DNA and Protein Analyses to Confirm the Absence of Cross-Contamination and Support the Clinical Reliability of Extensively Hydrolysed Diets for Adverse Food Reaction-Pets
Abstract
1. Introduction
- To confirm the specific protein composition of these diets and the extensive level of hydrolysis of their feather protein source
- To confirm the effectiveness of Royal Canin’s cross-contamination risk management process for these diets through a specially designed DNA-based testing strategy.
2. Material and Methods
2.1. Analyses of Protein Composition
2.1.1. Protein Separation by Protein Gel Electrophoresis and Chromatography
2.1.2. Protein Identification by Mass-Spectrometry
2.2. Analyses for the Detection of Cross-Contaminating Proteins
2.2.1. DNA to Protein Calibration
2.2.2. Analysis of Finished Product for Cross-Contaminating Proteins
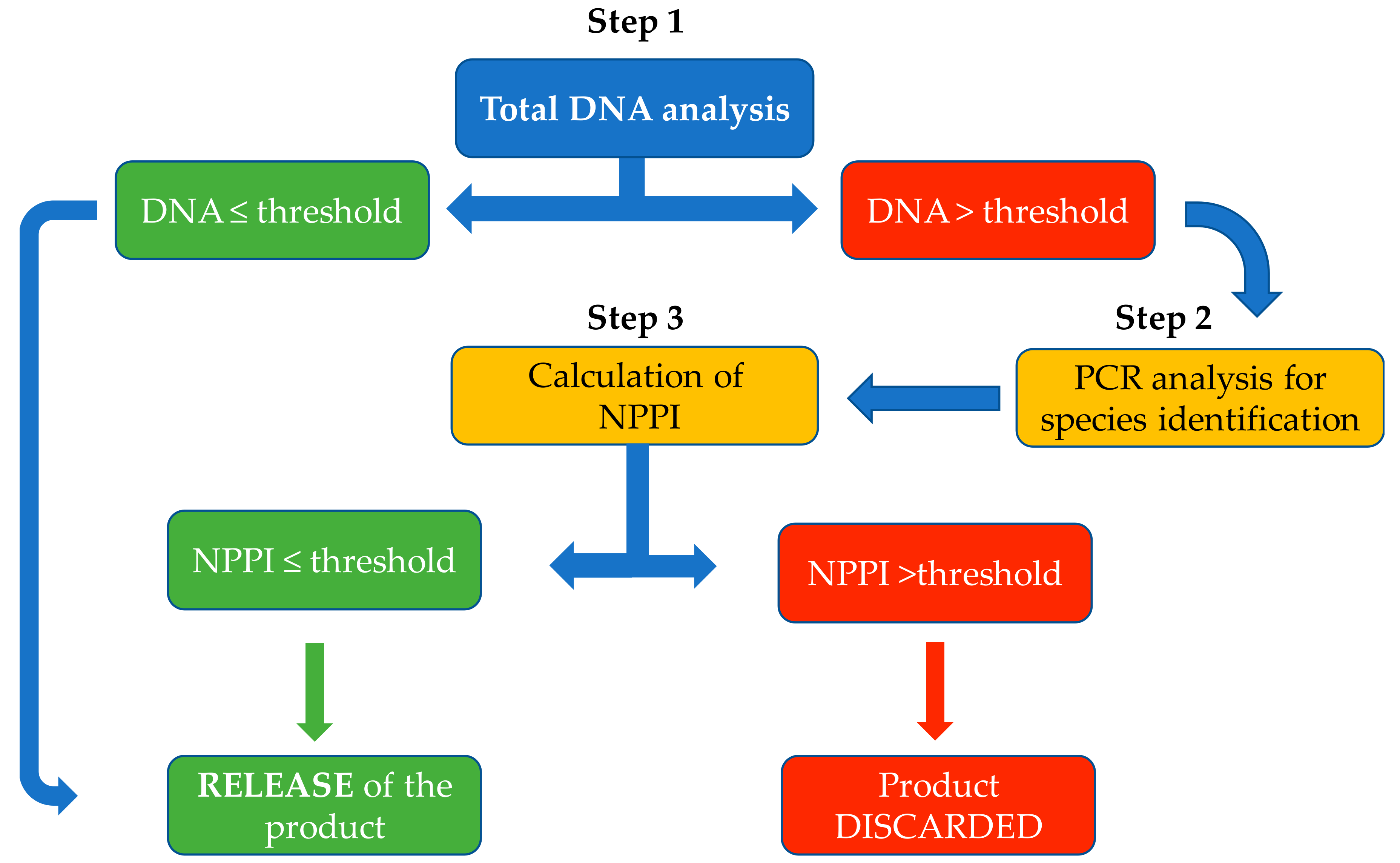
- Step 1: The total content of DNA in the diets was measured by the method described above and compared with the DNA conformity threshold. The sample was considered to be within specification if the total measured DNA was ≤1.2 µg/g.
