The Effects of Dietary Manganese and Selenium on Growth and the Fecal Microbiota of Nursery Piglets
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Care and Experimental Design
2.2. Fecal Collection and Storage
2.3. DNA Extraction and Sequencing
2.4. Volatile Fatty Acid Analysis
2.5. Statistical Analyses
3. Results
3.1. Animal Performance
3.2. Volatile Fatty Acids and Alpha Diversity
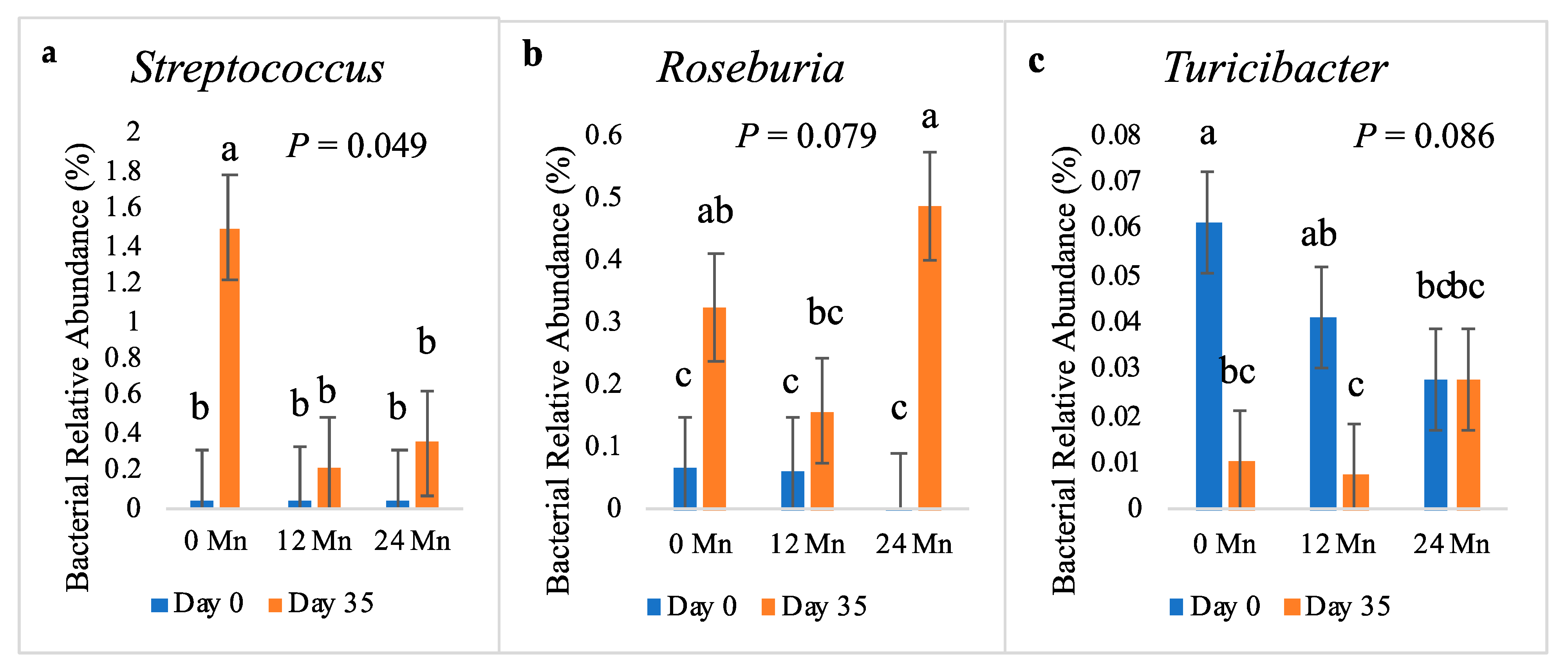
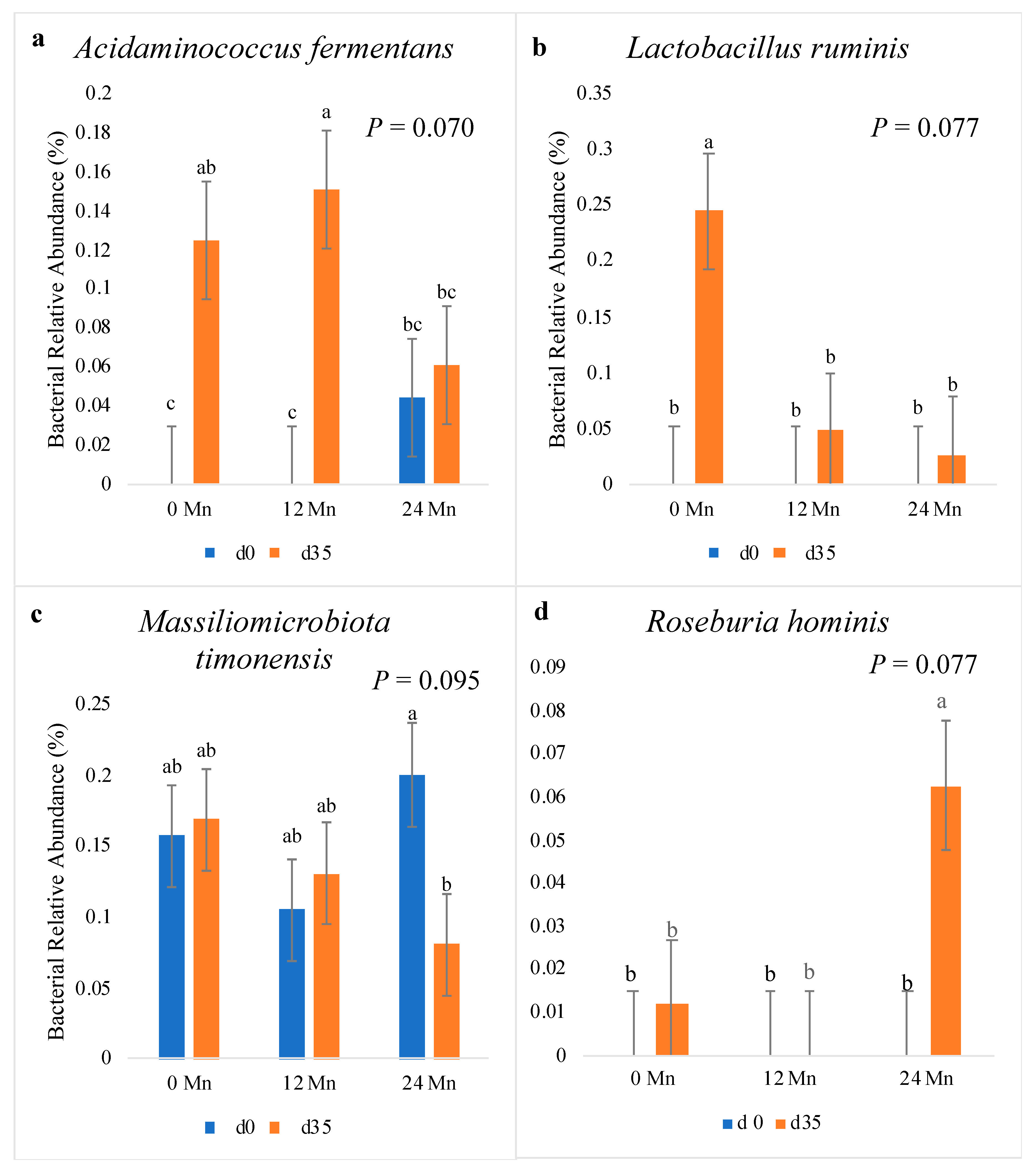
3.3. Microbial Populations
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cao, S.T.; Wang, C.C.; Wu, H.; Zhang, Q.H.; Jiao, L.F.; Hu, C.H. Weaning Disrupts Intestinal Antioxidant Status, Impairs Intestinal Barrier and Mitochondrial Function, and Triggers Mitophagy in Piglets. J. Anim. Sci. 2018, 96, 1073–1083. [Google Scholar] [CrossRef] [PubMed]
- Lu, T.; Harper, A.F.; Dibner, J.J.; Scheffler, J.M.; Corl, B.A.; Estienne, M.J.; Zhao, J.; Dalloul, R.A. Supplementing Antioxidants to Pigs Fed Diets High in Oxidants: II. Effects on Carcass Characteristics, Meat Quality, and Fatty Acid Profile. J. Anim. Sci. 2014, 92, 5464–5475. [Google Scholar] [CrossRef] [PubMed]
- Miriyala, S.; Spasojevic, I.; Tovmasyan, A.; Salvemini, D.; Vujaskovic, Z.; Clair, D.S.; Batinic-Haberle, I. Manganese Superoxide Dismutase, MnSOD and Its Mimics. Biochim. Biophys. Acta Mol. Basis Dis. 2012, 1822, 794–814. [Google Scholar] [CrossRef] [PubMed]
- Richards, J.D.; Zhao, J.; Harreil, R.J.; Atwell, C.A.; Dibner, J.J. Trace Mineral Nutrition in Poultry and Swine. Asian-Australas. J. Anim. Sci. 2010, 23, 1527–1534. [Google Scholar] [CrossRef]
- Waris, G.; Ahsan, H. Reactive Oxygen Species: Role in the Development of Cancer and Various Chronic Conditions. J. Carcinog. 2006, 5, 14. [Google Scholar] [CrossRef]
- Holley, A.K.; Bakthavatchalu, V.; Velez-Roman, J.M.; St. Clair, D.K.S. Manganese Superoxide Dismutase: Guardian of the Powerhouse. Int. J. Mol. Sci. 2011, 12, 7114–7162. [Google Scholar] [CrossRef]
- Zhu, L.H.; Zhao, K.L.; Chen, X.L.; Xu, J.X. Impact of Weaning and an Antioxidant Blend on Intestinal Barrier Function and Antioxidant Status in Pigs. J. Anim. Sci. 2012, 90, 2581–2589. [Google Scholar] [CrossRef]
