Simple Summary
Molecular genetic approaches have been developed over the last half-century to identify genetic variation associated with economically important growth performance and carcass characteristics. In the livestock sector, identifying and localizing the genes responsible for each trait, as well as selecting beneficial alleles based on live animal experiments, meat production, and nutritional quality, have played an essential role in improving productivity. The purpose of this study is to examine the association between IGF1 5′UTR polymorphisms and various growth and carcass parameters of meat-type sheep breeds raised in Turkey. A total of eight nucleotide changes were identified that were able to characterize three IGF1 5′UTR variants, and certain variants were associated with variations in chest width and leg circumference. The P1 variants had a leaner profile and the P2 variants had a higher percentage of rack and loin. Nucleotide sequence variations in IGF1 5′UTR could, thus, be exploited for marker-assisted selection in order to enhance growth and production attributes and carcass quality.
Abstract
This investigation was conducted to determine how the growth and carcass traits of meat-type sheep breeds raised in Turkey are associated with IGF1 5′UTR polymorphisms. Overall, 202 lambs from five breeds were evaluated. We identified eight nucleotide changes (seven substitutions and one deletion) in three variants of IGF1 5′UTR by SSCP analysis and nucleotide sequencing. It was found that the P1 variants had a unique deletion (g.171328230 delT), while the P2 variants were identified by SNPs rs401028781, rs422604851, and g.171328404C > Y. The P3 variants possessed one heterozygous substitution (g.171328260G > R) and three homozygous substitutions (g.171328246T > A, g.171328257T > G, g.171328265T > C) not observed in P1 or P2. Based on the growth and production traits, a statistically significant difference was found only in chest width at weaning (p < 0.01) and leg circumferences at yearling (p < 0.05). The P1 variants showed a leaner profile with a higher Musculus longissimus dorsi, but the differences were not significant (p > 0.05). The P2 variants had a higher percentage of rack (p < 0.01) and loin (p > 0.05). Moreover, there was no discernible difference between variants, even though the P3 variants had a higher percentage of neck and leg and the P1 variants had a higher percentage of the shoulder. It is concluded that nucleotide changes in IGF1 5′UTR could be exploited utilizing a marker-assisted selection technique to increase growth and production attributes, as well as carcass quality traits.
1. Introduction
A recent report on global population trends stated that the number of people will reach 9.7 billion in 2050 and 10.9 billion in 2100 [1]. As developing countries around the world become more Westernized, the demand for meat, milk, and other animal products is also expected to increase [2]. However, this seems unlikely to be accomplished due to the limited resources available. In addition, it is difficult to control the quality and nutritional value of these protein sources in order to satisfy consumer demands, especially when this goal depends on several factors simultaneously [3]. As an example, meat is affected by a number of factors, including water-holding capacity (WHC), color, tenderness, texture, carcass composition and conformation, animal environments, breeds, stunning, slaughtering, storage conditions, and gene expression [3].
In the livestock sector, advances in molecular genetics have allowed for the identification of genes and sequence variation associated with various production traits [4,5]. Consequently, many scientists are advocating a marker-assisted selection (MAS) program as a means of predicting muscle mass gain in farm animals and determining the most productive individuals [6,7].
One of the promising candidate genes to be used in the MAS program is insulin-like growth factor type-1 (IGF1) [8]. A mammal IGF1 consists of six exons separated by five introns and spans more than 80 kb [9]. While species share a commonality in the sequence and length of exons 1–4, there is considerable variation in exons 5 and 6. The mature IGF1 peptides that serve as receptor-binding ligands are specified by exons 3 and 4, while exons 1 and 2 determine the type of protein and signal peptides that are encoded for cellular localization following translation [10].
It is widely acknowledged that IGF1 plays an important role in the regulation of normal growth, placental and fetal development, immunity, reproduction, and metabolism [11,12,13]. In addition, previous studies have shown that the polymorphism in the IGF1 gene influences growth and production traits in a variety of farm animals, including chickens [14,15], pigs [16,17], goats [18,19], sheep [20,21], and cattle [22,23]. IGF1 has been shown to stimulate the proliferation and differentiation of myocytes during myogenesis, which may contribute to muscle growth and repair, as well as muscle regeneration and repair [24,25]. Fat deposition and carcass quality in New Zealand Romney sheep have been shown to be affected by six single nucleotide polymorphism (SNPs) in the exon 3, exon 4, and 5′ flanking region of ovine IGF1 [10]. Similarly, Trukhachev et al. [20] investigated the IGF1 polymorphism in the Russian Soviet Merino breed and concluded that the identification of SNPs c.-91A > C could be used to predict its parameters of superior meat production. The IGF1 polymorphism is also associated with lactation persistence in dairy sheep breeds [26], as well as wool and hair production in sheep [27] and goats [28].
A mutation in IGF1 5′UTR might possibly have an effect on the functional features of the protein due to the role that it plays in selecting the type of protein and signal peptides that are encoded for cellular localization after translation [29,30]. As discussed in our previous studies [31,32], Kıvırcık (K), Karacabey Merino (KM), German Black-Head Mutton × Kıvırcık (GBK), Hampshire Down × Merino (HM), and Ramlıç (Rambouillet × Daglıç, R) are the main meat-type sheep breeds widely grown in the western part of Turkey in order to meet market demands. To the best of our knowledge, there are no known data in Turkey on the association between the IGF1 polymorphism with growth, body size, slaughter, and meat quality traits in those meat-type sheep breeds. As a result, the purpose of this study was to determine the impact of the IGF1 5′UTR polymorphism on a variety of production variables, including body measurements, slaughter and carcass features, and meat quality.
2. Materials and Methods
2.1. Ethical Approval
All of the experiments on animals were done in accordance with the Declaration of Helsinki and were approved by the Ethics Committee of the Sheep Breeding Research Institute in Turkey (approval number:13360037). The animals were handled and cared for in the experimental farm unit of the same institute from January to June 2018.
2.2. Animals and Feeding Schedules
The animal population and feeding schedules used in the current study were based on our studies that were carried out earlier [31,32,33,34,35]. Figure 1 illustrates the breeds of sheep used in the study and their distribution according to gender. The lambs were kept with their dams in separate barns during the preweaning period, and at 15 days old, they were given access to ad libitum high-quality alfalfa hay and commercial starter feed. Weaned lambs of different breeds were grouped together and fed a diet consisting of concentrated feed (600 g per lamb per day), alfalfa hay (100 g per lamb per day), and a vetches−wheat mixture (300 g per lamb per day) until slaughter. After slaughter, male and female lambs were separated in order to prevent unwanted mating. Between the ages of weaning and yearlings, they were allowed to roam freely on pasture when the weather permitted, and were provided with a diet of alfalfa hay, vetches−wheat mixed hay, concentrate feed, and wheat straw ad libitum. In our previous studies, we provided detailed information about the chemical composition of the roughages and concentrates that were used in this study [31,32].

Figure 1.
The breeds of sheep used in the study and their distribution by gender: Kıvırcık (a), Karacabey Merino (b), Ramlıç (c), German Black-Head Mutton × Kıvırcık (d), Hampshire Down × Merino (e).
