Trichobezoars in Captive-Bred Fat-Tailed Dunnarts and Potential Preventative Protocols
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Enclosures
2.3. Diet
2.4. Records
2.5. Necropsies
- In-house necropsies were carried out by experienced laboratory technicians and included an assessment of body condition, nutritional and hydration state, pregnancy status, and a systemic evaluation of major body systems.
- Cadavers were transferred to Cerberus Science, an ISO accredited specialist animal laboratory pathology lab, where qualified veterinary pathologist carried out full necropsies.
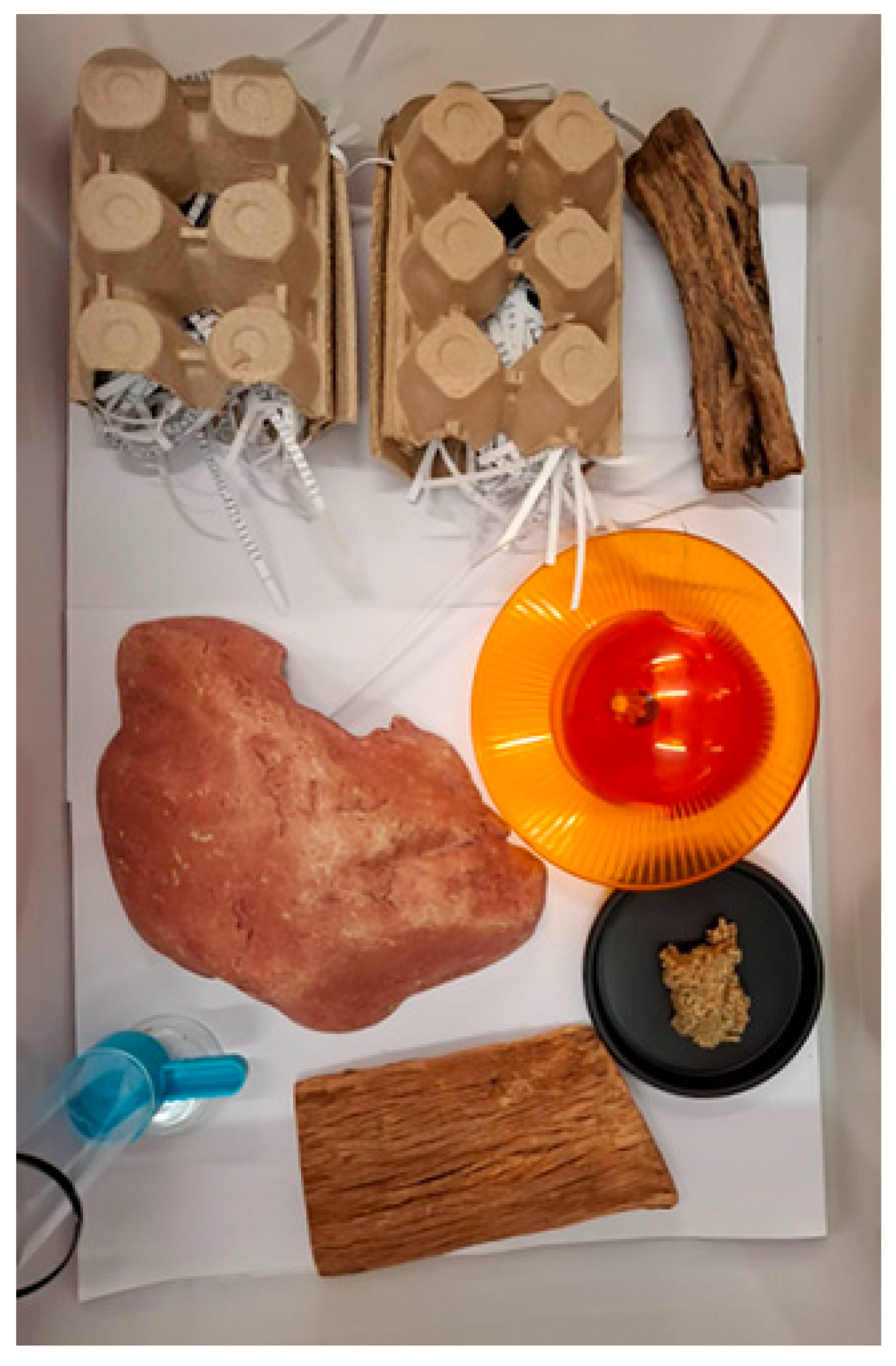
2.6. Husbandry Interventions
3. Results
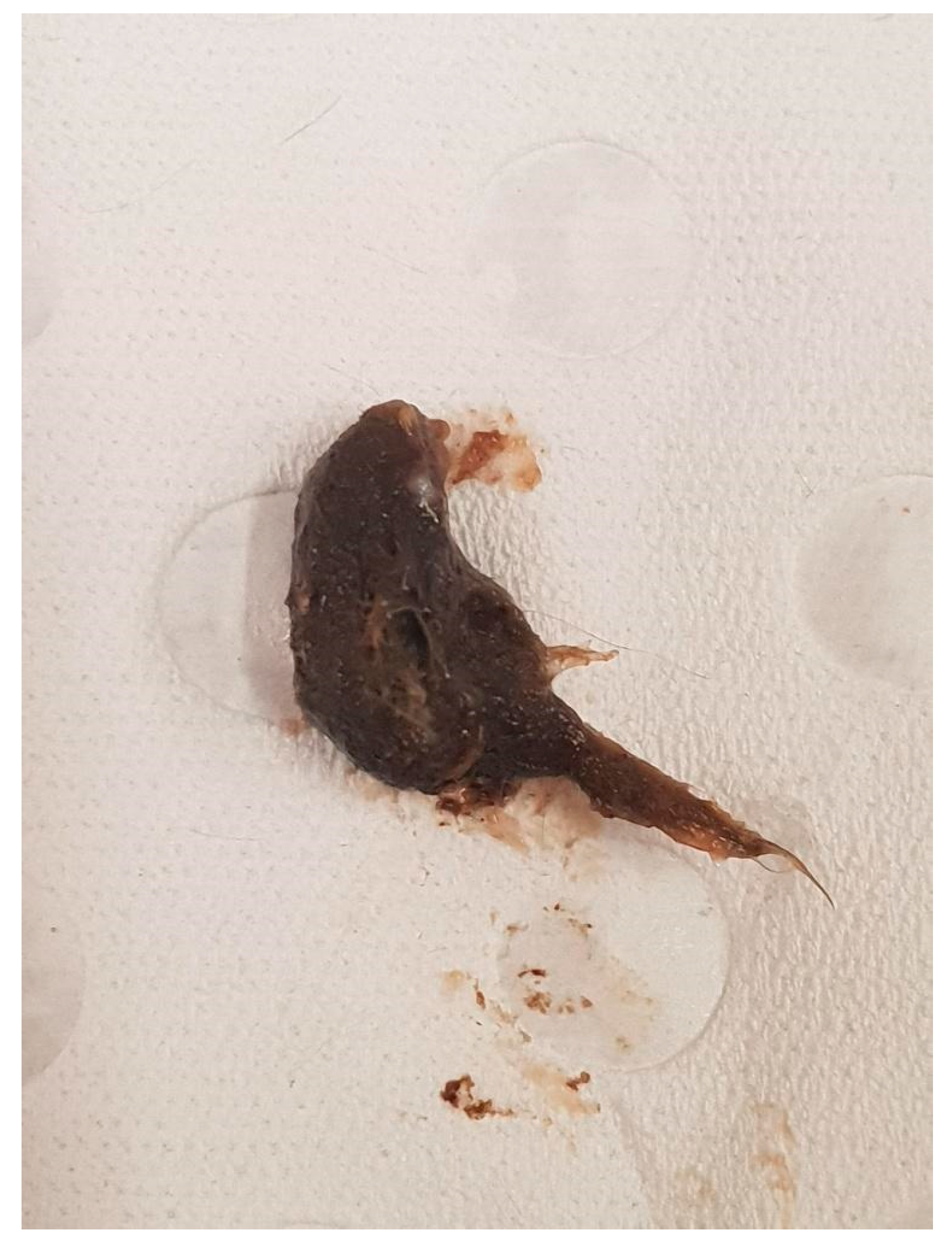
3.1. Animal A (My291)
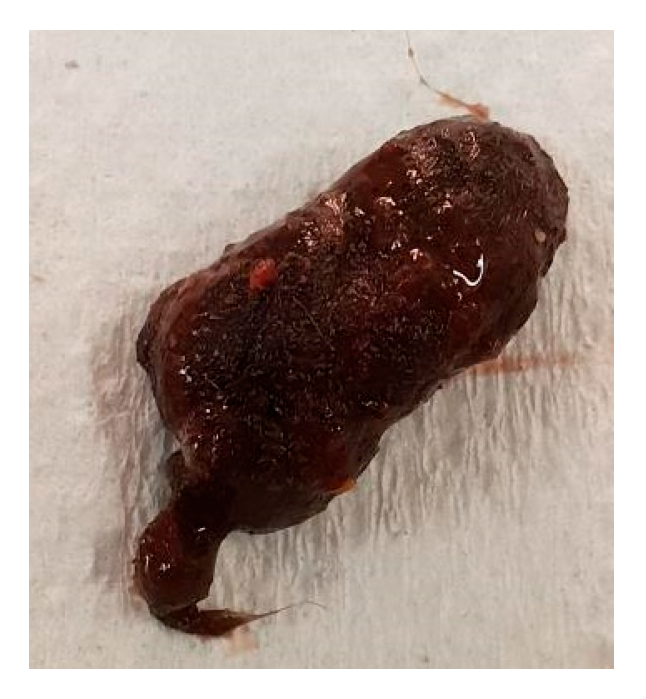
3.2. Animal B (868)
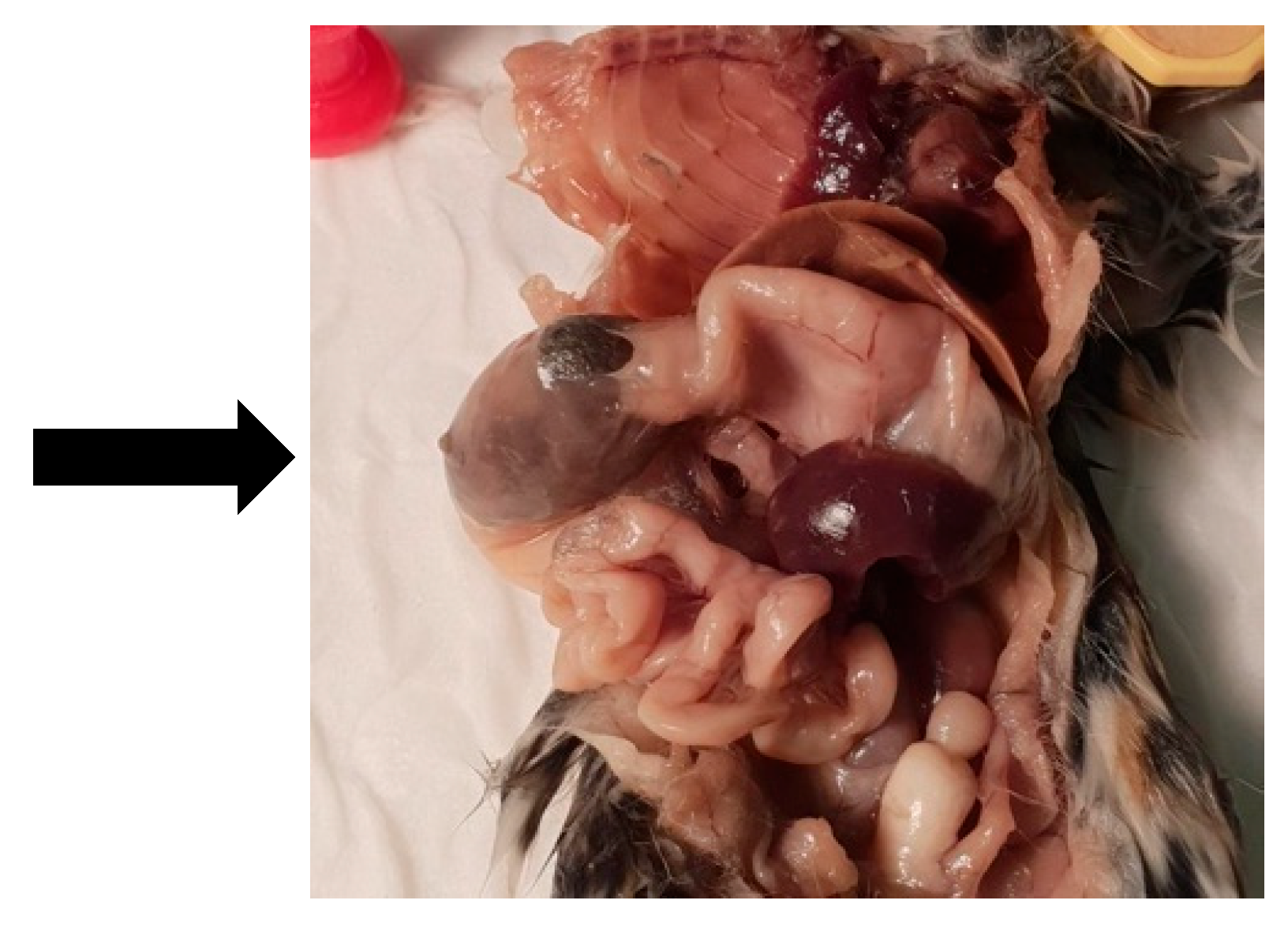
3.3. Animal C (768)
3.4. Post-Intervention Trichobezoar Deaths
4. Discussion
4.1. Diet Considerations and the Use of Gastric Lubricants
4.2. Behaviour and Environmental Considerations
4.3. Alternative Treatments
4.4. Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
Diet
- Ingredients:
- 500 g Wombaroo (Glen Osmond, South Australia, Australia) small carnivore diet.
- 400 g of Advance Kitten (Bathurst, New South Wales, Australia) dry cat biscuits—chicken
- 2× tins (156 g each) of Hills AD (Sydney, New South Wales, Australia) adult cat science diet
- 100 mL Wombaroo (Glen Osmond, South Australia, Australia) —The Good Oil
- 15 mL Vetsense Paraffin Oil (Mulgrave, Victoria, Australia)
- 2 L of boiling hot water, let dry biscuits soak for at least an hour as this will allow the biscuit to swell (add more water if necessary).