- Step 2: When the concentration of total DNA exceeded 1.2 µg/g, polymerase chain reaction (PCR) analyses were conducted to identify the source of the contamination. Species detection was carried out by targeted quantitative reverse transcriptase PCR conducted by an accredited laboratory.
- Step 3: The calibration curves were then used to determine the NPPI for the specific protein source. The NPPI was considered to be acceptable if it was ≤0.5% (corresponding to a DNA concentration for example of ≤4.4 µg/g for duck protein sources). In this way, the calibration curves allowed a more refined assessment of protein contamination in those samples that contained more than the threshold level of total DNA.
3. Results
3.1. Protein Composition of the Diets
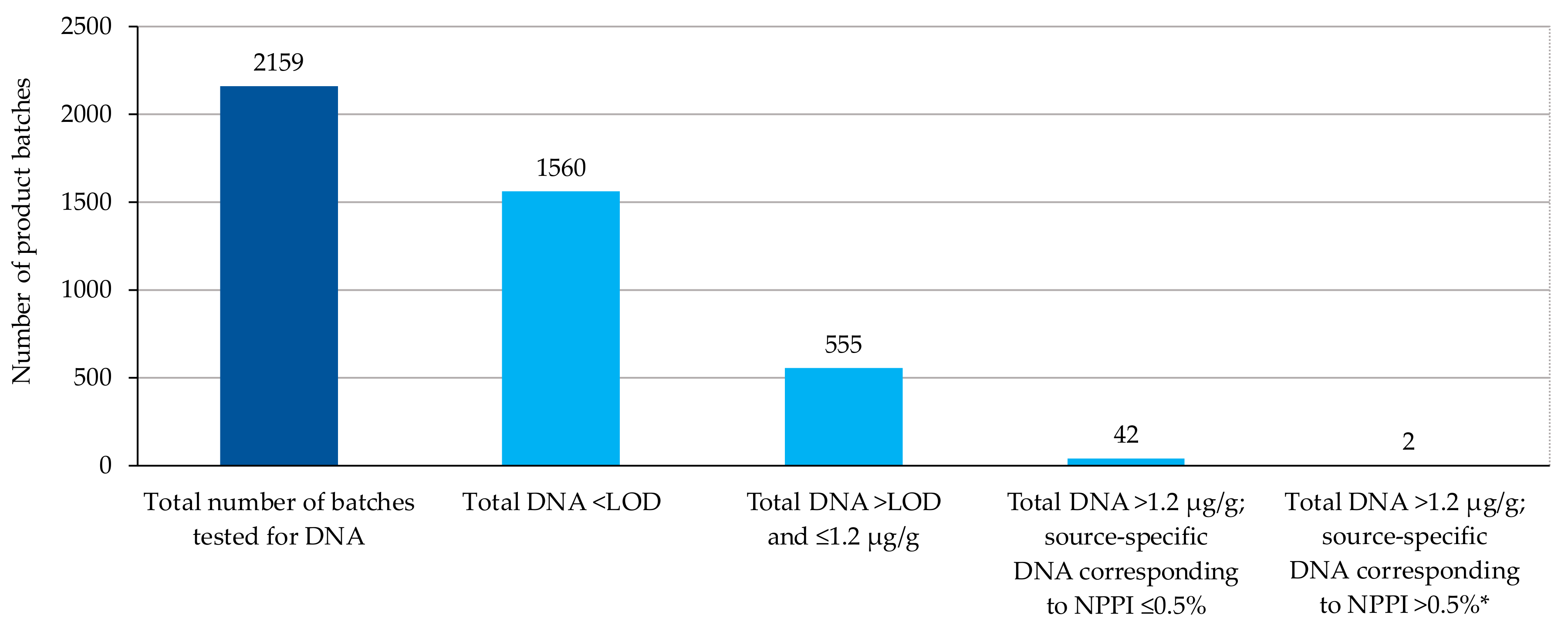
3.2. Cross-Contamination Risk Management
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Glossary
| AFR | adverse food reaction(s) |
| CADESI | Canine Atopic Dermatitis Extent and Severity Index |
| CAFR | cutaneous adverse food reaction(s) |
| CCD | cross-reactive carbohydrate determinant |
| FIAD | Food-Induced Allergic Dermatitis |
| GBSS | granule-bound starch synthase |
| LTP | lipid-transfer proteins |
| nsLTP | non-specific lipid-transfer protein |
| MALDI | Matrix Assisted Laser Desorption Ionisation |
| NPPI | no protein pollution index |
| TOF | Time Of Flight detector |
References
- Picco, F.; Zini, E.; Nett, C.; Naegeli, C.; Bigler, B.; Rüfenacht, S.; Roosje, P.; Gutzwiller, M.E.; Wilhelm, S.; Pfister, J.; et al. A prospective study on canine atopic dermatitis and food-induced allergic dermatitis in Switzerland. Vet. Dermatol. 2008, 19, 150–155. [Google Scholar] [CrossRef] [PubMed]
- Outerbridge, C.A. Nutritional Management of Skin Diseases. In Applied Veterinary Clinical Nutrition; Fascetti, A.J., Delaney, S.J., Eds.; Wiley & Sons Ltd.: Hoboken, NJ, USA, 2012; ISBN 978-0-813-80657-0. [Google Scholar]
- Chesney, C.J. Systematic review of evidence for the prevalence of food sensitivity in dogs. Vet. Rec. 2001, 148, 445–448. [Google Scholar] [CrossRef] [PubMed]
- Verlinden, A.; Hesta, M.; Millet, S.; Janssens, G.P. Food allergy in dogs and cats: A review. Crit. Rev. Food Sci. Nutr. 2006, 46, 259–273. [Google Scholar] [CrossRef] [PubMed]