2.3. Measurement of Live Weight, Linear Body Measurements, and In Vivo Ultrasound
Lambs born within 10 days of the end of the lambing season were employed in the study, and they were weaned after a mean of 90.5 ± 5.7 days (mean ± SD) [32]. Within 12 h of birth, the birth weight (BW) was assessed, and on the 90th, 180th, and 360th days of the research, the live weight (LW), linear body measurements, and ultrasound measurements were obtained. LW was recorded before morning feeding to avoid the effects of a full stomach on the accuracy of the measurement. A skilled technician used a flexible calibrated tape and callipers to obtain linear measurements of the lamb while it was standing with its head raised, including the following: body length (BL), back height (BH), rump height (RH), withers height (WH), chest depth (CD), chest width (CW), rump width (RW), chest circumference (CC), leg circumference (LC), and cannon bone perimeter (CBP) [36].
Lambs’ fat thickness (FT), skin thickness (ST), and Musculus longissimus dorsi depth (MLDD) were measured between the 12th and 13th ribs using a Mindray DP-20 real-time ultrasound system and Mindray 75L50EAV linear veterinary ultrasound transducers [37]. These measurements were taken after linear body measurements were performed at each specified interval. We used ultrasonic gel as a couplant and manually immobilized the lambs so that we could separate their wool between their 12th and 13th ribs. Taking care not to compress the fat, measurements were collected on the left side, 4 cm from the vertebral column [31]. After the scanned image was taken, MLDD, FT, and ST were measured using the scanner’s electronic callipers.
2.4. Assessment of Slaughter and Carcass Characteristics and Meat Quality
Ten male lambs were chosen at random from each breed to be used in our analysis of slaughter and carcass features and meat quality. Before slaughter, the lambs were provided with fresh water and were fasted for 12 h in the institute slaughterhouse. The slaughter weight (SW) of the lambs was noted and then they were slaughtered according to commercial standards. For the purposes of determining the hot carcass weight (HCW), we skinned the animals and then removed the heads, feet, lungs, liver, heart, spleen, gastrointestinal tract, and testicles. The hot dressing percentage (HDP) was calculated by dividing HCW by SW. Following chilling the carcasses at 4 °C for 24 h, the cold carcass weight (CCW) and cold dressing percentage (CDP) were calculated.
Each carcass was split into five portions (neck, shoulder, rack, loin, and leg), and the Longissimus thoracis et lumborum (LTL) muscle was taken between the 5th and 12th thoracic vertebra for additional laboratory investigation [38]. The Fiji image measurement program was used to measure the MLD area (MLDA), MLD perimeter (MLDP), MLD depth (MLDD), MLD width (MLDW), MLD fat thickness (MLDFT), the body fatness (BF) of the chilled carcasses (Version 1.52d) [39]. Color parameters of LTL were measured for 0, 48, and 168 h after storage at 4 °C using a portable colorimeter (Chroma Meter CR-410; Konica Minolta, Tokyo, Japan) with three replicates of samples. The filter-paper method was employed to determine WHC, according to Honikel and Hamm [40]. In accordance with previously published studies by Choi and Kim [41] and Gonzales-Barron et al. [42], we evaluated a sample of LTL for thawing loss (TL) and cooking loss (CL), respectively. The shear force (SF) of the cooked LTL samples was measured using a texture analyzer (TA.HDplus, Stable Micro Systems Ltd., Surrey, UK) and Warner Bratzler blade (HDP/WBV, Stable Micro Systems Ltd., Surrey, UK).
2.5. Extraction of DNA, Primer Design, PCR Amplification, SSCP Analysis, and Sequencing of DNA
Blood samples were taken from each of the lambs’ vena jugularis and placed into sterile EDTA tubes before the male lambs were slaughtered. Genomic DNA was extracted from blood samples collected from lambs using the GeneAll® DNA extraction kit and Bio-Rad T100 thermal cycler.
Polymorphisms in 265 bp of IGF1 5′UTR were discovered to alter growth and carcass features [29,30]; hence, a set of PCR primers designed by Behzadi et al. [29] was employed to amplify this region. Using GeneAll® 2XAmpMaster, DNA was amplified in a 20 µL reaction with each primer weighing 100 ng. The PCR conditions for amplification of the 265 bp promoter region of the IGF1 5′UTR were as follows: the initial extension was 3 min at 95 °C, 35 cycles 45 s at 95 °C, 40 s at 60 °C, 50 s at 72 °C, and the final extension was 10 min at 72 °C.
The PCR products were mixed with a loading dye that contained 98% formamide and denatured at 95 °C for 7 min. Following rapid cooling of the samples on wet ice, the samples were separated by acrylamide−bisacrylamide gels (29:11) according to Green and Sambrook [43]. According to the method described by Byun et al. [44], vertical electrophoresis was performed for 4 h at 350 V. As part of the staining technique, the formaldehyde ratio was modified to 2% before determining how visible the electrophoresis bands were.
We utilized a genetic analyzer (ABI PRISM 3500, Applied Biosystems, Foster City, CA, USA) to detect various band patterns within the DNA sequences of the samples. By removing noisy sequences and aligning clear sequences, we were able to visualize the alignment of chromatograms using the Bioedit Sequence Alignment Editor. In order to determine the location of SNPs on the chromosomes, we compared our DNA sequences with those found in the sheep genome (Oar_v3.1, GCA_000298735.1) using the Ensembl Genome Database. Note that two of the SNPs obtained in this study had previously been identified in the reference genome.
According to Falconer and Mackay [45], the genotypes and variant frequencies were calculated for each SNP.
2.6. Statistical Analysis
A GLM procedure was employed in Minitab [46] to determine whether the IGF1 5′UTR polymorphism influenced the studied traits. To compare LW, body measurements, and in vivo development of MLD throughout different time periods, the fixed effect of breed (K, KM, R, GBK and HM), gender (female and male), type of birth (single and twin), age of dam (2–7+), and variants of IFG1 promotor-exon 1 (P1-P3; considering one variation at a time), as well as their interactions were taken into account (Model 1). Gender was removed from the model in the analysis of the slaughter and carcass characteristics, retail carcass percentage, and meat quality features, as only male lambs were slaughtered in this study (Model 2). The predicted number of animals for Models 1 and 2 in each group is shown in Table S2 based on the Power analysis of Cohen’s f values (0.10, 0.25, and 0.40 as small, medium, and large values, respectively). To ascertain whether there were statistically significant differences between the color parameters, a repeat-measures analysis of variance was also utilized. Duncan’s multiple range test was used to compare the means, with a significance level of p < 0.05 indicating a statistically significant difference.
3. Results
In the case of IGF1 5′UTR, 21 of the samples did not amplify, which may be the result of a mutation in the major alignment region present in these animals. Thus, 181 animals’ worth of information was used in the association analysis.
In polyacrylamide gels, three different variants in PCR products were discovered using the PCR-SSCP approach. Three distinct PCR-based SSCP patterns (P1, P2, and P3) were deposited in the NCBI GeneBank OQ197497-OQ197499 (Figure 2a). Alignment of the sanger sequencing’s complementary sequences was performed using the Bioedit’s Clustal W algorithm (Figure 2b).
