Clinical Effects of Faecal Microbiota Transplantation as Adjunctive Therapy in Dogs with Chronic Enteropathies—A Retrospective Case Series of 41 Dogs
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Ethics Approval
2.2. Animals
2.3. FMT Procedure
2.4. CIBDAI, Definition of Clinical Response and Outcome
2.5. Storage and Shipping of Faecal Samples
2.6. Bacterial qPCR Analysis
2.7. Statistical Analysis
3. Results
3.1. Animals
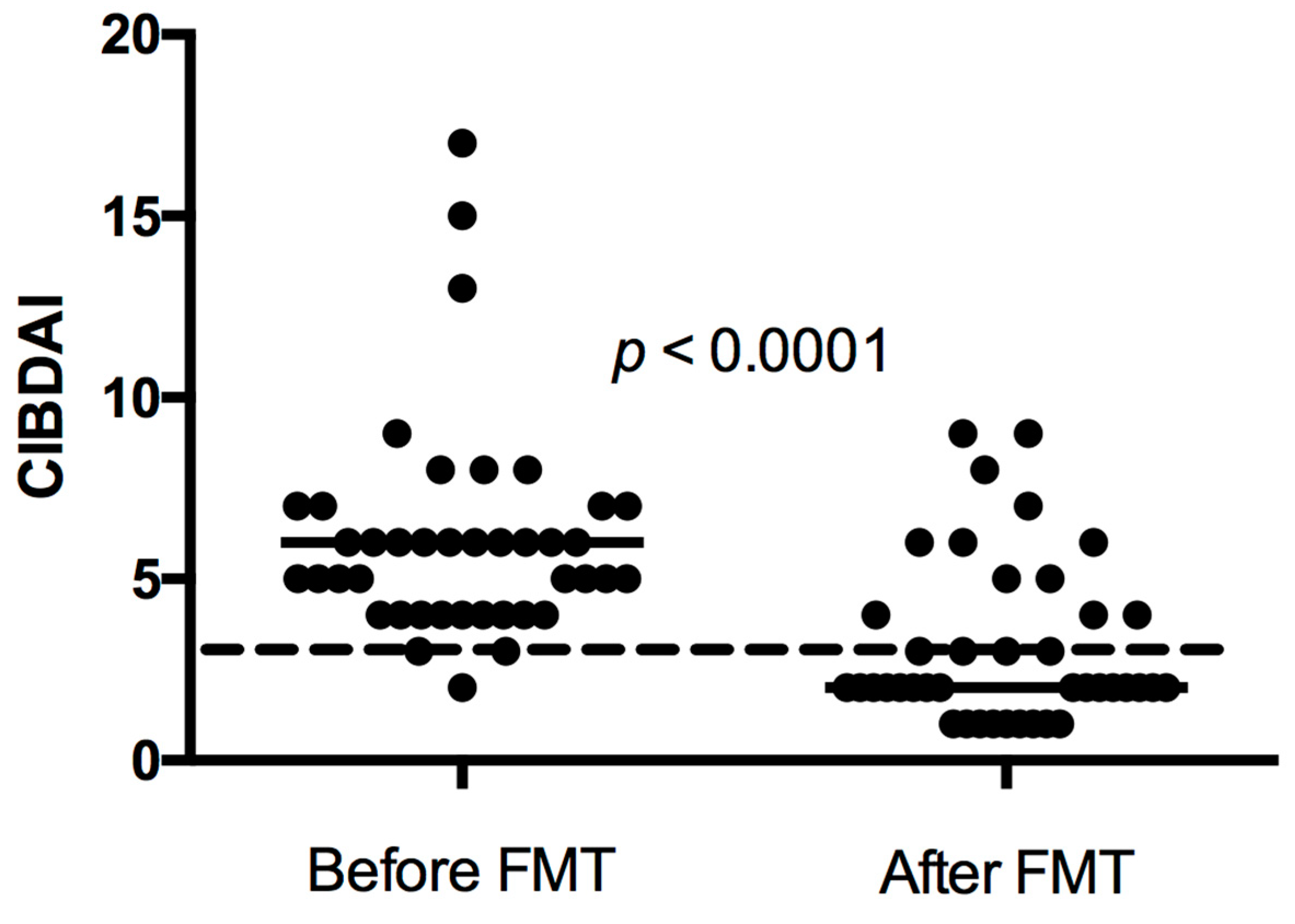
3.2. FMT and Clinical Response
3.3. Adverse Effects
3.4. Bacterial qPCR Results
3.5. Long-Term Outcome
3.5.1. Good Responders
3.5.2. Short-Lasting Responders
3.5.3. Non-Responders
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Makielski, K.; Cullen, J.; O’Connor, A.; Jergens, A.E. Narrative Review of Therapies for Chronic Enteropathies in Dogs and Cats. J. Vet. Intern. Med. 2019, 33, 11–22. [Google Scholar] [CrossRef]
- Craven, M.; Simpson, J.W.; Ridyard, A.E.; Chandler, M.L. Canine Inflammatory Bowel Disease: Retrospective Analysis of Diagnosis and Outcome in 80 Cases (1995–2002). J. Small Anim. Pract. 2004, 45, 336–342. [Google Scholar] [CrossRef] [PubMed]
- Allenspach, K.; Wieland, B.; Gröne, A.; Gaschen, F. Chronic Enteropathies in Dogs: Evaluation of Risk Factors for Negative Outcome. J. Vet. Intern. Med. 2007, 21, 700–708. [Google Scholar] [CrossRef]
- Félix, A.P.; Souza, C.M.M.; de Oliveira, S.G. Biomarkers of Gastrointestinal Functionality in Dogs: A Systematic Review and Meta-Analysis. Anim. Feed Sci. Technol. 2022, 283, 115183. [Google Scholar] [CrossRef]
- Chapman, B.C.; Moore, H.B.; Overbey, D.M.; Morton, A.P.; Harnke, B.; Gerich, M.E.; Vogel, J.D. Fecal Microbiota Transplant in Patients with Clostridium Difficile Infection: A Systematic Review. J. Trauma Acute Care Surg. 2016, 81, 756–764. [Google Scholar] [CrossRef] [PubMed]
- Paramsothy, S.; Kamm, M.A.; Kaakoush, N.O.; Walsh, A.J.; van den Bogaerde, J.; Samuel, D.; Leong, R.W.L.; Connor, S.; Ng, W.; Paramsothy, R.; et al. Multidonor Intensive Faecal Microbiota Transplantation for Active Ulcerative Colitis: A Randomised Placebo-Controlled Trial. Lancet Lond. Engl. 2017, 389, 1218–1228. [Google Scholar] [CrossRef]