- Olivry, T.; Saridomichelakis, M.; Nuttall, T.; Bensignor, E.; Griffin, C.E.; Hill, P.B.; International Committee on Allergic Diseases of Animals (ICADA). Validation of the Canine Atopic Dermatitis Extent and Severity Index (CADESI)-4, a simplified severity scale for assessing skin lesions of atopic dermatitis in dogs. Vet. Dermatol. 2014, 25, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Plant, J.D.; Gortel, K.; Kovalik, M.; Polissar, N.L.; Neradilek, M.B. Development and validation of the Canine Atopic Dermatitis Lesion Index.; a scale for the rapid scoring of lesion severity in canine atopic dermatitis. Vet. Dermatol. 2012, 23. [Google Scholar] [CrossRef] [PubMed]
- Willemse, T. Adverse Food Reactions in cats. In Proceedings of the South European Veterinary Congress, Barcelona, Spain, 17–19 October 2008. [Google Scholar]
- Halliwell, R.E.W. The diagnostic approach to pruritus. In Proceedings of the 8th World Congress of Veterinary Dermatology, Bordeaux, France, 31 May–4 June 2016. [Google Scholar]
- Linek, M. Specificities of management of the pruritic cat. In Proceedings of the Royal Canin Veterinary Symposium, New Orleans, LA, USA, 12–15 November 2015. [Google Scholar]
- Mueller, R.; Olivry, T. Critically appraised topic on adverse food reactions of companion animals (4): Can we diagnose adverse food reactions in dogs and cats with in vivo or in vitro tests? BMC Vet. Res. 2017, 13, 275. [Google Scholar] [CrossRef] [PubMed]
- Olivry, T.; Bizikova, P. A systematic review of the evidence of reduced allergenicity and clinical benefit of food hydrolysates in dogs with cutaneous adverse food reactions. Vet. Dermatol. 2010, 21, 32–41. [Google Scholar] [CrossRef] [PubMed]
- Horvath-Ungerboeck, C.; Widmann, K.; Handl, S. Detection of DNA from undeclared animal species in commercial elimination diets for dogs using PCR. Vet. Dermatol. 2017, 28, 373–e86. [Google Scholar] [CrossRef] [PubMed]
- Roitel, O.; Bonnard, L.; Stella, A.; Schiltz, O.; Maurice, D.; Douchin, G.; Jacquenet, S.; Favrot, C.; Bihain, B.E.; Couturier, N. Detection of IgE-reactive proteins in hydrolysed dog foods. Vet. Dermatol. 2017, 28. [Google Scholar] [CrossRef] [PubMed]
- Ricci, R.; Granato, A.; Vascellari, M.; Boscarato, M.; Palagiano, C.; Andrighetto, I.; Diez, M.; Mutinelli, F. Identification of undeclared sources of animal origin in canine dry foods used in dietary elimination trials. J. Anim. Physiol. Anim. Nutr. 2013, 97 (Suppl. 1), 32–38. [Google Scholar] [CrossRef] [PubMed]
