Equine Colostrum-Derived Mesenchymal Stromal Cells: A Potential Resource for Veterinary Regenerative Medicine
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Samples
2.3. Equine Colostrum-Derived MSCs’ Isolation and Culture
2.4. Characterization of Colostrum-Derived MSCs
2.4.1. Population Doubling Time (PDT) Analysis
2.4.2. CFU (Colony-Forming Unit) Assay
2.4.3. Adhesion and Migration Assays
2.4.4. Multi-Lineage In Vitro Differentiation
2.4.5. Molecular Characterization
2.4.6. Statistical Analysis
3. Results
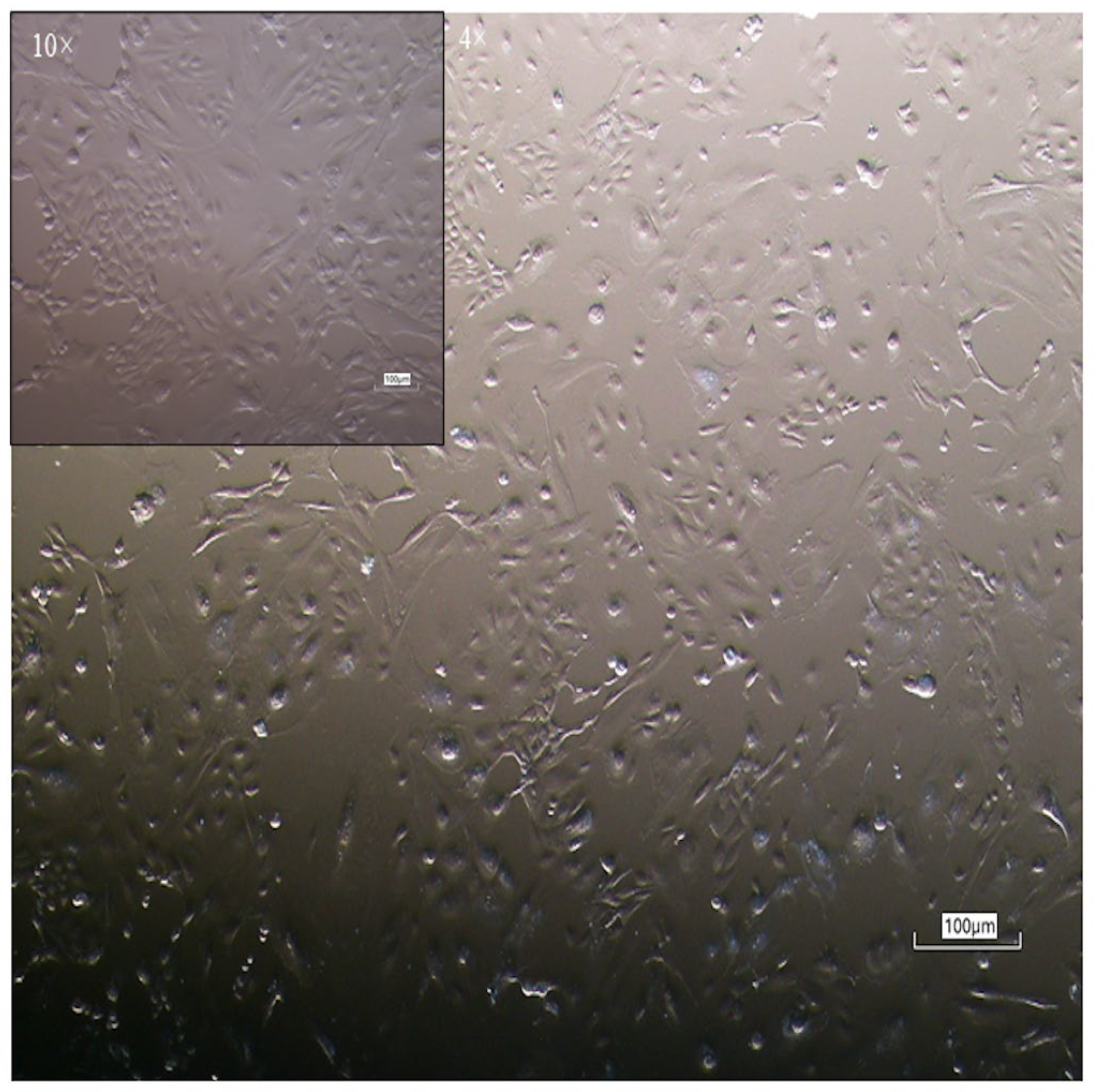
3.1. Isolation and Morphological Characterization of Equine Colostrum-Derived Cells
3.2. Characterization of Colostrum-Derived MSCs
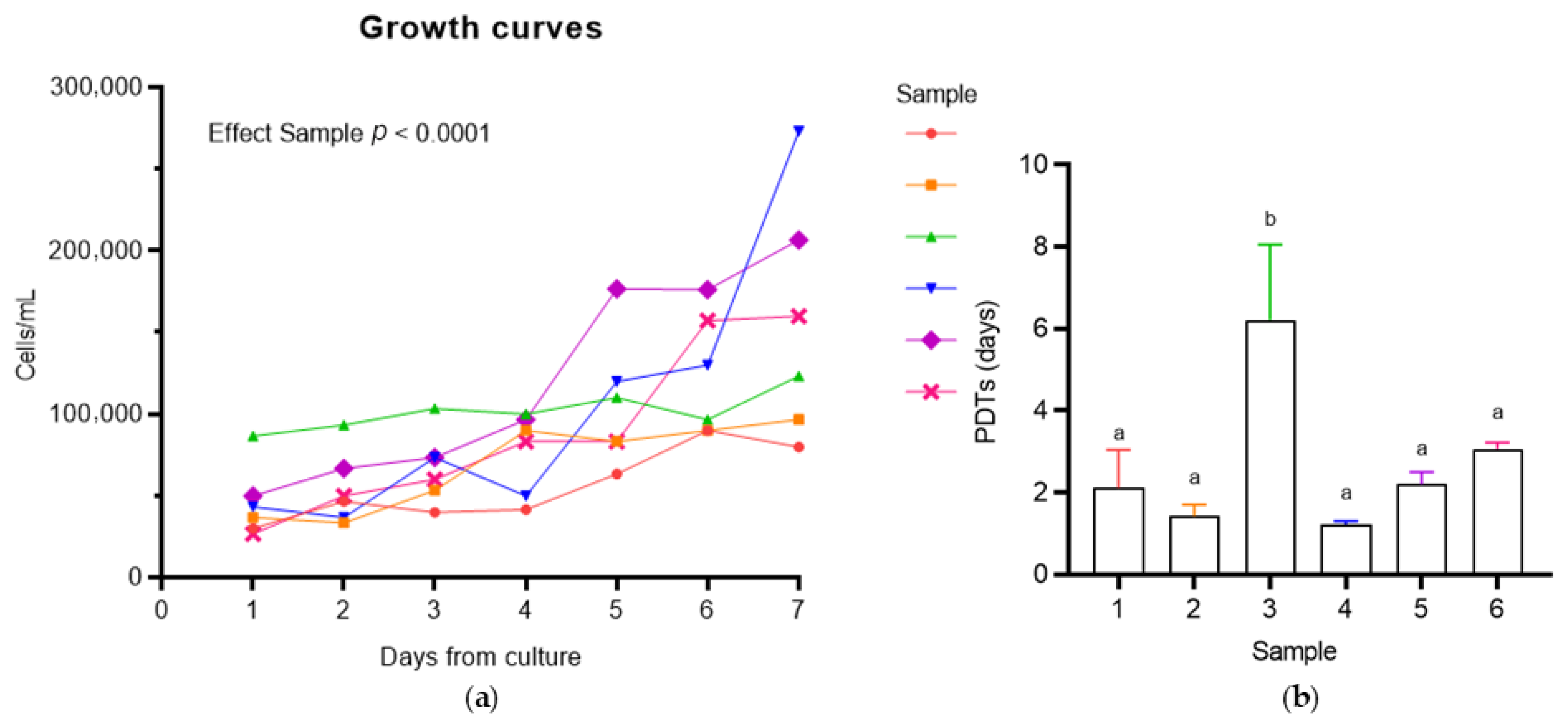
3.2.1. Population Doubling Time (PDT) Analysis
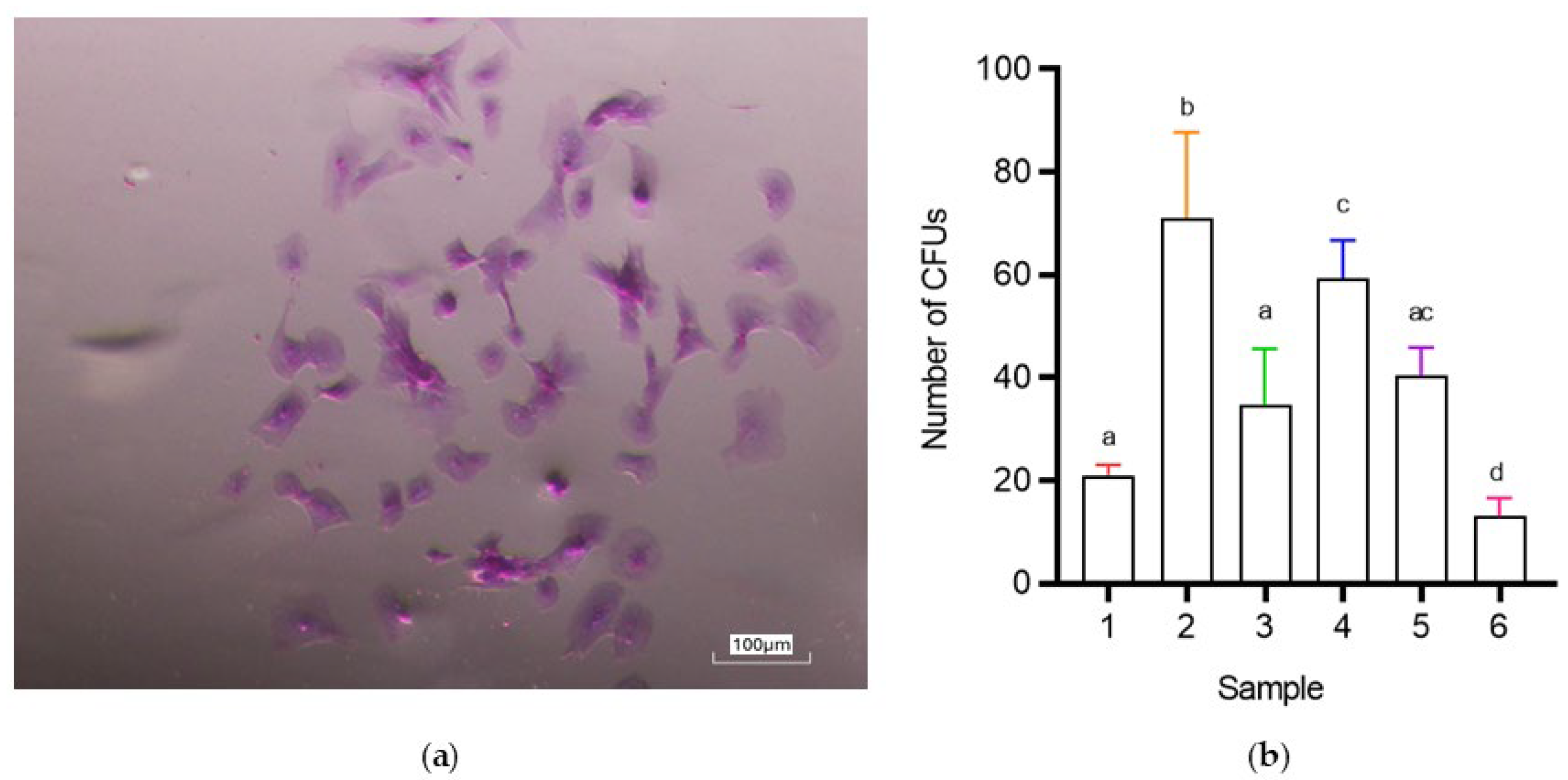
3.2.2. CFU (Colony-Forming Unit) Assay
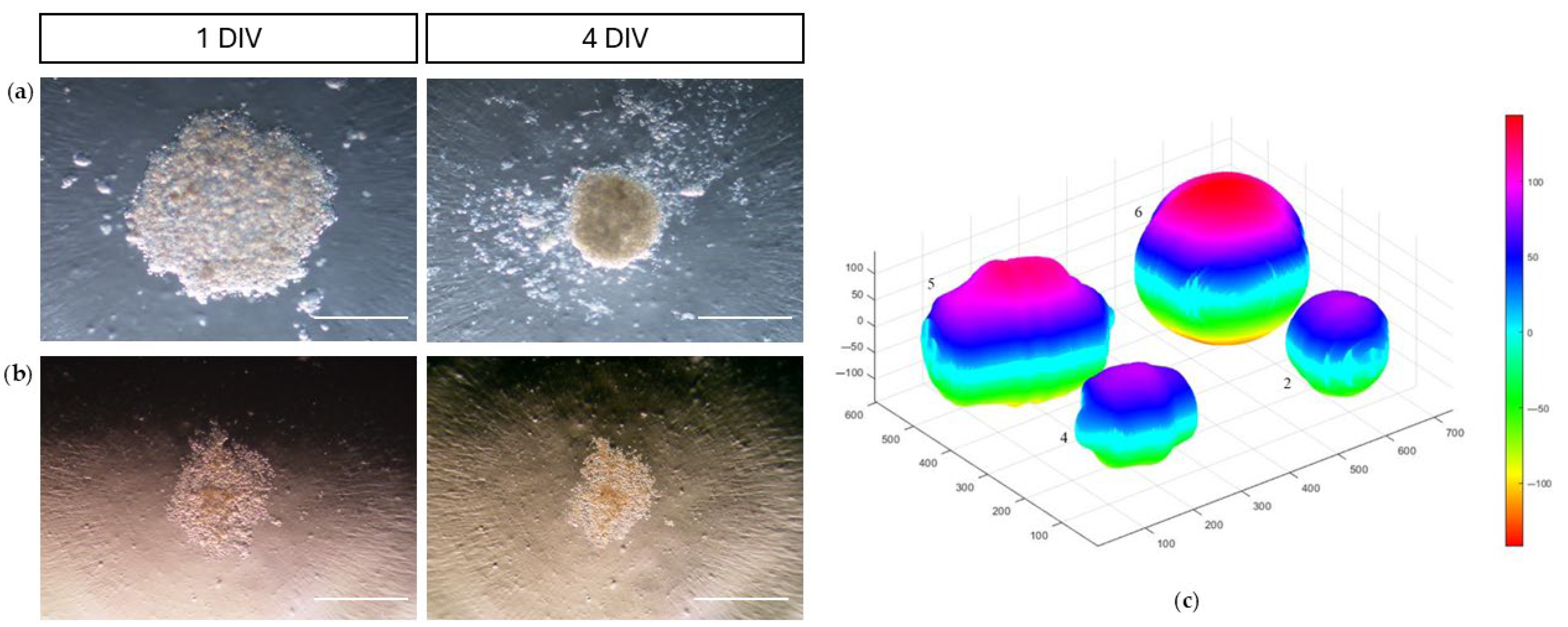
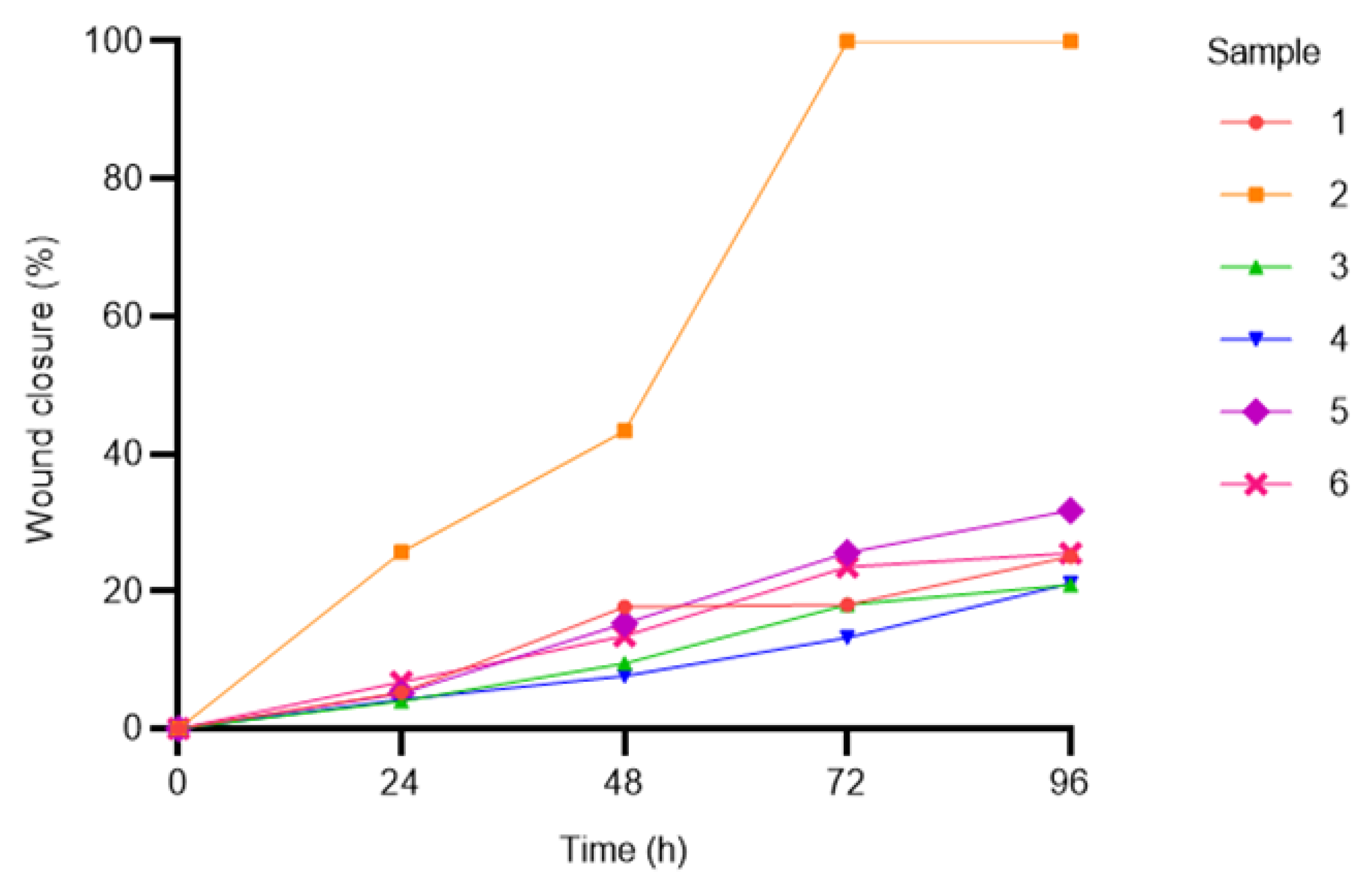
3.2.3. Adhesion and Migration Assays
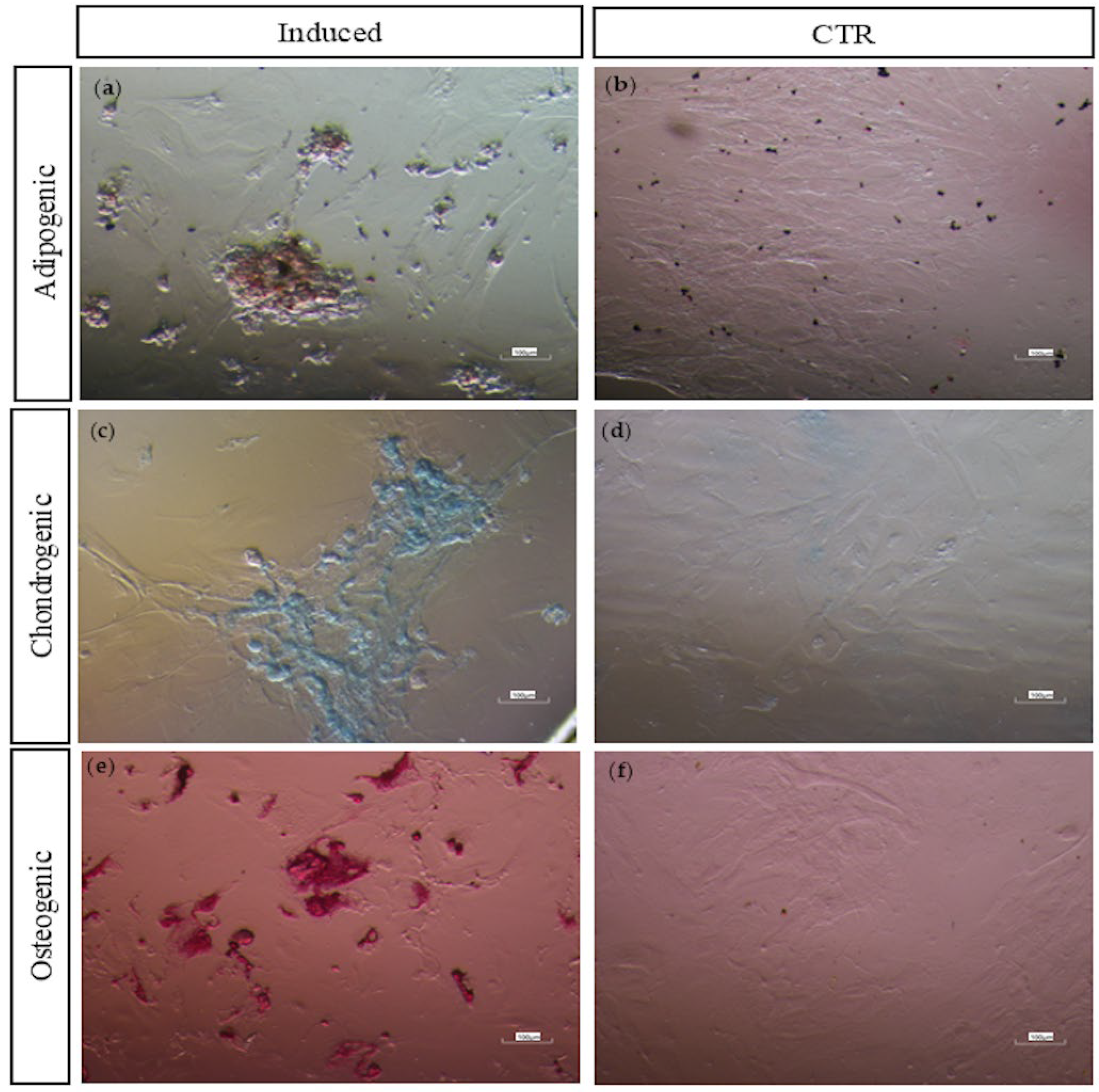
3.2.4. Multi-Lineage In Vitro Differentiation
3.2.5. Molecular Characterization
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MSCs | Mesenchymal Stem/Stromal Cells |
| C-MSCs | Colostrum-derived MSCs |
| PDT | Population Doubling Time |
| CFU | Colony-Forming Unit |
| RT-PCR | Reverse Transcription-Polymerase Chain Reaction |
| MHC | Major Histocompatibility Complex |
| MaSCs | Mammary Stem/Stromal Cells |
| CK5 | Cytokeratin 5 |
| BSCs | Breastmilk Stem Cells |
| OCT4 | Octamer-binding Transcription Factor 4 |
| SOX2 | SRY-Box Transcription Factor 2 |
| NANOG | Homeobox Protein NANOG |
| KLF4 | Kruppel-like Factor 4 |
| REX1 | Reduced Expression 1 |
| GDF3 | Growth Differentiation Factor 3 |
| SMA | Smooth Muscle Actin |
| ISCT | International Society for Cell & Gene Therapy |
| CD | Cluster of Differentiation |
| HLA-DR | Human Leukocyte Antigen—DR isotype |
| FBS | Fetal Bovine Serum |
| IgG | Immunoglobulin G |
| DPBS | Dulbecco’s polyphosphate-buffered saline |
| DMEM F-12 | Dulbecco’s Modified Eagle Medium F-12 |
| EDTA | Ethylenediaminetetraacetic Acid |
| DMSO | Dimethyl sulfoxide |
| P | Passage (number of cell subcultures) |
| RT | Room Temperature |
| IBMX | Isobutylmethylxanthine, |
| DXM | Dexamethasone, |
| hTGF-β1 | Human transforming growth factor-β1, |
| AA2P | Ascorbic acid 2-phosphate, |
| BGP | Beta-glycerophosphate. |
| DR | Differentiation Ratio |
| GAPDH | Glyceraldehyde-3-phosphate dehydrogenase |
| SD | Standard deviation |
| EMT | Epithelial-to-mesenchymal transition |
| WJ-MSCs | Wharton’s jelly MSCs |
| BM-MSCs | Bone Marrow MSCs |
| AF-MSCs | Amniotic Fluid MSCs |
| AT-MSCs | Adipose Tissue MSCs |
| UCB-MSCs, | Umbilical Cord Blood MSCs |
| AM-MSCs | Amniotic Membrane MSCs |
References
- McCue, P.M. Colostrum Banking. Equine Reproductive Procedures; Wiley: Hoboken, NJ, USA, 2014; pp. 299–301. [Google Scholar] [CrossRef]
- de Lima, T.C.; de Sobral, G.G.; de França Queiroz, A.E.S.; Chinelate, G.C.B.; Porto, T.S.; Oliveira, J.T.C.; Carneiro, G.F. Characterization of Lyophilized Equine Colostrum. J. Equine Vet. Sci. 2024, 132, 104975. [Google Scholar] [CrossRef] [PubMed]
- Perkins, G.A.; Goodman, L.B.; Wimer, C.; Freer, H.; Babasyan, S.; Wagner, B. Maternal T-Lymphocytes in Equine Colostrum Express a Primarily Inflammatory Phenotype. Vet. Immunol. Immunopathol. 2014, 161, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Dukes, H.H.; Reece, W.O. Fisiologia Dos Animais Domésticos; Guanabara Koogan: Rio de Janeiro, Brazil, 2019. [Google Scholar]
- Pichiri, G.; Lanzano, D.; Piras, M.; Dessì, A.; Reali, A.; Puddu, M.; Noto, A.; Fanos, V.; Coni, C.; Faa, G.; et al. Human Breast Milk Stem Cells: A New Challenge for Perinatologists. J. Pediatr. Neonatal Individ. Med. 2016, 5, e050120-1. [Google Scholar] [CrossRef]
