Effects of Chestnut Hydrolysable Tannin on Intake, Digestibility, Rumen Fermentation, Milk Production and Somatic Cell Count in Crossbred Dairy Cows
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Welfare Statement
2.2. Animals, Diets and Experimental Design
2.3. Sampling Procedures and Analysis Methods
2.4. Statistical Analysis
3. Results
3.1. Nutrient Composition of the Experimental Diets
3.2. Feed Intake, Nutrient Intake and Nutrient Digestibility
3.3. Rumen Fermentation Patterns and Blood Metabolites
3.4. Milk Yield and Milk Composition
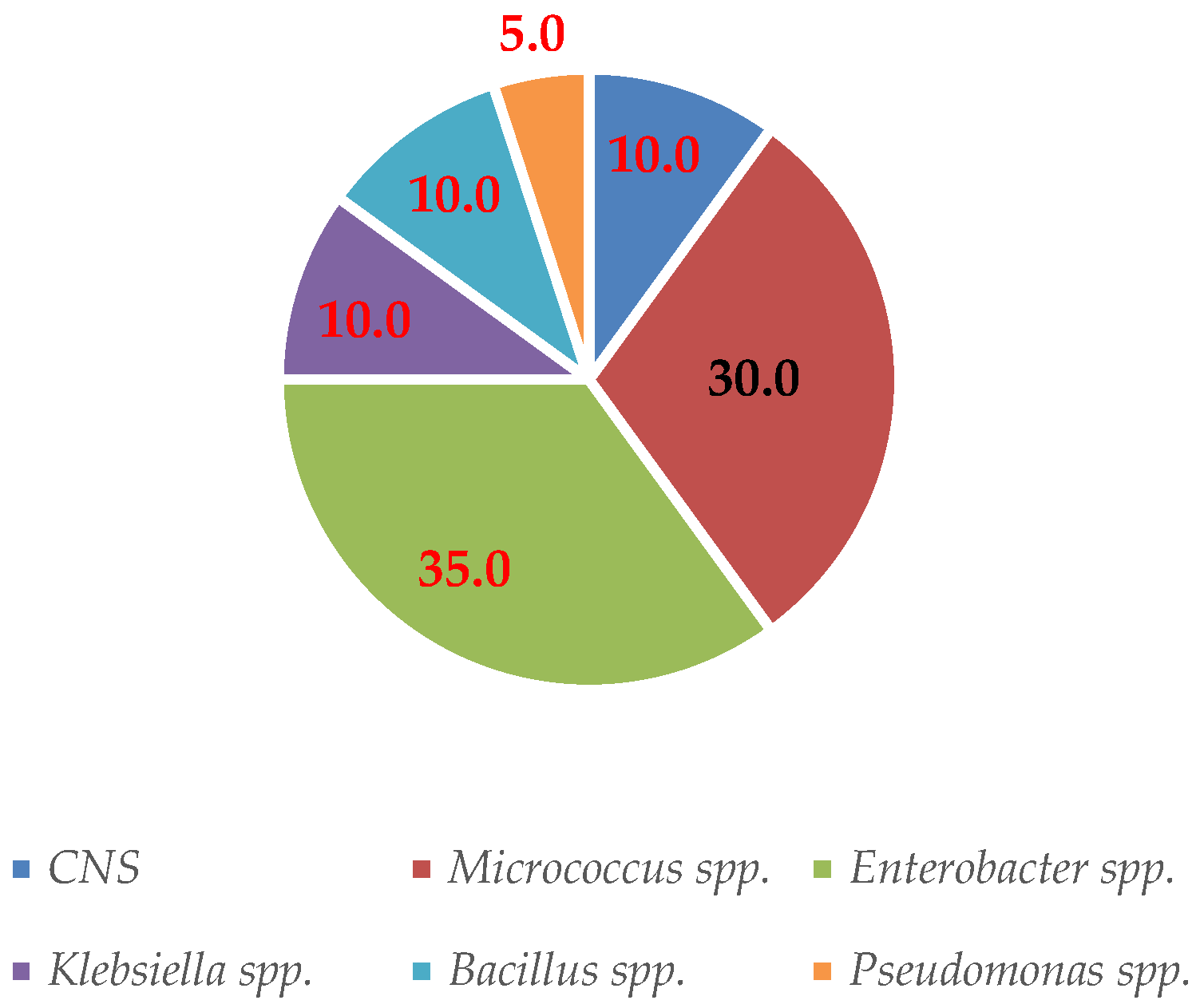
3.5. Identification of Mastitis-Causing Bacteria
4. Discussion
4.1. Dry Matter Intake, Nutrient Intake and Nutrient Digestibility
4.2. Rumen Fermentation Patterns and Blood Metabolites
4.3. Milk Yield and Milk Composition
4.4. Identification of Mastitis-Causing Bacteria
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lauzon, K.; Zhao, X.; Bouetard, A.; Delbecchi, L.; Paquette, B.; Lacasse, P. Antioxidants to prevent bovine neutrophil induced mammary epithelial cell damage. J. Dairy Sci. 2005, 88, 4295–4303. [Google Scholar] [CrossRef] [PubMed]
- Lejonklev, J.; Kidmose, U.; Jensen, S.; Petersen, M.A.; Helwing, A.L.F.; Mortensen, G.; Weisbjerg, M.R.; Larsen, M.K. Short communication: Effect of oregano and caraway essential oils on the production and flavor of cow milk. J. Dairy Sci. 2016, 99, 7898–7903. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.; Hristov, A.N. Effects of plant-derived bio-active compounds on rumen fermentation, nutrient utilization, immune response, and productivity of ruminant animals. In Medicinal and Aromatic Crops: Production, Phytochemistry, and Utilization; Jeliazkov, V.D., Cantrell, C.L., Eds.; American Chemical Society Publications: Washington, DC, USA, 2016; pp. 167–186. [Google Scholar]
- Oh, J.; Wall, E.H.; Bravo, D.M.; Hristov, A.N. Host-mediated effects of phytonutrients in ruminants: A review. J. Dairy Sci. 2017, 100, 5974–5983. [Google Scholar] [CrossRef]
- Patra, A.K.; Saxena, J. Exploitation of dietary tannin to improve rumen metabolism and ruminant nutrition. J. Sci. Food Agric. 2011, 91, 24–37. [Google Scholar] [CrossRef]
- Aboagye, I.A.; Oba, M.; Castillo, A.R.; Koenig, K.M.; Iwaasa, A.D.; Beauchemin, K.A. Effects of hydrolysable tannin with or without condensed tannin on methane emissions, nitrogen use, and performance of beef cattle fed a high-forage diet. J. Anim. Sci. 2018, 96, 5276–5286. [Google Scholar] [CrossRef]
- Rivera-Méndez, C.; Plascencia, A.; Torrentera, N.; Zinn, R.A. Effect of level and source of supplemental tannin on growth performance of steers during the late finishing phase. J. Appl. Anim. Res. 2016, 45, 199–203. [Google Scholar] [CrossRef]
- Aguerre, M.J.; Duval, B.; Powell, J.M.; Vadas, P.A.; Wattiaux, M.A. Effects of feeding a quebracho–chestnut tannin extract on lactating cow performance and nitrogen utilization efficiency. J. Dairy Sci. 2020, 103, 2264–2271. [Google Scholar] [CrossRef] [PubMed]