- Brière, J.-J.; Favier, J.; Gimenez-Roqueplo, A.-P.; Rustin, P. Tricarboxylic Acid Cycle Dysfunction as a Cause of Human Diseases and Tumor Formation. Am. J. Physiol. Physiol. 2006, 291, C1114–C1120. [Google Scholar] [CrossRef]
- Valko, M.; Rhodes, C.J.; Moncol, J.; Izakovic, M.; Mazur, M. Free Radicals, Metals and Antioxidants in Oxidative Stress-Induced Cancer. Chem. Biol. Interact. 2006, 160, 1–40. [Google Scholar] [CrossRef]
- Mates, J.M.; Perez-Gomes, C.; Nunez De Castro, I. Antioxidant Enzymes and Human Diseases. Clin. Biochem. 1999, 32, 595–603. [Google Scholar] [CrossRef]
- Haikarainen, T.; Frioux, C.; Zhnag, L.Q.; Li, D.C.; Papageorgiou, A.C. Crystal Structure and Biochemical Characterization of a Manganese Superoxide Dismutase from Chaetomium Thermophilum. Biochim. Biophys. Acta Proteins Proteom. 2014, 1844, 422–429. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Yan, T.; Yang, J.; Oberley, T.D.; Oberley, L.W. The Role of Cellular Glutathione Peroxidase Redox Regulation in the Suppression of Tumor. Cancer Res. 2000, 60, 3927–3939. [Google Scholar] [PubMed]
- Dalto, D.B.; Lapointe, J.; Matte, J.J. Assessment of Antioxidative and Selenium Status by Seleno-Dependent Glutathione Peroxidase Activity in Different Blood Fractions Using a Pig Model: Issues for Clinical Nutrition and Research. J. Anim. Physiol. Anim. Nutr. 2018, 102, 184–193. [Google Scholar] [CrossRef] [PubMed]
- Pluske, J.R. Feed- and Feed Additives-Related Aspects of Gut Health and Development in Weanling Pigs. J. Anim. Sci. Biotechnol. 2013, 4, 1. [Google Scholar] [CrossRef] [PubMed]
- Looft, T.; Allen, H.K.; Casey, T.A.; Alt, D.P.; Stanton, T.B. Carbadox Has Both Temporary and Lasting Effects on the Swine Gut Microbiota. Front. Microbiol. 2014, 5, 276. [Google Scholar] [CrossRef]
- Lourenco, J.M.; Hampton, R.S.; Johnson, H.M.; Callaway, T.R.; Rothrock, M.J.; Azain, M.J. The Effects of Feeding Antibiotic on the Intestinal Microbiota of Weanling Pigs. Front. Vet. Sci. 2021, 8, 601394. [Google Scholar] [CrossRef]
- Hansen, S.L.; Spears, J.W.; Lloyd, K.E.; Whisnant, C.S. Feeding a Low Manganese Diet to Heifers During Gestation Impairs Fetal Growth and Development. J. Dairy Sci. 2006, 89, 4305–4311. [Google Scholar] [CrossRef]
