Environmental Cadmium Exposure Perturbs Gut Microbial Dysbiosis in Ducks
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Sample Acquisition
2.2. 16S rDNA Amplicon Sequencing
2.3. Bioinformatics and Data Analysis
3. Results
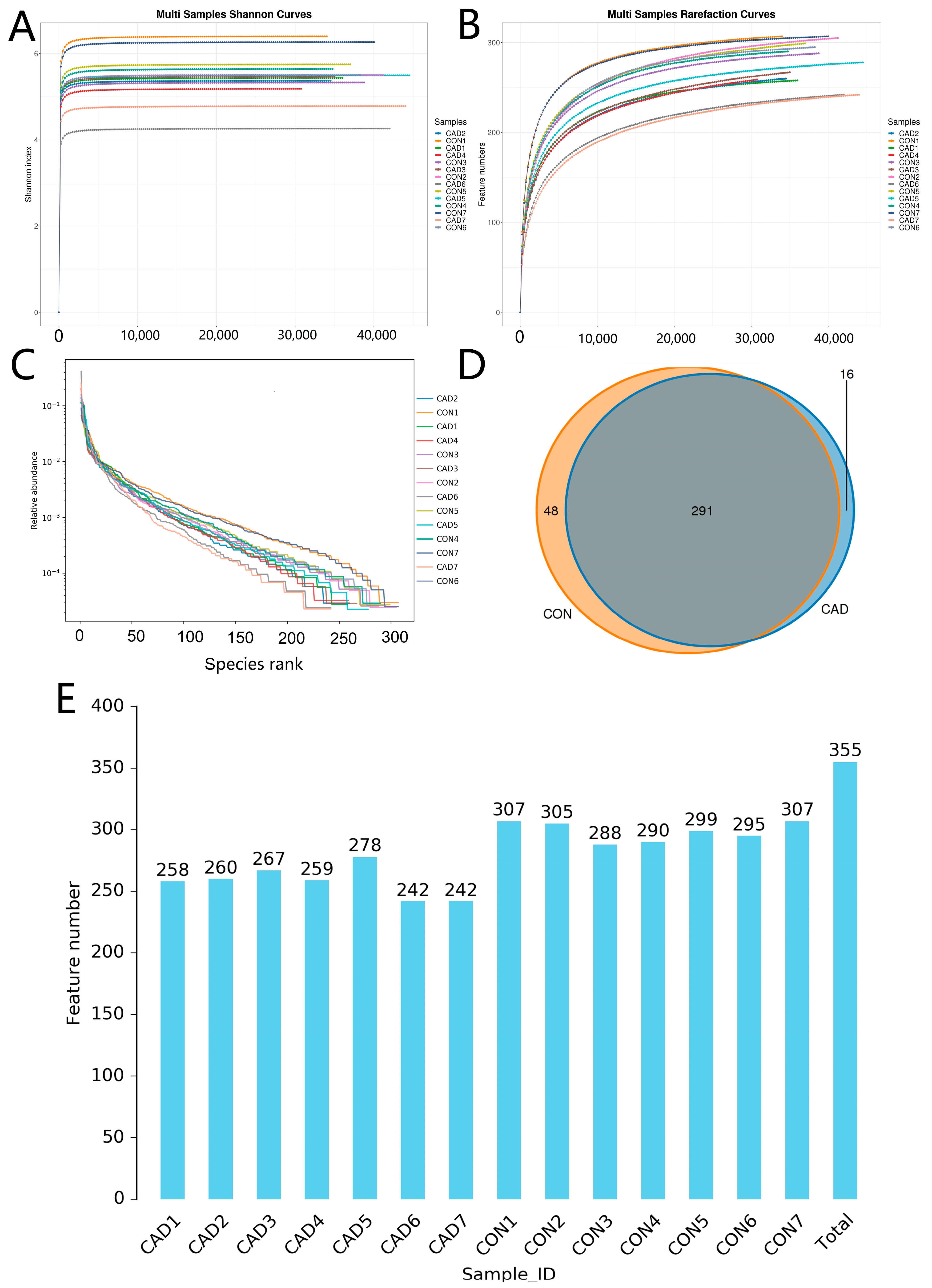
3.1. Data Acquisition and Analysis
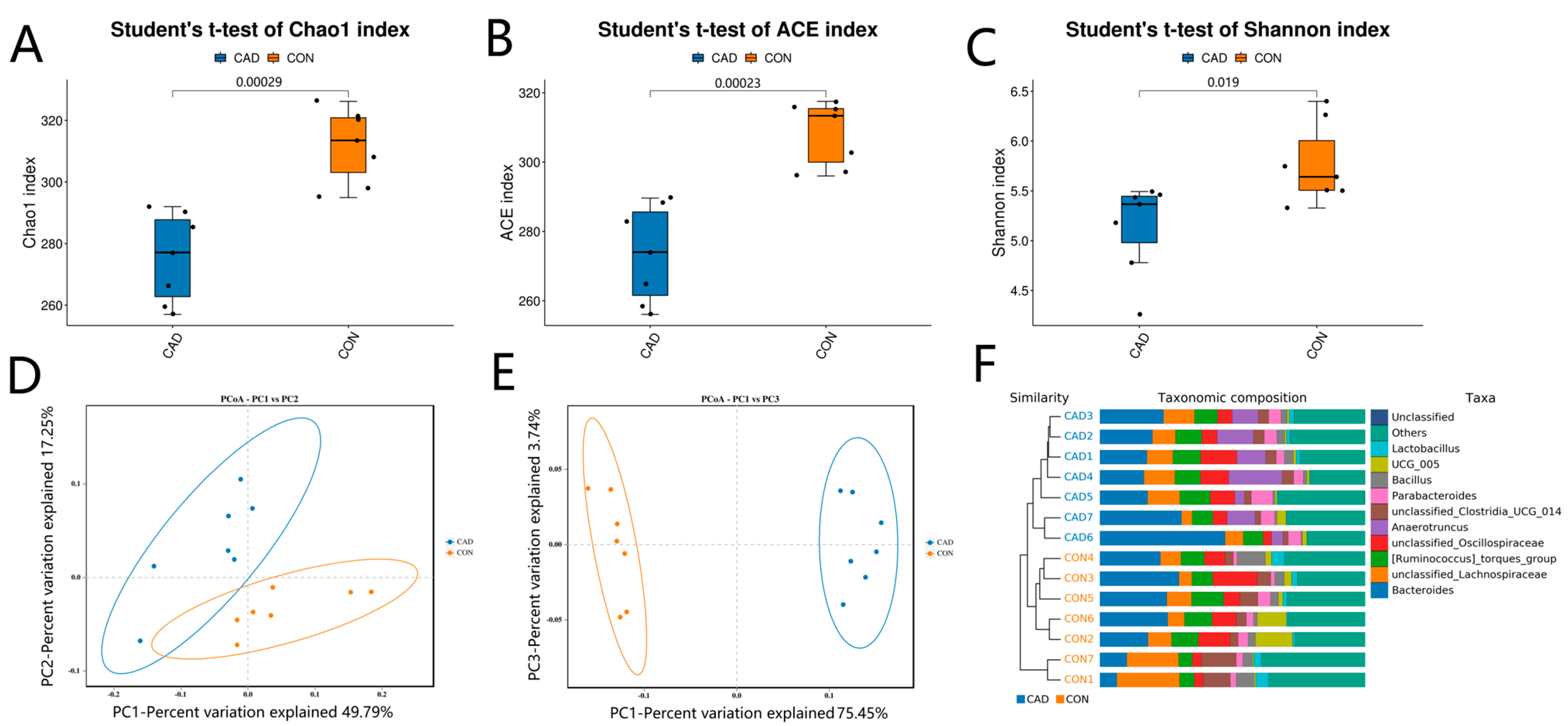
3.2. Alterations in the Diversity Index Associated with Cadmium Exposure
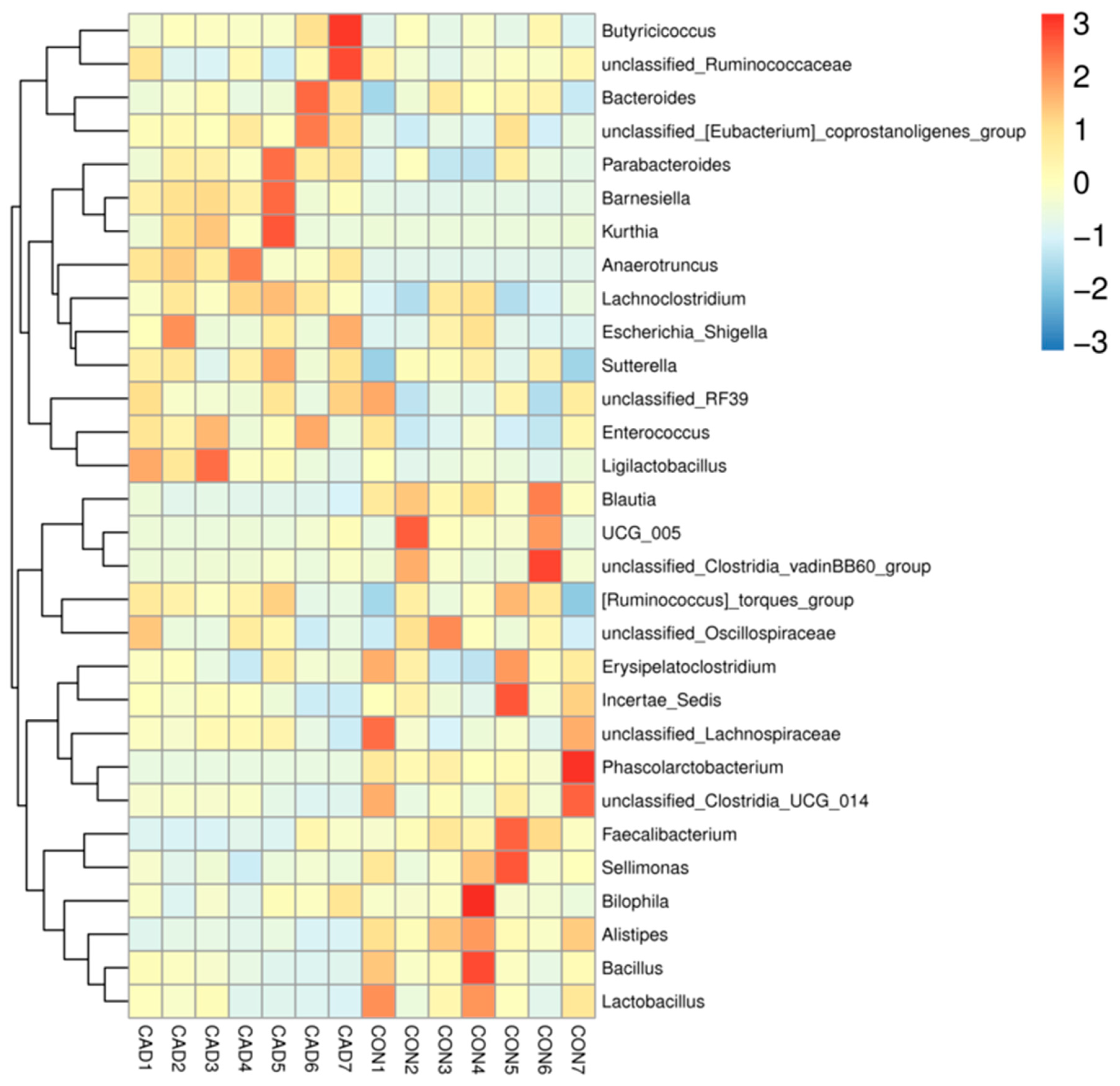
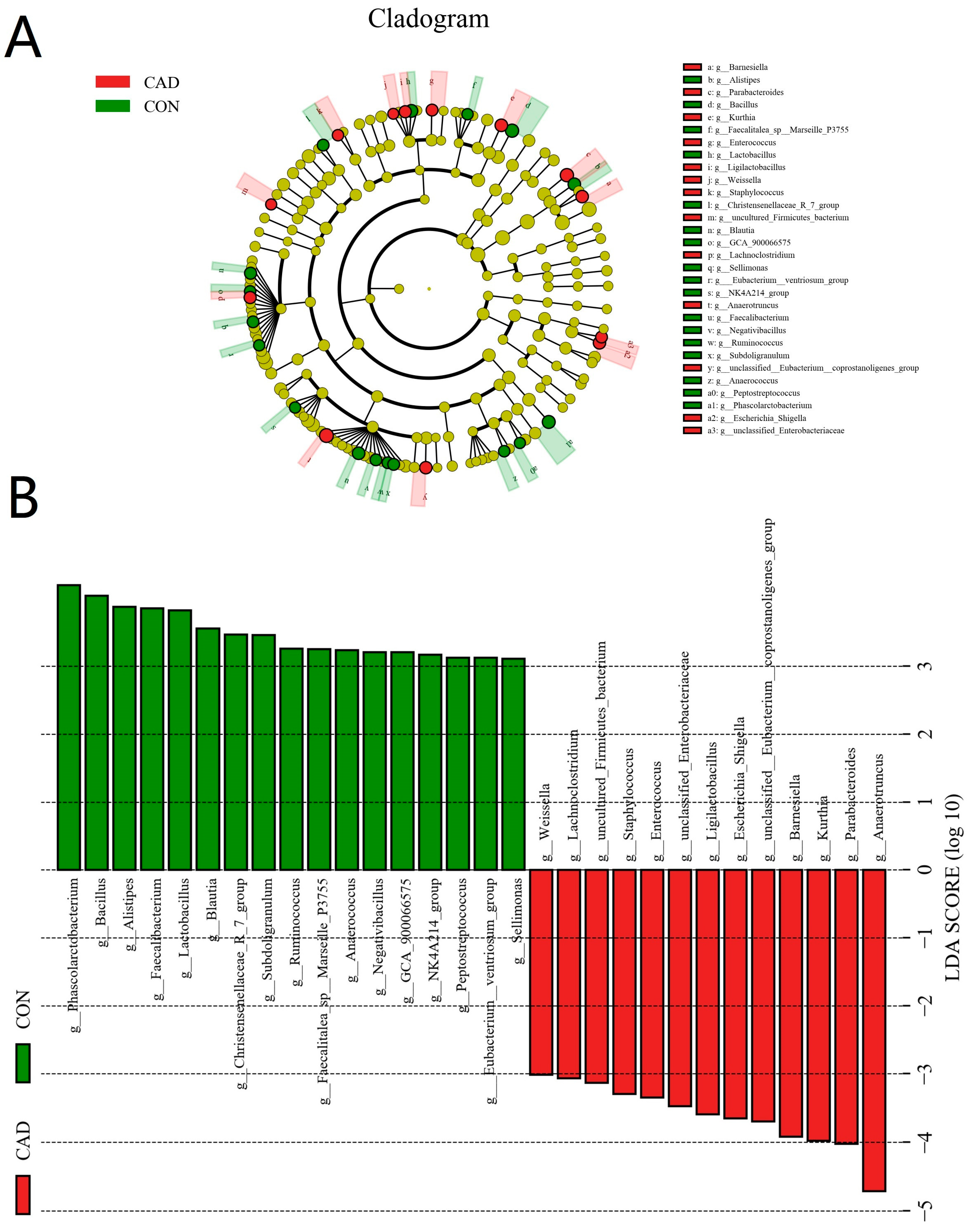
3.3. Alterations in the Gut Microbial Composition after Cadmium Exposure
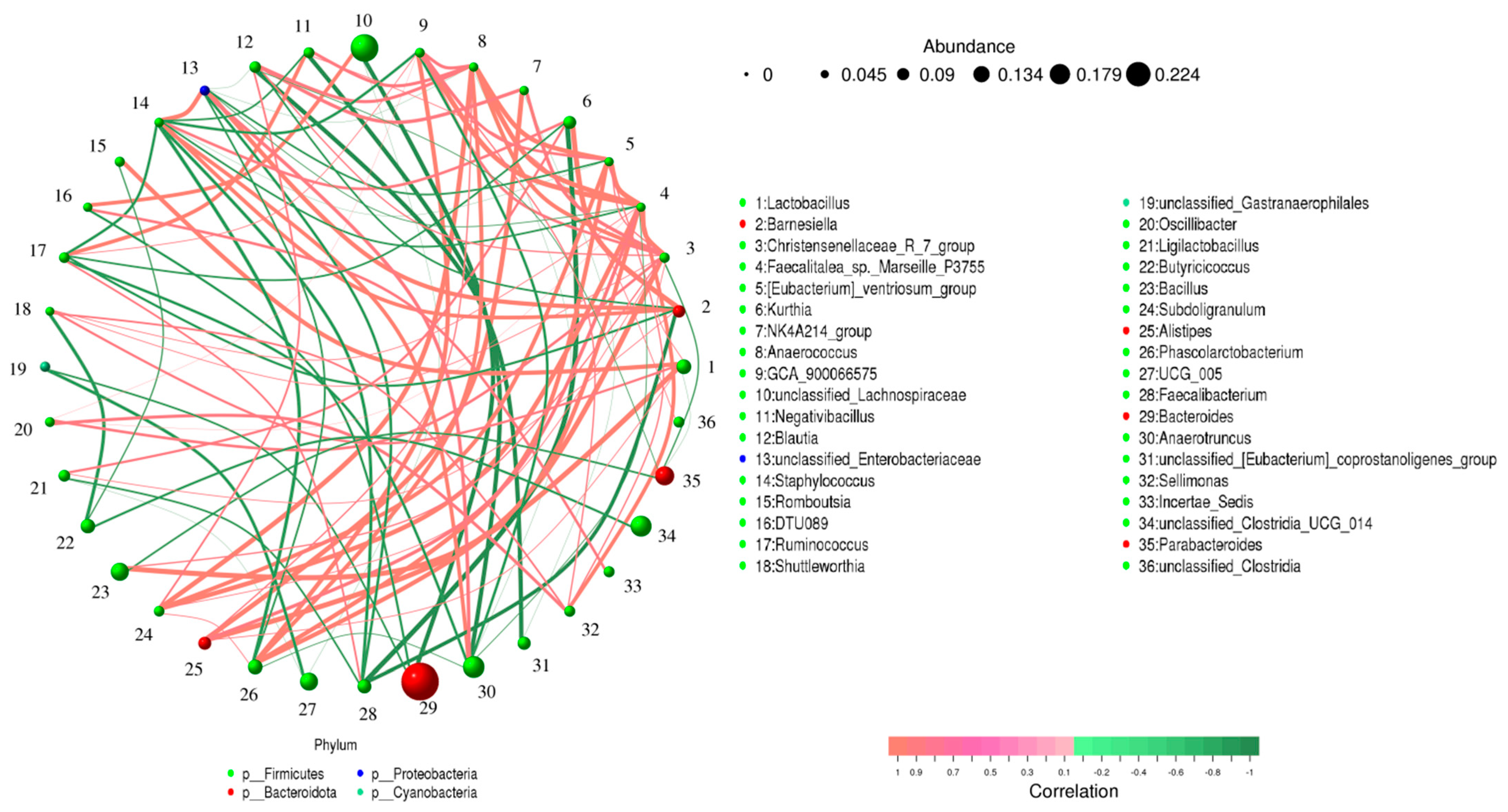
3.4. Correlation Network Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ouyang, J.; Li, Y.; Wu, Y.; Tang, H.; Zheng, S.; Xiong, Y.; Wang, L.; Wang, C.; Luo, K.; Gao, Y.; et al. Microbial diversity and community composition of fecal microbiota in dual-purpose and egg type ducks. Front. Microbiol. 2023, 14, 1092100. [Google Scholar] [CrossRef] [PubMed]
- Tellez, G.; Eisenreich, W.; Petrone, V.; Hernandez-Velasco, X.; Castellanos-Huerta, I.; Tellez, G., Jr.; Latorre, J.; Bottje, W.; Senas-Cuesta, R.; Coles, M.; et al. Effects of chronic stress and intestinal inflammation on commercial poultry health and performance: A review. Ger. J. Vet. Res. 2023, 3, 38–57. [Google Scholar] [CrossRef]
- Abouelezz, K.; Abou-Hadied, M.; Yuan, J.; Elokil, A.A.; Wang, G.; Wang, S.; Wang, J.; Bian, G. Nutritional impacts of dietary oregano and Enviva essential oils on the performance, gut microbiota and blood biochemicals of growing ducks. Animal 2019, 13, 2216–2222. [Google Scholar] [CrossRef]
- Song, X.; Wang, Y.; Guan, R.; Ma, N.; Yin, L.; Zhong, M.; Wang, T.; Shi, L.; Geng, Y. Effects of pine pollen wall on gut microbiota and biomarkers in mice with dyslipidemia. Phytother. Res. 2021, 35, 2057–2073. [Google Scholar] [CrossRef]
- Wang, R.; Yang, X.; Liu, J.; Zhong, F.; Zhang, C.; Chen, Y.; Sun, T.; Ji, C.; Ma, D. Gut microbiota regulates acute myeloid leukaemia via alteration of intestinal barrier function mediated by butyrate. Nat. Commun. 2022, 13, 2522. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.J.; Wang, H.J.; Ma, X.J.; Li, Y.; Yang, H.J.; Li, H.; Su, J.R.; Zhang, C.E.; Huang, L.Q. Modulation of gut microbiota and intestinal barrier function during alleviation of antibiotic-associated diarrhea with Rhizoma Zingiber officinale (Ginger) extract. Food Funct. 2020, 11, 10839–10851. [Google Scholar] [CrossRef] [PubMed]
- Qiu, P.; Ishimoto, T.; Fu, L.; Zhang, J.; Zhang, Z.; Liu, Y. The Gut Microbiota in Inflammatory Bowel Disease. Front. Cell. Infect. Microbiol. 2022, 12, 733992. [Google Scholar] [CrossRef] [PubMed]