- Instructions:
Appendix B
Raw Data
| Date | Animal ID | Sex | Necropsied | Pathology |
|---|---|---|---|---|
| 16 April 2018 | Mg57 | Male | NO | |
| 14 May 2018 | FG118 | Female | YES | Organs looked liquified |
| 24 May 2018 | My100 | Male | NO | |
| 18 December 2018 | Fr155 | Female | NO | |
| 11 March 2019 | Mg189 | Male | YES | No abnormalities detected |
| 8 April 2019 | Mr239 | Male | NO | |
| 14 April 2019 | Mg189 | Male | YES | No abnormalities detected |
| 3 June 2019 | Mr240 | Male | NO | |
| 6 August 2019 | Fw262 | Female | NO | |
| 21 August 2019 | Fb199 | Female | NO | |
| 8 September 2019 | Fy249 | Female | YES | Enlarged uterus |
| 9 December 2019 | Mg244 | Male | NO | |
| 10 April 2020 | Fr258 | Female | NO | |
| 10 September 2020 | My300 | Male | NO | |
| 7 November 2020 | My291 | Male | YES | 2 cm furball lodged in small intestine |
| 28 November 2020 | Mg311 | Male | NO | |
| 16 December 2020 | F760 | Female | NO | |
| 6 January 2021 | Female | NO | ||
| 27 May 2021 | Female | NO | ||
| 27 August 2021 | Female | YES | Gastrointestinal tract blockage unknown size | |
| 4 October 2021 | 768 | Female | YES | Trichobezoar in stomach unknown size |
| 8 December 2021 | Male | NO |
References
- Scicluna, E.L.; Gill, B.P.; Robert, K.A. Fat-tailed dunnarts (Sminthopsis crassicaudata) of the Werribee grasslands: A case study of a species in decline. Aust. J. Zool. 2021, 69, 27–32. [Google Scholar] [CrossRef]
- Stannard, H.J.; McAllan, B.M.; Old, J.M. Dietary composition and nutritional outcomes in two marsupials, Sminthopsis macroura and S. crassicaudata. J. Mammal. 2014, 95, 503–515. [Google Scholar] [CrossRef]
- Morton, S. An Ecological Study of Sminthopsis crassicaudata (Marsupialia: Dasyuridae) I. Distribution, Study Areas and Methods. Wildl. Res. 1978, 5, 151–162. [Google Scholar] [CrossRef]
- Phillips, C.; Davies, E.; Lisle, A. Housing systems to meet the behavioural needs of a solitary mammal with an extensive home range: The Julia Creek dunnart (Sminthopsis douglasi). Appl. Anim. Behav. Sci. 2012, 141, 36–42. [Google Scholar] [CrossRef]
- Woolley, P.; Watson, M. Observations on a captive outdoor breeding colony of a small dasyurid marsupial, Sminthopsis crassicaudata. Wildl. Res. 1984, 11, 249–254. [Google Scholar] [CrossRef]
- Bennett, J.H.; BreedB, W.G.; HaymanA, D.L.; HopeA, R.M. Reproductive and Genetical Studies with a Laboratory Colony of the Dasyurid Marsupial Sminthopsis crassicaudata. Austalian J. Zool. 1989, 37, 207–222. [Google Scholar] [CrossRef]
- GODFREY, G.K.; CROWCROFT, P. Breeding the Fat-tailed marsupial mouse in captivity. Int. Zoo Yearb. 1971, 11, 33–38. [Google Scholar] [CrossRef]
- Scicluna, E.L.; Newton, A.H.; Hutchison, J.C.; Dimovski, A.M.; Fanson, K.V.; D’Souza, G.; Whitehead, S.; Pask, A.J. Breeding fat-tailed dunnarts (Sminthopsis crassicaudata) in captivity: Revised practices to minimize stress whilst maintaining considerations of wild biology. Dev. Dyn. 2025, 254, 189–204. [Google Scholar] [CrossRef]
