Unusual Case of Biliary Peritonitis in a Dog Secondary to a Gastric Perforation
Abstract
Simple Summary
Abstract
1. Introduction
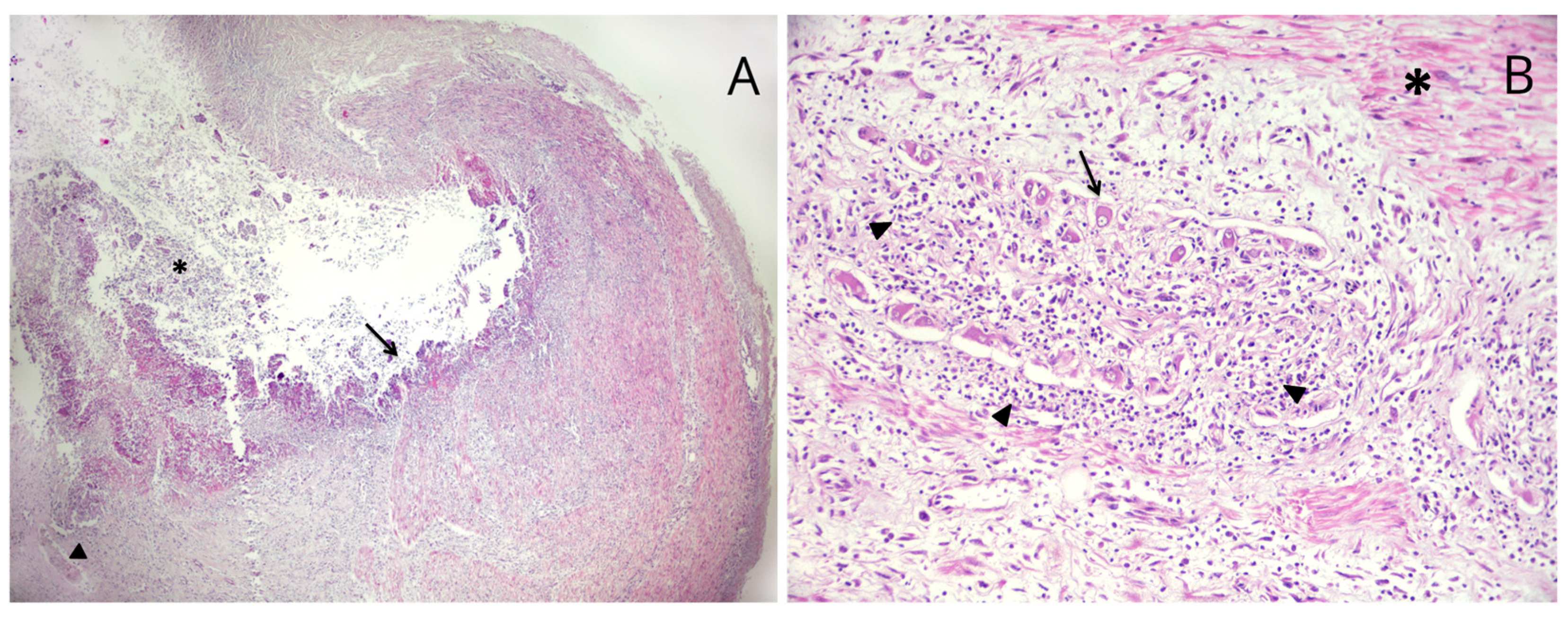
2. Case Presentation
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Thompson, B.J.; Sherman, R.A. Comprehensive Review of Biliary Peritonitis. Top. Companion Anim. Med. 2021, 44, 100532. [Google Scholar] [CrossRef]
- Mehler, S.J. Complications of the extra hepatic biliary surgery in companion animals. Vet. Clin. N. Am. Small Anim. Pract. 2011, 41, 949–967. [Google Scholar] [CrossRef]
- Crews, L.J.; Feeney, D.A.; Jessen, C.R.; Rose, N.D.; Matise, I. Clinical, ultrasonographic, and laboratory findings associated with gallbladder disease and rupture findings: 45 cases (1997–2007). J. Am. Vet. Med. Assoc. 2009, 234, 359–366. [Google Scholar] [CrossRef]
- Church, E.M.; Matthiesen, D.T. Surgical treatment of 23 dogs with necrotizing cholecystitis. J. Am. Anim. Hosp. Assoc. 1988, 24, 305–310. [Google Scholar]
- Mutsaers, S.E. The mesothelial cell. Int. J. Biochem. Cell Biol. 2004, 36, 9–16. [Google Scholar] [CrossRef]
- Ludwig, L.L.; McLoughlin, M.A.; Graves, T.K.; Crisp, M.S. Surgical treatment of bile peritonitis in 24 dogs and 2 cats: A retrospective study (1987–1994). Vet. Surg. 1997, 26, 90–98. [Google Scholar] [CrossRef]
- MacLeod, A.N.; Reichle, J.K.; Szabo, D.; Cohen, E.B.; Artiles, C.; Fulkerson, C.V.; Kurihara, M.; Mattoon, J. Ultrasonographic Appearance of Gallbladder Neoplasia in 14 Dogs and 1 Cat. Vet. Radiol. Ultrasound, 2023; ahead of print. [Google Scholar] [CrossRef]
- Harrison, J.; Turek, B.; Brown, D.; Bradley, C.; Clark, J.C. Cholangitis and Cholangiohepatitis in Dogs: A Descriptive Study of 54 Cases Based on Histopathologic Diagnosis (2004–2014). J. Vet. Intern. Med. 2018, 32, 172–180. [Google Scholar] [CrossRef]
- Wilkinson, A.R.; DeMonaco, S.M.; Panciera, D.L.; Otoni, C.C.; Leib, M.S.; Larson, M.M. Bile duct obstruction associated with pancreatitis in 46 dogs. J. Vet. Intern. Med. 2020, 34, 1794–1800. [Google Scholar] [CrossRef]
- Hamura, R.; Haruki, K.; Tsutsumi, J.; Takayama, S.; Shiba, H.; Yanaga, K. Spontaneous biliary peritonitis with common bile duct stones: Report of a case. Surg. Case Rep. 2016, 2, 103. [Google Scholar] [CrossRef] [PubMed]
- Shukla, R.M.; Roy, D.; Mukherjee, P.P.; Saha, K.; Mukhopadhyay, B.; Mandal, K.C.; SahaBasu, K.; Barman, S.S. Spontaneous gall bladder perforation: A rare condition in the differential diagnosis of acute abdomen in children. J. Pediatr. Surg. 2011, 46, 241–243. [Google Scholar] [CrossRef]
- Andersson, R.; Tranberg, K.G.; Bengmark, S. Roles of bile and bacteria in biliary peritonitis. Br. J. Surg. 1990, 77, 36–39. [Google Scholar] [CrossRef] [PubMed]