- Haifer, C.; Paramsothy, S.; Kaakoush, N.O.; Saikal, A.; Ghaly, S.; Yang, T.; Luu, L.D.W.; Borody, T.J.; Leong, R.W. Lyophilised Oral Faecal Microbiota Transplantation for Ulcerative Colitis (LOTUS): A Randomised, Double-Blind, Placebo-Controlled Trial. Lancet Gastroenterol. Hepatol. 2022, 7, 141–151. [Google Scholar] [CrossRef] [PubMed]
- Imdad, A.; Nicholson, M.R.; Tanner-Smith, E.E.; Zackular, J.P.; Gomez-Duarte, O.G.; Beaulieu, D.B.; Acra, S. Fecal Transplantation for Treatment of Inflammatory Bowel Disease. Cochrane Database Syst. Rev. 2018, 11, CD012774. [Google Scholar] [CrossRef] [PubMed]
- Cheng, F.; Huang, Z.; Wei, W.; Li, Z. Fecal Microbiota Transplantation for Crohn’s Disease: A Systematic Review and Meta-Analysis. Technol. Coloproctology 2021, 25, 495–504. [Google Scholar] [CrossRef]
- Fehily, S.R.; Basnayake, C.; Wright, E.K.; Kamm, M.A. Fecal Microbiota Transplantation Therapy in Crohn’s Disease: Systematic Review. J. Gastroenterol. Hepatol. 2021, 36, 2672–2686. [Google Scholar] [CrossRef]
- Pereira, G.Q.; Gomes, L.A.; Santos, I.S.; Alfieri, A.F.; Weese, J.S.; Costa, M.C. Fecal Microbiota Transplantation in Puppies with Canine Parvovirus Infection. J. Vet. Intern. Med. 2018, 32, 707–711. [Google Scholar] [CrossRef]
- Chaitman, J.; Ziese, A.-L.; Pilla, R.; Minamoto, Y.; Blake, A.B.; Guard, B.C.; Isaiah, A.; Lidbury, J.A.; Steiner, J.M.; Unterer, S.; et al. Fecal Microbial and Metabolic Profiles in Dogs With Acute Diarrhea Receiving Either Fecal Microbiota Transplantation or Oral Metronidazole. Front. Vet. Sci. 2020, 7, 192. [Google Scholar] [CrossRef] [PubMed]
- Gal, A.; Barko, P.C.; Biggs, P.J.; Gedye, K.R.; Midwinter, A.C.; Williams, D.A.; Burchell, R.K.; Pazzi, P. One Dog’s Waste Is Another Dog’s Wealth: A Pilot Study of Fecal Microbiota Transplantation in Dogs with Acute Hemorrhagic Diarrhea Syndrome. PLoS ONE 2021, 16, e0250344. [Google Scholar] [CrossRef]
- Niina, A.; Kibe, R.; Suzuki, R.; Yuchi, Y.; Teshima, T.; Matsumoto, H.; Kataoka, Y.; Koyama, H. Improvement in Clinical Symptoms and Fecal Microbiome After Fecal Microbiota Transplantation in a Dog with Inflammatory Bowel Disease. Vet. Med. Auckl. NZ 2019, 10, 197–201. [Google Scholar] [CrossRef]