- Mlambo, V.; Smith, T.; Owen, E.; Mould, F.L.; Sikosana, J.L.N.; Mueller-Harvey, I. Tanniniferous dichrostachys cinerea fruits do not require detoxification for goat nutrition: In sacco and in vivo evaluations. Livest. Prod. Sci. 2004, 90, 135–144. [Google Scholar] [CrossRef]
- Lavrenčič, A. Effect of hydrolyzable tannin extract on bovine milk production and composition. Sustainable grassland productivity. In Proceedings of the 21st Ed. General Meeting of the European Grassland Federation, Badajoz, Spain, 3–6 April 2006; pp. 586–588. [Google Scholar]
- Liu, H.W.; Zhou, D.W.; Li, K. Effects of chestnut tannin on performance and antioxidative status of transition dairy cows. J. Dairy Sci. 2013, 96, 5901–5907. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.; Mehboob, H.A.; Mirza, M.A.; Raza, H.; Osredkar, M. Effect of hydrolysable tannin supplementation on production performance of dairy crossbred cows. J. Anim. Plant Sci. 2017, 27, 1088–1093. [Google Scholar]
- Funatogawa, K.; Hayashi, S.; Shimomura, H.; Yoshida, T.; Hatano, T.; Ito, H.; Iría, Y. Antibacterial activity of hydrolysable tannin derived from medicinal plants against helicobacter pylori. Microbiol. Immunol. 2004, 48, 251–261. [Google Scholar] [CrossRef] [PubMed]
- Scalbert, A. Antimicrobial properties of tannin. Phytochemistry 1991, 30, 3875–3883. [Google Scholar] [CrossRef]
- Buzzini, P.; Arapitsas, P.; Goretti, M.; Branda, E.; Turchetti, B.; Pinelli, P.; Ieri, F.; Romani, A. Antimicrobial and antiviral activity of hydrolysable tannin. Mini-Rev. Med. Chem. 2008, 8, 1179–1187. [Google Scholar] [CrossRef] [PubMed]
- Maisak, H.; Sasibha, J.; Mintra, L.; Janenuj, W. Antibacterial Activity of Tannin from Sweet Chestnut Wood Against Aeromonas and Streptococcal Pathogens of Tilapia (Oreochromis niloticus). Thai J. Vet. Med. 2013, 43, 105–111. [Google Scholar]
- Lopes Júnior, J.E.F.; Lange, C.C.; Brito, M.A.V.P.; Santos, F.R.; Silva, M.A.S.; de Moraes, L.C.D.; de Souza, G.N. Relationship between total bacteria counts and somatic cell counts from mammary quarters infected by mastitis pathogens. Ciência Rural. 2012, 42, 691–696. [Google Scholar] [CrossRef]
- Prapaiwong, T.; Srakaew, W.; Wachirapakorn, C.; Jarassaeng, C. Effects of hydrolyzable tannin extract obtained from sweet chestnut wood (Castanea sativa Mill.) against bacteria causing subclinical mastitis in Thai Friesian dairy cows. Veterinary World 2021, 14, 2427–2433. [Google Scholar] [CrossRef]
- NRC. Nutrient Requirements of Dairy Cattle, 7th ed.; National Academy Press: Washington, DC, USA, 2001. [Google Scholar]
- AOAC. The Association of Official Analytical Chemists, 18th ed.; AOAC: Arlington, TX, USA, 2005. [Google Scholar]
- Goering, H.K.; Van Soest, P.J. Forage Fiber Analysis (Apparatus, Reagents, Procedures and Some Applications). In Agricultural Handbook No. 379; ARSUSDA: Washington, DC, USA, 1970. [Google Scholar]