- Widmann, K.; Handl, S.; Horvath-Ungerboeck, C. Detection of food antigens in commercial elimination diets for dogs using PCR. Vet. Dermatol. 2014, 25, 389. [Google Scholar]
- Olivry, T.; Mueller, R. Critically appraised topic on adverse food reactions of companion animals (5): Discrepancies between ingredients and labeling in commercial pet foods. BMC Vet. Res. 2018, 14, 24. [Google Scholar] [CrossRef] [PubMed]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randa, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [PubMed]
- Friedman, M. Applications of the Ninhydrin Reaction for Analysis of Amino Acids, Peptides and Proteins to Agricultural and Biomedical Sciences. J. Agric. Food Chem. 2004, 52, 385–406. [Google Scholar] [CrossRef] [PubMed]
- Moore, S.; Stein, W.H. A modified ninhydrin reagent for the photometric determination of amino acids and related compounds. J. Biol. Chem. 1954, 211, 907–913. [Google Scholar] [PubMed]
- Mougeot, I.; Weese, H.; Sauve, F.; Valentine, B.; Feugier, A.; Biourge, V. Clinical efficacy of a highly hydrolyzed poultry feather protein-based diet for canine AFR diagnosis and dietary management: A 12 case pilot study. In Proceedings of the Waltham International Nutritional Science Symposium, Portland, OR, USA, 1–4 October 2013; p. 70. [Google Scholar]
- Olivry, T.; Paps, J.S.; Bizikova, P.; Murphy, K.M.; Jackson, H.A.; Zebala, J. A pilot open trial evaluating the efficacy of low-dose aminopterin in the canine homologue of human atopic dermatitis. Br. J. Dermatol. 2007, 157, 1040–1042. [Google Scholar] [CrossRef] [PubMed]
- Roitel, O.; Maurice, D.; Douchin, G.; Jacquenet, S.; Bihain, B.; Favrot, C.; Couturier, N. High molecular weight proteins in hydrolysed dog foods. Vet. Dermatol. 2015, 26, 304. [Google Scholar]
- Pastorello, E.A.; Farioli, L.; Pravettoni, V.; Ispano, M.; Scibola, E.; Trambaioli, C.; Giuffrida, M.G.; Ansaloni, R.; Godovac-Zimmermann, J.; Conti, A.; et al. The maize major allergen, which is responsible for food-induced allergic reactions, is a lipid transfer protein. J. Allergy Clin. Immunol. 2000, 106, 744–751. [Google Scholar] [CrossRef] [PubMed]