- Sugita, K.; Shima, A.; Takahashi, K.; Matsuda, Y.; Miyajima, M.; Hirokawa, M.; Kondo, H.; Kimura, J.; Ishihara, G.; Ohmori, K. Successful Outcome after a Single Endoscopic Fecal Microbiota Transplantation in a Shiba Dog with Non-Responsive Enteropathy during the Treatment with Chlorambucil. J. Vet. Med. Sci. 2021, 83, 984–989. [Google Scholar] [CrossRef]
- Niina, A.; Kibe, R.; Suzuki, R.; Yuchi, Y.; Teshima, T.; Matsumoto, H.; Kataoka, Y.; Koyama, H. Fecal Microbiota Transplantation as a New Treatment for Canine Inflammatory Bowel Disease. Biosci. Microbiota Food Health 2021, 40, 98–104. [Google Scholar] [CrossRef]
- Cerquetella, M.; Marchegiani, A.; Rossi, G.; Trabalza-Marinucci, M.; Passamonti, F.; Isidori, M.; Rueca, F. Case Report: Oral Fecal Microbiota Transplantation in a Dog Suffering From Relapsing Chronic Diarrhea-Clinical Outcome and Follow-Up. Front. Vet. Sci. 2022, 9, 893342. [Google Scholar] [CrossRef] [PubMed]
- Innocente, G.; Patuzzi, I.; Furlanello, T.; Di Camillo, B.; Bargelloni, L.; Giron, M.C.; Facchin, S.; Savarino, E.; Azzolin, M.; Simionati, B. Machine Learning and Canine Chronic Enteropathies: A New Approach to Investigate FMT Effects. Vet. Sci. 2022, 9, 502. [Google Scholar] [CrossRef]
- Jergens, A.E.; Schreiner, C.A.; Frank, D.E.; Niyo, Y.; Ahrens, F.E.; Eckersall, P.D.; Benson, T.J.; Evans, R. A Scoring Index for Disease Activity in Canine Inflammatory Bowel Disease. J. Vet. Intern. Med. 2003, 17, 291–297. [Google Scholar] [CrossRef] [PubMed]
- Salavati Schmitz, S. Observational Study of Small Animal Practitioners’ Awareness, Clinical Practice and Experience With Fecal Microbiota Transplantation in Dogs. Top. Companion Anim. Med. 2022, 47, 100630. [Google Scholar] [CrossRef]
- AlShawaqfeh, M.K.; Wajid, B.; Minamoto, Y.; Markel, M.; Lidbury, J.A.; Steiner, J.M.; Serpedin, E.; Suchodolski, J.S. A Dysbiosis Index to Assess Microbial Changes in Fecal Samples of Dogs with Chronic Inflammatory Enteropathy. FEMS Microbiol. Ecol. 2017, 93, fix136. [Google Scholar] [CrossRef]
- Fecal Scoring Chart. Available online: https://www.proplanveterinarydiets.ca/sites/g/files/auxxlc696/files/2021-02/180107_PPPVD-Fecal-Scoring-Chart-UPDATE-EN-FINAL.pdf (accessed on 15 December 2022).
