Cutaneous Hypersensitivity Dermatoses in the Feline Patient: A Review of Allergic Skin Disease in Cats
Abstract
:1. Introduction
2. Flea Bite Hypersensitivity and Other Insect Bite Allergies
2.1. Flea Bite Hypersensitivity (Flea Allergy Dermatitis)
2.2. Mosquito Bite Hypersensitivity
3. Food Induced Hypersensitivity Dermatitis (Cutaneous Adverse Food Reaction; Food Allergy)
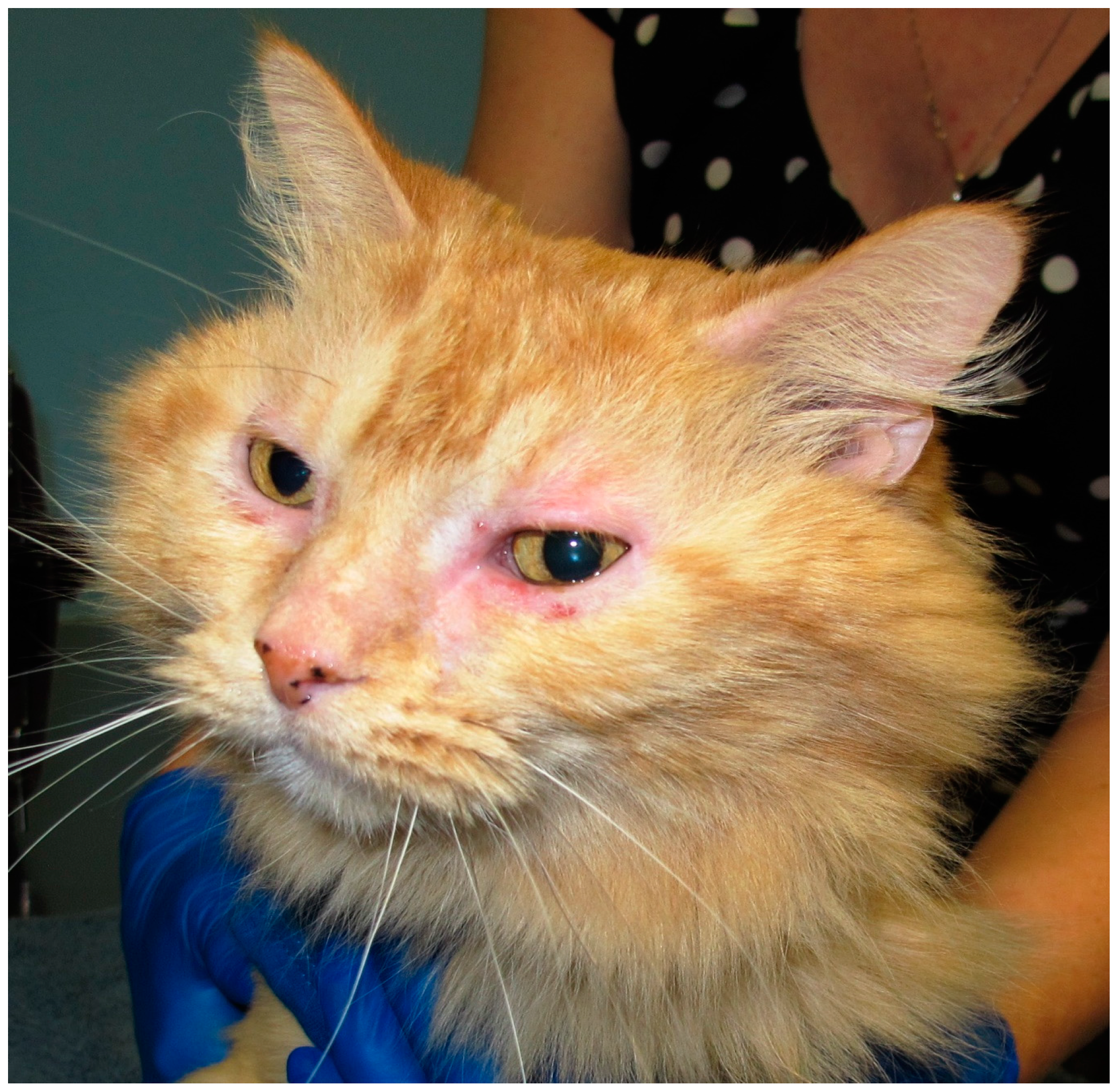
4. Non-Flea, Non-Food Hypersensitivity Dermatitis (Feline Atopic Syndrome)
5. Conclusions
Conflicts of Interest
Abbreviations
| IgE | immunoglobulin E |
| ENFNFHD | non-flea, non-food hypersensitivity |
| FAD | flea allergy dermatitis |
| FSA1 | flea salivary antigen 1 |
| DNA | deoxyribonucleic acid |
| DEET | N,N-diethyl-m-toluamide |
| CAFR | cutaneous adverse food reaction |
| AD | atopic dermatitis |
| CD | cluster of differentiation molecule |
References
- Hobi, S.; Linek, M.; Marignac, G.; Olivry, T.; Beco, L.; Nett, C.; Fontaine, J.; Roosje, P.; Bergvall, K.; Belova, S.; et al. Clinical characteristics and causes of pruritus in cats: A multicentre study on feline hypersensitivity-associated dermatoses. Vet. Dermatol. 2011, 22, 406–413. [Google Scholar] [CrossRef] [PubMed]
- Reinero, C.R. Feline immunoglobulin E: Historical perspective, diagnostics and clinical relevance. Vet. Immunol. Immunopathol. 2009, 132, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Miller, W.H.; Griffin, C.E.; Campbell, K.L. Hypersensitivity disorders. In Muller and Kirk’s Small Animal Dermatology, 7th ed.; Elsevier: St. Louis, MS, USA, 2013; pp. 363–431. [Google Scholar]
- Bond, R.; Riddle, A.; Mottram, L.; Beugnet, F.; Stevenson, R. Survey of flea infestation in dogs and cats in the United Kingdom during 2005. Vet. Rec. 2007, 160, 503–506. [Google Scholar] [CrossRef] [PubMed]
- Colombini, S.; Hodgin, E.C.; Foil, C.S.; Hosgood, G.; Foil, L.D. Induction of feline flea allergy dermatitis and the incidence and histopathological characteristics of concurrent indolent lip ulcers. Vet. Dermatol. 2001, 12, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Bond, R. Pathogenesis (Flea Bite Allergy). In Veterinary Allergy; Noli, C., Foster, A., Rosenkrantz, W., Eds.; Wiley Blackwell: West Sussex, UK, 2014; pp. 249–251. [Google Scholar]
- O’Dair, H.A.; Markwell, P.J.; Maskell, I.E. An open prospective investigation into aetiology in a group of cats with suspected allergic skin disease. Vet. Dermatol. 1996, 7, 193–202. [Google Scholar] [CrossRef]
- McCall, C.A.; Stedman, K.E.; Bevier, D.E.; Kunkle, G.A.; Foil, C.S.; Foil, L.D. Correlation of feline IgE, determined by FcεRIα-based ELISA technology, and IDST to Ctenocephalides felis salivary antigens in a feline model of flea bite allergic dermatitis. Compend. Contin. Educ. Pract. Vet. 1997, 19, 29–32. [Google Scholar]