- Indumathi, S.; Dhanasekaran, M.; Rajkumar, J.S.; Sudarsanam, D. Exploring the Stem Cell and Non-Stem Cell Constituents of Human Breast Milk. Cytotechnology 2013, 65, 385–393. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Chong, Y.S.; Choolani, M.A.; Cregan, M.D.; Chan, J.K.Y. Unravelling the Mystery of Stem/Progenitor Cells in Human Breast Milk. PLoS ONE 2010, 5, e14421. [Google Scholar] [CrossRef] [PubMed]
- Visvader, J.E. Keeping Abreast of the Mammary Epithelial Hierarchy and Breast Tumorigenesis. Genes Dev. 2009, 23, 2563–2577. [Google Scholar] [CrossRef] [PubMed]
- Visvader, J.E.; Lindeman, G.J. Mammary Stem Cells and Mammopoiesis. Cancer Res. 2006, 66, 9798–9801. [Google Scholar] [CrossRef] [PubMed]
- Patki, S.; Kadam, S.; Chandra, V.; Bhonde, R. Human Breast Milk Is a Rich Source of Multipotent Mesenchymal Stem Cells. Hum. Cell 2010, 23, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Lochter, A. Plasticity of Mammary Epithelia during Normal Development and Neoplastic Progression. Biochem. Cell Biol. 2011, 76, 997–1008. [Google Scholar] [CrossRef]
- Hassiotou, F.; Geddes, D.T.; Hartmann, P.E. Cells in Human Milk: State of the Science. J. Hum. Lact. 2013, 29, 171–182. [Google Scholar] [CrossRef] [PubMed]
- Somasundaram, I.; Kaingade, P.; Bhonde, R. Breast Milk-Derived Mesenchymal Stem-Like Cells: History and Mystery. In Stem Cell and Non-Stem Cell Components of Breast Milk; Springer Nature: Singapore, 2023; pp. 45–53. [Google Scholar] [CrossRef]
- Cregan, M.D.; Fan, Y.; Appelbee, A.; Brown, M.L.; Klopcic, B.; Koppen, J.; Mitoulas, L.R.; Piper, K.M.E.; Choolani, M.A.; Chong, Y.S.; et al. Identification of Nestin-Positive Putative Mammary Stem Cells in Human Breastmilk. Cell Tissue Res. 2007, 329, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Thomas, E.; Zeps, N.; Rigby, P.; Hartmann, P. Reactive Oxygen Species Initiate Luminal but Not Basal Cell Death in Cultured Human Mammary Alveolar Structures: A Potential Regulator of Involution. Cell Death Dis. 2011, 2, e189. [Google Scholar] [CrossRef] [PubMed]
- Hassiotou, F.; Beltran, A.; Chetwynd, E.; Stuebe, A.M.; Twigger, A.J.; Metzger, P.; Trengove, N.; Lai, C.L.; Filgueira, L.; Blancafort, P.; et al. Breastmilk Is a Novel Source of Stem Cells with Multilineage Differentiation Potential. Stem Cells 2012, 30, 2164–2174. [Google Scholar] [CrossRef] [PubMed]
- Twigger, A.J.; Hepworth, A.R.; Tat Lai, C.; Chetwynd, E.; Stuebe, A.M.; Blancafort, P.; Hartmann, P.E.; Geddes, D.T.; Kakulas, F. Gene Expression in Breastmilk Cells Is Associated with Maternal and Infant Characteristics. Sci. Rep. 2015, 5, 12933. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, S.M.; Talaei-Khozani, T.; Sani, M.; Owrangi, B. Differentiation of Human Breast-Milk Stem Cells to Neural Stem Cells and Neurons. Neurol. Res. Int. 2014, 2014, 807896. [Google Scholar] [CrossRef] [PubMed]
- Abd Allah, S.H.; Shalaby, S.M.; El-Shal, A.S.; El Nabtety, S.M.; Khamis, T.; Abd El Rhman, S.A.; Ghareb, M.A.; Kelani, H.M. Breast Milk MSCs: An Explanation of Tissue Growth and Maturation of Offspring. IUBMB Life 2016, 68, 935–942. [Google Scholar] [CrossRef] [PubMed]
- Briere, C.E.; McGrath, J.M.; Jensen, T.; Matson, A.; Finck, C. Breast Milk Stem Cells. Adv. Neonatal Care 2016, 16, 410–419. [Google Scholar] [CrossRef] [PubMed]
- Hassiotou, F.; Hartmann, P.E. At the Dawn of a New Discovery: The Potential of Breast Milk Stem Cells. Adv. Nutr. 2014, 5, 770–778. [Google Scholar] [CrossRef] [PubMed]
- Kaingade, P.; Somasundaram, I.; Sharma, A.; Patel, D.; Marappagounder, D. Cellular Components, Including Stem-Like Cells, of Preterm Mother’s Mature Milk as Compared with Those in Her Colostrum: A Pilot Study. Breastfeed. Med. 2017, 12, 446–449. [Google Scholar] [CrossRef] [PubMed]
- Kaingade, P.M.; Somasundaram, I.; Nikam, A.B.; Sarang, S.A.; Patel, J.S. Assessment of Growth Factors Secreted by Human Breastmilk Mesenchymal Stem Cells. Breastfeed. Med. 2016, 11, 26–31. [Google Scholar] [CrossRef] [PubMed]
- Sam Vijay Kumar, J.; Rajkumar, H.; Nagasamy, S.; Ghose, S.; Subramanian, B.; Adithan, C. Human Colostrum Is a Rich Source of Cells with Stem Cell-Like Properties. SBV J. Basic Clin. Appl. Health Sci. 2017, 1, 26–31. [Google Scholar] [CrossRef]