- Day, M.J.; Bilzer, T.; Mansell, J.; Wilcock, B.; Hall, E.J.; Jergens, A.; Minami, T.; Willard, M.; Washabau, R.; World Small Animal Veterinary Association Gastrointestinal Standardization Group. Histopathological Standards for the Diagnosis of Gastrointestinal Inflammation in Endoscopic Biopsy Samples from the Dog and Cat: A Report from the World Small Animal Veterinary Association Gastrointestinal Standardization Group. J. Comp. Pathol. 2008, 138 (Suppl. 1), S1–S43. [Google Scholar] [CrossRef]
- Kitahara, M.; Takamine, F.; Imamura, T.; Benno, Y. Clostridium Hiranonis Sp. Nov., a Human Intestinal Bacterium with Bile Acid 7alpha-Dehydroxylating Activity. Int. J. Syst. Evol. Microbiol. 2001, 51 Pt 1, 39–44. [Google Scholar] [CrossRef]
- Guard, B.C.; Honneffer, J.B.; Jergens, A.E.; Jonika, M.M.; Toresson, L.; Lawrence, Y.A.; Webb, C.B.; Hill, S.; Lidbury, J.A.; Steiner, J.M.; et al. Longitudinal Assessment of Microbial Dysbiosis, Fecal Unconjugated Bile Acid Concentrations, and Disease Activity in Dogs with Steroid-Responsive Chronic Inflammatory Enteropathy. J. Vet. Intern. Med. 2019, 33, 1295–1305. [Google Scholar] [CrossRef] [PubMed]
- Toresson, L.; Steiner, J.M.; Suchodolski, J.S. Cholestyramine Treatment in Two Dogs with Presumptive Bile Acid Diarrhoea: A Case Report. Canine Med. Genet. 2021, 8, 1. [Google Scholar] [CrossRef]
- Nocerino, A.; Nguyen, A.; Agrawal, M.; Mone, A.; Lakhani, K.; Swaminath, A. Fatigue in Inflammatory Bowel Diseases: Etiologies and Management. Adv. Ther. 2020, 37, 97–112. [Google Scholar] [CrossRef]
- Shiha, M.G.; Aziz, I. Review Article: Physical and Psychological Comorbidities Associated with Irritable Bowel Syndrome. Aliment. Pharmacol. Ther. 2021, 54 (Suppl. 1), S12–S23. [Google Scholar] [CrossRef]
- Wei, Y.; Zhu, W.; Gong, J.; Guo, D.; Gu, L.; Li, N.; Li, J. Fecal Microbiota Transplantation Improves the Quality of Life in Patients with Inflammatory Bowel Disease. Gastroenterol. Res. Pract. 2015, 2015, 517597. [Google Scholar] [CrossRef] [PubMed]
- El-Salhy, M.; Hatlebakk, J.G.; Gilja, O.H.; Bråthen Kristoffersen, A.; Hausken, T. Efficacy of Faecal Microbiota Transplantation for Patients with Irritable Bowel Syndrome in a Randomised, Double-Blind, Placebo-Controlled Study. Gut 2020, 69, 859–867. [Google Scholar] [CrossRef] [PubMed]
- Johnsen, P.H.; Hilpüsch, F.; Valle, P.C.; Goll, R. The Effect of Fecal Microbiota Transplantation on IBS Related Quality of Life and Fatigue in Moderate to Severe Non-Constipated Irritable Bowel: Secondary Endpoints of a Double Blind, Randomized, Placebo-Controlled Trial. EBioMedicine 2020, 51, 102562. [Google Scholar] [CrossRef]
- Chen, Y.; Xu, J.; Chen, Y. Regulation of Neurotransmitters by the Gut Microbiota and Effects on Cognition in Neurological Disorders. Nutrients 2021, 13, 2099. [Google Scholar] [CrossRef]
- Gershon, M.D. 5-Hydroxytryptamine (Serotonin) in the Gastrointestinal Tract. Curr. Opin. Endocrinol. Diabetes Obes. 2013, 20, 14–21. [Google Scholar] [CrossRef]
- Margolis, K.G.; Cryan, J.F.; Mayer, E.A. The Microbiota-Gut-Brain Axis: From Motility to Mood. Gastroenterology 2021, 160, 1486–1501. [Google Scholar] [CrossRef]
- Elkholly, D.A.; Brodbelt, D.C.; Church, D.B.; Pelligand, L.; Mwacalimba, K.; Wright, A.K.; O’Neill, D.G. Side Effects to Systemic Glucocorticoid Therapy in Dogs Under Primary Veterinary Care in the UK. Front. Vet. Sci. 2020, 7, 515. [Google Scholar] [CrossRef]
- Suchodolski, J.S.; Dowd, S.E.; Westermarck, E.; Steiner, J.M.; Wolcott, R.D.; Spillmann, T.; Harmoinen, J.A. The Effect of the Macrolide Antibiotic Tylosin on Microbial Diversity in the Canine Small Intestine as Demonstrated by Massive Parallel 16S RRNA Gene Sequencing. BMC Microbiol. 2009, 9, 210. [Google Scholar] [CrossRef] [PubMed]
- Manchester, A.C.; Webb, C.B.; Blake, A.B.; Sarwar, F.; Lidbury, J.A.; Steiner, J.M.; Suchodolski, J.S. Long-term Impact of Tylosin on Fecal Microbiota and Fecal Bile Acids of Healthy Dogs. J. Vet. Intern. Med. 2019, 33, 2605–2617. [Google Scholar] [CrossRef] [PubMed]