- Hansen, S.L.; Spears, J.W.; Lloyd, K.E.; Whisnant, C.S. Growth, Reproductive Performance, and Manganese Status of Heifers Fed Varying Concentrations of Manganese. J. Anim. Sci 2006, 84, 3375–3380. [Google Scholar] [CrossRef]
- Leibholz, J.M.; Speer, V.C.; Hays, V.W. Effect of Dietary Manganese on Baby Pig Performance and Tissue Manganese Levels. J. Anim. Sci. 1962, 21, 772–776. [Google Scholar] [CrossRef]
- Spears, J.W. Boron, Chromium, Manganese, and Nickel in Agricultural Animal Production. Biol. Trace Elem. Res. 2019, 188, 35–44. [Google Scholar] [CrossRef]
- Mahan, D.C.; Azain, M.; Crenshaw, T.D.; Cromwell, G.L.; Dove, C.R.; Kim, S.W.; Lindemann, M.D.; Miller, P.S.; Pettigrew, J.E.; Stein, H.H.; et al. Supplementation of Organic and Inorganic Selenium to Diets Using Grains Grown in Various Regions of the United States with Differing Natural Se Concentrations and Fed to Grower–Finisher Swine. J. Anim. Sci. 2014, 92, 4991–4997. [Google Scholar] [CrossRef] [PubMed]
- Mahan, D.C.; Moxon, A.L. Effects of Adding Inorganic or Organic Selenium Sources to the Diets of Young Swine. J. Anim. Sci. 1978, 47, 456–466. [Google Scholar] [CrossRef]
- Edmunds, C.E.; Seidel, D.S.; Welch, C.B.; Lee, E.A.; Azain, M.J.; Callaway, T.R.; Dove, C.R. The Effect of Varying Dietary Manganese and Selenium Levels on the Growth Performance and Manganese-Superoxide Dismutase Activity in Nursery Pigs. Livest. Sci. 2022, 265, 105100. [Google Scholar] [CrossRef]
- NRC. Nutrient Requirements of Swine; NRC: Ottawa, ON, Canada, 2012. [Google Scholar]
- Welch, C.B.; Lourenco, J.M.; Davis, D.B.; Krause, T.R.; Carmichael, M.N.; Rothrock, M.J.; Pringle, T.D.; Callaway, T.R. The Impact of Feed Efficiency Selection on the Ruminal, Cecal, and Fecal Microbiomes of Angus Steers from a Commercial Feedlot. J. Anim. Sci. 2020, 98, skaa230. [Google Scholar] [CrossRef]
- Lourenco, J.M.; Kieran, T.J.; Seidel, D.S.; Glenn, T.C.; Da Silveira, M.F.; Callaway, T.R.; Stewart, R.L. Comparison of the Ruminal and Fecal Microbiotas in Beef Calves Supplemented or Not with Concentrate. PLoS ONE 2020, 15, e0231533. [Google Scholar] [CrossRef]
- Grummer, R.H.; Bentley, O.G.; Phillips, P.H.; Bohstedt, G. The Role of Manganese in Growth, Reproduction, and Lactation of Swine. J. Anim. Sci. 1950, 9, 170–175. [Google Scholar] [CrossRef]