- Moriello, K.A.; McMurdy, M.A. The prevalence of positive intradermal skin test reactions to flea extract in clinically normal cats. Companion Anim. Pract. 1989, 19, 28–30. [Google Scholar]
- Jin, J.; Ding, Z.; Meng, F.; Liu, Q.; Ng, T.; Hu, Y.; Zhao, G.; Zhai, B.; Chu, H.J.; Wang, B. An immunotherapeutic treatment against flea allergy dermatitis in cats by co-immunization of DNA and protein vaccines. Vaccine 2010, 28, 1997–2004. [Google Scholar] [CrossRef] [PubMed]
- Nagata, M. Mosquito bite. In Veterinary Allergy; Noli, C., Foster, A., Rosenkrantz, W., Eds.; Wiley Blackwell: West Sussex, UK, 2014; pp. 267–270. [Google Scholar]
- Wilkinson, G.T.; Bate, M.J. A possible further clinical manifestation of the feline eosinophilic granuloma complex. JAAHA 1984, 20, 325–331. [Google Scholar]
- Olivry, T.; Mueller, R.S. Critically appraised topic on adverse food reactions of companion animals (3): Prevalence of cutaneous adverse food reactions in dogs and cats. BMC Vet. Res. 2017, 13, 51. [Google Scholar] [CrossRef] [PubMed]
- Hillier, A.; Griffin, C.E. The ACVD task force on canine atopic dermatitis (X): Is there a relationship between canine atopic dermatitis and cutaneous adverse food reactions? Vet. Immunol. Immunopathol. 2001, 81, 227–231. [Google Scholar] [CrossRef]
- Gilbert, S.; Halliwell, R.E. The effects of endoparasitism on the immune response to orally administered antigen in cats. Vet. Immunol. Immunopathol. 2005, 106, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Friberg, C. Feline facial dermatoses. Vet. Clin. North Am. Small Anim. Pract. 2006, 36, 115–140. [Google Scholar] [CrossRef] [PubMed]
- Guilford, W.G.; Markwell, P.J.; Jones, B.R.; Harte, J.G.; Wills, J.M. Prevalence and causes of food sensitivity in cats with chronic pruritus, vomiting, or diarrhea. J Nutr. 1998, 128, 2790S–2791S. [Google Scholar] [PubMed]
- Guilford, W.G.; Jones, B.R.; Markwell, P.J.; Arthur, D.G.; Collett, M.G.; Harte, J.G. Food sensitivity in cats with chronic idiopathic gastrointestinal problems. J. Vet. Intern. Med. 2001, 15, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Carlotti, D.N.; Remy, I.; Prost, C. Food allergy in dogs and cats. A review and report of 43 cases. Vet. Dermatol. 1990, 1, 55–62. [Google Scholar] [CrossRef]
- Kennis, R.A. Food allergies: Update of pathogenesis, diagnoses, and management. Vet. Clin. North Am. Small Anim. Pract. 2006, 36, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Foster, A.P.; Knowles, T.G.; Moore, A.H.; Cousins, P.D.; Day, M.J.; Hall, E.J. Serum IgE and IgG responses to food antigens in normal and atopic dogs, and dogs with gastrointestinal disease. Vet. Immunol. Immunopathol. 2003, 92, 113–124. [Google Scholar] [CrossRef]
- Olivry, T.; Mueller, R.S.; Prélaud, P. critically appraised topic on adverse food reactions of companion animals (1): Duration of elimination diets. BMC Vet. Res. 2015, 11, 225. [Google Scholar] [CrossRef] [PubMed]