- Van Keulen, J.; Young, B.A. Evaluation of acid insoluble ash as a neutral marker in ruminant digestibility studies. J. Anim. Sci. 1977, 44, 282–287. [Google Scholar] [CrossRef]
- Mathew, S.; Sagathevan, S.; Thomas, J.; Mathen, G. An HPLC method for estimation of volatile fatty acids in ruminal fluid. Indian J. Anim. 1997, 67, 805–807. [Google Scholar]
- Ali, A.K.A.; Shook, G.E. An optimum transformation for somatic cell concentration in milk. J. Dairy Sci. 1980, 63, 487–490. [Google Scholar] [CrossRef]
- National Mastitis Council (NMC). Current Concepts of Bovine Mastitis; National Mastitis Council, Inc.: Washington, DC, USA, 1978. [Google Scholar]
- Quinn, P.J.; Carter, M.E.; Markey, B.K.; Carter, G.R. Clinical Veterinary Microbiology; Mosby Ltd.: Wolfe, TX, USA, 1994; p. 648. [Google Scholar]
- SAS. User’s Guide: Statistic; SAS Institute Inc.: Cary, NC, USA, 2002. [Google Scholar]
- Kearl, L.C. Nutrient Requirements of Ruminants in Developing Countries. International Feed Stuffs Institute; Utah Agriculture Experimental Station, Utah State University: Logon, UT, USA, 1982. [Google Scholar]
- IDF (International Dairy Federation). A Common Carbon Footprint Approach for Dairy. In The IDF Guide to Standard Lifecycle Assessment Methodology for the Dairy Sector; Bulletin IDF No. 479/2010; IDF: Brussels, Belgium, 2015. [Google Scholar]
- Broderick, G.A.; Stevenson, M.J.; Patton, R.A. Effect of dietary protein concentration and degradability on response to rumen-protected methionine in lactating dairy cows. J. Dairy Sci. 2009, 92, 2719–2728. [Google Scholar] [CrossRef]
- Kumar, R.; Singh, M. Tannins: Their adverse role in ruminant nutrition. J. Agric. Food Chem. 1984, 32, 447–453. [Google Scholar] [CrossRef]
- Gemeda, B.S.; Hassen, A. The potential of tropical tannin rich browses in reduction of enteric methane. Approaches Poult. Dairy Vet. Sci. 2018, 2, 154–162. [Google Scholar] [CrossRef]
- Kapp-Bitter, A.N.; Dickhoefer, U.; Suglo, E.; Baumgartner, L.; Kreuzer, M.; Leiber, F. Graded supplementation of chestnut tannins to dairy cows fed protein-rich spring pasture: Effects on indicators of protein utilization. J. Anim. Feed Sci. 2020, 29, 97–104. [Google Scholar] [CrossRef]
- Aguerre, M.J.; Capozzolo, M.C.; Lencioni, P.; Cabral, C.; Wattiaux, M.A. Effect of quebracho-chestnut tannin extracts at 2 dietary crude protein levels on performance, rumen fermentation, and nitrogen partitioning in dairy cows. J. Dairy Sci. 2016, 99, 4476–4486. [Google Scholar] [CrossRef]
- Sliwinski, B.J.; Kreuzer, M.; Sutter, F.; Machmuller, A.; Weststein, H.R. Performance, body nitrogen conversion and nitrogen emission from manure of dairy cows fed diets supplemented with different plant extracts. J. Anim. Feed Sci. 2004, 13, 73–91. [Google Scholar] [CrossRef]