- Pilla, R.; Gaschen, F.P.; Barr, J.W.; Olson, E.; Honneffer, J.; Guard, B.C.; Blake, A.B.; Villanueva, D.; Khattab, M.R.; AlShawaqfeh, M.K.; et al. Effects of Metronidazole on the Fecal Microbiome and Metabolome in Healthy Dogs. J. Vet. Intern. Med. 2020, 34, 1853–1866. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, L.H.; Örtqvist, A.K.; Cao, Y.; Simon, T.G.; Roelstraete, B.; Song, M.; Joshi, A.D.; Staller, K.; Chan, A.T.; Khalili, H.; et al. Antibiotic Use and the Development of Inflammatory Bowel Disease: A National Case-Control Study in Sweden. Lancet Gastroenterol. Hepatol. 2020, 5, 986–995. [Google Scholar] [CrossRef]
- Belas, A.; Menezes, J.; Gama, L.T.; Pomba, C. Sharing of Clinically Important Antimicrobial Resistance Genes by Companion Animals and Their Human Household Members. Microb. Drug Resist. Larchmt. N 2020, 26, 1174–1185. [Google Scholar] [CrossRef]
- Toombs-Ruane, L.J.; Benschop, J.; French, N.P.; Biggs, P.J.; Midwinter, A.C.; Marshall, J.C.; Chan, M.; Drinković, D.; Fayaz, A.; Baker, M.G.; et al. Carriage of Extended-Spectrum-Beta-Lactamase- and AmpC Beta-Lactamase-Producing Escherichia Coli Strains from Humans and Pets in the Same Households. Appl. Environ. Microbiol. 2020, 86, e01613-20. [Google Scholar] [CrossRef]
- Gómez-Sanz, E.; Ceballos, S.; Ruiz-Ripa, L.; Zarazaga, M.; Torres, C. Clonally Diverse Methicillin and Multidrug Resistant Coagulase Negative Staphylococci Are Ubiquitous and Pose Transfer Ability Between Pets and Their Owners. Front. Microbiol. 2019, 10, 485. [Google Scholar] [CrossRef] [PubMed]
- Jackson, C.R.; Fedorka-Cray, P.J.; Barrett, J.B.; Ladely, S.R. Effects of Tylosin Use on Erythromycin Resistance in Enterococci Isolated from Swine. Appl. Environ. Microbiol. 2004, 70, 4205–4210. [Google Scholar] [CrossRef]
- Cazer, C.L.; Eldermire, E.R.B.; Lhermie, G.; Murray, S.A.; Scott, H.M.; Gröhn, Y.T. The Effect of Tylosin on Antimicrobial Resistance in Beef Cattle Enteric Bacteria: A Systematic Review and Meta-Analysis. Prev. Vet. Med. 2020, 176, 104934. [Google Scholar] [CrossRef]
- Manchester, A.C.; Dogan, B.; Guo, Y.; Simpson, K.W. Escherichia Coli-Associated Granulomatous Colitis in Dogs Treated According to Antimicrobial Susceptibility Profiling. J. Vet. Intern. Med. 2021, 35, 150–161. [Google Scholar] [CrossRef]
- European Medicines Agency. Advice on the Designation of Antimicrobials or Groups of Antimicrobials Reserved for Treatment of Certain Infections in Humans—in Relation to Implementing Measures under Article 37(5) of Regulation (EU) 2019/6 on Veterinary Medicinal Products. Available online: https://food.ec.europa.eu/system/files/2022-03/ah_vet-med_imp-reg-2019-06_ema-advice_art-37-5.pdf (accessed on 22 November 2022).
- He, Z.; Li, P.; Zhu, J.; Cui, B.; Xu, L.; Xiang, J.; Zhang, T.; Long, C.; Huang, G.; Ji, G.; et al. Multiple Fresh Fecal Microbiota Transplants Induces and Maintains Clinical Remission in Crohn’s Disease Complicated with Inflammatory Mass. Sci. Rep. 2017, 7, 4753. [Google Scholar] [CrossRef]
- Bhutiani, N.; Schucht, J.E.; Miller, K.R.; McClave, S.A. Technical Aspects of Fecal Microbial Transplantation (FMT). Curr. Gastroenterol. Rep. 2018, 20, 30. [Google Scholar] [CrossRef]
- Ianiro, G.; Bibbò, S.; Porcari, S.; Settanni, C.R.; Giambò, F.; Curta, A.R.; Quaranta, G.; Scaldaferri, F.; Masucci, L.; Sanguinetti, M.; et al. Fecal Microbiota Transplantation for Recurrent C. Difficile Infection in Patients with Inflammatory Bowel Disease: Experience of a Large-Volume European FMT Center. Gut Microbes 2021, 13, 1994834. [Google Scholar] [CrossRef]
- Gerbec, Z. Evaluation of Therapeutic Potential of Restoring Gastrointestinal Homeostasis by a Fecal Microbial Transplant in Dogs. Master’s Thesis, University of Ljubljana, Ljubljana, Slovenia, 2016. Available online: https://www.semanticscholar.org/paper/EVALUATION-OF-THERAPEUTIC-POTENTIAL-OF-RESTORING-BY-Ljubljani-Farmacijo/80793d51d8748651683c53de1406870d9ba73d6a (accessed on 12 December 2022).