- Yue, S.J.; Liu, J.; Wang, W.X.; Wang, A.T.; Yang, X.Y.; Guan, H.S.; Wang, C.Y.; Yan, D. Berberine treatment-emergent mild diarrhea associated with gut microbiota dysbiosis. Biomed. Pharmacother. 2019, 116, 109002. [Google Scholar] [CrossRef]
- Du, F.; Huang, R.; Lin, D.; Wang, Y.; Yang, X.; Huang, X.; Zheng, B.; Chen, Z.; Huang, Y.; Wang, X.; et al. Resveratrol Improves Liver Steatosis and Insulin Resistance in Non-alcoholic Fatty Liver Disease in Association with the Gut Microbiota. Front. Microbiol. 2021, 12, 611323. [Google Scholar] [CrossRef] [PubMed]
- Chi, Y.; Lin, Y.; Lu, Y.; Huang, Q.; Ye, G.; Dong, S. Gut microbiota dysbiosis correlates with a low-dose PCB126-induced dyslipidemia and non-alcoholic fatty liver disease. Sci. Total Environ. 2019, 653, 274–282. [Google Scholar] [CrossRef]
- Xu, Y.; Kong, X.; Zhu, Y.; Xu, J.; Mao, H.; Li, J.; Zhang, J.; Zhu, X. Contribution of gut microbiota toward renal function in sepsis. Front. Microbiol. 2022, 13, 985283. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Li, Y.; Iqbal, M.; Tang, Z.; Zhang, H. Thiram exposure in environment: A critical review on cytotoxicity. Chemosphere 2022, 295, 133928. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.Y.; Wang, Y.J.; Iqbal, M.; Mehmood, K.; Li, Y.; Tang, Z.X.; Zhang, H. Challenges of fluoride pollution in environment: Mechanisms and pathological significance of toxicity—A review. Environ. Pollut. 2022, 304, 119241. [Google Scholar] [CrossRef]
- Brila, I.; Lavrinienko, A.; Tukalenko, E.; Ecke, F.; Rodushkin, I.; Kallio, E.R.; Mappes, T.; Watts, P.C. Low-level environmental metal pollution is associated with altered gut microbiota of a wild rodent, the bank vole (Myodes glareolus). Sci. Total Environ. 2021, 790, 148224. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Yin, X.; Gao, S.; Jiang, T.; Ma, C. In vitro oral bioaccessibility investigation and human health risk assessment of heavy metals in wheat grains grown near the mines in North China. Chemosphere 2020, 252, 126522. [Google Scholar] [CrossRef]
- Sun, Z.; Sun, W.; An, J.; Xu, H.; Liu, Y.; Yan, C. Copper and chlorpyrifos stress affect the gut microbiota of chironomid larvae (Propsilocerus akamusi). Ecotox. Environ. Safe 2022, 244, 114027. [Google Scholar] [CrossRef] [PubMed]
- Yi, J.; Liao, J.; Bai, T.; Wang, B.; Yangzom, C.; Ahmed, Z.; Mehmood, K.; Abbas, R.Z.; Li, Y.; Tang, Z.; et al. Battery wastewater induces nephrotoxicity via disordering the mitochondrial dynamics. Chemosphere 2022, 303, 135018. [Google Scholar] [CrossRef]
- Li, A.; Wang, Y.; Wang, Y.; Dong, H.; Wu, Q.; Mehmood, K.; Chang, Z.; Li, Y.; Chang, Y.; Shi, L.; et al. Microbiome analysis reveals soil microbial community alteration with the effect of animal excretion contamination and altitude in Tibetan Plateau of China. Int. Soil Water Conserv. Res. 2021, 9, 639–648. [Google Scholar] [CrossRef]