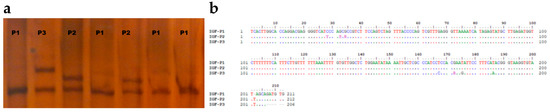
Figure 2.
The variation of sequences in meat-type sheep breeds. Different sequences in the region of IGF1 5′UTR are found using PCR-SSCP analysis and DNA sequencing (a) Alignment of the IGF1 5′UTR variant sequences with the NCBI reference sequence GCA_000298735.1 using the Clustal W algorithm (b).
A comparison of the clear sequences obtained with the reference genome revealed that they were located on the third chromosome and the IGF1 gene was located within the promoter region of the first exon. On the basis of the difference between the three variants, nucleotide changes were detected in eight nucleotide positions (Table 1). It was only P1 that showed deletion within the nucleotide sequences (c.202delT). In P2, a total of three heterozygous substitutions (g.171328398 G > R(A/G), g.171328400 G > S(G/C), g.171328404 C > Y(C/T)) were observed, of which two had already been identified (rs401028781 and rs422604851). Furthermore, three homozygotes (g.171328246 T > A, g.171328257 T > G, g.171328265 T > C) and one heterozygote (g.171328260 G > R(A/G)) were identified.

Table 1.
Variation in the sequence of IGF1 5′UTR.
The frequency of the wild-type variant (P1) was the greatest in each breed and gender (Table 2). The P1 variant was identified in all male and female R lambs. Although neither KM nor HM male lambs carried the P2 variant, neither did R male lambs. While the P3 variant was not found in female KM and R lambs, it was also not found in female HM and K lambs. GBK lambs, on the other hand, were the only breed in which all three variants were found in both males and females.

Table 2.
Frequency of IGF1 5′UTR variants by race and gender.
In all breeds, the wild-type variant was more common than the mutant variant in all SNPs, except at position c.202 (Table S2). For all SNP sites in the GBK and KM breeds, two genotypes were observed: wild-type homozygous and mutant heterozygous. Homozygous wild genotypes were found in the R breed at positions c.28, c.32, and c.34, whereas they were found in the HM and K breeds at positions c.167, c.172, c.175, and c.186. The positions of c.8, c.32, and c.34 had the highest frequency of mutant variants in the K breed, whereas positions c.167, c.172, c.175, and c.186 had the highest frequency of mutant variants in the GBK breed. For all breeds except GBK, the deletion rate of the variant T at position c.202 was higher than in the wild type variant. All of the reported SNP variant frequencies in both wild-type and mutant-type homozygotes deviated from the Hardy Weinberg distribution (p > 0.05).
There were no significant differences between the male and female variants of IGF1 5′UTR in terms of BW or LW measured at the different developmental stages (p > 0.05, Figure 3). In the P3 variant, males had a lower BW and weaning weight (LW90d) than females, but this changed when measuring 180 days after birth.
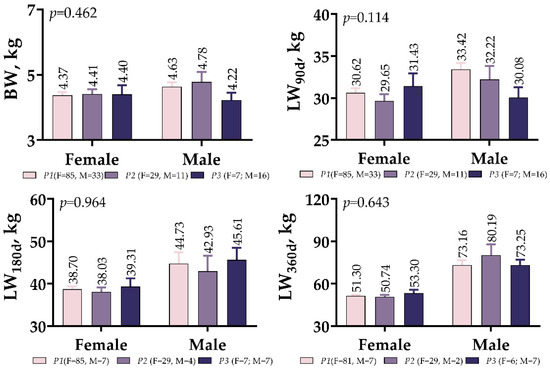
Figure 3.
IGF1 5′UTR polymorphism in meat-type lambs: effect on weight at different ages.
Figure S1 offers a visual representation of how the IGF1 5′UTR polymorphism influences the body measurements of meat-type lambs. We found statistically significant differences only in CW between the IGF1 5′UTR variants at weaning (Figure S1a; p < 0.01) and in LC at yearling (Figure S1c; p < 0.05). At six months, however, none of the other body measurements had significantly changed (Figure S1b, p > 0.05). In the weaning period, males and females of variants P3 showed the highest and lowest CW values (18.95 cm and 17.14 cm, respectively). It was observed, however, that the difference in CW disappeared after six months. Moreover, the LC of female lambs was comparable between variants; however, the variant P2 had 24.55–25.57 cm greater LC than the other variants at yearling, and the difference was statistically significant (Figure S1c; p < 0.05).
As shown in Figure 4, the effects of the IGF1 5′UTR polymorphism on the development of MLD in meat-type lambs were not different between males and females. The male variants of P2 showed the lowest MLDD values at weaning and 180 days (2.27 and 2.20 cm, respectively), while the male variants of P3 exhibited the highest MLDD values (2.50 and 2.73 cm, respectively). However, in the 360-day measurements, this difference in MLDD between male variants no longer existed. On both the 90th and 180th day measurements, male lambs had lower FT values than female lambs; however, the 360th day measurements were very similar.
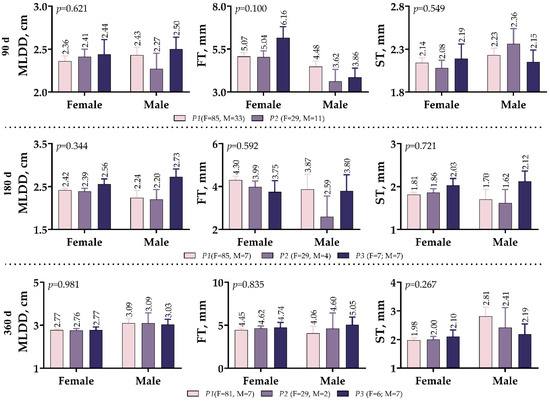
Figure 4.
IGF1 5′UTR polymorphism in meat-type lambs: effect on Musculus longissimus dorsi muscle development.
Figure 5 provides an overview of the data from the carcass parameters of the IGF1 5′UTR variants. The results indicate that there was no significant relationship between the IGF1 5′UTR variants and any of the parameters examined (p > 0.05). Although SW and both carcass weights (HCW and CCW) were higher in variant P1, the dressing percentages (HDP and CDP) were higher in variant P2. In the variants, ChL, T45m, T24h, pH45m, and pH24h values ranged from 1.49–1.55%, 35.98–36.73 °C, 6.57–7.01 °C, 6.34–6.42, and 5.44–5.57, respectively. Likewise, no statistical differences were observed between any of the MLD values obtained using the image processing technique. Compared with the other variants, the P1 variant had 5.11–7.51% higher MLDD and 5.03–7.84% higher MLDA values and exhibited a leaner profile.