- Jayanegara, A.; Goel, G.; Makkar, H.P.S.; Becker, K. Divergence between purified hydrolysable and condensed tannin effects on methane emission, rumen fermentation and microbial population in vitro. Anim. Feed Sci. Technol. 2015, 209, 60–68. [Google Scholar] [CrossRef]
- Mannelli, F.; Daghio, M.; Alves, S.P.; Bessa, R.J.B.; Minieri, S.; Giovannetti, L.; Conte, G.; Mele, M.; Messini, A.; Rapaccini, S.; et al. Effects of chestnut tannin extract, vescalagin and gallic acid on the dimethyl acetals profile and microbial community composition in rumen liquor: An in vitro study. Microorganisms 2019, 7, 202. [Google Scholar] [CrossRef]
- Deaville, E.R.; Givens, D.I.; Mueller-Harvey, I. Chestnut and mimosa tannin silages: Effects in sheep differ for apparent digestibility, nitrogen utilization and losses. Anim. Feed Sci. Technol. 2010, 157, 129–138. [Google Scholar] [CrossRef]
- Carrasco, J.M.D.; Cabral, C.; Redondo, L.M.; Viso, N.D.; Colombatto, D.; Farber, M.D.; Miyakawa, M.E.F. Impact of chestnut and quebracho tannin on rumen microbiota of bovines. BioMed Res Int. 2017, 2017, 9610810. [Google Scholar] [CrossRef]
- Zhang, J.; Xu, X.; Cao, Z.; Wang, Y.; Yang, H.; Azarfar, A.; Li, S. Effect of different tannin sources on nutrient intake, digestibility, performance, nitrogen utilization, and blood parameters in dairy cows. Animals 2019, 9, 507. [Google Scholar] [CrossRef]
- Calabrò, S.; Moniello, G.; Piccolo, C.; Bovera, F.; Infascelli, F.; Tudisco, R.; Cutrignelli, M.I. Rumen fermentation and degradability in buffalo and cattle using the in vitro gas production technique. J. Anim. Physiol. Anim. Nutr. 2008, 92, 356–362. [Google Scholar] [CrossRef] [PubMed]
- Wanapat, M.; Cherdthong, A. Use of real-time PCR technique in studying rumen cellulolytic bacteria population as affected by level of roughage in swamp buffalo. Current Microbiol. 2009, 58, 294–299. [Google Scholar] [CrossRef]
- Costa, M.; Alves, S.P.; Cappucci, A.; Cook, S.R.; Duarte, A.; Caldeira, R.M.; McAllister, T.A.; Bessa, R.J.B. Effects of condensed and hydrolyzable tannin on rumen metabolism with emphasis on the biohydrogenation of unsaturated fatty acids. J. Agric. Food Chem. 2018, 66, 3367–3377. [Google Scholar] [CrossRef]
- Herremans, S.; Decruyenaere, V.; Cantalapiedra-Hijar, G.; Beckers, Y.; Froidmont, E. Effects of hydrolysable tannin-treated grass silage on milk yield and composition, nitrogen partitioning and nitrogen isotopic discrimination in lactating dairy cows. Animals 2020, 14, 771–779. [Google Scholar] [CrossRef] [PubMed]
- Anantasook, N.; Wanapat, M. Influence of rain tree pod meal supplementation on rice straw-based diets using in vitro gas fermentation technique. Asian J. Appl. Sci. 2012, 25, 325–334. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, J.; Zsédely, E. Nutrition of Ruminants. 2011. Available online: https://www.tankonyvtar.hu/en/tartalom/tamop425/0059_ruminants_nutrition/0059_ruminants_nutrition.pdf (accessed on 13 August 2022).