- Ma, Y.; Ran, D.; Zhao, H.; Song, R.; Zou, H.; Gu, J.; Yuan, Y.; Bian, J.; Zhu, J.; Liu, Z. Cadmium exposure triggers osteoporosis in duck via P2X7/PI3K/AKT-mediated osteoblast and osteoclast differentiation. Sci. Total Environ. 2021, 750, 141638. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, X.; Pi, S.; Wei, Z.; Wang, C.; Yang, F.; Li, G.; Nie, G.; Hu, G. Cadmium and molybdenum co-exposure triggers autophagy via CYP450s/ROS pathway in duck renal tubular epithelial cells. Sci. Total Environ. 2021, 759, 143570. [Google Scholar] [CrossRef]
- Mwelwa, S.; Chungu, D.; Tailoka, F.; Beesigamukama, D.; Tanga, C. Biotransfer of heavy metals along the soil-plant-edible insect-human food chain in Africa. Sci. Total Environ. 2023, 881, 163150. [Google Scholar] [CrossRef]
- Etesami, H. Bacterial mediated alleviation of heavy metal stress and decreased accumulation of metals in plant tissues: Mechanisms and future prospects. Ecotox. Environ. Safe 2018, 147, 175–191. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Fan, Y.; Tao, C.; Yan, W.; Huang, Y.; Qian, H.; Xu, Q.; Wan, T.; Chen, Y.; Qin, Y.; et al. Association between exposure to cadmium and risk of all-cause and cause-specific mortality in the general US adults: A prospective cohort study. Chemosphere 2022, 307, 136060. [Google Scholar] [CrossRef]
- Zhao, L.; Zhu, N.W.; Wang, X.H. Comparison of bio-dissolution of spent Ni-Cd batteries by sewage sludge using ferrous ions and elemental sulfur as substrate. Chemosphere 2008, 70, 974–981. [Google Scholar] [CrossRef]
- Turner, A.; Filella, M. Hazardous metal additives in plastics and their environmental impacts. Environ. Int. 2021, 156, 106622. [Google Scholar] [CrossRef] [PubMed]
- Butt, A.; Rehman, K.; Khan, M.X.; Hesselberg, T. Bioaccumulation of cadmium, lead, and zinc in agriculture-based insect food chains. Environ. Monit. Assess. 2018, 190, 698. [Google Scholar] [CrossRef]
- Hamid, Y.; Tang, L.; Sohail, M.I.; Cao, X.; Hussain, B.; Aziz, M.Z.; Usman, M.; He, Z.L.; Yang, X. An explanation of soil amendments to reduce cadmium phytoavailability and transfer to food chain. Sci. Total Environ. 2019, 660, 80–96. [Google Scholar] [CrossRef]
- Shi, L.; Cao, H.; Luo, J.; Liu, P.; Wang, T.; Hu, G.; Zhang, C. Effects of molybdenum and cadmium on the oxidative damage and kidney apoptosis in Duck. Ecotox. Environ. Safe 2017, 145, 24–31. [Google Scholar] [CrossRef]
- Wang, C.; Nie, G.; Yang, F.; Chen, J.; Zhuang, Y.; Dai, X.; Liao, Z.; Yang, Z.; Cao, H.; Xing, C.; et al. Molybdenum and cadmium co-induce oxidative stress and apoptosis through mitochondria-mediated pathway in duck renal tubular epithelial cells. J. Hazard Mater. 2020, 383, 121157. [Google Scholar] [CrossRef] [PubMed]