- Newton, A.H.; Hutchison, J.C.; Farley, E.R.; Scicluna, E.L.; Youngson, N.A.; Liu, J.; Menzies, B.R.; Hildebrandt, T.B.; Lawrence, B.M.; Sutherland, A.H. Embryology of the fat-tailed dunnart (Sminthopsis crassicaudata): A marsupial model for comparative mammalian developmental and evolutionary biology. Dev. Dyn. 2025, 254, 142–157. [Google Scholar] [CrossRef]
- Bennett, J.; Smith, M.; Hope, R.; Chesson, C. The establishment and maintenance of a laboratory colony of Sminthopsis crassicaudata (Gould). Manag. Aust. Mamm. Captiv. 1982, 14, 38–44. [Google Scholar]
- Walker, K. Husbandry Guidelines Fat-Tailed Dunnart (Sminthopsis crassicaudata); Western Sydney TAFE.2012: Richmond, Australia, 2012. [Google Scholar]
- Mejido, D.C.; Dick, E.J., Jr.; Williams, P.C.; Sharp, R.M.; Andrade, M.C.; DiCarlo, C.; Hubbard, G.B. Trichobezoars in baboons. J. Med. Primatol. 2009, 38, 302–309. [Google Scholar] [CrossRef] [PubMed]
- Anderson, D.E.; Rings, M. Current Veterinary Therapy: Food Animal Practice; Elsevier Health Sciences: Amsterdam, The Netherlands, 2008. [Google Scholar]
- Cannon, M. Hair Balls in Cats: A normal nuisance or a sign that something is wrong? J. Feline Med. Surg. 2013, 15, 21–29. [Google Scholar] [CrossRef]
- Woerde, D.J.; Hoffmann, K.L.; Kicinski, A.; Brown, N.L. Oesophageal obstruction due to trichobezoars in two cats. J. Feline Med. Surg. Open Rep. 2019, 5, 2055116918823581. [Google Scholar] [CrossRef] [PubMed]
- Kottwitz, J.; Munsterman, A.S. Pyloric trichobezoar in a Canadian lynx (Lynx canadensis). J. Zoo Wildl. Med. 2013, 44, 1111–1114. [Google Scholar] [CrossRef]
- Akhtardanesh, B.; Kheirandish, R.; Nadimi, N.; Nakhaei, A.; Shademan, R. Report of trichobezoar causing peritonitis in a captive African lion (Panthera leo). Iran. Vet. J. 2020, 16, 99–103. [Google Scholar]
- Podhade, D.N.; Ranjeet Harne, R.H.; Jagtap, H.V. Therapeutic management of trichobezoar in tiger (Panthera tigris). Vet. Pract. 2014, 15, 128–129. [Google Scholar]
- Husa, L.; Mercer, C. Hairball Problem in Rabbits. Can. Vet. J. 1988, 29, 553. [Google Scholar]
- Mondal, D.; Risam, K.S.; Sharma, S.R.; Kumar, D. Prevalence of trichobezoars in Angora rabbits in sub-temperate Himalayan conditions. World Rabbit Sci. 2010, 14, 33–38. [Google Scholar] [CrossRef][Green Version]
- Hoefer, H.L. Gastrointestinal Diseases of Ferrets. In Ferrets, Rabbits, and Rodents; Elsevier: Amsterdam, The Netherlands, 2011. [Google Scholar]
- Theus, M.; Bitterli, F.; Foldenauer, U. Successful treatment of a gastric trichobezoar in a Peruvian guinea pig (cavia aperea porcellus). J. Exot. Pet Med. 2008, 17, 148–151. [Google Scholar] [CrossRef]
- Lindsay, S.A. Characterisation of Experimentally Induced and Spontaneously Occurring Disease Within Captive Bred Dasyurids. Master’s Thesis, University of Sydney, Camperdown, Australia, 2014. [Google Scholar]
- Minor, R.L.; Huckins, G.; Hawkins, S.; Townsend, A.; Sample, S.; Tolliver, S.; Loeber, S.; Brandão, J.; Doss, G.A. Diagnosis and surgical correction of gastrointestinal obstruction secondary to an intestinal trichophytobezoar in a red-necked wallaby (Notamacropus rufogriseus). J. Exot. Pet Med. 2023, 46, 1–4. [Google Scholar] [CrossRef]
- Suckow, M.; Terril-Robb, L.; Grigdesby, C. Gastric trichobezoar in a banner-tailed kangaroo rat (Dipodomys spectabilis). Lab. Anim. 1996, 30, 383–385. [Google Scholar] [CrossRef]