- Naar, J.; Lesponne, I. Total DNA quantification to confirm the absence of cross-contamination in veterinary-prescribed diets for animals with Adverse Food Reactions (AFR). In Proceedings of the 8th International Symposium on Recent Advances in Food Analysis, Prague, Czech Republic, 7–10 November 2017. [Google Scholar]
- Lesponne, I.; Naar, J.; Montano, M.; Garzino, B.; Boutigny, I.; Servet, E.; Danset, G.; Molina, L.; Filippini, M.; Le Bozec, A.; et al. DNA and protein analyses support the clinical reliability of an extensively hydrolysed diet. Vet. Dermatol. 2017, 28, 11. [Google Scholar] [CrossRef]
- Jackson, H.A. Food allergy in dogs—Clinical signs and diagnosis. Eur. J. Companion Anim. Pract. 2009, 19, 230–233. [Google Scholar]
- Biourge, V. Protein hydrolysates: Do they really work? In Proceedings of the European Veterinary Conference Voorjaarsdagen, Amsterdam, The Netherlands, 24–26 April 2005. [Google Scholar]
- Hill, D.J.; Murch, S.H.; Rafferty, K.; Wallis, P.; Green, C.J. The efficacy of amino acid-based formulas in relieving the symptoms of cow’s milk allergy: A systematic review. Clin. Exp. Allergy 2007, 37, 808–822. [Google Scholar] [CrossRef] [PubMed]
- Cave, N.J. Hydrolyzed protein diets for dogs and cats. Vet. Clin. North Am. Small Anim. Pract. 2006, 36, 1251–1268. [Google Scholar] [CrossRef] [PubMed]
- Bush, R.K.; Hefle, S.L. Food allergens. Crit. Rev. Food Sci. Nutr. 1996, 36, S119–S163. [Google Scholar] [CrossRef] [PubMed]
- Giampietro, P.G.; Kjellman, N.I.; Oldaeus, G.; Wouters-Wesseling, W.; Businco, L. Hypoallergenicity of an extensively hydrolyzed whey formula. Pediatr. Allergy Immunol. 2001, 12, 83–86. [Google Scholar] [CrossRef] [PubMed]
- Olivry, T.; Bexley, J.; Mougeot, I. Extensive protein hydrolyzation is indispensable to prevent IgE-mediated poultry allergen recognition in dogs and cats. BMC Vet. Res. 2017, 13, 251. [Google Scholar] [CrossRef] [PubMed]
- Salminen, T.A.; Blomqvist, K.; Edqvist, J. Lipid transfer proteins: Classification, nomenclature, structure and function. Planta 2016, 244, 971–997. [Google Scholar] [CrossRef] [PubMed]
- Salcedo, G.; Sanchez-Monge, R.; Barber, D.; Diaz-Perales, A. Plant non-specific lipid transfer proteins: An interface between plant defence and human allergy. Biochim. Biophys. Acta 2007, 1771, 781–791. [Google Scholar] [CrossRef] [PubMed]
- Bublin, M.; Eiwegger, T.; Breiteneder, H. Do lipids influence the allergic sensitization process? J. Allergy Clin. Immunol. 2014, 134, 521–529. [Google Scholar] [CrossRef] [PubMed]