- Chaitman, J.; Guard, B.C.; Sarwar, F.; Lidbury, J.A.; Suchodolski, J.S. Fecal Microbial Transplantation Decreases the Dysbiosis Index in Dogs Presenting with Chronic Diarrhea (Abstract). J. Vet. Intern. Med. 2017, 31, 1287. [Google Scholar]
- Paramsothy, S.; Nielsen, S.; Kamm, M.A.; Deshpande, N.P.; Faith, J.J.; Clemente, J.C.; Paramsothy, R.; Walsh, A.J.; van den Bogaerde, J.; Samuel, D.; et al. Specific Bacteria and Metabolites Associated With Response to Fecal Microbiota Transplantation in Patients With Ulcerative Colitis. Gastroenterology 2019, 156, 1440–1454.e2. [Google Scholar] [CrossRef] [PubMed]
- Sokol, H.; Landman, C.; Seksik, P.; Berard, L.; Montil, M.; Nion-Larmurier, I.; Bourrier, A.; Le Gall, G.; Lalande, V.; De Rougemont, A.; et al. Fecal Microbiota Transplantation to Maintain Remission in Crohn’s Disease: A Pilot Randomized Controlled Study. Microbiome 2020, 8, 12. [Google Scholar] [CrossRef] [PubMed]
- Kazemian, N.; Ramezankhani, M.; Sehgal, A.; Khalid, F.M.; Kalkhoran, A.H.Z.; Narayan, A.; Wong, G.K.-S.; Kao, D.; Pakpour, S. The Trans-Kingdom Battle between Donor and Recipient Gut Microbiome Influences Fecal Microbiota Transplantation Outcome. Sci. Rep. 2020, 10, 18349. [Google Scholar] [CrossRef]
- Wang, S.; Martins, R.; Sullivan, M.C.; Friedman, E.S.; Misic, A.M.; El-Fahmawi, A.; De Martinis, E.C.P.; O’Brien, K.; Chen, Y.; Bradley, C.; et al. Diet-Induced Remission in Chronic Enteropathy Is Associated with Altered Microbial Community Structure and Synthesis of Secondary Bile Acids. Microbiome 2019, 7, 126. [Google Scholar] [CrossRef] [PubMed]
- Pilla, R.; Guard, B.C.; Blake, A.B.; Ackermann, M.; Webb, C.; Hill, S.; Lidbury, J.A.; Steiner, J.M.; Jergens, A.E.; Suchodolski, J.S. Long-Term Recovery of the Fecal Microbiome and Metabolome of Dogs with Steroid-Responsive Enteropathy. Anim. Open Access J. MDPI 2021, 11, 2498. [Google Scholar] [CrossRef] [PubMed]
- Smith, P.M.; Howitt, M.R.; Panikov, N.; Michaud, M.; Gallini, C.A.; Bohlooly, -Y.M.; Glickman, J.N.; Garrett, W.S. The Microbial Metabolites, Short-Chain Fatty Acids, Regulate Colonic Treg Cell Homeostasis. Science 2013, 341, 569–573. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhang, H.; Chen, T.; Shi, L.; Wang, D.; Tang, D. Regulatory Role of Short-Chain Fatty Acids in Inflammatory Bowel Disease. Cell Commun. Signal. CCS 2022, 20, 64. [Google Scholar] [CrossRef]
- Kang, J.D.; Myers, C.J.; Harris, S.C.; Kakiyama, G.; Lee, I.-K.; Yun, B.-S.; Matsuzaki, K.; Furukawa, M.; Min, H.-K.; Bajaj, J.S.; et al. Bile Acid 7α-Dehydroxylating Gut Bacteria Secrete Antibiotics That Inhibit Clostridium Difficile: Role of Secondary Bile Acids. Cell Chem. Biol. 2019, 26, 27–34.e4. [Google Scholar] [CrossRef]
- Duboc, H.; Rajca, S.; Rainteau, D.; Benarous, D.; Maubert, M.-A.; Quervain, E.; Thomas, G.; Barbu, V.; Humbert, L.; Despras, G.; et al. Connecting Dysbiosis, Bile-Acid Dysmetabolism and Gut Inflammation in Inflammatory Bowel Diseases. Gut 2013, 62, 531–539. [Google Scholar] [CrossRef]
- Jergens, A.E.; Heilmann, R.M. Canine Chronic Enteropathy-Current State-of-the-Art and Emerging Concepts. Front. Vet. Sci. 2022, 9, 923013. [Google Scholar] [CrossRef] [PubMed]
- Sicard, J.-F.; Le Bihan, G.; Vogeleer, P.; Jacques, M.; Harel, J. Interactions of Intestinal Bacteria with Components of the Intestinal Mucus. Front. Cell. Infect. Microbiol. 2017, 7, 387. [Google Scholar] [CrossRef]