- Dey, A.; De, P.S. Influence of condensed tannin from Ficus bengalensis leaves on feed utilization, milk production and antioxidant status of crossbred cows. Asian Australas J. Anim. Sci. 2014, 27, 342–348. [Google Scholar] [CrossRef] [PubMed]
- Allam, A.M.; Nagadi, S.A.; Bakhashwain, A.A.; Sallam, S.M.A. Impact of sub-tropical grass grown in arid region on methane emission, milk yield and composition in dairy cows. J. Food Agric. Environ. 2013, 1, 620–625. [Google Scholar]
- Ougi, T. Description of urea nitrogen in blood and milk. Dairy Japan. 1994, 39, 26–29. [Google Scholar]
- Dschaak, C.M.; Williams, C.M.; Holt, M.S.; Eun, J.S.; Young, A.J.; Min, B.R. Effects of supplementing condensed tannin extract on intake, digestion, ruminal fermentation, and milk production of lactating dairy cows. J. Dairy Sci. 2011, 94, 2508–2519. [Google Scholar] [CrossRef]
- Penner, G.B.; Taniguchi, M.; Guan, L.L.; Beauchemin, K.A.; Oba, M. Effect of dietary forage to concentrate ratio on volatile fatty acid absorption and the expression of genes related to volatile fatty acid absorption and metabolism in ruminal tissue. J. Dairy Sci. 2009, 92, 2767–2781. [Google Scholar] [CrossRef]
- Wang, L.; Guangning, Z.; Yang, L.; Yonggen, Z. Effects of High Forage/Concentrate Diet on Volatile Fatty Acid Production and the Microorganisms Involved in VFA Production in Cow Rumen. Animals 2020, 10, 223. [Google Scholar] [CrossRef]
- Buccioni, A.; Pauselli, M.; Viti, C.; Minieri, S.; Pallara, G.; Roscini, V.; Rapaccini, S.; Marinucci, M.T.; Lupi, P.; Conte, G.; et al. Milk fatty acid composition, rumen microbial population, and animal performances in response to diets rich in linoleic acid supplemented with chestnut or quebracho tannin in dairy ewes. J. Dairy Sci. 2015, 98, 1145–1156. [Google Scholar] [CrossRef] [PubMed]
- Bhatta, R.; Uyeno, Y.; Tajima, K.; Takenaka, A.; Yabumoto, Y.; Nonaka, I.; Enishi, O.; Kurihara, M. Difference in the nature of tannin on in vitro ruminal methane and volatile fatty acid production and on methanogenic archaea and protozoal populations. J. Dairy Sci. 2009, 92, 5512–5522. [Google Scholar] [CrossRef] [PubMed]
- Hervás, G.; Frutos, P.; Giráldez, F.J.; Mantecón, A.R.; Álvarez Del Pino, M.C. Effect of different doses of quebracho tannin extract on rumen fermentation in ewes. Anim. Feed Sci. Technol. 2003, 109, 65–78. [Google Scholar] [CrossRef]
- Liu, H.; Vaddella, V.; Zhou, D. Effects of chestnut tannin and coconut oil on growth performance, methane emission, ruminal fermentation, and microbial populations in sheep. J. Dairy Sci. 2011, 94, 6069–6077. [Google Scholar] [CrossRef] [PubMed]
- Toral, P.; Hervàs, G.; Bichi, E.; Belenguer, A.; Fruitos, P. Tannin as feed additives to modulate ruminal biohydrogenation: Effects on animal performance, milk fatty acid composition and ruminal fermentation in dairy ewes fed a diet containing sunflower oil. Anim. Feed Sci. Technol. 2011, 164, 199–206. [Google Scholar] [CrossRef]
- Beauchemin, K.; McGinn, S.M.; Martinez, T.F.; McAllis-ter, T.A. Use of condensed tannin extract from quebracho trees to reduce methane emissions from cattle. J. Anim. Sci. 2007, 85, 1990–1996. [Google Scholar] [CrossRef]