- Sani, M.; Hosseini, S.M.; Salmannejad, M.; Aleahmad, F.; Ebrahimi, S.; Jahanshahi, S.; Talaei-Khozani, T. Origins of the Breast Milk-Derived Cells; an Endeavor to Find the Cell Sources. Cell Biol. Int. 2015, 39, 611–618. [Google Scholar] [CrossRef] [PubMed]
- Witkowska-Zimny, M.; Kaminska-El-Hassan, E. Cells of Human Breast Milk. Cell Mol. Biol. Lett. 2017, 22, 11. [Google Scholar] [CrossRef] [PubMed]
- Hassiotou, F.; Heath, B.; Ocal, O.; Filgueira, L.; Geddes, D.; Hartmann, P.; Wilkie, T. Breastmilk Stem Cell Transfer from Mother to Neonatal Organs (216.4). FASEB J. 2014, 28, 216.4. [Google Scholar] [CrossRef]
- Aydın, M.Ş.; Yiğit, E.N.; Vatandaşlar, E.; Erdoğan, E.; Öztürk, G. Transfer and Integration of Breast Milk Stem Cells to the Brain of Suckling Pups. Sci. Rep. 2018, 8, 14289. [Google Scholar] [CrossRef] [PubMed]
- Barinaga, M. Cells Exchanged during Pregnancy Live On. Science 2002, 296, 2169–2172. [Google Scholar] [CrossRef] [PubMed]
- Hanson, L.Å.; Silfverdal, S.A.; Strömbäck, L.; Erling, V.; Zaman, S.; Olcén, P.; Telemo, E. The Immunological Role of Breast Feeding. Pediatr. Allergy Immunol. 2001, 12 (Suppl. S14), 15–19. [Google Scholar] [CrossRef] [PubMed]
- Hamosh, M. Bioactive Factors in Human Milk. Pediatr. Clin. N. Am. 2001, 48, 69–86. [Google Scholar] [CrossRef] [PubMed]
- Ranera, B.; Lyahyai, J.; Romero, A.; Vázquez, F.J.; Remacha, A.R.; Bernal, M.L.; Zaragoza, P.; Rodellar, C.; Martín-Burriel, I. Immunophenotype and Gene Expression Profiles of Cell Surface Markers of Mesenchymal Stem Cells Derived from Equine Bone Marrow and Adipose Tissue. Vet. Immunol. Immunopathol. 2011, 144, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Alipour, F.; Parham, A.; Mehrjerdi, H.K.; Dehghani, H. Equine Adipose-Derived Mesenchymal Stem Cells: Phenotype and Growth Characteristics, Gene Expression Profile and Differentiation Potentials. Cell J. 2015, 16, 456. [Google Scholar] [CrossRef] [PubMed]
- Venugopal, P.; Balasubramanian, S.; Majumdar, A.S.; Ta, M. Isolation, Characterization, and Gene Expression Analysis of Wharton’s Jelly-Derived Mesenchymal Stem Cells under Xeno-Free Culture Conditions. Stem Cells Cloning 2011, 4, 39–50. [Google Scholar] [CrossRef] [PubMed][Green Version]
- in’t Anker, P.S.; Scherjon, S.A.; Kleijburg-van der Keur, C.; de Groot-Swings, G.M.J.S.; Claas, F.H.J.; Fibbe, W.E.; Kanhai, H.H.H. Isolation of Mesenchymal Stem Cells of Fetal or Maternal Origin from Human Placenta. Stem Cells 2004, 22, 1338–1345. [Google Scholar] [CrossRef] [PubMed]
- Mihu, C.M.; Rus Ciucă, D.; Soritău, O.; Suşman, S.; Mihu, D. Isolation and Characterization of Mesenchymal Stem Cells from the Amniotic Membrane. Rom. J. Morphol. Embryol. 2009, 50, 73–77. [Google Scholar] [PubMed]
- In ’t Anker, P.S.; Scherjon, S.A.; Kleijburg-van der Keur, C.; Noort, W.A.; Claas, F.H.J.; Willemze, R.; Fibbe, W.E.; Kanhai, H.H.H. Amniotic Fluid as a Novel Source of Mesenchymal Stem Cells for Therapeutic Transplantation. Blood 2003, 102, 1548–1549. [Google Scholar] [CrossRef] [PubMed]
- Horwitz, E.M.; Le Blanc, K.; Dominici, M.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.C.; Deans, R.J.; Krause, D.S.; Keating, A. Clarification of the Nomenclature for MSC: The International Society for Cellular Therapy Position Statement. Cytotherapy 2005, 7, 393–395. [Google Scholar] [CrossRef] [PubMed]
- Le Blanc, K.; Davies, L.C. Mesenchymal Stromal Cells and the Innate Immune Response. Immunol. Lett. 2015, 168, 140–146. [Google Scholar] [CrossRef] [PubMed]
- Caplan, A.I. MSCs: The Sentinel and Safe-Guards of Injury. J. Cell Physiol. 2016, 231, 1413–1416. [Google Scholar] [CrossRef] [PubMed]
