRef]
- Oldford, S.A.; Marshall, J.S. Mast cells as targets for immunotherapy of solid tumors. Mol. Immunol. 2015, 63, 113–124. [Google Scholar] [CrossRef] [PubMed]
- Ribatti, D. Mast cells as therapeutic target in cancer. Eur. J. Pharmacol. 2016, 778, 152–157. [Google Scholar] [CrossRef]
- Palmieri, C.; Lean, F.Z.; Akter, S.H.; Romussi, S.; Grieco, V. A retrospective analysis of 111 canine prostatic samples: Histopathological findings and classification. Res. Vet. Sci. 2014, 97, 568–573. [Google Scholar] [CrossRef] [PubMed]
- Im, K.-S.; Kim, J.-H.; Yhee, J.-Y.; Yu, C.-H.; Kim, N.-H.; Nho, W.-G.; Sur, J.-H. Tryptase-Positive Mast Cells Correlate with Angiogenesis in Canine Mammary Carcinoma. J. Comp. Pathol. 2011, 144, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Fonseca-Alves, C.E.; Kobayashi, P.E.; Palmieri, C.; Laufer-Amorim, R. Investigation of c-KIT and Ki67 expression in normal, preneoplastic and neoplastic canine prostate. BMC Vet. Res. 2017, 13, 380. [Google Scholar] [CrossRef] [PubMed]
- Thamm, D.H.; Kurzman, I.D.; Clark, M.A.; Ehrhart, E.J.; Kraft, S.L.; Gustafson, D.L.; Vail, D.M. Preclinical Investigation of PEGylated Tumor Necrosis Factor α in Dogs with Spontaneous Tumors: Phase I Evaluation. Clin. Cancer Res. 2010, 16, 1498–1508. [Google Scholar] [CrossRef] [PubMed]
- Weidner, N.; Carroll, P.R.; Flax, J.; Blumenfeld, W.; Folkman, J. Tumor angiogenesis correlates with metastasis in invasive prostate carcinoma. Am. J. Pathol. 1993, 143, 401–409. [Google Scholar] [PubMed]
- Woldemeskel, M.; Mann, E.; Whittington, L. Tumor microvessel density-associated mast cells in canine nodal lymphoma. SAGE Open Med. 2014, 2. [Google Scholar] [CrossRef]
- Marech, I.; Ammendola, M.; Leporini, C.; Patruno, R.; Luposella, M.; Zizzo, N.; Passantino, G.; Sacco, R.; Farooqi, A.A.; Zuccalà, V.; et al. C-Kit receptor and tryptase expressing mast cells correlate with angiogenesis in breast cancer patients. Oncotarget 2017, 9, 7918–7927. [Google Scholar] [CrossRef]
- Amir, T.; Pai, R.R.; Raghuveer, C.V. Mast cell profile in prostatic lesions. Indian J. Med. Sci. 1998, 52, 507–513. [Google Scholar]
- Taverna, G.; Giusti, G.; Seveso, M.; Hurle, R.; Colombo, P.; Stifter, S.; Grizzi, F. Mast Cells as a Potential Prognostic Marker in Prostate Cancer. Available online: https://www.hindawi.com/journals/dm/2013/478303/abs/ (accessed on 14 October 2018).
- Aydin, O.; Dusmez, D.; Cinel, L.; Doruk, E.; Kanik, A. Immunohistological analysis of mast cell numbers in the intratumoral and peritumoral regions of prostate carcinoma compared to benign prostatic hyperplasia. Pathol. Res. Pract. 2002, 198, 267–271. [Google Scholar] [CrossRef]
- Auxilia, S.T.; Hill, P.B. Mast cell distribution, epidermal thickness and hair follicle density in normal canine skin: Possible explanations for the predilection sites of atopic dermatitis? Vet. Dermatol. 2000, 11, 247–254. [Google Scholar] [CrossRef]
- Frangogiannis, N.G.; Burns, A.R.; Michael, L.H.; Entman, M.L. Histochemical and Morphological Characteristics of Canine Cardiac Mast Cells. Histochem. J. 1999, 31, 221–229. [Google Scholar] [CrossRef] [PubMed]
- Fujita, T. Characteristic distribution of mast cells in dog liver: A consideration on the mechanism of anaphylactic shock. Arch. Histol. Jpn. 1964, 24, 435–445. [Google Scholar] [CrossRef] [PubMed]
- Myles, A.D.; Halliwell, R.E.W.; Ballauf, B.; Miller, H.R.P. Mast cell tryptase levels in normal canine tissues. Vet. Immunol. Immunopathol. 1995, 46, 223–235. [Google Scholar] [CrossRef]