- Benchaar, C.; McAllister, T.A.; Chouinard, P.Y. Digestion, ruminal fermentation, ciliate protozoal populations, and milk production from dairy cows fed cinnamaldehyde, quebracho con-densed tannin, or Yucca schidigera saponin extract. J. Dairy Sci. 2008, 91, 4765–4777. [Google Scholar] [CrossRef]
- Colombini, S.; Colombari, G.; Crovetto, G.M.; Galassi, G.; Rapetti, L. Tannin treated Lucerne silage in dairy cow feeding. Italian. J. Anim. Sci. 2010, 8, 289–291. [Google Scholar]
- Dubey, D. Studies on Degradation of Tannin from Acacia Nilotica Pods and Their Influence on Nutrient Utilization, Milk Production and Reproduction in Dairy Animals. Ph.D. Thesis, NDRI (Deemed University), Karnal, Haryana, 2007. [Google Scholar]
- Errante, J.; Barbera, S.; Baldi, C. Use of hydrolysable tannin in high productive dairy cattle diets. Krmiva 1998, 40, 257–261. [Google Scholar]
- Cook-Mills, J.M.; Fraker, P.J. The role of metals in the production of toxic oxygen metabolites by mononuclear phagocytes. In Nutrient Modulation of the Immune Response; Cunningham-Rundles, S., Ed.; Marcel Dekker Inc.: New York, NY, USA, 1993; pp. 127–140. [Google Scholar]
- Craven, N.; Williams, M.R. Defences of the bovine mammary gland against infection and prospects for their enhancement. Vet. Immunol. Immunopathol. 1985, 10, 71–127. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.B.; Ding, Y.S.; Zhang, Y.; Chen, J.B.; Cui, B.S.; Bai, J.Y.; Lin, M.B.; Hou, Q.; Zhang, P.C.; Li, S. Anti-inflammatory hydrolyzable tannin from Myricaria bracteata. J. Nat. Prod. 2015, 78, 1015–1025. [Google Scholar] [CrossRef] [PubMed]
- Ekambaram, S.P.; Perumal, S.S.; Balakrishnan, A. Scope of hydrolysable tannin as possible antimicrobial agent. Phytother. Res. 2016, 30, 1035–1045. [Google Scholar] [CrossRef] [PubMed]
- Birkinshaw, A.; Schwarm, A.; Marquardt, S.; Kreuzer, M.; Terranova, M. Rapid responses in bovine milk fatty acid composition and phenol content to various tanniferous forages. J. Anim. Feed Sci. 2020, 29, 297–305. [Google Scholar] [CrossRef]
- Türkyilmaz, S.; Yildiz, Ö.; Oryaşin, E.; Kaynarca, S.; Bozdoğan, B. Molecular identification of bacteria isolated from dairy herds with mastitis. Kafkas Univ Vet Fak Derg. 2010, 16, 1025–1032. [Google Scholar]
- Hillerton, J.E.; Berry, E.A. The management and treatment of environmental streptococcal mastitis. Veterinary Clinics of North America. Vet. Clin. Food Anim. Pract. 2003, 19, 157–169. [Google Scholar] [CrossRef]
- Panneum, S.; Iniam, K.; Choorut, P.; Bumrungkit, K.; Sroynum, A.; Pinyopummin, A. Identification of clinical mastitis pathogens and antibiotic sensitivity in dairy cattle during years 2004–2006 in Kanchanaburi, Nakhonpathom and Ratchburi. In Proceedings of the 45th Kasetsart University Annual Conference: Animals and Veterinary Medicine, Bangkok, Thailand, 30 January–2 February 2007. [Google Scholar]
- Juangphanich, S.; Kiatyingangsulee, T.; Worarach, A.; Ketphan, W.; Kumpusiri, N.; Arunvipas, P. Incidence of subclinical mastitis and antimicrobial susceptibility test: The case study of crossbred Holstein Friesian dairy cow, Chong Sarika Subdistrict, Phatthana Nikhom District, Lopburi Province. Thai-NIAH Ejournal 2018, 13, 9–15. [Google Scholar]