- Bernardo, M.E.; Fibbe, W.E. Mesenchymal Stromal Cells: Sensors and Switchers of Inflammation. Cell Stem Cell 2013, 13, 392–402. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, J.R.; Teixeira, G.Q.; Santos, S.G.; Barbosa, M.A.; Almeida-Porada, G.; Gonçalves, R.M. Mesenchymal Stromal Cell Secretome: Influencing Therapeutic Potential by Cellular Pre-Conditioning. Front. Immunol. 2018, 9, 425936. [Google Scholar] [CrossRef] [PubMed]
- Serra, M.; Cunha, B.; Peixoto, C.; Gomes-Alves, P.; Alves, P.M. Advancing Manufacture of Human Mesenchymal Stem Cells Therapies: Technological Challenges in Cell Bioprocessing and Characterization. Curr. Opin. Chem. Eng. 2018, 22, 226–235. [Google Scholar] [CrossRef]
- Sutton, M.T.; Fletcher, D.; Ghosh, S.K.; Weinberg, A.; Van Heeckeren, R.; Kaur, S.; Sadeghi, Z.; Hijaz, A.; Reese, J.; Lazarus, H.M.; et al. Antimicrobial Properties of Mesenchymal Stem Cells: Therapeutic Potential for Cystic Fibrosis Infection, and Treatment. Stem Cells Int. 2016, 2016, 5303048. [Google Scholar] [CrossRef] [PubMed]
- Vizoso, F.J.; Eiro, N.; Cid, S.; Schneider, J.; Perez-Fernandez, R. Mesenchymal Stem Cell Secretome: Toward Cell-Free Therapeutic Strategies in Regenerative Medicine. Int. J. Mol. Sci. 2017, 18, 1852. [Google Scholar] [CrossRef] [PubMed]
- Coni, P.; Piras, M.; Piludu, M.; Lachowicz, J.I.; Matteddu, A.; Coni, S.; Reali, A.; Fanos, V.; Jaremko, M.; Faa, G.; et al. Exploring Cell Surface Markers and Cell-Cell Interactions of Human Breast Milk Stem Cells. J. Public. Health Res. 2023, 12, 22799036221150332. [Google Scholar] [CrossRef] [PubMed]
- Goudarzi, N.; Shabani, R.; Ebrahimi, M.; Baghestani, A.; Dehdashtian, E.; Vahabzadeh, G.; Soleimani, M.; Moradi, F.; Katebi, M. Comparative Phenotypic Characterization of Human Colostrum and Breast Milk-Derived Stem Cells. Hum. Cell 2020, 33, 308–317. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Zhang, L.; Zhou, Q.; Jiang, S.; Yang, Y.; Cao, Y. Characterization of Stem Cells and Immune Cells in Preterm and Term Mother’s Milk. J. Hum. Lact. 2019, 35, 528–534. [Google Scholar] [CrossRef] [PubMed]
- Vijay Tukaram Mali, S.M.P. Cytology of the Human Milk in the First Post Partum Week—A Clinical Perspective. J. Cytol. Histol. 2014, 4, 1–3. [Google Scholar] [CrossRef]
- Zaitsev, K.V.; Mezheritskii, S.A.; Stepanenko, N.P.; Gostyukhina, A.A.; Zhukova, O.B.; Kondrat’eva, E.I.; Stepanov, I.A.; Dzyuman, A.N.; Nikolaevskaya, E.E.; Vorob’ev, V.A.; et al. Immunological and Phenotypic Characterization of Cell Constituents of Breast Milk. Cell Tissue Biol. 2016, 10, 410–415. [Google Scholar] [CrossRef]
- Twigger, A.J.; Engelbrecht, L.K.; Bach, K.; Schultz-Pernice, I.; Pensa, S.; Stenning, J.; Petricca, S.; Scheel, C.H.; Khaled, W.T. Transcriptional Changes in the Mammary Gland during Lactation Revealed by Single Cell Sequencing of Cells from Human Milk. Nat. Commun. 2022, 13, 562. [Google Scholar] [CrossRef] [PubMed]
- Thomas, E.; Zeps, N.; Cregan, M.; Hartmann, P.; Martin, T. 14-3-3σ (Sigma) Regulates Proliferation and Differentiation of Multipotent P63-Positive Cells Isolated from Human Breastmilk. Cell Cycle 2011, 10, 278–284. [Google Scholar] [CrossRef] [PubMed]
- Nageeb, M.M.; Saadawy, S.F.; Attia, S.H. Breast Milk Mesenchymal Stem Cells Abate Cisplatin-Induced Cardiotoxicity in Adult Male Albino Rats via Modulating the AMPK Pathway. Sci. Rep. 2022, 12, 17554. [Google Scholar] [CrossRef] [PubMed]
- Pisano, C.; Galley, J.; Elbahrawy, M.; Wang, Y.; Farrell, A.; Brigstock, D.; Besner, G.E. Human Breast Milk-Derived Extracellular Vesicles in the Protection Against Experimental Necrotizing Enterocolitis. J. Pediatr. Surg. 2020, 55, 54–58. [Google Scholar] [CrossRef] [PubMed]
- Borhani-Haghighi, M.; Navid, S.; Mohamadi, Y. The Therapeutic Potential of Conditioned Medium from Human Breast Milk Stem Cells in Treating Spinal Cord Injury. Asian Spine J. 2020, 14, 131. [Google Scholar] [CrossRef] [PubMed]
- Khamis, T.; Abdelalim, A.F.; Abdallah, S.H.; Saeed, A.A.; Edress, N.M.; Arisha, A.H. Breast Milk MSCs Transplantation Attenuates Male Diabetic Infertility Via Immunomodulatory Mechanism in Rats. Adv. Anim. Vet. Sci. 2019, 7, 145–153. [Google Scholar] [CrossRef]