- Noviana, D.; Mamba, K.; Makimura, S.; Horii, Y. Distribution, histochemical and enzyme histochemical characterization of mast cells in dogs. J. Mol. Histol. 2004, 35, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Mukaratirwa, S.; Chikafa, L.; Dliwayo, R.; Moyo, N. Mast cells and angiogenesis in canine melanomas: Malignancy and clinicopathological factors. Vet. Dermatol. 2006, 17, 141–146. [Google Scholar] [CrossRef] [PubMed]
- Sfacteria, A.; Lanteri, G.; Grasso, G.; Macrì, B.; Mazzullo, G. Mast cells in canine mammary gland tumour: Number, distribution and EPOR positivity. Vet. Comparat. Oncol. 2011, 9, 310–315. [Google Scholar] [CrossRef] [PubMed]
- Mukaratirwa, S.; Chiwome, T.; Chitanga, S.; Bhebhe, E. Canine Transmissible Venereal Tumour: Asessment of Mast Cell Numbers as Indicators of the Growth Phase. Vet. Res. Commun. 2006, 30, 613–621. [Google Scholar] [CrossRef] [PubMed]
- Woldemeskel, M.; Rajeev, S. Mast cells in canine cutaneous hemangioma, hemangiosarcoma and mammary tumors. Vet. Res. Commun. 2010, 34, 153–160. [Google Scholar] [CrossRef]
- Sfanos, K.S.; Yegnasubramanian, S.; Nelson, W.G.; Marzo, A.M.D. The inflammatory microenvironment and microbiome in prostate cancer development. Nat. Rev. Urol. 2018, 15, 11–24. [Google Scholar] [CrossRef] [PubMed]
- Tuxhorn, J.A.; Ayala, G.E.; Rowley, D.R. Reactive stroma in prostate cancer progression. J. Urol. 2001, 166, 2472–2483. [Google Scholar] [CrossRef]
- Marichal, T.; Tsai, M.; Galli, S.J. Mast Cells: Potential Positive and Negative Roles in Tumor Biology. Cancer Immunol. Res. 2013, 1, 269–279. [Google Scholar] [CrossRef] [PubMed]
- Khazaie, K.; Blatner, N.R.; Khan, M.W.; Gounari, F.; Gounaris, E.; Dennis, K.; Bonertz, A.; Tsai, F.-N.; Strouch, M.J.; Cheon, E.; et al. The significant role of mast cells in cancer. Cancer Metast. Rev. 2011, 30, 45–60. [Google Scholar] [CrossRef] [PubMed]
- Theoharides, T.C.; Conti, P. Mast cells: The Jekyll and Hyde of tumor growth. Trends Immunol. 2004, 25, 235–241. [Google Scholar] [CrossRef] [PubMed]
- De Nunzio, C.; Kramer, G.; Marberger, M.; Montironi, R.; Nelson, W.; Schröder, F.; Sciarra, A.; Tubaro, A. The Controversial Relationship Between Benign Prostatic Hyperplasia and Prostate Cancer: The Role of Inflammation. Eur. Urol. 2011, 60, 106–117. [Google Scholar] [CrossRef] [PubMed]
- Robert, G.; Descazeaud, A.; Nicolaïew, N.; Terry, S.; Sirab, N.; Vacherot, F.; Maillé, P.; Allory, Y.; Taille, A. De la Inflammation in benign prostatic hyperplasia: A 282 patients’ immunohistochemical analysis. Prostate 2009, 69, 1774–1780. [Google Scholar] [CrossRef]
- Nechushtan, H. The complexity of the complicity of mast cells in cancer. Int. J. Biochem. Cell Biol. 2010, 42, 551–554. [Google Scholar] [CrossRef]
- Galinsky, D.S.T.; Nechushtan, H. Mast cells and cancer—No longer just basic science. Crit. Rev. Oncol. Hematol. 2008, 68, 115–130. [Google Scholar] [CrossRef]
- Di Lorenzo, G.; Autorino, R.; D’Armiento, F.P.; Mignogna, C.; De Laurentiis, M.; De Sio, M.; D’Armiento, M.; Damiano, R.; Vecchio, G.; De Placido, S. Expression of proto-oncogene c-kit in high risk prostate cancer. Eur. J. Surg. Oncol. (EJSO) 2004, 30, 987–992. [Google Scholar] [CrossRef]
- Ribatti, D.; Ranieri, G. Tryptase, a novel angiogenic factor stored in mast cell granules. Exp. Cell Res. 2015, 332, 157–162. [Google Scholar] [CrossRef] [PubMed]