- Howell, S.R.; Schlack, C.; McCay, C.; Taylor, B. Prevention of Trichobezoar in the Cotton Rat, Sigmodon hispidus hispidus. Science 1948, 107, 424–425. [Google Scholar] [CrossRef]
- Donadelli, R.A.; Aldrich, C.G. The effects of diets varying in fibre sources on nutrient utilization, stool quality and hairball management in cats. J. Anim. Physiol. Anim. Nutr. 2020, 104, 715–724. [Google Scholar] [CrossRef] [PubMed]
- Loureiro, B.A.; Monti, M.; Pedreira, R.S.; Vitta, A.; Pacheco, P.D.G.; Putarov, T.C.; Carciofi, A.C. Beet pulp intake and hairball faecal excretion in mixed-breed shorthaired cats. J. Anim. Physiol. Anim. Nutr. 2017, 101, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Miltenburg, T.Z.; Peralta, R.M.; de Oliveira, C.A.L.; Janeiro, V.; Pereira, E.Q.; de Nicolau, J.T.S.; Ribeiro, L.B.; Vasconcellos, R.S. Effects of combined use of keratinolytic enzymes and sugarcane fibre on the hairball excretion in cats. J. Anim. Physiol. Anim. Nutr. 2021, 105, 129–137. [Google Scholar] [CrossRef]
- Harrenstien, L. Gastrointestinal Diseases of Pet Rabbits. Semin. Avian Exot. Pet Med. 1999, 8, 83–89. [Google Scholar] [CrossRef]
- Fukumura, K.; Haneda, R.; Endoh, T.; Takano, M.; Mizoguchi, Y.; Matsuoka, T.; Asano, Y. Gastric hairballs in rabbits: Significance in developmental toxicity study. Congenit. Anom. 2012, 52, 104–105. [Google Scholar] [CrossRef] [PubMed]
- Gamble, K.C. Marsupial care and husbandry. Vet. Clin. Exot. Anim. Pract. 2004, 7, 283–298. [Google Scholar] [CrossRef]
- Dann, J.R.; Adler, M.A.; Duffy, K.L.; Giffard, C.J. A potential nutritional prophylactic for the reduction of feline hairball symptoms. J. Nutr. 2004, 134, 2124S–2125S. [Google Scholar] [CrossRef]


| Date of Death | Animal Number | Sex | Age | Pathology |
|---|---|---|---|---|
| 7 November 2020 | My291 | Male | 1 year 4 months | Furball lodged in small intestine |
| 27 August 2021 | 868 | Female | 0 years 8 months | Furball |
| 4 October 2021 | 768 | Female | 1 year 7 months | Furball in stomach |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moschos, C.; Cohen, S.; Scicluna, E.L.; Frankenberg, S.; Pask, A.J.; Chow, K. Trichobezoars in Captive-Bred Fat-Tailed Dunnarts and Potential Preventative Protocols. Vet. Sci. 2025, 12, 625. https://doi.org/10.3390/vetsci12070625
Moschos C, Cohen S, Scicluna EL, Frankenberg S, Pask AJ, Chow K. Trichobezoars in Captive-Bred Fat-Tailed Dunnarts and Potential Preventative Protocols. Veterinary Sciences. 2025; 12(7):625. https://doi.org/10.3390/vetsci12070625
Chicago/Turabian StyleMoschos, Christine, Shari Cohen, Emily L. Scicluna, Stephen Frankenberg, Andrew J. Pask, and Keshuan Chow. 2025. "Trichobezoars in Captive-Bred Fat-Tailed Dunnarts and Potential Preventative Protocols" Veterinary Sciences 12, no. 7: 625. https://doi.org/10.3390/vetsci12070625
APA StyleMoschos, C., Cohen, S., Scicluna, E. L., Frankenberg, S., Pask, A. J., & Chow, K. (2025). Trichobezoars in Captive-Bred Fat-Tailed Dunnarts and Potential Preventative Protocols. Veterinary Sciences, 12(7), 625. https://doi.org/10.3390/vetsci12070625