- Goliáš, J.; Humlová, Z.; Halada, P.; Hábová, V.; Janatková, I.; Tučková, L. Identification of rice proteins recognized by the IgE antibodies of patients with food allergies. J. Agric. Food Chem. 2013, 61, 8851–8860. [Google Scholar] [CrossRef] [PubMed]
- Miao, H.; Sun, P.; Liu, W.; Xu, B.; Jin, Z. Identification of Genes Encoding Granule-Bound Starch Synthase Involved in Amylose Metabolism in Banana Fruit. PLoS ONE 2014, 9, e88077. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, H.B.; Chen, M.H. Identification of an abundant 56 kDa protein implicated in food allergy as granule-bound starch synthase. J. Agric. Food Chem. 2013, 61, 5404–5409. [Google Scholar] [CrossRef] [PubMed]
- Raemakers, K.; Schreuder, M.; Suurs, L.; Furrer-Verhorst, H.; Vincken, J.-P.; de Vetten, N.; Jacobsen, E.; Visser, R.G.F. Improved Cassava Starch by Antisense Inhibition of Granule-bound Starch Synthase I. Mol. Breed. 2005, 16, 163–172. [Google Scholar] [CrossRef]
- Otani, M.; Hamada, T.; Katayama, K.; Kitahara, K.; Kim, S.H.; Takahata, Y.; Suganuma, T.; Shimada, T. Inhibition of the gene expression for granule-bound starch synthase I by RNA interference in sweet potato plants. Plant Cell Rep. 2007, 26, 1801–1807. [Google Scholar] [CrossRef] [PubMed]
- Vrinten, P.L.; Nakamura, T. Wheat Granule-Bound Starch Synthase I and II Are Encoded by Separate Genes That Are Expressed in Different Tissues. Plant Physiol. 2000, 122, 255–264. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Huang, Y.; Huang, R.; Wu, Y.; Wang, W. The genetic architecture of amylose biosynthesis in maize kernel. Plant Biotechnol. J. 2018, 16, 688–695. [Google Scholar] [CrossRef] [PubMed]
- Favrot, C. New options for allergen identification in allergic patients. In Proceedings of the 29th European Society of Veterinary Dermatology-European College of Veterinary Dermatology Congress, Lausanne, Switzerland, 7–9 September 2017. [Google Scholar]
- Levy, B.J.; DeBoer, D.J. IgE against cross-reactive carbohydrate determinants in client-owned dogs with atopic dermatitis. In Proceedings of the 29th European Society of Veterinary Dermatology-European College of Veterinary Dermatology Congress, Lausanne, Switzerland, 7–9 September 2017. [Google Scholar]