- Bergstrom, K.S.B.; Xia, L. Mucin-Type O-Glycans and Their Roles in Intestinal Homeostasis. Glycobiology 2013, 23, 1026–1037. [Google Scholar] [CrossRef]
- Fyderek, K.; Strus, M.; Kowalska-Duplaga, K.; Gosiewski, T.; Wedrychowicz, A.; Jedynak-Wasowicz, U.; Sładek, M.; Pieczarkowski, S.; Adamski, P.; Kochan, P.; et al. Mucosal Bacterial Microflora and Mucus Layer Thickness in Adolescents with Inflammatory Bowel Disease. World J. Gastroenterol. 2009, 15, 5287–5294. [Google Scholar] [CrossRef] [PubMed]
- Nagata, N.; Ohta, H.; Yokoyama, N.; Teoh, Y.B.; Sasaki, N.; Nakamura, K.; Takiguchi, M. Characterization of Mucin Gene Expression and Goblet Cell Proportion in Inflammatory Colorectal Polyps in Miniature Dachshunds. J. Vet. Med. Sci. 2022, 84, 872–876. [Google Scholar] [CrossRef] [PubMed]
- Guilford, W.G. Nutritional Management of Gastrointestinal Tract Diseases of Dogs and Cats. J. Nutr. 1994, 124 (Suppl. 12), 2663S–2669S. [Google Scholar] [CrossRef] [PubMed]
- Nery, J.; Biourge, V.; Tournier, C.; Leray, V.; Martin, L.; Dumon, H.; Nguyen, P. Influence of Dietary Protein Content and Source on Fecal Quality, Electrolyte Concentrations, and Osmolarity, and Digestibility in Dogs Differing in Body Size. J. Anim. Sci. 2010, 88, 159–169. [Google Scholar] [CrossRef]
- Hang, I.; Heilmann, R.M.; Grützner, N.; Suchodolski, J.S.; Steiner, J.M.; Atroshi, F.; Sankari, S.; Kettunen, A.; de Vos, W.M.; Zentek, J.; et al. Impact of Diets with a High Content of Greaves-Meal Protein or Carbohydrates on Faecal Characteristics, Volatile Fatty Acids and Faecal Calprotectin Concentrations in Healthy Dogs. BMC Vet. Res. 2013, 9, 201. [Google Scholar] [CrossRef]
- Kong, L.; Lloyd-Price, J.; Vatanen, T.; Seksik, P.; Beaugerie, L.; Simon, T.; Vlamakis, H.; Sokol, H.; Xavier, R.J. Linking Strain Engraftment in Fecal Microbiota Transplantation With Maintenance of Remission in Crohn’s Disease. Gastroenterology 2020, 159, 2193–2202.e5. [Google Scholar] [CrossRef] [PubMed]
- Tang, G.; Yin, W.; Liu, W. Is Frozen Fecal Microbiota Transplantation as Effective as Fresh Fecal Microbiota Transplantation in Patients with Recurrent or Refractory Clostridium Difficile Infection: A Meta-Analysis? Diagn. Microbiol. Infect. Dis. 2017, 88, 322–329. [Google Scholar] [CrossRef]
- Gillespie, S.H. The Epidemiology of Toxocara Canis. Parasitol. Today Pers. Ed 1988, 4, 180–182. [Google Scholar] [CrossRef]
- Robertson, L.J.; Gjerde, B.K. Effects of the Norwegian Winter Environment on Giardia Cysts and Cryptosporidium Oocysts. Microb. Ecol. 2004, 47, 359–365. [Google Scholar] [CrossRef]
- Schurer, J.; Davenport, L.; Wagner, B.; Jenkins, E. Effects of Sub-Zero Storage Temperatures on Endoparasites in Canine and Equine Feces. Vet. Parasitol. 2014, 204, 310–315. [Google Scholar] [CrossRef]




| Parameter | Result (Range (Median) or Number) |
|---|---|
| Age (years) | 0.6–13.0 (5.8) |
| Breeds a | GSD 4, GR 3, MB 3, LR 2, RT 2, WH 2, STDPDL 2, ESS 2, MISC 21 |
| Treated for CE (months) | 1–110 (20) |
| Immunosuppressive treatment | CS b 15, CS + mycophenolate 8, CS + cyclosporine 7, CS + chlorambucil 6, CS + azathioprine 2 |
| Miscellaneous treatment c | Probiotics c 18, olsalazine 13, cobalamin 11, prebiotics d 11, cisapride 3, sucralfate 3, metronidazole 2, mirtazapine 2 |
| Diet e | Hydrolysed protein 28, single protein 11, GI f 2 |