- Hagnestam-Nielsen, C.; Emanuelson, U.; Berglund, B.; Strandberg, E. Relationship between somatic cell count and milk yield in different stages of lactation. J. Dairy Sci. 2009, 92, 3124–3133. [Google Scholar] [CrossRef]
| Item | Concentrate | Rice Straw | Chestnut Hydrolysable Tannin Extract Powder |
|---|---|---|---|
| Ingredients, %DM | |||
| Cassava chip | 47.00 | ||
| Corn meal | 7.00 | ||
| Rice bran | 5.00 | ||
| Soybean meal | 20.00 | ||
| Peanut hulls | 4.00 | ||
| Palm kernel meal | 9.50 | ||
| Residue sugar | 3.00 | ||
| Urea | 2.50 | ||
| Dicalcium phosphate | 1.00 | ||
| Salt | 0.50 | ||
| Premix | 0.50 | ||
| Total | 100.00 | ||
| Chemical composition (%DM) | |||
| Dry matter, % | 90.73 | 92.83 | 91.72 |
| OM | 93.32 | 88.13 | 97.75 |
| CP | 21.68 | 3.21 | 9.63 |
| EE | 3.20 | 1.80 | 0.75 |
| Ash | 6.68 | 11.87 | 2.25 |
| NDF | 19.14 | 90.63 | - |
| ADF | 14.40 | 57.48 | - |
| ME, Mcal/kgDM | 2.62 | 1.53 | - |
| Items | Chestnut Hydrolysable Tannin, g/day | SEM c | p Value d | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 0 | 3.15 | 6.30 | 9.45 | Control vs. CHT | L | Q | C | ||
| Avg. BW, kg | 458.4 | 464.6 | 480.9 | 481.4 | 10.54 | 0.21 | 0.12 | 0.79 | 0.60 |
| DM intake, | |||||||||
| Concentrate | 11.0 | 10.8 | 11.7 | 12.0 | 0.51 | 0.39 | 0.12 | 0.63 | 0.47 |
| Rice straw | 5.1 ab | 6.0 a | 5.4 ab | 4.7 b | 0.27 | 0.39 | 0.18 | * | 0.31 |
| Total, kg/day | 16.0 | 16.8 | 17.1 | 16.7 | 0.72 | 0.35 | 0.49 | 0.44 | 0.90 |
| %BW | 3.49 | 3.60 | 3.56 | 3.47 | 0.09 | 0.62 | 0.80 | 0.29 | 0.83 |
| g/kg W0.75 | 161.5 | 167.2 | 166.8 | 162.4 | 4.81 | 0.50 | 0.92 | 0.33 | 0.93 |
| Nutrient intake, kg/day | |||||||||
| OM | 14.6 | 15.2 | 15.7 | 15.3 | 0.64 | 0.33 | 0.44 | 0.44 | 0.79 |
| CP | 3.1 | 3.0 | 3.3 | 3.3 | 0.14 | 0.36 | 0.13 | 0.78 | 0.50 |
| EE | 0.5 | 0.5 | 0.5 | 0.5 | 0.03 | 0.70 | 0.42 | 0.62 | 0.89 |
| NDF | 7.0 | 1.6 | 1.2 | 6.8 | 0.38 | 0.63 | 0.58 | 0.22 | 0.52 |
| ADF | 4.4 | 5.2 | 4.6 | 4.6 | 0.25 | 0.17 | 0.93 | 0.13 | 0.12 |
| Apparent digestibility, % | |||||||||
| DM | 68.1 a | 70.6 ab | 72.7 ab | 75.8 b | 1.35 | * | ** | 0.81 | 0.87 |
| OM | 71.6 a | 73.4 a | 75.4 ab | 78.5 b | 1.44 | * | * | 0.68 | 0.91 |
| CP | 77.7 a | 81.0 ab | 81.2 ab | 84.6 b | 1.76 | 0.07 | * | 0.97 | 0.44 |
| EE | 81.0 a | 83.3 ab | 91.1 a | 85.3 ab | 1.81 | * | * | 0.07 | 0.06 |
| NDF | 47.3 | 50.6 | 48.1 | 55.8 | 2.16 | 0.29 | 0.15 | 0.51 | 0.30 |
| ADF | 37.5 a | 44.9 ab | 40.9 a | 53.2 b | 3.18 | * | * | 0.47 | 0.10 |
| Energy intake 1 | |||||||||
| Mcal ME/day | 42.2 | 41.9 | 45.2 | 44.2 | 1.88 | 0.49 | 0.31 | 0.84 | 0.39 |
| Items | Chestnut Hydrolysable Tannin, g/day | SEM c | p Value d | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 0 | 3.15 | 6.30 | 9.45 | Control vs. CHT | L | Q | C | ||
| Rumen end-products | |||||||||
| Ruminal pH | 6.92 ab | 6.98 ab | 6.81 b | 6.87 ab | 0.03 | 0.37 | * | 0.93 | 0.01 |
| Ruminal NH3-N, mg/dL | 13.6 | 16.4 | 17.8 | 13.1 | 1.67 | 0.30 | 0.99 | 0.06 | 0.54 |
| Total VFAs, mM | 89.3 | 99.6 | 102.8 | 105.1 | 4.62 | * | * | 0.42 | 0.77 |
| VFA profiles, mol/100 mol | |||||||||
| Acetic acid (C2) | 64.7 | 66.7 | 67.2 | 65.4 | 1.14 | 0.22 | 0.61 | 0.14 | 0.89 |
| Propionic acid (C3) | 22.5 | 21.6 | 21.3 | 23.0 | 0.77 | 0.55 | 0.77 | 0.13 | 0.65 |
| Butyric acid (C4) | 12.8 | 11.7 | 11.6 | 11.6 | 0.58 | 0.12 | 0.20 | 0.34 | 0.75 |