- Khamis, T.; Abdelalim, A.F.; Saeed, A.A.; Edress, N.M.; Nafea, A.; Ebian, H.F.; Algendy, R.; Hendawy, D.M.; Arisha, A.H.; Abdallah, S.H. Breast Milk MSCs Upregulated β-Cells PDX1, Ngn3, and PCNA Expression via Remodeling ER Stress/Inflammatory/Apoptotic Signaling Pathways in Type 1 Diabetic Rats. Eur. J. Pharmacol. 2021, 905, 174188. [Google Scholar] [CrossRef] [PubMed]
- Khamis, T.; Abdelalim, A.F.; Abdallah, S.H.; Saeed, A.A.; Edress, N.M.; Arisha, A.H. Early Intervention with Breast Milk Mesenchymal Stem Cells Attenuates the Development of Diabetic-Induced Testicular Dysfunction via Hypothalamic Kisspeptin/Kiss1r-GnRH/GnIH System in Male Rats. Biochim. Biophys. Acta Mol. Basis Dis. 2020, 1866, 165577. [Google Scholar] [CrossRef] [PubMed]
- Pipino, C.; Mandatori, D.; Buccella, F.; Lanuti, P.; Preziuso, A.; Castellani, F.; Grotta, L.; Di Tomo, P.; Marchetti, S.; Di Pietro, N.; et al. Identification and Characterization of a Stem Cell-Like Population in Bovine Milk: A Potential New Source for Regenerative Medicine in Veterinary. Stem Cells Dev. 2018, 27, 1587–1597. [Google Scholar] [CrossRef] [PubMed]
- Marquez-Curtis, L.A.; Dorobantu, L.S.; Sauvageau, D.; Elliott, J.A.W. Cryopreservation of Swine Colostrum-Derived Cells. Cryobiology 2020, 97, 168–178. [Google Scholar] [CrossRef] [PubMed]
- Danev, N.; Harman, R.M.; Oliveira, L.; Huntimer, L.; Van de Walle, G.R. Bovine Milk-Derived Cells Express Transcriptome Markers of Pluripotency and Secrete Bioactive Factors with Regenerative and Antimicrobial Activity. Sci. Rep. 2023, 13, 12600. [Google Scholar] [CrossRef] [PubMed]
- Spaas, J.H.; Chiers, K.; Bussche, L.; Burvenich, C.; Van De Walle, G.R. Stem/Progenitor Cells in Non-Lactating Versus Lactating Equine Mammary Gland. Stem Cells Dev. 2012, 21, 3055–3067. [Google Scholar] [CrossRef] [PubMed]
- Bussche, L.; Rauner, G.; Antonyak, M.; Syracuse, B.; McDowell, M.; Brown, A.M.C.; Cerione, R.A.; Van De Walle, G.R. Microvesicle-Mediated Wnt/β-Catenin Signaling Promotes Interspecies Mammary Stem/Progenitor Cell Growth. J. Biol. Chem. 2016, 291, 24390–24405. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.L.; Kanke, M.; Rauner, G.; Bakhle, K.M.; Sethupathy, P.; Van de Walle, G.R. Comparative Analysis of MicroRNAs That Stratify in Vitro Mammary Stem and Progenitor Activity Reveals Functionality of Human MiR-92b-3p. J. Mammary Gland. Biol. Neoplasia 2022, 27, 253–269. [Google Scholar] [CrossRef] [PubMed]
- Bartlett, A.P.; Harman, R.M.; Weiss, J.R.; Van de Walle, G. Establishment and Characterization of Equine Mammary Organoids Using a Method Translatable to Other Non-Traditional Model Species. Development 2022, 149, dev200412. [Google Scholar] [CrossRef] [PubMed]
- Harman, R.M.; Das, S.P.; Kanke, M.; Sethupathy, P.; Van de Walle, G.R. MiRNA-214-3p Stimulates Carcinogen-Induced Mammary Epithelial Cell Apoptosis in Mammary Cancer-Resistant Species. Commun. Biol. 2023, 6, 1006. [Google Scholar] [CrossRef] [PubMed]
- Vaala, W.E.; Sertich, P.L. Perinatology. In The Equine Manual; Elsevier: Amsterdam, The Netherlands, 2006; pp. 803–804. [Google Scholar] [CrossRef]
- Cash, R.S.G. Colostral Quality Determined by Refractometry. Equine Vet. Educ. 1999, 11, 36–38. [Google Scholar] [CrossRef]
- Magalhaes, H.B.; Canisso, I.F. Colostrum Conductivity, PH and Brix Index as Predictors of Passive Immunity Transfer in Foals. Equine Vet. J. 2024, 57, 898–903. [Google Scholar] [CrossRef] [PubMed]
- Widjaja, S.L.; Salimo, H.; Yulianto, I. Soetrisno Proteomic Analysis of Hypoxia and Non-Hypoxia Secretome Mesenchymal Stem-like Cells from Human Breastmilk. Saudi J. Biol. Sci. 2021, 28, 4399–4407. [Google Scholar] [CrossRef] [PubMed]
- Merlo, B.; Pirondi, S.; Iacono, E.; Rossi, B.; Ricci, F.; Mari, G. Viability, in Vitro Differentiation and Molecular Characterization of Equine Adipose Tissue-Derived Mesenchymal Stem Cells Cryopreserved in Serum and Serum-Free Medium. Cryoletters 2016, 37, 243–252. [Google Scholar] [PubMed]