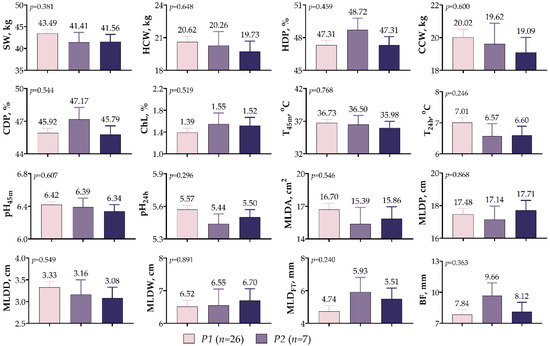
Figure 5.
IGF1 5′UTR polymorphism in meat-type lambs: effect on carcass traits.
In Figure 6, it is shown that the effects of the IGF1 5′UTR polymorphism on non-carcass components were not significant, except for the weight of the testicles (p = 0.001). The mean testicular weight was the lowest for the P2 variants (159.7 g), while it was similar for the P1 and P3 variants (296.8 g vs. 306.8 g). Although not statistically significant, the P1 variant had the highest weights for skin (4.46 kg), feet (1.04 kg), liver (784.4 g), kidney (124.2 g), full stomach (6.58 kg), empty stomach (1.40 kg), full intestine (3.81 kg), empty intestine (2.02 kg), and omental and mesenteric fat (488.9 g). Furthermore, the P2 variant was characterized by a head weight of 2.49 kg and a heart weight of 277.6 g, while the P3 variant was characterized by a spleen weight of 146.7 g and a red offal weight of 246.6 g.
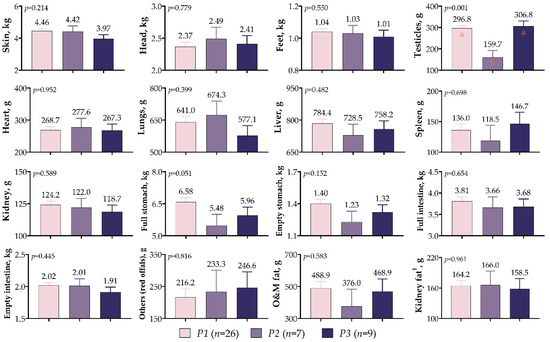
Figure 6.
IGF1 5′UTR polymorphism in meat-type lambs: effects on non-carcass components. The values with different letters (a–b) in each graph are statistically different (p < 0.05).
The polymorphisms in IGF1 5′UTR had a significant effect only on the rack proportion of meat-type lamb (p < 0.01, Figure 7). Lambs of the P2 variant exhibited higher rack ratios than those of the P1 and P3 variants by 25.06% and 21.51%, respectively. There was also a high loin (14.54%) proportion in the P2 variant; however, the differences were not statistically significant (p > 0.05). The difference between variants was insignificant despite the fact that the shoulder proportions were high in P1 variant lambs, and the neck and leg proportions were high in the P3 variant lambs (p > 0.05).
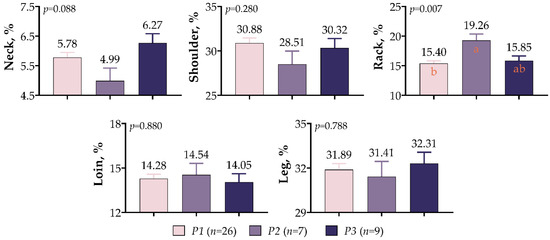
Figure 7.
IGF1 5′UTR polymorphism in meat-type lambs: effects on retail carcass percentage. The values with different letters (a–b) in each graph are statistically different (p < 0.05).
A comparison of meat quality assessments in meat-type lambs based on IGF1 5′UTR variants is presented in Figure 8. A statistical difference was not observed between variants, despite the fact that SF (7.61 kg), WHC (20.49%), and TL (9.54%) were higher in P2 variants and CL (27.23%) was higher in P3 variants (p > 0.05).
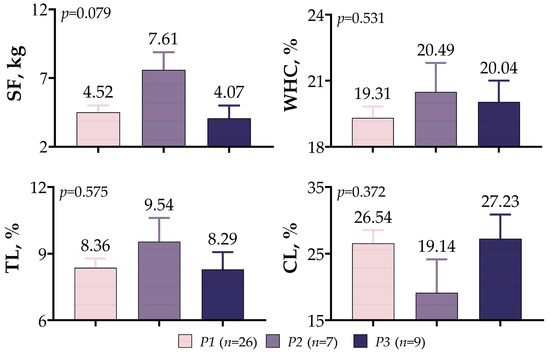
Figure 8.
IGF1 5′UTR polymorphism in meat-type lambs: effect on meat quality.
As can be seen in Figure 9, there were no statistically significant interactions between the genotype and storage time for any of the measured color characteristics (p > 0.05). A definite upward trend could be seen in terms of the values of L* and h across all of the variants. Unlike the other variants, a decrease in the a* and C values was observed in the P2 variant after 48 h of storage. Higher L*, a*, and h values were observed in the P3 after 168 h of storage, but greater b* and C values were observed in the P2 variation.
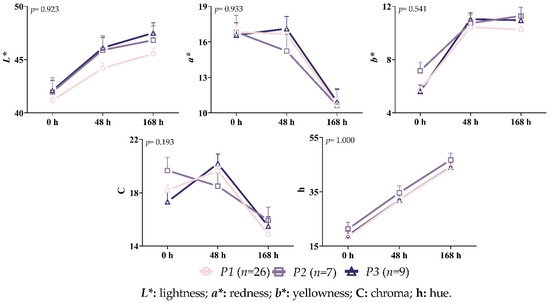
Figure 9.
Interaction between variant and time on the color characteristics of the Longissimus thoracis and lumborum muscle during storage.
4. Discussion
There is compelling evidence that IGF1, one of the family of IGFs, plays a critical role in cell differentiation, embryogenesis, metabolism, reproductive development, and fetal development, making it a potential candidate gene for characteristics of sheep that are both productive and economical [47]. Yet, there is inconsistent information about the effects of the IGF1 polymorphism on the growth and production qualities in different sheep breeds. For example, while several studies have associated the IGF1 polymorphism in the 5′-flanking region with growth traits in Makooei [48], Baluchi [49], and Makui [50] sheep, no such correlations have been discovered in Indian Madras Red sheep [51], Baluchi sheep [52], and Zandi sheep [53]. Moreover, two SNPs in IGF1 exon 2 (c.144G > A and c.150T > C) and a single SNP in IGF1 exon 5 (c.495G > A) were demonstrated to be significantly associated with four growth traits (CC at 4 and 9 months of age, BL at 4 months of age, and average daily gain (ADG) from 4 to 9 months of age) in Hulun Buir sheep [21]. In another investigation, Machado et al. [54] found that Santa Inês sheep with IGF1 haplotype replacements possessed ADG, BL, RH, WH, CC, CW, and LC. In this research, there was a significant difference in CW only during the weaning stage and in LC at the yearling stage between the IGF1 5′UTR variants characterized; hence, the IGF1 polymorphism seen in the 5′-flanking region and SNPs detected in IGF1 exon 2 and IGF1 exon 5 exhibited comparable findings with the studies considered [21,49,50,51]. In female lambs, the g.171328260 G > R(A/G) nucleotide substitution in the P3 may result in a wider CW, depending on the gender of the individual at weaning. Three heterozygous substitutions in P2 (g.171328398 G > R(A/G), g.171328400 G > S(G/C), g.171328404 C > Y(C/T)), on the other hand, may have contributed to the increase in LC in adulthood. Oberbauer [55] made the claim that altering the promoter region might have an impact on the structure and operation of the transcription activator binding region of the gene, in line with our findings. However, it should not be assumed that nucleotide modifications in this area have an influence on meat production characteristics, but may be confined as a single component. As part of the same process, other genes and environmental factors must be considered; for instance, McLellan et al. [56] revealed that transcription factors that bind to the E box, such as MyoD1, promote the transcription of class 1 transcripts and play a crucial role in muscle cell differentiation.