- Li, A.; Wang, Y.; Kulyar, M.F.; Iqbal, M.; Lai, R.; Zhu, H.; Li, K. Environmental microplastics exposure decreases antioxidant ability, perturbs gut microbial homeostasis and metabolism in chicken. Sci. Total Environ. 2023, 856, 159089. [Google Scholar] [CrossRef]
- Huang, S.; Rehman, M.; Lan, Y.; Qiu, G.; Zhang, H.; Iqbal, M.; Luo, H.; Mehmood, K.; Zhang, L.; Li, J. Tibial dyschondroplasia is highly associated with suppression of tibial angiogenesis through regulating the HIF-1α/VEGF/VEGFR signaling pathway in chickens. Sci. Rep. 2017, 7, 9089. [Google Scholar] [CrossRef]
- Geng, H.X.; Wang, L. Cadmium: Toxic effects on placental and embryonic development. Environ. Toxicol. Phar. 2019, 67, 102–107. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Yan, L.; Chen, X.; Lam, S.S.; Rinklebe, J.; Yu, Q.; Yang, Y.; Peng, W.; Sonne, C. Phytoremediation of cadmium from soil, air and water. Chemosphere 2023, 320, 138058. [Google Scholar] [CrossRef] [PubMed]
- Larson-Casey, J.L.; Liu, S.; Pyles, J.M.; Lapi, S.E.; Saleem, K.; Antony, V.B.; Gonzalez, M.L.; Crossman, D.K.; Carter, A.B. Impaired PPARgamma activation by cadmium exacerbates infection-induced lung injury. JCI Insight. 2023, 8, e166608. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Zhang, Y.; Zeng, L.; Wang, X.; Mu, H.; Wang, M.; Pan, H.; Su, P. Paternal cadmium exposure affects testosterone synthesis by reducing the testicular cholesterol pool in offspring mice. Ecotox. Environ. Safe 2022, 242, 113947. [Google Scholar] [CrossRef]
- Wang, X.; Cui, W.; Wang, M.; Liang, Y.; Zhu, G.; Jin, T.; Chen, X. The association between life-time dietary cadmium intake from rice and chronic kidney disease. Ecotox. Environ. Safe 2021, 211, 111933. [Google Scholar] [CrossRef]
- Basiouni, S.; Tellez-Isaias, G.; Latorre, J.D.; Graham, B.D.; Petrone-Garcia, V.M.; El-Seedi, H.R.; Yalcin, S.; El-Wahab, A.A.; Visscher, C.; May-Simera, H.L.; et al. Anti-Inflammatory and Antioxidative Phytogenic Substances against Secret Killers in Poultry: Current Status and Prospects. Vet. Sci. 2023, 10, 55. [Google Scholar] [CrossRef]
- Orisakwe, O.E.; Oladipo, O.O.; Ajaezi, G.C.; Udowelle, N.A. Horizontal and Vertical Distribution of Heavy Metals in Farm Produce and Livestock around Lead-Contaminated Goldmine in Dareta and Abare, Zamfara State, Northern Nigeria. J. Environ. Public Health 2017, 2017, 3506949. [Google Scholar] [CrossRef] [PubMed]
- Li, A.; Liu, B.; Li, F.; He, Y.; Wang, L.; Fakhar-E-Alam, K.M.; Li, H.; Fu, Y.; Zhu, H.; Wang, Y.; et al. Integrated Bacterial and Fungal Diversity Analysis Reveals the Gut Microbial Alterations in Diarrheic Giraffes. Front. Microbiol. 2021, 12, 712092. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, S.; Mazumder, M.; Al-Attas, O.; Husain, T. Heavy metals in drinking water: Occurrences, implications, and future needs in developing countries. Sci. Total Environ. 2016, 569–570, 476–488. [Google Scholar] [CrossRef]