| A:P ratio | 2.90 | 3.13 | 3.18 | 2.99 | 0.90 | 0.22 | 0.57 | 0.15 | 0.90 |
| Blood metabolites | |||||||||
| SUN, mg/dL | 21.8 a | 21.1 a | 20.5 ab | 15.4 b | 1.16 | 0.09 | ** | 0.10 | 0.41 |
| Items | Chestnut Hydrolysable Tannin, g/day | SEM c | p Value d | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 0 | 3.15 | 6.30 | 9.45 | Control vs. CHT | L | Q | C | ||
| Milk yield, kg/day | 18.2 | 17.9 | 19.9 | 20.5 | 1.17 | 0.34 | 0.13 | 0.73 | 0.50 |
| 4% FCM 1, kg/day | 19.0 | 19.5 | 20.8 | 21.6 | 1.47 | 0.38 | 0.21 | 0.91 | 0.84 |
| Milk composition | |||||||||
| Fat, % | 4.35 | 4.61 | 4.27 | 4.48 | 0.22 | 0.69 | 0.96 | 0.90 | 0.28 |
| Protein, % | 3.50 | 3.45 | 3.25 | 3.36 | 0.05 | 0.03 | 0.02 | 0.12 | 0.07 |
| Lactose, % | 4.89 | 4.94 | 5.27 | 5.16 | 0.09 | 0.06 | 0.02 | 0.38 | 0.11 |
| SNF, % | 9.08 | 9.08 | 9.23 | 9.22 | 0.06 | 0.22 | 0.08 | 0.92 | 0.29 |
| TS, % | 13.43 | 13.69 | 13.50 | 13.69 | 0.25 | 0.52 | 0.62 | 0.90 | 0.49 |
| Composition yield | |||||||||
| Fat, kg/d | 0.79 | 0.82 | 0.85 | 0.91 | 0.07 | 0.43 | 0.28 | 0.90 | 0.95 |
| Protein, kg/day | 0.63 | 0.62 | 0.65 | 0.69 | 0.04 | 0.60 | 0.23 | 0.53 | 0.77 |
| Lactose, kg/day | 0.90 | 0.89 | 1.05 | 1.06 | 0.07 | 0.26 | 0.09 | 0.91 | 0.36 |
| SNF, kg/d | 1.65 | 1.63 | 1.84 | 1.89 | 0.11 | 0.36 | 0.12 | 0.75 | 0.46 |
| TS, kg/d | 2.44 | 2.45 | 2.69 | 2.80 | 0.18 | 0.36 | 0.15 | 0.81 | 0.67 |
| ECM, kg | 18.9 | 19.2 | 20.8 | 21.6 | 1.44 | 0.38 | 0.19 | 0.87 | 0.77 |
| FPCM 2, kg | 19.1 | 19.3 | 20.6 | 21.5 | 1.38 | 0.41 | 0.22 | 0.83 | 0.85 |
| Fat:Protein ratio | 1.25 | 1.34 | 1.31 | 1.33 | 0.06 | 0.29 | 0.44 | 0.54 | 0.58 |
| Feed efficiency | 1.12 | 1.06 | 1.15 | 1.22 | 0.03 | 0.42 | 0.02 | 0.07 | 0.22 |
| NUE 3 | 0.20 | 0.20 | 0.19 | 0.20 | 0.004 | 0.40 | 0.69 | 0.17 | 0.26 |
| SCC, log10 | 5.66 a | 5.17 b | 5.09 b | 5.13 b | 0.06 | ** | ** | ** | 0.33 |
| SCS 4 | 5.18 a | 3.56 b | 3.30 b | 3.42 b | 0.21 | ** | ** | ** | 0.35 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prapaiwong, T.; Srakaew, W.; Poolthajit, S.; Wachirapakorn, C.; Jarassaeng, C. Effects of Chestnut Hydrolysable Tannin on Intake, Digestibility, Rumen Fermentation, Milk Production and Somatic Cell Count in Crossbred Dairy Cows. Vet. Sci. 2023, 10, 269. https://doi.org/10.3390/vetsci10040269
Prapaiwong T, Srakaew W, Poolthajit S, Wachirapakorn C, Jarassaeng C. Effects of Chestnut Hydrolysable Tannin on Intake, Digestibility, Rumen Fermentation, Milk Production and Somatic Cell Count in Crossbred Dairy Cows. Veterinary Sciences. 2023; 10(4):269. https://doi.org/10.3390/vetsci10040269
Chicago/Turabian StylePrapaiwong, Tipwadee, Wuttikorn Srakaew, Sukanya Poolthajit, Chalong Wachirapakorn, and Chaiwat Jarassaeng. 2023. "Effects of Chestnut Hydrolysable Tannin on Intake, Digestibility, Rumen Fermentation, Milk Production and Somatic Cell Count in Crossbred Dairy Cows" Veterinary Sciences 10, no. 4: 269. https://doi.org/10.3390/vetsci10040269
APA StylePrapaiwong, T., Srakaew, W., Poolthajit, S., Wachirapakorn, C., & Jarassaeng, C. (2023). Effects of Chestnut Hydrolysable Tannin on Intake, Digestibility, Rumen Fermentation, Milk Production and Somatic Cell Count in Crossbred Dairy Cows. Veterinary Sciences, 10(4), 269. https://doi.org/10.3390/vetsci10040269