- Plumlee, M.P.; Thrasher, D.M.; Beeson, W.M.; Andrews, F.N.; Parker, H.E. The Effects of a Manganese Deficiency Upon the Growth, Development, and Reproduction of Swine. J. Anim. Sci. 1956, 15, 352–368. [Google Scholar] [CrossRef]
- Edmunds, C.E.; Cornelison, A.S.; Farmer, C.; Rapp, C.; Ryman, V.E.; Schweer, W.P.; Wilson, M.E.; Dove, C.R. The Effect of Increasing Dietary Manganese from an Organic Source on the Reproductive Performance of Sows. Agriculture 2022, 12, 2168. [Google Scholar] [CrossRef]
- Patterson, M.J. Streptococcus. In Medical Microbiology; Baron, S., Ed.; University of Texas Medical Branch at Galveston: Galveston, TX, USA, 1996. [Google Scholar]
- Torres-Pitarch, A.; Gardiner, G.E.; Cormican, P.; Rea, M.; Crispie, F.; O’Doherty, J.V.; Cozannet, P.; Ryan, T.; Cullen, J.; Lawlor, P.G. Effect of Cereal Fermentation and Carbohydrase Supplementation on Growth, Nutrient Digestibility and Intestinal Microbiota in Liquid-Fed Grow-Finishing Pigs. Sci. Rep. 2020, 10, 13716. [Google Scholar] [CrossRef]
- Villagómez-Estrada, S.; Pérez, J.F.; Darwich, L.; Vidal, A.; Van Kuijk, S.; Melo-Durán, D.; Solà-Oriol, D. Effects of Copper and Zinc Sources and Inclusion Levels of Copper on Weanling Pig Performance and Intestinal Microbiota. J. Anim. Sci. 2020, 98, skaa117. [Google Scholar] [CrossRef]
- Cromwell, G.L.; Lindemann, M.D.; Monegue, H.J.; Hall, D.D.; Orr, D.E. Tribasic Copper Chloride and Copper Sulfate as Copper Sources for Weanling Pigs. J. Anim. Sci. 1998, 76, 118–123. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Dove, C.R. The Effect of Adding Copper and Various Fat Sources to the Diets of Weanling Swine on Growth Performance and Serum Fatty Acid Profiles. J. Anim. Sci. 1993, 71, 2187–2192. [Google Scholar] [CrossRef] [PubMed]
- Højberg, O.; Canibe, N.; Poulsen, H.D.; Hedemann, M.S.; Jensen, B.B. Influence of Dietary Zinc Oxide and Copper Sulfate on the Gastrointestinal Ecosystem in Newly Weaned Piglets. Appl. Environ. Microbiol. 2005, 71, 2267–2277. [Google Scholar] [CrossRef] [PubMed]
- Namkung, H.; Gong, J.; Yu, H.; De Lange, C.F.M. Effect of Pharmacological Intakes of Zinc and Copper on Growth Performance, Circulating Cytokines and Gut Microbiota of Newly Weaned Piglets Challenged with Coliform Lipopolysaccharides. Can. J. Anim. Sci. 2006, 86, 511–522. [Google Scholar] [CrossRef]