- Mueller, R.S.; Olivry, T.; Prélaud, P. Critically appraised topic on adverse food reactions of companion animals (2): Common food allergen sources in dogs and cats. BMC Vet. Res. 2016, 12, 9. [Google Scholar] [CrossRef] [PubMed]
- Biziokva, P.; Olivry, T. A randomized, double-blinded crossover trial testing the benefit of two hydrolysed poultry-based commercial diets for dogs with spontaneous pruritic chicken allergy. Vet. Dermatol. 2016, 27, 289-e70. [Google Scholar] [CrossRef] [PubMed]
- Reedy, L.M. Results of allergy testing and hyposensitization in selected feline skin diseases. J. Am. Anim. Hosp. Assoc. 1982, 18, 618–623. [Google Scholar]
- Moriello, K.A. Feline atopy in three littermates. Vet. Dermatol. 2001, 12, 177–181. [Google Scholar] [CrossRef] [PubMed]
- Prost, C. Les dermatoses allergiques du chat. Prat. Méd. Chir. Anim. Comp. 1993, 28, 151–153. [Google Scholar]
- Taglinger, K.; Day, M.J.; Foster, A.P. Characterization of inflammatory cell infiltration in feline allergic skin disease. J. Comp. Pathol. 2007, 137, 211–223. [Google Scholar] [CrossRef] [PubMed]
- Roosje, P.J.; van Kooten, P.J.; Thepen, T.; Bihari, I.C.; Rutten, V.P.; Koeman, J.P.; Willemse, T. Increased numbers of CD4+ and CD8+ T cells in lesional skin of cats with allergic dermatitis. Vet. Pathol. 1998, 35, 268–273. [Google Scholar] [CrossRef] [PubMed]
- Cardona, I.D.; Cho, S.H.; Leung, D.Y. Role of bacterial superantigens in atopic dermatitis: Implications for future therapeutic strategies. Am. J. Clin. Dermatol. 2006, 7, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Marsella, R.; Olivry, T. Animal models of atopic dermatitis. Clin. Dermatol. 2003, 21, 122–133. [Google Scholar] [CrossRef]
- Foster, A.P.; Roosje, P.J. Update on feline immunoglobulin E (IgE) and diagnostic recommendations for atopy. In Consultations in Feline Internal Medicine, 5th ed.; August, J.R., Ed.; Elsevier: St. Louis, MO, USA, 2006; pp. 229–238. [Google Scholar]
- Woolley, K.L.; Kelly, R.F.; Fazakerley, J.; Williams, N.J.; Nuttall, T.J.; McEwan, N.A. Reduced in vitro adherence of Staphylococcus species to feline corneocytes compared to canine and human corneocytes. Vet. Dermatol. 2008, 19, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Abraham, J.L.; Morris, D.O.; Griffeth, G.C.; Shofer, F.S.; Rankin, S.C. Surveillance of healthy cats and cats with inflammatory skin disease for colonization of the skin by methicillin-resistant coagulase-positive staphylococci and Staphylococcus schleiferi ssp. schleiferi. Vet. Dermatol. 2007, 18, 252–259. [Google Scholar] [CrossRef] [PubMed]
- Wildermuth, B.E.; Griffin, C.E.; Rosenkrantz, W.S. Feline pyoderma therapy. Clin. Tech. Small Anim. Pract. 2006, 21, 150–156. [Google Scholar] [CrossRef] [PubMed]