IGF1 is an important growth and development regulator in mammalian muscle tissues; together with insulin-like growth factor 2 (IGF2), growth hormone (GH), and growth hormone-releasing hormone (GHRH), it is an essential component of the somatotropic axis in the development of vertebrates [20]. IGF1 also plays a role in mediating the stimulatory effects of GH and testosterone on muscle growth and development [57]. In the current study, there were no differences in MLDD between weaning (90th day), six months (180th day), and one year (360th day); however, the nucleotide substitutions observed in the P2 had a significant positive effect on LC. This could be associated with an increase in IGFI expression, particularly in the leg muscles. As was previously observed, IGF1 overexpression was accompanied by an increased expression of the cytoskeletal actin gene, suggesting that IGF1 stimulates the protein production of structural components [58]. Therefore, the eight nucleotide changes we detected in our study may have caused an increase in LC. Our results were similar to the findings of previous studies showing that IGFI mRNA and protein were expressed and up-regulated in C2 and sheep myoblast and satellite cells throughout differentiation [57,59]. Additionally, IGFI has been shown to accelerate muscle differentiation after myoblasts exit the cell cycle by activating the mef2c and raising myogenin and MRF4, resulting in a net increase in structural gene expression and the development of bigger myotubes [60].
In research including Angus, Charolais, and hybrid beef cattle, an SNP (c.512C > T) within the promoter of IGF1 was investigated to determine the connection between fat deposition and carcass merit [61]. In the Angus cow population, the c.512C > T substitution was found to have a significant impact on carcass average BF, ultrasonography backfat thickness, and carcass lean meat output, with the “CC” genotype had a greater fat depth and lower lean meat yield than the “TT” genotype [61]. Another study revealed that nucleotide sequence in IGF1 exon 3, IGF1 exon 4, and the flanking region of IGF1 was associated with the depth of carcass fat at the 12th rib measured by video imaging and the percentage proportion of lean meat in the leg [10]. However, no difference was found in the current study between IGF1 5′UTR in MLDFT, BF measured after slaughter, and FT values from in vivo ultrasonography measurements taken on days 90, 180, and 360. Thus, the present study differs from those of Islam et al. [61] and Li et al. [10]. One possible explanation is that lambs born at the end of the lambing season were chosen for the research and maintained the same feeding regimen in a considerably more controlled environment. There was also a possibility that the diverse breeds used in this study could have contributed to the outcome, which was in accordance with Li et al. [10].
In a study by Hofmann et al. [62], the effects of maternal dietary restriction during late gestation on the development of female and male reproductive organs were investigated. The results showed that the restricted dam lambs had lower testicles than the lambs born to the control dams. The study also reached the conclusion that maternal dietary restrictions during late gestation decreased IGF1 mRNA levels during fetal life and interfered with fetal reproductive development, which could have a long-term negative effect on future reproductive success. The results of this study, which examined lambs born to unrestricted dams, showed that lambs with P2 had considerably reduced testicle weights, highlighting the significance of these genes’ regulated expression for healthy development and reproduction. It is known that the IGF system is essential to the proper development of male organs [63]. Therefore, changes in nucleotide substitutions may result in reduced circulating IGF1 levels and the mRNA expression of insulin-like growth factor 1 receptor (IGF1R), resulting in a lower testicular weight. Baker et al. [63] observed comparable results, finding a delay in Leydig cell differentiation causing interstitial space to comprise predominantly undifferentiated mesenchymal cells at 2 weeks after birth in a study evaluating the impact of IGF1 null mutation on reproductive organs.
The assessment of slaughter and carcass traits on a large scale is both time-consuming and costly. Thus, several researchers have emphasized the importance of molecular markers in order to achieve the breeding objective of improving the carcass quality and weight [31,64]. Previous research has linked carcass weight in sheep [29] and cattle [65] to nucleotide polymorphisms in the IGF1, but we did not find such a relationship in our study. This may be explained by differences in the breeds of sheep and the amplified region of the genes used in the study. In contrast, the IGH1 exon1 polymorphism had a substantial impact on the proportion of rack in a lamb’s carcass. One possible explanation for this might be the up-regulation of the IGF1 in rack muscle tissue of lambs possessing P2. In agreement with our findings, previous research has also shown that the IGF family binds to its receptor, modulating the pathways that promote muscle growth and blocking the physiological processes that promote muscle atrophy [66].
Grochowska et al. [64] genotyped the polymorphism (c.654G > A) in exon3 of the IGF1R and investigated its association with growth, body size, slaughter, and meat quality traits. They found that the polymorphisms of both IGF1 and IGF1R had a significant impact on the water-holding capabilities of lamb meat. In the current study, we found no significant variations in the evaluation of meat quality or LTL color between the IGH1 exon1. These results are consistent, to some extent, with those of Grochowska et al. [64], who found no statistically significant variations between the color parameters of genotypes.
Although there is persuasive evidence that IGF1 is essential for cell differentiation, embryogenesis, metabolism, and fetal development, our study’s limited sample size for each breed and the fact that 10% of animals did not amplify were major drawbacks. In addition, the fact that the sequence data provide Y, S, and R suggests that SSCP did not discriminate between all of the variants and that P2 likely represented more than one variation. Many environmental conditions and other genes had a significant influence on determining the features of meat production; hence our study’s findings cannot be regarded as definitive. Therefore, the study findings should be viewed with caution and more research is needed to confirm them, preferably with larger samples and considering additional factors.
5. Conclusions
A PCR-SSCP study was undertaken on meat-type sheep breeds raised in Turkey to determine the association between the IGF1 5′UTR polymorphism and a number of growth and carcass traits, including body measurements (CW and LC), MLD development, non-carcass components (testicles weight), and retail carcass percentage (rack percentage). The results show that nucleotide changes on IGF1 5′UTR may be used in a MAS strategy to improve the sheep carcass quality characteristics, and that differences between variants can be recognized. Furthermore, it is recommended that studies in larger populations be conducted to confirm the results obtained.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/vetsci10040270/s1. Table S1: Sample size for multigroup comparisons for optimum animal research. Table S2: Genotype and variant frequencies of IGF1 5′UTR. Figure S1: The effect of IGF1 5′UTR polymorphism on body measurements in meat-type lamb at various times [67].