- Hillman, E.T.; Kozik, A.J.; Hooker, C.A.; Burnett, J.L.; Heo, Y.; Kiesel, V.A.; Nevins, C.J.; Oshiro, J.M.K.I.; Robins, M.M.; Thakkar, R.D.; et al. Comparative Genomics of the Genus Roseburia Reveals Divergent Biosynthetic Pathways That May Influence Colonic Competition among Species. Microb. Genom. 2020, 6, 7–24. [Google Scholar] [CrossRef]
- Tamanai-Shacoori, Z.; Smida, I.; Bousarghin, L.; Loreal, O.; Meuric, V.; Fong, S.B.; Bonnaure-Mallet, M.; Jolivet-Gougeon, A. Roseburia spp.: A Marker of Health? Future Microbiol. 2017, 12, 157–170. [Google Scholar] [CrossRef]
- Chen, L.; Xu, Y.; Chen, X.; Fang, C.; Zhao, L.; Chen, F. The Maturing Development of Gut Microbiota in Commercial Piglets during the Weaning Transition. Front. Microbiol. 2017, 8, 1688. [Google Scholar] [CrossRef]
- Choudhury, R.; Middelkoop, A.; Boekhorst, J.; Gerrits, W.J.J.; Kemp, B.; Bolhuis, J.E.; Kleerebezem, M. Early Life Feeding Accelerates Gut Microbiome Maturation and Suppresses Acute Post-Weaning Stress in Piglets. Environ. Microbiol. 2021, 23, 7201–7213. [Google Scholar] [CrossRef]
- Zhong, Y.; Nyman, M.; Fåk, F. Modulation of Gut Microbiota in Rats Fed High-Fat Diets by Processing Whole-Grain Barley to Barley Malt. Mol. Nutr. Food Res. 2015, 59, 2066–2076. [Google Scholar] [CrossRef]
- Sun, Y.; Su, Y.; Zhu, W. Microbiome-Metabolome Responses in the Cecum and Colon of Pig to a High Resistant Starch Diet. Front. Microbiol. 2016, 7, 779. [Google Scholar] [CrossRef]
- Wang, X.; Tsai, T.; Deng, F.; Wei, X.; Chai, J.; Knapp, J.; Apple, J.; Maxwell, C.V.; Lee, J.A.; Li, Y.; et al. Longitudinal Investigation of the Swine Gut Microbiome from Birth to Market Reveals Stage and Growth Performance Associated Bacteria. Microbiome 2019, 7, 109. [Google Scholar] [CrossRef] [PubMed]
- Fuller, R. Some Morphological and Physiological Characteristics of Gram Negative Anaerobic Bacteria Isolated from the Alimentary Tract of the Pig. J. Appl. Bacteriol. 1966, 29, 375–379. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.J.; Pukall, R.; Saunders, E.; Lapidus, A.; Copeland, A.; Nolan, M.; del Rio, T.G.; Lucas, S.; Chen, F.; Tice, H.; et al. Complete Genome Sequence of Acidaminococcus Fermentans Type Strain (VR4 T). Stand. Genom. Sci. 2010, 3, 1–14. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Varel, V.H.; Yen, J.T. Microbial Perspective on Fiber Utilization by Swine. J. Anim. Sci. 1997, 75, 2715–2722. [Google Scholar] [CrossRef]