- Bezerra, A.F.; Alves, J.P.M.; Fernandes, C.C.L.; Cavalcanti, C.M.; Silva, M.R.L.; Conde, A.J.H.; Tetaping, G.M.; Ferreira, A.C.A.; Melo, L.M.; Rodrigues, A.P.R.; et al. Dyslipidemia Induced by Lipid Diet in Late Gestation Donor Impact on Growth Kinetics and in Vitro Potential Differentiation of Umbilical Cord Wharton’s Jelly Mesenchymal Stem Cells in Goats. Vet. Res. Commun. 2022, 46, 1259–1270. [Google Scholar] [CrossRef] [PubMed]
- Spaas, J.H.; De Schauwer, C.; Cornillie, P.; Meyer, E.; Van Soom, A.; Van de Walle, G.R. Culture and Characterisation of Equine Peripheral Blood Mesenchymal Stromal Cells. Vet. J. 2013, 195, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Bellotti, C.; Duchi, S.; Bevilacqua, A.; Lucarelli, E.; Piccinini, F. Long Term Morphological Characterization of Mesenchymal Stromal Cells 3D Spheroids Built with a Rapid Method Based on Entry-Level Equipment. Cytotechnology 2016, 68, 2479–2490. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Liu, G.; Halim, A.; Ju, Y.; Luo, Q.; Song, G. Mesenchymal Stem Cell Migration and Tissue Repair. Cells 2019, 8, 784. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.C.; Park, A.Y.; Guan, J.L. In Vitro Scratch Assay: A Convenient and Inexpensive Method for Analysis of Cell Migration In Vitro. Nat. Protoc. 2007, 2, 329–333. [Google Scholar] [CrossRef] [PubMed]
- Rossi, B.; Merlo, B.; Colleoni, S.; Iacono, E.; Tazzari, P.L.; Ricci, F.; Lazzari, G.; Galli, C. Isolation and in Vitro Characterization of Bovine Amniotic Fluid Derived Stem Cells at Different Trimesters of Pregnancy. Stem Cell Rev. Rep. 2014, 10, 712–724. [Google Scholar] [CrossRef] [PubMed]
- Mizuno, H.; Hyakusoku, H. Mesengenic Potential and Future Clinical Perspective of Human Processed Lipoaspirate Cells. J. Nippon. Med. Sch. 2003, 70, 300–306. [Google Scholar] [CrossRef] [PubMed]
- Heyman, E.; Meeremans, M.; Devriendt, B.; Olenic, M.; Chiers, K.; De Schauwer, C. Validation of a Color Deconvolution Method to Quantify MSC Tri-Lineage Differentiation across Species. Front. Vet. Sci. 2022, 9, 987045. [Google Scholar] [CrossRef] [PubMed]
- Iacono, E.; Pascucci, L.; Rossi, B.; Bazzucchi, C.; Lanci, A.; Ceccoli, M.; Merlo, B. Ultrastructural Characteristics and Immune Profile of Equine MSCs from Fetal Adnexa. Reproduction 2017, 154, 509–519. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Iacono, E.; Lanci, A.; Gugole, P.; Merlo, B. Shipping Temperature, Time and Media Effects on Equine Wharton’s Jelly and Adipose Tissue Derived Mesenchymal Stromal Cells Characteristics. Animals 2022, 12, 1967. [Google Scholar] [CrossRef] [PubMed]
- Merlo, B.; Teti, G.; Lanci, A.; Burk, J.; Mazzotti, E.; Falconi, M.; Iacono, E. Comparison between Adult and Foetal Adnexa Derived Equine Post-Natal Mesenchymal Stem Cells. BMC Vet. Res. 2019, 15, 277. [Google Scholar] [CrossRef] [PubMed]
- Mohanty, N.; Gulati, B.R.; Kumar, R.; Gera, S.; Kumar, P.; Somasundaram, R.K.; Kumar, S. Immunophenotypic Characterization and Tenogenic Differentiation of Mesenchymal Stromal Cells Isolated from Equine Umbilical Cord Blood. In Vitro. Cell Dev. Biol. Anim. 2014, 50, 538–548. [Google Scholar] [CrossRef] [PubMed]
- Corradetti, B.; Lange-Consiglio, A.; Barucca, M.; Cremonesi, F.; Bizzaro, D. Size-Sieved Subpopulations of Mesenchymal Stem Cells from Intervascular and Perivascular Equine Umbilical Cord Matrix. Cell Prolif. 2011, 44, 330–342. [Google Scholar] [CrossRef] [PubMed]
- Desmarais, J.A.; Demers, S.P.; Suzuki, J.; Laflamme, S.; Vincent, P.; Laverty, S.; Smith, L.C. Trophoblast Stem Cell Marker Gene Expression in Inner Cell Mass-Derived Cells from Parthenogenetic Equine Embryos. Reproduction 2011, 141, 321–332. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kumari, P.; Raval, A.; Rana, P.; Mahto, S.K. Regenerative Potential of Human Breast Milk: A Natural Reservoir of Nutrients, Bioactive Components and Stem Cells. Stem Cell Rev. Rep. 2023, 19, 1307–1327. [Google Scholar] [CrossRef] [PubMed]