Author Contributions
Conceptualization, V.K.E.; methodology, S.E. and V.K.E.; software, S.E. and V.K.E.; validation, S.E. and V.K.E.; formal analysis, V.K.E.; investigation, S.E. and V.K.E.; resources, V.K.E.; data curation, S.E. and V.K.E.; writing—original draft preparation, S.E. and V.K.E.; writing—review and editing, S.E. and V.K.E.; visualization, S.E. and V.K.E.; project administration, V.K.E.; funding acquisition, V.K.E. All authors have read and agreed to the published version of the manuscript.
Funding
The Ministry of Agriculture and Forestry, General Directorate of Agricultural Research of Turkey financed this research under grant number TAGEM/HAYSUD/B/18/A4/P2/308.
Institutional Review Board Statement
The ethics committee of the Sheep Breeding Research Institute in Balkesir, Turkey, authorized the animal study protocol (protocol code 13360037, date of approval 11 April 2018).
Informed Consent Statement
Informed consent was obtained from all of the subjects involved in the study.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- United Nations, Department of Economic and Social Affairs, Population Division. World Population Prospects 2019: Highlights (ST/ESA/SER.A/423). New York: United Nations. Available online: https://population.un.org/wpp/publications/files/wpp2019_highlights.pdf (accessed on 20 January 2023).
- Brameld, J.M.; Parr, T. Improving efficiency in meat production. Proc. Nutr. Soc. 2016, 75, 242–246. [Google Scholar] [CrossRef] [PubMed]
- Tolee, A.R.R.; Olga, E.; Ekaterina, C. Identification of CLPG gene polymorphism using PCR-RFLP of Iraq and Belarus population sheep breeds. Gene Rep. 2021, 22, 100974. [Google Scholar] [CrossRef]
- Margawati, E.T.; Raadsma, H.W.; Martojo, H. Quantitative Trait Loci (QTL) Analysis for Production Traits of Birth Weight and Weight 360 days in Backcross Sheep. HAYATI J. Biosci. 2006, 13, 31–35. [Google Scholar] [CrossRef]
- Nanekarani, S.; Goodarzi, M. Polymorphism of Candidate Genes for Meat Production in Lori Sheep. IERI Procedia 2014, 8, 18–23. [Google Scholar] [CrossRef]
- Karadag, O. Meat-Type Lambs in Turkey: The polymorphism of insulin-like growth factor-1 receptor (IGF-1R) gene in meat-type Lambs in Turkey. Small Rumin. Res. 2022, 215, 106765. [Google Scholar] [CrossRef]
- Kumar, S.; Dahiya, S.P.; Magotra, A.; Ratwan, P. Influence of single nucleotide polymorphism in the IGF-1 gene on performance and conformation traits in Munjal sheep. Zygote 2022, 31, 70–77. [Google Scholar] [CrossRef]
- Song, X.T.; Zhang, J.N.; Zhao, D.W.; Zhai, Y.F.; Lu, Q.; Qi, M.Y.; Lu, M.H.; Deng, S.L.; Han, H.B.; Yang, X.Q.; et al. Molecular cloning, expression, and functional features of IGF1 splice variants in sheep. Endocr. Connect. 2021, 10, 980–994. [Google Scholar] [CrossRef]
- Rotwein, P. Mapping the growth hormone--Stat5b--IGF-I transcriptional circuit. Trends Endocrinol. Metab. 2012, 23, 186–193. [Google Scholar] [CrossRef]
- Li, S.; Zhou, H.; Zhao, F.; Fang, Q.; Wang, J.; Liu, X.; Luo, Y.; Hickford, J.G.H. Nucleotide sequence variation in the insulin-like growth factor 1 gene affects growth and carcass traits in new zealand romney sheep. DNA Cell Biol. 2021, 40, 265–271. [Google Scholar] [CrossRef]
- He, J.N.; Zhang, B.Y.; Chu, M.X.; Wang, P.Q.; Feng, T.; Cao, G.L.; Di, R.; Fang, L.; Huang, D.W.; Tang, Q.Q.; et al. Polymorphism of insulin-like growth factor 1 gene and its association with litter size in Small Tail Han sheep. Mol. Biol. Rep. 2012, 39, 9801–9807. [Google Scholar] [CrossRef]
- D’Occhio, M.J.; Baruselli, P.S.; Campanile, G. Influence of nutrition, body condition, and metabolic status on reproduction in female beef cattle: A review. Theriogenology 2019, 125, 277–284. [Google Scholar] [CrossRef]
- Bedir, Ö.; Gram, A.; Dorsam, S.T.; Grazul-Bilska, A.T.; Kowalewski, M.P. Plane of nutrition and FSH-induced superovulation affect the expression of steroid hormone receptors and growth factors in caruncular tissue of non-pregnant sheep. Domest. Anim. Endocrinol. 2022, 78, 106683. [Google Scholar] [CrossRef] [PubMed]
- Kurokawa, M.; Sato, F.; Aramaki, S.; Soh, T.; Yamauchi, N.; Hattori, M.A. Monitor of the myostatin autocrine action during differentiation of embryonic chicken myoblasts into myotubes: Effect of IGF-I. Mol. Cell Biochem. 2009, 331, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Hosnedlova, B.; Vernerova, K.; Kizek, R.; Bozzi, R.; Kadlec, J.; Curn, V.; Kouba, F.; Fernandez, C.; Machander, V.; Horna, H. Associations between IGF1, IGFBP2 and tgfß3 genes polymorphisms and growth performance of broiler chicken lines. Animals 2020, 10, 800. [Google Scholar] [CrossRef]
- Zou, T.; He, D.; Yu, B.; Yu, J.; Mao, X.; Zheng, P.; He, J.; Huang, Z.; Chen, D. Moderate Maternal Energy Restriction during Gestation in Pigs Attenuates Fetal Skeletal Muscle Development Through Changing Myogenic Gene Expression and Myofiber Characteristics. Reprod. Sci. 2017, 24, 156–167. [Google Scholar] [CrossRef] [PubMed]
- Liao, W.; Wang, Y.; Qiao, X.; Zhang, X.; Deng, H.; Zhang, C.; Li, J.; Yuan, X.; Zhang, H. A Poly(dA:dT) Tract in the IGF1 Gene Is a Genetic Marker for Growth Traits in Pigs. Animals 2022, 12, 3316. [Google Scholar] [CrossRef] [PubMed]
- Zonaed Siddiki, A.M.A.M.; Miah, G.; Islam, M.S.; Kumkum, M.; Rumi, M.H.; Baten, A.; Hossain, M.A. Goat Genomic Resources: The Search for Genes Associated with Its Economic Traits. Int. J. Genom. 2020, 2020, 1–13. [Google Scholar] [CrossRef]
- Chalbi, S.; Dettori, M.L.; Djemali, M.N.; Vacca, G.M.; Petretto, E.; Pazzola, M.; Bedhiaf-Romdhani, S. Haplotype structure of MSTN, IGF1, and BMP2 genes in Tunisian goats (Capra hircus) and their association with morphometric traits. Trop. Anim. Health Prod. 2023, 55, 1–9. [Google Scholar] [CrossRef]