- Mueller, R.S.; Olivry, T.; Prélaud, T. Critically appraised topic on adverse food reactions of companion animals (2): Common food allergen sources in dogs and cats. BMC Vet. Res. 2016, 12, 9. [Google Scholar] [CrossRef] [PubMed]
- Richard, C.; Jacquenet, S.; Brulliard, M.; Bihain, B.E.; Favrot, C. Cooking and processing reduce IgE sensitization to foods in dogs. In Proceedings of the 29th European Society of Veterinary Dermatology-European College of Veterinary Dermatology Congress, Lausanne, Switzerland, 7–9 September 2017. abstract. [Google Scholar]
- Bizikova, P.; Olivry, T.A. Randomized, double-blinded crossover trial testing the benefit of two hydrolysed poultry-based commercial diets for dogs with spontaneous pruritic chicken allergy. Vet. Dermatol. 2016, 27. [Google Scholar] [CrossRef] [PubMed]
- Cadiergues, M.C.; Muller, A.; Bensignor, E.; Héripret, D.; Yaguiyan-Colliard, L.; Carlotti, D.-N.; Mougeot, I. Diagnostic value of home-cooked and an extensively hydrolysed diet (Anallergenic™, Royal Canin, France) in the diagnosis of canine adverse food reaction: A randomized prospective multicenter study in 72 dogs. In Proceedings of the 8th World Congress of Veterinary Dermatology, Bordeaux, France, 31 May–4 June 2016. [Google Scholar]
- Boutigny, L.; Lesponne, I.; Montreuil, C.; Roy, O. Evaluation of a new extensively hydrolysed poultry feather protein-based dry food for the dietary management of feline adverse food reaction (AFR): A 15 case pilot study. In Proceedings of the South European Veterinary Congress, Barcelona, Spain, 9–11 November 2017. [Google Scholar]
- Freiche, V.; Leclerc, A.; Dossin, O.; Dahan, J.; Toulza, O.; Mougeot, I.; Biourg, V. A very low molecular weight poultry feather hydrolyzed-based extruded diet allows idiopathic canine IBD clinical management without immunosuppressive treatment: A 13 case prospective pilot study. In Proceedings of the ECVIM Congress, Mainz, Germany, 4–6 September 2014. [Google Scholar]
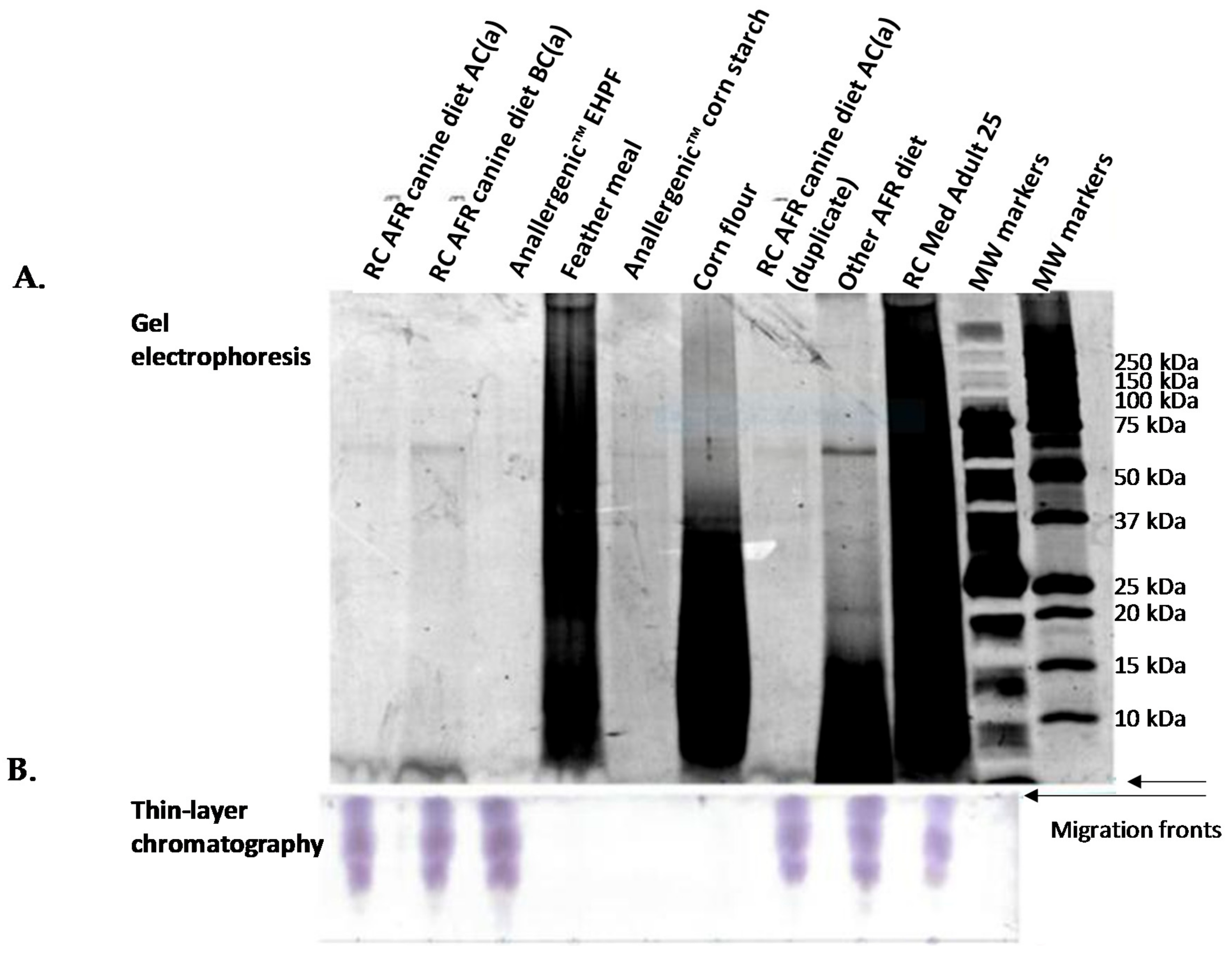
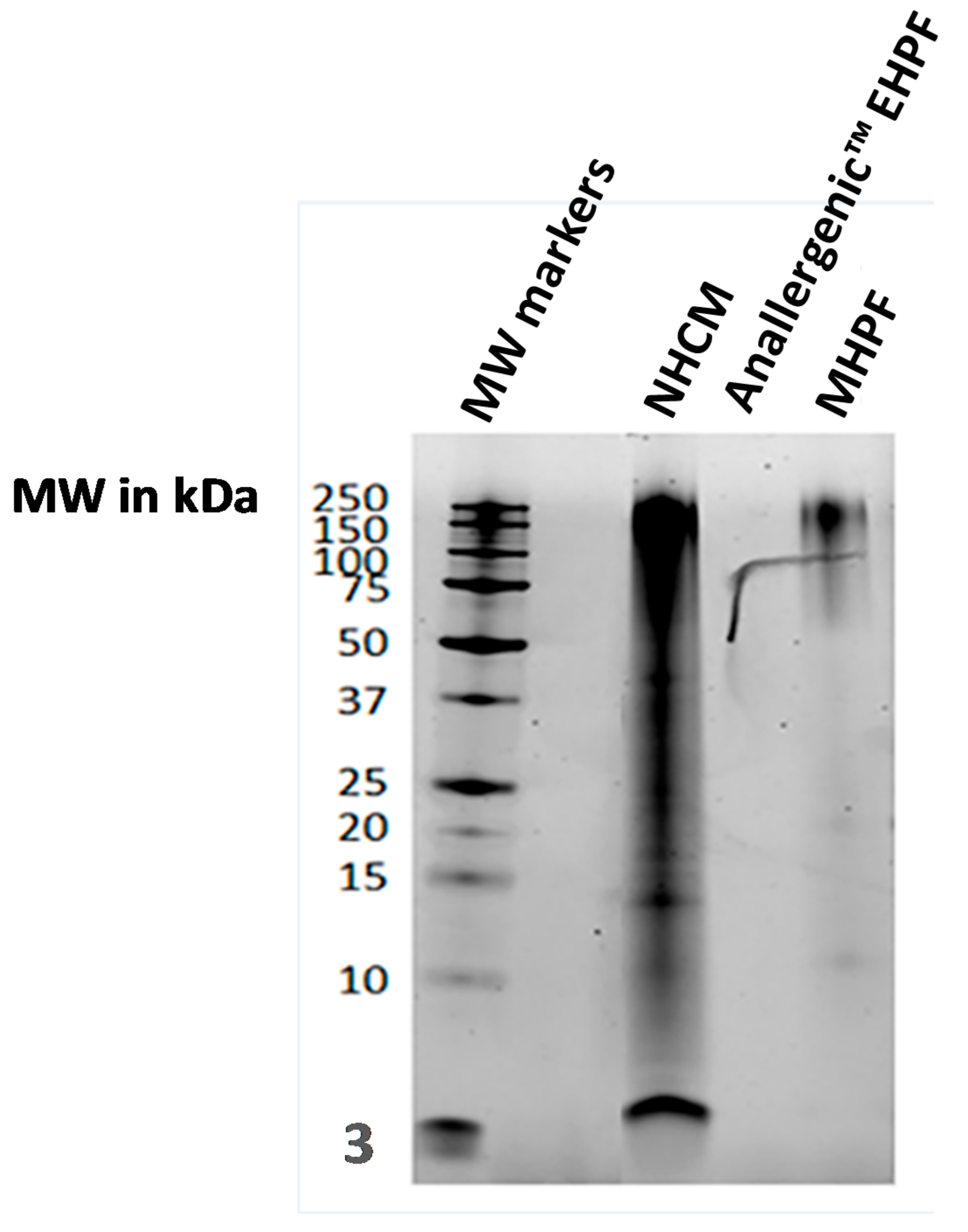
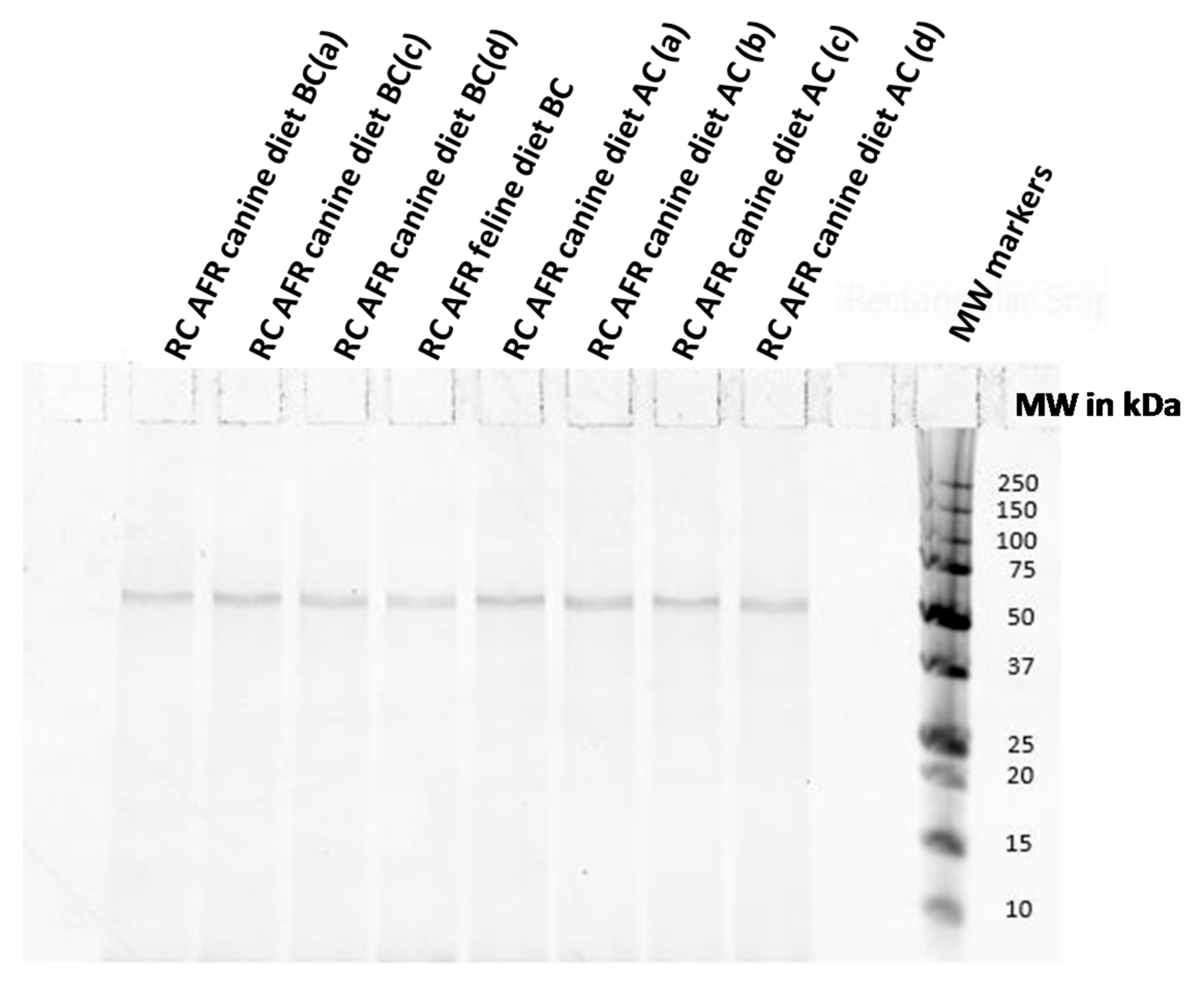
| Category | Code in Gels | Description | Comments |
|---|---|---|---|
| Complete Diets | RC AFR canine diet AC(a, b, c, d: different production batches) | Royal Canin Anallergenic™ canine diet—the finished product after final coating step | |