- Pan, L.; Li, G.; Li, J.; Gao, J.; Liu, Q.; Shi, B. Heavy metal enrichment in drinking water pipe scales and speciation change with water parameters. Sci. Total Environ. 2022, 806, 150549. [Google Scholar] [CrossRef] [PubMed]
- Elmassry, M.M.; Zayed, A.; Farag, M.A. Gut homeostasis and microbiota under attack: Impact of the different types of food contaminants on gut health. Crit. Rev. Food Sci. 2022, 62, 738–763. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Chen, W.; Sun, Y.; Liu, J.; Zhang, W. Effects of cadmium on organ function, gut microbiota and its metabolomics profile in adolescent rats. Ecotox. Environ. Safe 2021, 222, 112501. [Google Scholar] [CrossRef] [PubMed]
- Ba, Q.; Li, M.; Chen, P.; Huang, C.; Duan, X.; Lu, L.; Li, J.; Chu, R.; Xie, D.; Song, H.; et al. Sex-Dependent Effects of Cadmium Exposure in Early Life on Gut Microbiota and Fat Accumulation in Mice. Environ. Health Perspect. 2017, 125, 437–446. [Google Scholar] [CrossRef] [PubMed]
- Das, Q.; Shay, J.; Gauthier, M.; Yin, X.; Hasted, T.L.; Ross, K.; Julien, C.; Yacini, H.; Kennes, Y.M.; Warriner, K.; et al. Effects of Vaccination Against Coccidiosis on Gut Microbiota and Immunity in Broiler Fed Bacitracin and Berry Pomace. Front. Immunol. 2021, 12, 621803. [Google Scholar] [CrossRef]
- Kong, A.; Zhang, C.; Cao, Y.; Cao, Q.; Liu, F.; Yang, Y.; Tong, Z.; Rehman, M.U.; Wang, X.; Huang, S. The fungicide thiram perturbs gut microbiota community and causes lipid metabolism disorder in chickens. Ecotox. Environ. Safe 2020, 206, 111400. [Google Scholar] [CrossRef]
- Vaz-Moreira, I.; Nunes, O.C.; Manaia, C.M. Ubiquitous and persistent Proteobacteria and other Gram-negative bacteria in drinking water. Sci. Total Environ. 2017, 586, 1141–1149. [Google Scholar] [CrossRef]
- Shin, N.R.; Whon, T.W.; Bae, J.W. Proteobacteria: Microbial signature of dysbiosis in gut microbiota. Trends Biotechnol. 2015, 33, 496–503. [Google Scholar] [CrossRef]
- Ma, Z.; Gao, X.; Yang, X.; Lin, L.; Wei, X.; Wang, S.; Li, Y.; Peng, X.; Zhao, C.; Chen, J.; et al. Low-dose florfenicol and copper combined exposure during early life induced health risks by affecting gut microbiota and metabolome in SD rats. Ecotox. Environ. Safe 2022, 245, 114120. [Google Scholar] [CrossRef]
- Li, A.Y.; Wang, Y.L.; Hao, J.Y.; Wang, L.; Quan, L.T.; Duan, K.; Kulyar, M.; Ullah, K.; Zhang, J.B.; Wu, Y.; et al. Long-term hexavalent chromium exposure disturbs the gut microbial homeostasis of chickens. Ecotox. Environ. Safe 2022, 237, 113532. [Google Scholar] [CrossRef]
- Waters, J.L.; Ley, R.E. The human gut bacteria Christensenellaceae are widespread, heritable, and associated with health. BMC Biol. 2019, 17, 83. [Google Scholar] [CrossRef]