- Trukhachev, V.; Skripkin, V.; Kvochko, A.; Kulichenko, A.; Kovalev, D.; Pisarenko, S.; Volynkina, A.; Selionova, M.; Aybazov, M.; Shumaenko, S.; et al. Polymorphisms of the IGF1 gene in Russian sheep breeds and their influence on some meat production parameters. Slov. Vet. Res. 2016, 53, 77–83. [Google Scholar]
- Ding, N.; Tian, D.; Li, X.; Zhang, Z.; Tian, F.; Liu, S.; Han, B.; Liu, D.; Zhao, K. Genetic Polymorphisms of IGF1 and IGF1R Genes and Their Effects on Growth Traits in Hulun Buir Sheep. Genes 2022, 13, 666. [Google Scholar] [CrossRef]
- Mohammadabadi, M.; Bordbar, F.; Jensen, J.; Du, M.; Guo, W. Key genes regulating skeletal muscle development and growth in farm animals. Animals 2021, 11, 835. [Google Scholar] [CrossRef] [PubMed]
- Radovan, K.; Nina, M.; Barbora, O.; Gábor, M.; Luboš, V.; Hana, V.; Kristína, L.; Ján, P.; Juraj, C. The evaluation of genomic diversity and selection signals in the autochthonous Slovak Spotted cattle. Czech J. Anim. Sci. 2021, 66, 251–261. [Google Scholar] [CrossRef]
- Talebi, R.; Ghaffari, M.R.; Zeinalabedini, M.; Abdoli, R.; Mardi, M. Genetic basis of muscle-related traits in sheep: A review. Anim. Genet. 2022, 53, 723–739. [Google Scholar] [CrossRef]
- Zhang, Z.; Fan, Y.; Deng, K.; Liang, Y.; Zhang, G.; Gao, X.; El-Samahy, M.A.; Zhang, Y.; Deng, M.; Wang, F. Circular RNA circUSP13 sponges miR-29c to promote differentiation and inhibit apoptosis of goat myoblasts by targeting IGF1. FASEB J. 2022, 36, 1–18. [Google Scholar] [CrossRef]
- Scatà, M.C.; Catillo, G.; Annicchiarico, G.; De Matteis, G.; Napolitano, F.; Signorelli, F.; Moioli, B. Investigation on lactation persistency and IGF-I gene polymorphisms in dairy sheep. Small Rumin. Res. 2010, 89, 7–11. [Google Scholar] [CrossRef]
- Damak, S.; Su, H.; Jay, N.P.; Bullock, D.W. Improved Wool Production in Transgenic Sheep Expressing Insulin-like Growth Factor 1. Bio/Technology 1996, 14, 185–188. [Google Scholar] [CrossRef]
- Guo, X.D.; Yang, D.S.; Ao, X.D.; Wu, X.; Li, G.P.; Wang, L.L.; Bao, M.T.; Xue, L.; Bou, S.G. Production of transgenic cashmere goat embryos expressing red fluorescent protein and containing IGF1 hair-follicle-cell specific expression cassette by somatic cell nuclear transfer. Sci. China Ser. C Life Sci. 2009, 52, 390–397. [Google Scholar] [CrossRef]
- Behzadi, S.; Sadeghi, M.; Zamani, P.; Abdoli, R. Association of IGF-I Gene Polymorphisms with Carcass Traits in Iranian Mehraban Sheep Using SSCP Analysis. Iran. J. Appl. Anim. Sci. 2015, 5, 121–126. [Google Scholar]
- Qasimi, R.H.A.; Hassan, A.F.; Khudair, B.Y. Effect of IGF-1 and GH Genes Polymorphism on Weights and Body Measurements of Awassi Lambs in Different Ages. Basrah J. Agric. Sci. 2019, 32, 39–46. [Google Scholar] [CrossRef]
- Esen, V.K.; Elmacı, C. Effect of Growth Hormone Exon-5 Polymorphism on Growth Traits, Body Measurements, Slaughter and Carcass Characteristics, and Meat Quality in Meat-Type Lambs in Turkey. Ruminants 2022, 2, 420–434. [Google Scholar] [CrossRef]
- Kader Esen, V.; Esen, S.; Karadağ, O.; Önenç, A.; Elmaci, C. Slaughter and carcass characteristics of Kıvırcık, Karacabey Merino, Ramlıç, German Black-Head Mutton × Kıvırcık and Hampshire down × Merino crossbreed lambs reared under intensive conditions. Turk. J. Vet. Anim. Sci. 2020, 44, 1155–1163. [Google Scholar] [CrossRef]
- Kader Esen, V.; Elmacı, C. The Estimation of Live Weight from Body Measurements in Different Meat-Type Lambs. J. Agr. Sci.-Tarim Bili. 2021, 27, 469–475. [Google Scholar] [CrossRef]
- Kader-Esen, V.; Esen, S.; Karadag, O.; Elmaci, C. Genotypic characterization of meat-type lambs expressing the callipyge gene in Turkey: II. Effect on body indexes. Small Rumin. Res. 2022, 208, 106633. [Google Scholar] [CrossRef]
- Kader Esen, V.; Esen, S.; Karadağ, O.; Önenç, A.; Elmaci, C. Genotypic Characterization of Meat-Type Lambs Expressing the Callipyge Gene in Turkey: I. Carcass Characteristics and Retail Yield. Turk. J. Vet. Anim. Sci. 2022, 46, 1–8. [Google Scholar] [CrossRef]
- Yilmaz, O.; Cemal, I.; Karaca, O. Estimation of mature live weight using some body measurements in Karya sheep. Trop. Anim. Health Prod. 2012, 45, 397–403. [Google Scholar] [CrossRef] [PubMed]
- Selim, E.; Harun, K.; Cuneyt, K.; Huseyin, E. Effect of activated clinoptilolite and inactive brewer’s yeast mixture on loin eye muscle and body indexes in fattening period. Med. Weter. 2020, 76, 626–630. [Google Scholar] [CrossRef]
- Yakan, A.; Tayyar, C.; Alasahan, S.; Odabasioglu, F.; Unal, N. Damascus kids’ slaughter, carcass and meat quality traits in different production systems using antioxidant supplementation. Small Rumin. Res. 2016, 136, 43–53. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef]
- Honikel, K.O.; Hamm, R. Measurement of water-holding capacity and juiciness. In Quality Attributes and Their Measurement in Meat, Poultry and Fish Products; Pearson, A.M., Dutson, T.R., Eds.; Springer: Boston, MA, USA, 1994; pp. 125–161. [Google Scholar]
- Choi, Y.M.; Kim, B.C. Muscle fiber characteristics, myofibrillar protein isoforms, and meat quality. Livest. Sci. 2009, 122, 105–118. [Google Scholar] [CrossRef]
- Gonzales-Barron, U.; Santos-Rodrigues, G.; Piedra, R.B.; Coelho-Fernandes, S.; Osoro, K.; Celaya, R.; Maurício, R.S.; Pires, J.; Tolsdorf, A.; Geß, A.; et al. Quality attributes of lamb meat from European breeds: Effects of intrinsic properties and storage. Small Rumin. Res. 2021, 198, 106354. [Google Scholar] [CrossRef]
- Green, M.R.; Sambrook, J. Molecular Cloning: A Laboratory Manual, 4th ed.; Cold Spring Harbor Laboratory Press: New York, NY, USA, 2012. [Google Scholar]