| RC AFR canine/feline diet BC(a, b, c, d: different production batches) | Royal Canin Anallergenic™ canine or feline diet before final coating step | No palatability enhancers in these samples | |
| Other AFR diet | Other commercial hypoallergenic dry canine diet | Chicken liver hydrolysate as main protein source | |
| RC Med Adult 25 | Royal Canin® Medium Adult 25 | Diet not recommended for AFR but analysed for comparison | |
| Raw materials | Anallergenic™ EHPF | Extensively hydrolysed poultry feather | Anallergenic™ protein source (poultry: chicken, turkey, duck) |
| MHPF | Mildly hydrolysed poultry feather | Raw materials not included in Anallergenic™ diets but analysed for comparison | |
| NHCM | Non-hydrolysed chicken meal | ||
| Corn flour | Corn flour | ||
| Feather meal | Feather meal | ||
| Anallergenic™ corn starch | Anallergenic™ corn starch |
| Processed Animal Protein Used to Experimentally Cross-Contaminate Anallergenic™ Diets at an Inclusion Level (NPPI) of 0.5% | Corresponding DNA Content, µg/g (±0.2 µg/g) |
|---|---|
| Pork | 2.1 |
| Chicken | 3.0 |
| Beef | 3.1 |
| Duck | 4.4 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lesponne, I.; Naar, J.; Planchon, S.; Serchi, T.; Montano, M. DNA and Protein Analyses to Confirm the Absence of Cross-Contamination and Support the Clinical Reliability of Extensively Hydrolysed Diets for Adverse Food Reaction-Pets. Vet. Sci. 2018, 5, 63. https://doi.org/10.3390/vetsci5030063
Lesponne I, Naar J, Planchon S, Serchi T, Montano M. DNA and Protein Analyses to Confirm the Absence of Cross-Contamination and Support the Clinical Reliability of Extensively Hydrolysed Diets for Adverse Food Reaction-Pets. Veterinary Sciences. 2018; 5(3):63. https://doi.org/10.3390/vetsci5030063
Chicago/Turabian StyleLesponne, Isabelle, Jérôme Naar, Sébastien Planchon, Tommaso Serchi, and Mauricio Montano. 2018. "DNA and Protein Analyses to Confirm the Absence of Cross-Contamination and Support the Clinical Reliability of Extensively Hydrolysed Diets for Adverse Food Reaction-Pets" Veterinary Sciences 5, no. 3: 63. https://doi.org/10.3390/vetsci5030063
APA StyleLesponne, I., Naar, J., Planchon, S., Serchi, T., & Montano, M. (2018). DNA and Protein Analyses to Confirm the Absence of Cross-Contamination and Support the Clinical Reliability of Extensively Hydrolysed Diets for Adverse Food Reaction-Pets. Veterinary Sciences, 5(3), 63. https://doi.org/10.3390/vetsci5030063