- Liu, G.; Li, P.; Hou, L.; Niu, Q.; Pu, G.; Wang, B.; Du, T.; Kim, S.W.; Niu, P.; Li, Q.; et al. Metagenomic Analysis Reveals New Microbiota Related to Fiber Digestion in Pigs. Front. Microbiol. 2021, 12, 746717. [Google Scholar] [CrossRef]
- Li, X.; Højberg, O.; Canibe, N.; Jensen, B.B. Phylogenetic Diversity of Cultivable Butyrate-Producing Bacteria from Pig Gut Content and Feces. J. Anim. Sci. 2016, 94, 377–381. [Google Scholar] [CrossRef]
- Al Jassim, R.A.M. Lactobacillus Ruminis Is a Predominant Lactic Acid Producing Bacterium in the Caecum and Rectum of the Pig. Lett. Appl. Microbiol. 2003, 37, 213–217. [Google Scholar] [CrossRef]
- O’Donnell, M.M.; Harris, H.M.B.; Lynch, D.B.; Ross, R.P.; O’Toole, P.W. Lactobacillus Ruminis Strains Cluster According to Their Mammalian Gut Source. BMC Microbiol. 2015, 15, 80. [Google Scholar] [CrossRef]
- Fuller, R.; Crittenden, R.G.; MacCracken, V.J.; Gaskins, H.R.; Kullen, M.J.; Klaenhammer, T.; Mercenier, A.; O’Sullivan, D.J.; Reid, G.; Svensson, U.; et al. Probiotics: A Critical Review; Tannock, G.W., Ed.; Horizon Scientific Press: Norfolk, UK, 1999. [Google Scholar]
- Zhang, W.; Azevedo, M.S.P.; Gonzalez, A.M.; Saif, L.J.; Van Nguyen, T.; Wen, K.; Yousef, A.E.; Yuan, L. Influence of Probiotic Lactobacilli Colonization on Neonatal B Cell Responses in a Gnotobiotic Pig Model of Human Rotavirus Infection and Disease. Vet. Immunol. Immunopathol. 2008, 122, 175–181. [Google Scholar] [CrossRef]
- Neville, B.A.; Forde, B.M.; Claesson, M.J.; Darby, T.; Coghlan, A.; Nally, K.; Ross, R.P.; O’Toole, P.W. Characterization of Pro-Inflammatory Flagellin Proteins Produced by Lactobacillus ruminis and Related Motile Lactobacilli. PLoS ONE 2012, 7, e40592. [Google Scholar] [CrossRef]
- Yamashiro, K.; Tanaka, R.; Urabe, T.; Ueno, Y.; Yamashiro, Y.; Nomoto, K.; Takahashi, T.; Tsuji, H.; Asahara, T.; Hattori, N. Gut Dysbiosis Is Associated with Metabolism and Systemic Inflammation in Patients with Ischemic Stroke. PLoS ONE 2017, 12, e0171521. [Google Scholar] [CrossRef]