- Byun, S.O.; Fang, Q.; Zhou, H.; Hickford, J.G.H. An effective method for silver-staining DNA in large numbers of polyacrylamide gels. Anal. Biochem. 2009, 385, 174–175. [Google Scholar] [CrossRef] [PubMed]
- Falconer, D.S.; Mackay, T.E.C. Introduction to Quantitative Genetics, 4th ed.; Longman: Essex, UK, 1996. [Google Scholar]
- Minitab, I. Statistical Software for Windows, Release 17; Minitab Inc.: State College, PA, USA, 2014. [Google Scholar]
- Abousoliman, I.; Reyer, H.; Oster, M.; Muráni, E.; Mourad, M.; Rashed, M.A.S.; Mohamed, I.; Wimmers, K. Analysis of candidate genes for growth and milk performance traits in the Egyptian Barki sheep. Animals 2020, 10, 197. [Google Scholar] [CrossRef] [PubMed]
- Tahmoorespur, M.; Taheri, A.; Gholami, H.; Ansary, M. PCR-SSCP variation of GH and STAT5A genes and their association with estimated breeding values of growth traits in baluchi sheep. Anim. Biotechnol. 2011, 22, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Tahmoorespur, M.; Valeh, M.V.; Nassiry, M.R.; Moussavi, A.H.; Ansary, M. Association of the polymorphism in the 5′ flanking region of the ovine IGF-I gene with growth traits in the Baluchi sheep. South African J. Anim. Sci. 2009, 39, 97–101. [Google Scholar] [CrossRef]
- Hajihosseinlo, A.; Hashemi, A.; Razavi, S.A.; Pirany, N. Association of the polymorphism in the 5′ flanking region of the ovine IGF-I gene with growth and development traits in Markui sheep of Iran. Eur. J. Zool. Res. 2013, 2, 19–24. [Google Scholar]
- Ramasamy, C. Association of IGF1 Gene Polymorphism with Growth Rates in Madras Red Sheep. Int. J. Livest. Res. 2018, 8, 131. [Google Scholar] [CrossRef]
- Tahmoorespur, M.; Taheri, A.; Valeh, M.V.; Saghi, D.A.; Ansary, M. Assessment relationship between leptin and ghrelin genes polymorphisms and estimated breeding values (EBVs) of growth traits in Baluchi sheep. J. Anim. Vet. Adv. 2010, 9, 2460–2465. [Google Scholar]
- Nazari, F.; Noshary, A.; Hemati, B. Association between insulin-like growth factor I polymorphism and early growth traits in Iranian Zandi sheep, found polymerase chain reaction restriction fragment length polymorphism (PCR-RFLP). Iran. J. Appl. Anim. Sci. 2016, 6, 665–669. [Google Scholar]
- Machado, A.L.; Meira, A.N.; Juc, F.; Azevedo, H.C.; Muniz, E.N.; Coutinho, L.L.; Mour, G.B.; Pedrosa, V.B.; Pinto, F.B. Variants in GH, IGF1, and LEP Genes Associated with Body Traits in Santa Inês Sheep. Sci. Agric. 2021, 78, 1–9. [Google Scholar] [CrossRef]
- Oberbauer, A.M. The regulation of IGF-1 gene transcription and splicing during development and aging. Front. Endocrinol. 2013, 4, 1–9. [Google Scholar] [CrossRef]
- Mclellan, A.S.; Kealey, T.; Langlands, K. An E box in the exon 1 promoter regulates insulin-like growth factor-I expression in differentiating muscle cells. Am. J. Physiol. Cell Physiol. 2023, 291, 300–307. [Google Scholar] [CrossRef] [PubMed]
- Oksbjerg, N.; Gondret, F.; Vestergaard, M. Basic principles of muscle development and growth in meat-producing mammals as affected by the insulin-like growth factor (IGF) system. Domest. Anim. Endocrinol. 2004, 27, 219–240. [Google Scholar] [CrossRef]
- McKoy, G.; Ashley, W.; Mander, J.; Yu Yang, S.; Williams, N.; Russell, B.; Goldspink, G. Expression of insulin growth factor-1 splice variants and structural genes in rabbit skeletal muscle induced by stretch and stimulation. J. Physiol. 1999, 516, 583–592. [Google Scholar] [CrossRef]
- Tollefsen, S.E.; Lajara, R.; McCusker, R.H.; Clemmons, D.R.; Rotwein, P. Insulin-like Growth Factors (IGF) in Muscle Development. J. Biol. Chem. 1989, 264, 13810–13817. [Google Scholar] [CrossRef]
- Engert, J.C.; Berglund, E.B.; Rosenthal, N. Proliferation precedes differentiation in IGF-I-stimulated myogenesis. J. Cell Biol. 1996, 135, 431–440. [Google Scholar] [CrossRef] [PubMed]
- Islam, K.K.; Vinsky, M.; Crews, R.E.; Okine, E.; Moore, S.S.; Crews, D.H.; Li, C. Association analyses of a SNP in the promoter of IGF1 with fat deposition and carcass merit traits in hybrid, Angus and Charolais beef cattle. Anim. Genet. 2009, 40, 766–769. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, F.; Boretto, E.; Vitale, S.; Gonzalez, V.; Vidal, G.; Pardo, M.F.; Flores, M.F.; Garcia, F.; Bagnis, G.; Queiroz, O.C.M.; et al. Maternal nutritional restriction during late gestation impairs development of the reproductive organs in both male and female lambs. Theriogenology 2018, 108, 331–338. [Google Scholar] [CrossRef]
- Baker, J.; Hardy, M.P.; Zhou, J.; Bondy, C.; Lupu, F.; Bellvé, A.R.; Efstratiadis, A. Effects of an Igf1 gene null mutation on mouse reproduction. Mol. Endocrinol. 1996, 10, 903–918. [Google Scholar] [CrossRef]
- Grochowska, E.; Lisiak, D.; Akram, M.Z.; Adeniyi, O.O.; Lühken, G.; Borys, B. Association of a polymorphism in exon 3 of the IGF1R gene with growth, body size, slaughter and meat quality traits in Colored Polish Merino sheep. Meat Sci. 2021, 172, 108314. [Google Scholar] [CrossRef]
- Curi, R.A.; De Oliveira, H.N.; Silveira, A.C.; Lopes, C.R. Association between IGF-I, IGF-IR and GHRH gene polymorphisms and growth and carcass traits in beef cattle. Livest. Prod. Sci. 2005, 94, 159–167. [Google Scholar] [CrossRef]
- Girnita, L.; Worrall, C.; Takahashi, S.I.; Seregard, S.; Girnita, A. Something old, something new and something borrowed: Emerging paradigm of insulin-like growth factor type 1 receptor (IGF-1R) signaling regulation. Cell Mol. Life Sci. 2014, 71, 2403–2427. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences, 2nd ed.; Lawrence Erlbaum: Hillsdale, NJ, USA, 1988. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).