- Ming, J.; Yu, X.; Xu, X.; Wang, L.; Ding, C.; Wang, Z.; Xie, X.; Li, S.; Yang, W.; Luo, S.; et al. Effectiveness and safety of Bifidobacterium and berberine in human hyperglycemia and their regulatory effect on the gut microbiota: A multi-center, double-blind, randomized, parallel-controlled study. Genome Med. 2021, 13, 125. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Tello, J.; Schofield, Z.; Kiu, R.; Dalby, M.J.; van Sinderen, D.; Le Gall, G.; Sferruzzi-Perri, A.N.; Hall, L.J. Maternal gut microbiota Bifidobacterium promotes placental morphogenesis, nutrient transport and fetal growth in mice. Cell Mol. Life Sci. 2022, 79, 386. [Google Scholar] [CrossRef]
- Dong, J.; Ping, L.; Cao, T.; Sun, L.; Liu, D.; Wang, S.; Huo, G.; Li, B. Immunomodulatory effects of the Bifidobacterium longum BL-10 on lipopolysaccharide-induced intestinal mucosal immune injury. Front. Immunol. 2022, 13, 947755. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Luo, L.; Liang, W.; Yin, Q.; Guo, J.; Rush, A.M.; Lv, Z.; Liang, Q.; Fischbach, M.A.; Sonnenburg, J.L.; et al. Bifidobacterium alters the gut microbiota and modulates the functional metabolism of T regulatory cells in the context of immune checkpoint blockade. Proc. Natl. Acad. Sci. USA 2020, 117, 27509–27515. [Google Scholar] [CrossRef]
- Yang, B.; Huang, Z.; He, Z.; Yue, Y.; Zhou, Y.; Ross, R.P.; Stanton, C.; Zhang, H.; Zhao, J.; Chen, W. Protective effect of Bifidobacterium bifidum FSDJN7O5 and Bifidobacterium breve FHNFQ23M3 on diarrhea caused by enterotoxigenic Escherichia coli. Food Funct. 2021, 12, 7271–7282. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Liang, S.; Zhao, J.; Li, X.; Guo, J.; Xin, B.; Li, B.; Huo, G.; Ma, W. Bifidobacterium animalis subsp. lactis XLTG11 improves antibiotic-related diarrhea by alleviating inflammation, enhancing intestinal barrier function and regulating intestinal flora. Food Funct. 2022, 13, 6404–6418. [Google Scholar] [CrossRef]
- Walls, L.E.; Otoupal, P.; Ledesma-Amaro, R.; Velasquez-Orta, S.B.; Gladden, J.M.; Rios-Solis, L. Bioconversion of cellulose into bisabolene using Ruminococcus flavefaciens and Rhodosporidium toruloides. Bioresour. Technol. 2023, 368, 128216. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.K.; Chae, J.P.; Pajarillo, E.; Kim, S.H.; Kwak, M.J.; Eun, J.S.; Chee, S.W.; Whang, K.Y.; Kim, S.H.; Kang, D.K. Association between the body weight of growing pigs and the functional capacity of their gut microbiota. Anim. Sci. J. 2020, 91, e13418. [Google Scholar] [CrossRef]
- Lenoir, M.; Martin, R.; Torres-Maravilla, E.; Chadi, S.; Gonzalez-Davila, P.; Sokol, H.; Langella, P.; Chain, F.; Bermudez-Humaran, L.G. Butyrate mediates anti-inflammatory effects of Faecalibacterium prausnitzii in intestinal epithelial cells through Dact3. Gut. Microbes 2020, 12, 1–16. [Google Scholar] [CrossRef]