- Ndongo, S.; Khelaifia, S.; Fournier, P.E.; Raoult, D. “Massiliomicrobiota Timonensis”, A New Bacterial Species Isolated from the Human Gut. New Microbes New Infect. 2016, 13, 25–26. [Google Scholar] [CrossRef] [PubMed]
| Ingredients, % | Phase I a | Phase II a | Phase III a |
|---|---|---|---|
| Corn | 22.80 | 40.20 | 57.34 |
| Soybean meal 47.5% | 15.00 | 21.00 | 28.14 |
| Whey | 15.00 | 10.00 | 7.00 |
| Oats | 10.00 | 5.00 | |
| Hamlet protein 1 | 10.00 | 7.50 | |
| Lactose | 10.00 | 3.00 | |
| Fish meal | 5.00 | 3.00 | 3.00 |
| Blood plasma | 3.00 | 1.50 | |
| Soybean Oil | 5.24 | 4.38 | 0.46 |
| L-Lysine | 0.32 | 0.40 | 0.30 |
| DL Methionine | 0.20 | 0.22 | 0.14 |
| L-Threonine | 0.12 | 0.16 | 0.12 |
| Dicalcium phosphate | 1.48 | 1.74 | 1.34 |
| Limestone | 0.32 | 0.38 | 0.64 |
| Salt | 0.26 | 0.26 | 0.26 |
| UGA vitamin premix 2 | 0.26 | 0.26 | 0.26 |
| Trace mineral premix 3,4,5 | 1.00 | 1.00 | 1.00 |
| Nutrient Composition 5 | |||
| ME 6,7, kcal/kg | 3511 | 3500 | 3319 |
| Crude protein, % | 21.1 | 21.2 | 21.3 |
| Lysine 7, % | 1.76 | 1.66 | 1.32 |
| Crude fat, % | 7.1 | 6.9 | 3.3 |
| Ash, % | 6.3 | 6.2 | 5.6 |
| Crude fiber, % | 1.6 | 7.4 | 7.7 |
| Phosphorus (total), % | 0.74 | 0.82 | 0.74 |
| P (avail) 7, % | 0.55 | 0.45 | 0.37 |
| Calcium, % | 0.97 | 1.1 | 0.93 |
| Treatments 1 Se, mg/kg | 0.1 | 0.3 | p-Values 2 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mn, mg/kg | 0 | 12 | 24 | 0 | 12 | 24 | SEM | Mn | Se | Mn × Se |
| Body weight, kg | ||||||||||
| d 0 | 5.12 | 5.33 | 5.19 | 5.19 | 5.26 | 5.24 | 0.7 | |||
| d 35 | 18.81 | 20.23 | 19.60 | 19.02 | 20.77 | 6.64 | 0.7 | 0.216 | 0.455 | 0.914 |
| Performance d 0–35 | ||||||||||
| ADG (g/pig/day) | 393 b | 429 ab | 414 ab | 397 b | 445 a | 442 a | 13 | 0.007 | 0.140 | 0.661 |
| ADFI (g/pig/day) | 541 | 584 | 583 | 547 | 559 | 581 | 22 | 0.221 | 0.702 | 0.767 |
| Gain:Feed | 0.73 | 0.74 | 0.71 | 0.71 | 0.80 | 0.76 | 0.03 | 0.144 | 0.141 | 0.191 |
| Treatments 1 Se, mg/kg | 0.1 | 0.3 | SEM | ||||
|---|---|---|---|---|---|---|---|
| Mn, mg/kg | 0 | 12 | 24 | 0 | 12 | 24 | |
| VFA Concentration | |||||||
| Acetate (MP 2) 3,4 | |||||||
| d 0 | 76.06 a | 68.48 ab | 61.16 c | 70.64 ab | 74.00 ab | 72.61 ab | 2.4 |
| d 35 | 55.14 cd | 55.71 cd | 54.19 d | 54.17 d | 55.65 cd | 57.79 cd | 2.4 |
| Propionate (MP 2) 3,4 | |||||||