- Hui, W.Y.; Vogelnest, L.J. Feline superficial pyoderma: A retrospective study of 52 cases (2001–2011). Vet. Dermatol. 2012, 23, 448–486. [Google Scholar]
- Mauldin, E.A.; Morris, D.O.; Goldschmidt, M.H. Retrospective study: The presence of Malassezia in feline skin biopsies. A clinicopathological study. Vet. Dermatol. 2002, 13, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Sierra, P.; Guillot, J.; Jacob, H.; Bussiéras, S.; Chermette, R. Fungal flora on cutaneous and mucosal surfaces of cats infected with feline immunodeficiency virus or feline leukemia virus. Am. J. Vet. Res. 2000, 61, 158–161. [Google Scholar] [CrossRef] [PubMed]
- Ordeix, L.; Galeotti, F.; Scarampella, F.; Dedola, C.; Bardagi, M.; Romano, E.; Fondati, A. Malassezia spp. overgrowth in allergic cats. Vet. Dermatol. 2007, 18, 316–323. [Google Scholar] [CrossRef] [PubMed]
- Masuda, K.; Kurata, K.; Sakaguchi, M.; Yamashita, K.; Hasegawa, A.; Ohno, K.; Tsujimoto, H. Seasonal rhinitis in a cat sensitized to Japanese cedar (Cryptomeria japonica) pollen. J. Vet. Med. Sci. 2001, 63, 79–81. [Google Scholar] [CrossRef] [PubMed]
- Carlotti, D.; Prost, C. L’atopie feline. Point Vét. 1988, 20, 777. [Google Scholar]
- Thiry, E.; Addie, D.; Belák, S.; Boucraut-Baralon, C.; Egberink, H.; Frymus, T.; Gruffydd-Jones, T.; Hartmann, K.; Hosie, M.J.; Lloret, A.; et al. Feline herpes virus infection. ABCD guidelines on prevention and management. J. Feline Med. Surg. 2009, 11, 547–555. [Google Scholar] [CrossRef] [PubMed]
- Sjödahl-Essén, T.; Tidholm, A.; Thorén, P.; Persson-Wadman, A.; Bölske, G.; Aspán, A.; Berndtsson, L.T. Evaluation of different sampling methods and results of real-time PCR for detection of feline herpes virus-1, Chlamydophila felis and Mycoplasma felis in cats. Vet. Ophth. 2008, 11, 375–380. [Google Scholar] [CrossRef] [PubMed]
- Norris Reinero, C.R.; Decile, K.C.; Berghaus, R.D.; Williams, K.J.; Leutenegger, C.M.; Walby, W.F.; Schelegle, E.S.; Hyde, D.M.; Gershwin, L.J. An experimental model of allergic asthma in cats sensitized to house dust mite or bermuda grass allergen. Int. Arch. Allergy Immunol. 2004, 135, 117–131. [Google Scholar] [CrossRef] [PubMed]
- Moriello, K.A.; Stepien, R.L.; Henik, R.A.; Wenholz, L.J. Pilot study: Prevalence of positive aeroallergen reactions in 10 cats with small-airway disease without concurrent skin disease. Vet. Dermatol. 2007, 18, 94–100. [Google Scholar] [CrossRef] [PubMed]
- Reinero, C.R.; Cohn, L.A.; Delgado, C.; Spinka, C.M.; Schooley, E.K.; DeClue, A.E. Adjuvanted rush immunotherapy using CpG oligodeoxynucleotides in experimental feline allergic asthma. Vet. Immunol. Immunopathol. 2008, 121, 241–250. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, R.E.W. Efficacy of hyposensitization in feline allergic diseases based upon results of in vitro testing for allergen-specific immunoglobulin E. J. Am. Anim. Hosp. Assoc. 1997, 33, 282–288. [Google Scholar] [CrossRef] [PubMed]