- Zhang, Z.; Taylor, L.; Shommu, N.; Ghosh, S.; Reimer, R.; Panaccione, R.; Kaur, S.; Hyun, J.E.; Cai, C.; Deehan, E.C.; et al. A Diversified Dietary Pattern Is Associated with a Balanced Gut Microbial Composition of Faecalibacterium and Escherichia/Shigella in Patients with Crohn’s Disease in Remission. J. Crohns Colitis 2020, 14, 1547–1557. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Bao, L.; Qiu, M.; Wu, K.; Zhao, Y.; Feng, L.; Xiang, K.; Zhang, N.; Hu, X.; Fu, Y. Commensal cow Roseburia reduces gut-dysbiosis-induced mastitis through inhibiting bacterial translocation by producing butyrate in mice. Cell Rep. 2022, 41, 111681. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; Liao, Y.; Li, J.; Pei, Z.; Wang, L.; Shi, Y.; Peng, H.; Tan, Y.; Li, C.; Bai, H.; et al. Lactobacillus plantarum GX17 benefits growth performance and improves functions of intestinal barrier/intestinal flora among yellow-feathered broilers. Front. Immunol. 2023, 14, 1195382. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yang, L.; Zhang, J.; Huang, K.; Sun, X.; Yang, Y.; Wang, T.; Zhang, Q.; Zou, Z.; Jin, M. Oral or intranasal immunization with recombinant Lactobacillus plantarum displaying head domain of Swine Influenza A virus hemagglutinin protects mice from H1N1 virus. Microb. Cell Fact. 2022, 21, 185. [Google Scholar] [CrossRef]
- Yu, L.; Zhai, Q.; Zhu, J.; Zhang, C.; Li, T.; Liu, X.; Zhao, J.; Zhang, H.; Tian, F.; Chen, W. Dietary Lactobacillus plantarum supplementation enhances growth performance and alleviates aluminum toxicity in tilapia. Ecotox. Environ. Safe 2017, 143, 307–314. [Google Scholar] [CrossRef]
- Qu, T.; Yang, L.; Wang, Y.; Jiang, B.; Shen, M.; Ren, D. Reduction of serum cholesterol and its mechanism by Lactobacillus plantarum H6 screened from local fermented food products. Food Funct. 2020, 11, 1397–1409. [Google Scholar] [CrossRef]
- Liu, P.Y.; Wang, Y.B.; Yang, G.; Zhang, Q.H.; Meng, L.B.; Xin, Y.; Jiang, X. The role of short-chain fatty acids in intestinal barrier function, inflammation, oxidative stress, and colonic carcinogenesis. Pharmacol. Res. 2021, 165, 105420. [Google Scholar] [CrossRef]
- Mirzaei, R.; Dehkhodaie, E.; Bouzari, B.; Rahimi, M.; Gholestani, A.; Hosseini-Fard, S.R.; Keyvani, H.; Teimoori, A.; Karampoor, S. Dual role of microbiota-derived short-chain fatty acids on host and pathogen. Biomed. Pharmacother. 2022, 145, 112352. [Google Scholar] [CrossRef]
- Liu, X.F.; Shao, J.H.; Liao, Y.T.; Wang, L.N.; Jia, Y.; Dong, P.J.; Liu, Z.Z.; He, D.D.; Li, C.; Zhang, X. Regulation of short-chain fatty acids in the immune system. Front. Immunol. 2023, 14, 1186892. [Google Scholar] [CrossRef]