- Jaffey, J.A.; Graham, A.; VanEerde, E.; Hostnik, E.; Alvarez, W.; Arango, J.; Jacobs, C.; DeClue, A.E. Gallbladder mucocele: Variables associated with outcome and the utility of ultrasonography to identify gallbladder rupture in 219 dogs (2007–2016). J. Vet. Intern. Med. 2018, 32, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Besso, J.G.; Wrigley, R.H.; Gliatto, J.M.; Webster, C.R. Ultrasonographic appearance and clinical findings in 14 dogs with gallbladder mucocele. Vet. Radiol. Ultrasound 2000, 41, 261–271. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Kim, A.; Keh, S.; Oh, J.; Kim, H.; Yoon, J. Comparison between ultrasonographic and clinical findings in 43 dogs with gallbladder mucoceles. Vet. Radiol. Ultrasound 2014, 55, 202–207. [Google Scholar] [CrossRef]
- Escobar, M.C.; Neel, J.A. Pathology in practice. J. Am. Vet. Med. Assoc. 2011, 239, 65–67. [Google Scholar] [CrossRef]
- Amsellem, P.M.; Seim, H.B., 3rd; MacPhail, C.M.; Bright, R.M.; Twedt, D.C.; Wrigley, R.H.; Monnet, E. Long-term survival and risk factors associated with biliary surgery in dogs: 34 cases. J. Am. Vet. Med. Assoc. 2006, 229, 1451–1457. [Google Scholar] [CrossRef]
- Norwich, A. Gallbladder mucocele in a 12 year-old cocker spaniel. Can. Vet. J. 2011, 52, 319–321. [Google Scholar]
- O’Brien, P.J.; Lumsden, J.H. The cytologic examination of body cavity fluids. Semin. Vet. Med. Surg. (Small Anim.) 1988, 3, 140–156. [Google Scholar]
- Bonczynski, J.J.; Ludwig, L.L.; Barton, L.J.; Loar, A.; Peterson, M.E. Comparison of peritoneal fluid and peripheral blood pH, bicarbonate, glucose, and lactate concentration as a diagnostic tool for septic peritonitis in dogs and cats. Vet. Surg. 2003, 32, 161–166. [Google Scholar] [CrossRef]
- Levin, G.M.; Bonczynski, J.J.; Ludwig, L.L.; Barton, L.J.; Loar, A.S. Lactate as a diagnostic test for septic peritoneal effusions in dogs and cats. J. Am. Anim. Hosp. Assoc. 2004, 40, 364–371. [Google Scholar] [CrossRef]
- Dempsey, S.M.; Ewing, P.J. A review of the pathophysiology, classification, and analysis of canine and feline cavitary effusions. J. Am. Anim. Hosp. Assoc. 2011, 47, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Fry, M.M. Diagnosis of uroabdomen. In Nephrology and Urology of Small Animals; Bartges, J., Polzin, D.J., Eds.; Wiley-Blackwell: Ames, IA, USA, 2011; pp. 73–74. [Google Scholar]
- Schmiedt, C.; Tobias, K.M.; Otto, C.M. Evaluation of abdominal fluid: Peripheral blood creatinine and potassium ratios for diagnosis of uroperitoneum in dogs. J. Vet. Emerg. Crit. Care 2011, 11, 275–280. [Google Scholar] [CrossRef]
- Gupta, R.K.; Johnston, P.S.; Naran, S.; Lallu, S.; Fauck, R. Cytological findings in a sample of peritoneal aspirate from a case of bile peritonitis. Diagn. Cytopathol. 2005, 32, 35–37. [Google Scholar] [CrossRef] [PubMed]
- Aumann, M.; Worth, L.T.; Drobatz, K.J. Uroperitoneum in cats: 26 cases (1986–1995). J. Am. Anim. Hosp. Assoc. 1998, 34, 315–324. [Google Scholar] [CrossRef]