- Ranera, B.; Ordovás, L.; Lyahyai, J.; Bernal, M.L.; Fernandes, F.; Remacha, A.R.; Romero, A.; Vázquez, F.J.; Osta, R.; Cons, C.; et al. Comparative Study of Equine Bone Marrow and Adipose Tissue-Derived Mesenchymal Stromal Cells. Equine Vet. J. 2012, 44, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Iacono, E.; Brunori, L.; Pirrone, A.; Pagliaro, P.P.; Ricci, F.; Tazzari, P.L.; Merlo, B. Isolation, Characterization and Differentiation of Mesenchymal Stem Cells from Amniotic Fluid, Umbilical Cord Blood and Wharton’s Jelly in the Horse. Reproduction 2012, 143, 455–468. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.; Zhang, N.; Marsano, A.; Vunjak-Novakovic, G.; Zhang, Y.; Lopez, M.J. In Vitro Mesenchymal Trilineage Differentiation and Extracellular Matrix Production by Adipose and Bone Marrow Derived Adult Equine Multipotent Stromal Cells on a Collagen Scaffold. Stem Cell Rev. Rep. 2013, 9, 858–872. [Google Scholar] [CrossRef] [PubMed]
- Bhat, S.; Viswanathan, P.; Chandanala, S.; Prasanna, S.J.; Seetharam, R.N. Expansion and Characterization of Bone Marrow Derived Human Mesenchymal Stromal Cells in Serum-Free Conditions. Sci. Rep. 2021, 11, 3403. [Google Scholar] [CrossRef] [PubMed]
- Mehrian, M.; Lambrechts, T.; Marechal, M.; Luyten, F.P.; Papantoniou, I.; Geris, L. Predicting in Vitro Human Mesenchymal Stromal Cell Expansion Based on Individual Donor Characteristics Using Machine Learning. Cytotherapy 2020, 22, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.Y.; Lee, E.J.; Kim, R.E.; Kil, T.Y.; Kim, M.K. Evaluation of Stability and Safety of Equine Mesenchymal Stem Cells Derived from Amniotic Fluid for Clinical Application. Front. Vet. Sci. 2024, 11, 1330009. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, C.; Leicht, U.; Rothe, C.; Drosse, I.; Luibl, V.; Röcken, M.; Schieker, M. Effects of Different Media on Proliferation and Differentiation Capacity of Canine, Equine and Porcine Adipose Derived Stem Cells. Res. Vet. Sci. 2012, 93, 457–462. [Google Scholar] [CrossRef] [PubMed]
- Guerrero, E.N.; Vega, S.; Fu, C.; De León, R.; Beltran, D.; Solis, M.A. Increased Proliferation and Differentiation Capacity of Placenta-Derived Mesenchymal Stem Cells from Women of Median Maternal Age Correlates with Telomere Shortening. Aging 2021, 13, 24542–24559. [Google Scholar] [CrossRef] [PubMed]
- Baxter, M.A.; Wynn, R.F.; Jowitt, S.N.; Wraith, J.E.; Fairbairn, L.J.; Bellantuono, I. Study of Telomere Length Reveals Rapid Aging of Human Marrow Stromal Cells Following In Vitro Expansion. Stem Cells 2004, 22, 675–682. [Google Scholar] [CrossRef] [PubMed]
- Vidal, M.A.; Walker, N.J.; Napoli, E.; Borjesson, D.L. Evaluation of Senescence in Mesenchymal Stem Cells Isolated from Equine Bone Marrow, Adipose Tissue, and Umbilical Cord Tissue. Stem Cells Dev. 2012, 21, 273–283. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, R.; Bassiouny, M.; Badawy, A.; Darwish, A.; Yahia, S.; El-Tantawy, N. Maternal and Neonatal Factors’ Effects on Wharton’s Jelly Mesenchymal Stem Cell Yield. Sci. Rep. 2024, 14, 24376. [Google Scholar] [CrossRef] [PubMed]
- Lange-Consiglio, A.; Corradetti, B.; Meucci, A.; Perego, R.; Bizzaro, D.; Cremonesi, F. Characteristics of Equine Mesenchymal Stem Cells Derived from Amnion and Bone Marrow: In Vitro Proliferative and Multilineage Potential Assessment. Equine Vet. J. 2013, 45, 737–744. [Google Scholar] [CrossRef] [PubMed]
- Hillmann, A.; Ahrberg, A.B.; Brehm, W.; Heller, S.; Josten, C.; Paebst, F.; Burk, J. Comparative Characterization of Human and Equine Mesenchymal Stromal Cells: A Basis for Translational Studies in the Equine Model. Cell Transplant. 2016, 25, 109–124. [Google Scholar] [CrossRef] [PubMed]
- Somasundaram, I.; Kaingade, P.; Bhonde, R. Applications of Breast Milk-Derived Cell Components: Present and Future Perspectives. In Stem Cell and Non-Stem Cell Components of Breast Milk; Springer Nature: Singapore, 2023; pp. 71–77. [Google Scholar] [CrossRef]