| d 0 | 14.91 | 18.51 | 16.79 | 18.29 | 17.00 | 16.6 | 1.4 |
| d 35 | 28.54 | 29.00 | 29.67 | 29.95 | 29.47 | 28.16 | 1.4 |
| Butyrate (MP 2) 3,4 | |||||||
| d 0 | 4.92 | 8.22 | 9.54 | 6.79 | 5.37 | 6.45 | 1.49 |
| d 35 | 11.55 | 10.78 | 10.98 | 10.90 | 10.40 | 9.87 | 1.49 |
| Valerate (MP 2) 3,4 | |||||||
| d 0 | 4.11 | 4.79 | 12.52 | 4.28 | 3.64 | 4.34 | 1.74 |
| d 35 | 4.78 | 4.51 | 5.16 | 4.97 | 4.48 | 4.19 | 1.74 |
| A:P 3,4 | |||||||
| d 0 | 5.40 | 3.75 | 3.87 | 4.19 | 4.40 | 4.87 | 0.46 |
| d 35 | 1.92 | 1.89 | 1.81 | 1.78 | 1.86 | 2.04 | 0.46 |
| Total VFAs 3,4 | |||||||
| d 0 | 38.98 | 49.70 | 48.59 | 39.62 | 39.00 | 37.18 | 12 |
| d 35 | 153.15 | 128.58 | 137.23 | 147.53 | 115.45 | 122.43 | 12 |
| Alpha Diversity | |||||||
| OTUs 3,4 | |||||||
| d 0 | 638 | 618 | 595 | 683 | 596 | 638 | 56 |
| d 35 | 665 | 644 | 628 | 529 | 691 | 725 | |
| Shannon 3,4 | |||||||
| d 0 | 7.84 | 7.51 | 7.79 | 7.87 | 7.73 | 7.85 | 0.21 |
| d 35 | 7.79 | 7.72 | 7.49 | 7.42 | 7.70 | 8.00 | |
| Evenness 3,4 | |||||||
| d 0 | 0.84 | 0.81 | 0.86 | 0.84 | 0.85 | 0.84 | 0.02 |
| d 35 | 0.84 | 0.83 | 0.81 | 0.83 | 0.82 | 0.84 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Edmunds, C.E.; Welch, C.B.; Lourenco, J.M.; Callaway, T.R.; Pringle, T.D.; Dove, C.R. The Effects of Dietary Manganese and Selenium on Growth and the Fecal Microbiota of Nursery Piglets. Vet. Sci. 2023, 10, 650. https://doi.org/10.3390/vetsci10110650
Edmunds CE, Welch CB, Lourenco JM, Callaway TR, Pringle TD, Dove CR. The Effects of Dietary Manganese and Selenium on Growth and the Fecal Microbiota of Nursery Piglets. Veterinary Sciences. 2023; 10(11):650. https://doi.org/10.3390/vetsci10110650
Chicago/Turabian StyleEdmunds, Clint E., Christina B. Welch, Jeferson M. Lourenco, Todd R. Callaway, T. Dean Pringle, and C. Robert Dove. 2023. "The Effects of Dietary Manganese and Selenium on Growth and the Fecal Microbiota of Nursery Piglets" Veterinary Sciences 10, no. 11: 650. https://doi.org/10.3390/vetsci10110650
APA StyleEdmunds, C. E., Welch, C. B., Lourenco, J. M., Callaway, T. R., Pringle, T. D., & Dove, C. R. (2023). The Effects of Dietary Manganese and Selenium on Growth and the Fecal Microbiota of Nursery Piglets. Veterinary Sciences, 10(11), 650. https://doi.org/10.3390/vetsci10110650