- Webb, C.B.; Twedt, D.C. Canine gastritis. Vet. Clin. N. Am. Small Anim. 2003, 33, 969–985. [Google Scholar] [CrossRef]
- Eagon, J.C.; Miedema, B.W.; Kelly, K.A. Postgastrectomy syndromes. Surg. Clin. N. Am. 1992, 72, 445–465. [Google Scholar] [CrossRef]
- Camilleri, M. Gastrointestinal hormones and regulation of gastric emptying. Curr. Opin. Endocrinol. Diabetes Obes. 2019, 26, 3–10. [Google Scholar] [CrossRef]
- Duane, W.C.; Wiegand, D.M. Mechanism by which bile salt disrupts the gastric mucosal barrier in the dog. J. Clin. Investig. 1980, 203, 537–544. [Google Scholar] [CrossRef]
- Ritchie, W.P. Alkaline reflux gastritis. Late results on a controlled trial of diagnosis and treatment. Ann. Surg. 1986, 203, 537–544. [Google Scholar] [CrossRef]
- Naylor, A.; Axon, A. Role of bacterial overgrowth in the stomach as an additional risk factor for gastritis. Can. J. Gastroenterol. 2003, 17 (Suppl. b), 13B–17B. [Google Scholar] [CrossRef]
- Shi, X.; Chen, Z.; Yang, Y.; Yan, S. Bile Reflux Gastritis: Insights into Pathogenesis, Relevant Factors, Carcinomatous Risk, Diagnosis, and Management. Gastroenterol. Res. Pract. 2022, 2022, 2642551. [Google Scholar] [CrossRef] [PubMed]
- Henderson, A.K.; Webster, C.R.L. An in-depth look: Disruption of the gastric mucosal barrier in dogs. Compendium 2006, 28, 340–357. [Google Scholar]
- Bertolini, A.; Ottani, A.; Sandrini, M. Dual acting anti-inflammatory drugs: A reappraisal. Pharmacol. Res. 2001, 44, 437–450. [Google Scholar] [CrossRef] [PubMed]
- Cariou, M.P.; Halfacree, Z.J.; Lee, K.C.; Baines, S.J. Successful surgical management of spontaneous gastric perforations in three cats. J. Feline Med. Surg. 2010, 12, 36–41. [Google Scholar] [CrossRef] [PubMed]
- Bernardin, F.; Martinez Rivera, L.; Ragetly, G.; Gomes, E.; Hernandez, J. Spontaneous gastrointestinal perforation in cats: A retrospective study of 13 cases. J. Feline Med. Surg. 2015, 17, 873–879. [Google Scholar] [CrossRef]
- Prabhu, V.; Shivani, A. An overview of history, pathogenesis and treatment of perforated peptic ulcer disease with evaluation of prognostic scoring in adults. Ann. Med. Health Sci. Res. 2014, 39, 421–424. [Google Scholar] [CrossRef]
- Forsyt, S.F.; Guilford, W.G.; Haslett, S.J.; Godfrey, J. Endoscopy of the gastroduodenal mucosa after carprofen, meloxicam and ketoprofen administration in dogs. J. Small Anim. Pract. 1998, 39, 421–424. [Google Scholar] [CrossRef]
- Lascelles, B.D.; Blikslager, A.T.; Fox, S.M.; Reece, D. Gastrointestinal tract perforation in dogs treated with a selective cycloxygenase-2 inhibitor: 29 cases (2002–2003). J. Am. Vet. Med. Assoc. 2005, 227, 1112–1117. [Google Scholar] [CrossRef]
- Runk, A.; Kyles, A.E.; Downs, M.O. Duodenal perforation in a cat following the administration of nonsteroidal anti-inflammatory medication. J. Am. Anim. Hosp. Assoc. 1999, 35, 52–55. [Google Scholar] [CrossRef]