- Reinero, C.R.; Byerly, J.R.; Berghaus, R.D.; Berghaus, L.J.; Schelegle, E.S.; Hyde, D.M.; Gershwin, L.J. Rush immunotherapy in an experimental model of feline allergic asthma. Vet. Immunol. Immunopathol. 2006, 110, 141–153. [Google Scholar] [CrossRef] [PubMed]
- Lee-Fowler, T.M.; Cohn, L.A.; DeClue, A.E.; Spinka, C.M.; Reinero, C.R. Evaluation of subcutaneous versus mucosal (intranasal) allergen-specific rush immunotherapy in experimental feline asthma. Vet. Immunol. Immunopathol. 2009, 129, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Diesel, A.B. Symptomatic treatments. In Veterinary Allergy; Noli, C., Foster, A., Rosenkrantz, W., Eds.; Wiley Blackwell: West Sussex, UK, 2014; pp. 228–233. [Google Scholar]
- Favrot, C. Feline non-flea induced hypersensitivity dermatitis: Clinical features, diagnosis and treatment. J. Feline Med. Surg. 2013, 15, 778–784. [Google Scholar] [CrossRef] [PubMed]
- Diesel, A.; Moriello, K.A. A busy clinician’s review of cyclosporine. Vet. Med. 2008, 103, 266–273. [Google Scholar]
- Ortalda, C.; Noli, C.; Colombo, S.; Borio, S. Oclacitinib in feline nonflea-, nonfood-induced hypersensitivity dermatitis: Results of a small prospective pilot study of client-owned cats. Vet. Dermatol. 2015, 26, 235-e52. [Google Scholar] [CrossRef] [PubMed]
- Hansel, T.T.; Kropshofer, H.; Singer, T.; Mitchell, J.A.; George, A.J. The safety and side effects of monoclonal antibodies. Nat. Rev. Drug Discov. 2010, 9, 325–338. [Google Scholar] [CrossRef] [PubMed]
- Diesel, A.B. Allergen-specific immunotherapy. In Veterinary Allergy; Noli, C., Foster, A., Rosenkrantz, W., Eds.; Wiley Blackwell: West Sussex, UK, 2014; pp. 234–236. [Google Scholar]
- Trimmer, A.M.; Griffin, C.E.; Boord, M.J.; Rosenkrantz, W.S. Rush allergen specific immunotherapy protocol in feline atopic dermatitis: A pilot study. Vet. Dermatol. 2005, 16, 324–329. [Google Scholar] [CrossRef] [PubMed]
© 2017 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Diesel, A. Cutaneous Hypersensitivity Dermatoses in the Feline Patient: A Review of Allergic Skin Disease in Cats. Vet. Sci. 2017, 4, 25. https://doi.org/10.3390/vetsci4020025
Diesel A. Cutaneous Hypersensitivity Dermatoses in the Feline Patient: A Review of Allergic Skin Disease in Cats. Veterinary Sciences. 2017; 4(2):25. https://doi.org/10.3390/vetsci4020025
Chicago/Turabian StyleDiesel, Alison. 2017. "Cutaneous Hypersensitivity Dermatoses in the Feline Patient: A Review of Allergic Skin Disease in Cats" Veterinary Sciences 4, no. 2: 25. https://doi.org/10.3390/vetsci4020025
APA StyleDiesel, A. (2017). Cutaneous Hypersensitivity Dermatoses in the Feline Patient: A Review of Allergic Skin Disease in Cats. Veterinary Sciences, 4(2), 25. https://doi.org/10.3390/vetsci4020025