- Tretiakova, M.; Antic, T.; Binder, D.; Kocherginsky, M.; Liao, C.; Taxy, J.B.; Oto, A. Microvessel density is not increased in prostate cancer: Digital imaging of routine sections and tissue microarrays. Hum. Pathol. 2013, 44, 495–502. [Google Scholar] [CrossRef] [PubMed]
- Deering, R.E.; Bigler, S.A.; Brown, M.; Brawer, M.K. Microvascularity in benign prostatic hyperplasia. Prostate 1995, 26, 111–115. [Google Scholar] [CrossRef] [PubMed]
- Taverna, G.; Grizzi, F.; Colombo, P.; Graziotti, P. Is angiogenesis a hallmark of prostate cancer? Front. Oncol. 2013, 3, 15. [Google Scholar] [CrossRef] [PubMed]
- Theoharides, T.C.; Alysandratos, K.-D.; Angelidou, A.; Zhang, B. Mast Cells and Tumor Microenvironment. In The Tumor Microenvironment; Bagley, R.G., Ed.; Cancer Drug Discovery and Development; Springer: New York, NY, USA, 2010; pp. 353–370. ISBN 978-1-4419-6615-5. [Google Scholar]
- da Silva, E.Z.M.; Jamur, M.C.; Oliver, C. Mast Cell Function: A New Vision of an Old Cell. J. Histochem. Cytochem. 2014, 62, 698–738. [Google Scholar] [CrossRef] [PubMed]
- de la Taille, A.; Katz, A.E.; Bagiella, E.; Buttyan, R.; Sharir, S.; Olsson, C.A.; Burchardt, T.; Ennis, R.D.; Rubin, M.A. Microvessel Density as a Predictor of PSA Recurrence After Radical Prostatectomy A Comparison of CD34 and CD31. Am. J. Clin. Pathol. 2000, 113, 555–562. [Google Scholar] [CrossRef]
| MCD-Toluidine Blue | Normal | BPH | PC |
|---|---|---|---|
| Periglandular/peritumoral | 9.39 ± 7.38 | 4.89 ± 3.46 | 15.50 ± 5.81 |
| Intraglandular/intratumoral p-Value | 2.61 ± 2.47 0.031 | 4.27 ± 3.91 0.365 | 2.75 ± 2.89 0.007 |
| MCD-Tryptase | Normal | BPH | PC |
|---|---|---|---|
| Periglandular/peritumoral | 6.11 ± 1.55 | 4.84 ± 0.80 | 9.37 ± 1.89 |
| Intraglandular/intratumoral p-Value | 2.22 ± 0.98 0.003 | 2.82 ± 0.60 0.034 | 1.91 ± 0.80 0.031 |
| MCD-c-Kit | Normal | BPH | PC |
|---|---|---|---|
| Periglandular/peritumoral | 1.05 ± 0.49 | 3.20 ± 0.88 | 6.10 ± 1.40 |
| Intraglandular/intratumoral p-Value | 0.72 ± 0.30 0.875 | 1.29 ± 0.37 0.023 | 1.00 ± 0.35 0.004 |
| MVD-CD31 | Normal | BPH | PC |
|---|---|---|---|
| Periglandular/peritumoral | 18.06 ± 4.10 | 10.69 ± 4.70 | 8.00 ± 4.16 |
| Intraglandular/intratumoral p-Value | 31.30 ± 11.80 0.047 | 9.68 ± 5.24 0.193 | 7.16 ± 4.96 0.398 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Defourny, S.V.P.; Romanucci, M.; Grieco, V.; Quaglione, G.R.; Santolini, C.; Della Salda, L. Tumor–Microenvironment Interaction: Analysis of Mast Cell Populations in Normal Tissue and Proliferative Disorders of the Canine Prostate. Vet. Sci. 2019, 6, 16. https://doi.org/10.3390/vetsci6010016
Defourny SVP, Romanucci M, Grieco V, Quaglione GR, Santolini C, Della Salda L. Tumor–Microenvironment Interaction: Analysis of Mast Cell Populations in Normal Tissue and Proliferative Disorders of the Canine Prostate. Veterinary Sciences. 2019; 6(1):16. https://doi.org/10.3390/vetsci6010016
Chicago/Turabian StyleDefourny, Sabrina Vanessa Patrizia, Mariarita Romanucci, Valeria Grieco, Gina Rosaria Quaglione, Chiara Santolini, and Leonardo Della Salda. 2019. "Tumor–Microenvironment Interaction: Analysis of Mast Cell Populations in Normal Tissue and Proliferative Disorders of the Canine Prostate" Veterinary Sciences 6, no. 1: 16. https://doi.org/10.3390/vetsci6010016
APA StyleDefourny, S. V. P., Romanucci, M., Grieco, V., Quaglione, G. R., Santolini, C., & Della Salda, L. (2019). Tumor–Microenvironment Interaction: Analysis of Mast Cell Populations in Normal Tissue and Proliferative Disorders of the Canine Prostate. Veterinary Sciences, 6(1), 16. https://doi.org/10.3390/vetsci6010016