- Kamm, J.L.; Parlane, N.A.; Riley, C.B.; Gee, E.K.; Dittmer, K.E.; McIlwraith, C.W. Blood Type and Breed-Associated Differences in Cell Marker Expression on Equine Bone Marrow-Derived Mesenchymal Stem Cells Including Major Histocompatibility Complex Class II Antigen Expression. PLoS ONE 2019, 14, e0225161. [Google Scholar] [CrossRef] [PubMed]
- Schnabel, L.V.; Pezzanite, L.M.; Antczak, D.F.; Felippe, M.J.B.; Fortier, L.A. Equine Bone Marrow-Derived Mesenchymal Stromal Cells Are Heterogeneous in MHC Class II Expression and Capable of Inciting an Immune Response in Vitro. Stem Cell Res. Ther. 2014, 5, 13. [Google Scholar] [CrossRef] [PubMed]
- Joel, M.D.M.; Yuan, J.; Wang, J.; Yan, Y.; Qian, H.; Zhang, X.; Xu, W.; Mao, F. MSC: Immunoregulatory Effects, Roles on Neutrophils and Evolving Clinical Potentials. Am. J. Transl. Res. 2019, 11, 3890. [Google Scholar] [PubMed]

| Adipogenic | Chondrogenic | Osteogenic |
|---|---|---|
| DMEM | DMEM | DMEM |
| 10% FBS | 1% FBS | 10% FBS |
| 1 µM DXM 2 (removed after 6 days) | 0.1 µM DXM 2 | 0.1 µM DXM 2 |
| 0.5 mM IBMX 1 (removed after 3 days) | 50 nM AA2P 4 | 50 µM AA2P 4 |
| 10 µg/mL insulin | 6.25 µg/mL insulin | 10 mM BGP 5 |
| 0.1 mM indomethacin | 10 ng/mL hTGF-β1 3 |
| Primers | References | Sequences (5′ → 3′) | bp |
|---|---|---|---|
| MSC markers | |||
| CD90 | [83] | F: TGCGAACTCCGCCTCTCT R: GCTTATGCCCTCGCACTTG | 93 |
| CD73 | [83] | F: GGGATTGTTGGATACACTTCAAAAG R: GCTGCAACGCAGTGATTTCA | 90 |
| Hematopoietic markers | |||
| CD34 | [83] | F: CACTAAACCCTCTACATCATTTTCTCCTA R: GGCAGATACCTTGAGTCAATTTCA | 101 |
| CD45 | [83] | F: TGATTCCCAGAAATGACCATGTA R: ACATTTTGGGCTTGTCCTGTAAC | 101 |
| MHC markers | |||
| MHC-I | [84] | F: GGAGAGGAGCAGAGATACA R: CTGTCACTGTTTGCAGTCT | 218 |
| MHC-II | [85] | F: TCTACACCTGCCAAGTG R: CCACCATGCCCTTTCTG | 178 |
| Housekeeping | |||
| GAPDH | [85] | F: GTCCATGCCATCACTGCCAC R: CCTGCTTCACCACCTTCTTG | 262 |
| Mare/Sample | Age (Years) | Number of Pregnancies | Volume (mL) | Brix Index (%) | Cell Yield (×103 cells/mL) | Days to Confluence | Mean PDTs 1 (Days) | Number of CFUs 2 |
|---|---|---|---|---|---|---|---|---|
| 1 | 11 | 5 | 25 | 25 | 260 | 10 | 2.1 ± 0.9 | 21 ± 2 |
| 2 | 20 | 14 | 23 | 29.5 | 30 | 10 | 1.4 ± 0.3 | 71 ± 16.7 |
| 3 | 17 | 2 | 17 | 27 | 300 | 11 | 6.2 ± 1.8 | 34.7 ± 11 |
| 4 | 13 | 4 | 20 | 26 | 500 | 8 | 1.2 ± 0.1 | 59.3 ± 7.4 |
| 5 | 8 | 1 | 21 | 35 | 480 | 11 | 2.2 ± 0.3 | 40.3 ± 5.5 |
| 6 | 14 | 3 | 11 | 28 | 400 | 11 | 3.1 ± 0.2 | 13 ± 3.6 |
| Mean | 14.8 ± 4.26 | 5.17 ± 4.71 | 19.5 ± 4.97 | 28.2 ± 3.96 | 328.3 ± 174.4 | 10.17 ± 1.17 | 2.7 ± 0.6 | 39.9 ± 7.7 |
| Sample | MSC Markers | Hematopoietic Markers | MHC Markers | |||
|---|---|---|---|---|---|---|
| CD 90 | CD73 | CD34 | CD 45 | MHC-I | MHC-II | |
| 1 | + | + | - | - | - | |
| 2 | + | + | - | - | +/− | - |
| 3 | + | + | - | - | +/− | - |
| 4 | + | + | - | - | +/− | - |
| 5 | + | + | - | - | +/− | - |
| 6 | + | + | - | - | +/− | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Capone, A.; Merlo, B.; Begni, F.; Iacono, E. Equine Colostrum-Derived Mesenchymal Stromal Cells: A Potential Resource for Veterinary Regenerative Medicine. Vet. Sci. 2025, 12, 681. https://doi.org/10.3390/vetsci12070681
Capone A, Merlo B, Begni F, Iacono E. Equine Colostrum-Derived Mesenchymal Stromal Cells: A Potential Resource for Veterinary Regenerative Medicine. Veterinary Sciences. 2025; 12(7):681. https://doi.org/10.3390/vetsci12070681
Chicago/Turabian StyleCapone, Angelita, Barbara Merlo, Fabiana Begni, and Eleonora Iacono. 2025. "Equine Colostrum-Derived Mesenchymal Stromal Cells: A Potential Resource for Veterinary Regenerative Medicine" Veterinary Sciences 12, no. 7: 681. https://doi.org/10.3390/vetsci12070681
APA StyleCapone, A., Merlo, B., Begni, F., & Iacono, E. (2025). Equine Colostrum-Derived Mesenchymal Stromal Cells: A Potential Resource for Veterinary Regenerative Medicine. Veterinary Sciences, 12(7), 681. https://doi.org/10.3390/vetsci12070681