| Main reasons for FMT | Diarrhoea 32, lethargy 18, unable taper CS 13, abdominal pain 13, hyporexia 9, underweight 6, reduce use/need of antibiotics 3 |
| Location | Histopathological Findings | Severity | n |
|---|---|---|---|
| Stomach | LP a gastritis | Mild | 12 |
| Moderate | 5 | ||
| Severe | 2 | ||
| Eosinophilic gastritis | Mild | 3 | |
| LP and partially eosinophilic gastritis | Mild | 2 | |
| Moderate | 2 | ||
| Mixed cell type (eosinophils/LP/neutrophils) | Mild | 1 | |
| Moderate | 1 | ||
| Fibrosis without inflammation | Mild | 1 | |
| Normal stomach | N/A b | 3 | |
| Small intestine | LP enteritis | Mild | 9 |
| Moderate | 11 | ||
| LP enteritis with multiple erosions | Severe | 2 | |
| LP and partially eosinophilic enteritis | Moderate | 4 | |
| Eosinophilic enteritis | Mild | 3 | |
| Moderate | 2 | ||
| Normal small intestine | N/A | 3 | |
| Large intestine | LP colitis | Mild | 15 |
| Moderate | 1 | ||
| LP colitis w multiple erosions | Mild | 1 | |
| LP and partially eosinophilic colitis | Mild | 1 | |
| Moderate | 1 | ||
| LP and partially eosinophilic colitis w. erosions | Moderate | 1 | |
| Eosinophilic colitis | Mild | 1 | |
| Erosive/ulcerative colitis | Moderate | 1 | |
| Severe | 1 | ||
| Histiocytic ulcerative colitis | Severe | 1 | |
| Normal large intestine | N/A | 3 |
| Parameter (Number of Dogs) | Good Responders n = 26 | Short-Lasting Responders n = 5 | Non-Responders n = 10 |
|---|---|---|---|
| Euthanasia for refractory GI a dx b | 0 | 3 | 4 |
| Additional FMTs performed in | 16 | 1 | 0 |
| FMT due to recurrence of clinical signs | 10 | 15 | N/A |
| Adding a booster dose | 5 | 3 | N/A |
| Treating new clinical GI signs | 1 | 0 | N/A |
| Clinically stable > 4 w. c after additional FMT | 15 | 0 | N/A |
| Tapering of maintenance corticosteroid tx d | 9 | 1 | 0 |
| Clinically long-term stable on decreased doses of corticosteroids | 9 | 0 | N/A |
| Stopping or reducing antibiotics | 3/3 | 0/1 | N/A |
| Treatment with cholestyramine | 0 | 1 | 5 |
| Response to cholestyramine | N/A | 1 | 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Toresson, L.; Spillmann, T.; Pilla, R.; Ludvigsson, U.; Hellgren, J.; Olmedal, G.; Suchodolski, J.S. Clinical Effects of Faecal Microbiota Transplantation as Adjunctive Therapy in Dogs with Chronic Enteropathies—A Retrospective Case Series of 41 Dogs. Vet. Sci. 2023, 10, 271. https://doi.org/10.3390/vetsci10040271
Toresson L, Spillmann T, Pilla R, Ludvigsson U, Hellgren J, Olmedal G, Suchodolski JS. Clinical Effects of Faecal Microbiota Transplantation as Adjunctive Therapy in Dogs with Chronic Enteropathies—A Retrospective Case Series of 41 Dogs. Veterinary Sciences. 2023; 10(4):271. https://doi.org/10.3390/vetsci10040271
Chicago/Turabian StyleToresson, Linda, Thomas Spillmann, Rachel Pilla, Ulrika Ludvigsson, Josefin Hellgren, Gunilla Olmedal, and Jan S. Suchodolski. 2023. "Clinical Effects of Faecal Microbiota Transplantation as Adjunctive Therapy in Dogs with Chronic Enteropathies—A Retrospective Case Series of 41 Dogs" Veterinary Sciences 10, no. 4: 271. https://doi.org/10.3390/vetsci10040271
APA StyleToresson, L., Spillmann, T., Pilla, R., Ludvigsson, U., Hellgren, J., Olmedal, G., & Suchodolski, J. S. (2023). Clinical Effects of Faecal Microbiota Transplantation as Adjunctive Therapy in Dogs with Chronic Enteropathies—A Retrospective Case Series of 41 Dogs. Veterinary Sciences, 10(4), 271. https://doi.org/10.3390/vetsci10040271