- Sostres, C.; Gargano, C.J.; Lanas, A. Nonsteroidal anti-inflammatory drugs and upper and lower gastrointestinal mucosal damage. Arthritis Res. Ther. 2013, 15 (Suppl. S3), S3. [Google Scholar] [CrossRef]
- Luna, S.P.; Basilio, A.C.; Steagall, P.V.; Machado, L.P.; Moutinho, F.Q.; Takahira, R.K.; Brandão, C.V. Evaluation of adverse effects of long-term oral administration of carprofen, etodolac, flunixin meglumine, ketoprofen, and meloxicam in dogs. Am. J. Vet. Res. 2007, 68, 258–264. [Google Scholar] [CrossRef] [PubMed]
- Trevor, B.E.; Braun, L.D.; Kuzma, A.B. Gastrointestinal perforation in five dogs associated with the administration of meloxicam. J. Vet. Emerg. Crit. Care 2006, 16, 34–43. [Google Scholar]
- Wallace, J.L.; Vong, L. NSAID-induced gastrointestinal damage and the design of GI-sparing NSAIDs. Curr. Opin. Investig. Drugs 2008, 9, 1151–1156. [Google Scholar] [CrossRef] [PubMed]
- Senello, K.A.; Leib, M.S. Effects of deracoxib or buffered aspirin on the gastric mucosa of healthy dogs. J. Vet. Intern. Med. 2006, 20, 1291–1296. [Google Scholar] [CrossRef]
- Goodman, L.; Torres, B.; Punke, J.; Reynolds, L.; Speas, A.; Ellis, A.; Budsberg, S. Effects of firocoxib and tepoxalin on healing in a canine gastric mucosal injury model. J. Vet. Intern. Med. 2009, 23, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Wooten, J.G.; Blikslager, A.T.; Marks, S.L.; Law, J.M.; Graeber, E.C.; Lascelles, B.D. Effect of nonsteroidal anti-inflammatory drugs with varied cycloxygenase-2 selectivity on cycloxygenase protein and prostanoid concentrations in pyloric and duodenal, mucosa of dogs. Am. J. Vet. Res. 2009, 70, 1243–1249. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pavone, G.; Castellucci, B.; Pavone, S.; Stefanetti, V.; Vitolo, C.; Mangiaterra, S. Unusual Case of Biliary Peritonitis in a Dog Secondary to a Gastric Perforation. Vet. Sci. 2023, 10, 384. https://doi.org/10.3390/vetsci10060384
Pavone G, Castellucci B, Pavone S, Stefanetti V, Vitolo C, Mangiaterra S. Unusual Case of Biliary Peritonitis in a Dog Secondary to a Gastric Perforation. Veterinary Sciences. 2023; 10(6):384. https://doi.org/10.3390/vetsci10060384
Chicago/Turabian StylePavone, Giovanni, Barbara Castellucci, Silvia Pavone, Valentina Stefanetti, Chiara Vitolo, and Sara Mangiaterra. 2023. "Unusual Case of Biliary Peritonitis in a Dog Secondary to a Gastric Perforation" Veterinary Sciences 10, no. 6: 384. https://doi.org/10.3390/vetsci10060384
APA StylePavone, G., Castellucci, B., Pavone, S., Stefanetti, V., Vitolo, C., & Mangiaterra, S. (2023). Unusual Case of Biliary Peritonitis in a Dog Secondary to a Gastric Perforation. Veterinary Sciences, 10(6), 384. https://doi.org/10.3390/vetsci10060384