- Longhurst, W.D.; Sheele, J.M. Spontaneous methicillin-resistant Staphylococcus aureus (MRSA) meningitis. Am. J. Emerg Med. 2018, 36, 901–909. [Google Scholar] [CrossRef] [PubMed]
- Pickens, C.I.; Wunderink, R.G. Methicillin-Resistant Staphylococcus aureus Hospital-Acquired Pneumonia/Ventilator-Associated Pneumonia. Semin. Resp. Crit. Care 2022, 43, 304–309. [Google Scholar] [CrossRef] [PubMed]
- Lo, T.S.; Borchardt, S.M. Antibiotic-associated diarrhea due to methicillin-resistant Staphylococcus aureus. Diagn. Microbiol. Infect. Dis. 2009, 63, 388–389. [Google Scholar] [CrossRef] [PubMed]
- Azimirad, M.; Dezfulian, A.; Alebouyeh, M.; Bahreiny, E.R.; Shahrokh, S.; Zali, M.R. Infection with enterotoxigenic Staphylococcus aureus as a concern in patients with gastroenteritis. J. Glob. Antimicrob. Resist. 2017, 9, 111–114. [Google Scholar] [CrossRef] [PubMed]
- Tamai, K.; Akashi, Y.; Yoshimoto, Y.; Yaguchi, Y.; Takeuchi, Y.; Shiigai, M.; Igarashi, J.; Hirose, Y.; Suzuki, H.; Ohkusu, K. First case of a bloodstream infection caused by the genus Brachybacterium. J. Infect. Chemother. 2018, 24, 998–1003. [Google Scholar] [CrossRef]
- Tai, D.; Go, J.R.; Fida, M.; Saleh, O.A. Management and treatment of Aerococcus bacteremia and endocarditis. Int. J. Infect. Dis. 2021, 102, 584–589. [Google Scholar] [CrossRef]
- Chen, Y.J.; Wu, H.; Wu, S.D.; Lu, N.; Wang, Y.T.; Liu, H.N.; Dong, L.; Liu, T.T.; Shen, X.Z. Parasutterella, in association with irritable bowel syndrome and intestinal chronic inflammation. J. Gastroen. Hepatol. 2018, 33, 1844–1852. [Google Scholar] [CrossRef]
- Yamamuro, R.; Hosokawa, N.; Otsuka, Y.; Osawa, R. Clinical Characteristics of Corynebacterium Bacteremia Caused by Different Species, Japan, 2014–2020. Emerg. Infect. Dis. 2021, 27, 2981–2987. [Google Scholar] [CrossRef]
- Huang, X.Q.; Qiu, J.K.; Wang, C.H.; Pan, L.; Xu, J.K.; Pan, X.H.; Ji, X.B.; Mao, M.J. Sepsis secondary to multifocal Enterococcus faecium infection: A case report. Medicine 2020, 99, e19811. [Google Scholar] [CrossRef]
- Zhang, X.; Jiang, C.; Zhou, C. Diagnosis of Enterococcus faecalis meningitis associated with long-term cerebrospinal fluid rhinorrhoea using metagenomics next-generation sequencing: A case report. BMC Infect. Dis. 2021, 21, 1105. [Google Scholar] [CrossRef]
- Li, G.; Walker, M.J.; De Oliveira, D. Vancomycin Resistance in Enterococcus and Staphylococcus aureus. Microorganisms 2022, 11, 24. [Google Scholar] [CrossRef]


| Sample | Raw Reads | Clean Reads | Effective Reads | AvgLen (bp) | GC (%) | Q20 (%) | Q30 (%) | Effective (%) |
|---|---|---|---|---|---|---|---|---|
| CAD1 | 49,376 | 37,217 | 37,079 | 413 | 51.91 | 99.94 | 99.52 | 75.1 |
| CAD2 | 47,727 | 35,427 | 35,348 | 415 | 51.84 | 99.93 | 99.48 | 74.06 |
| CAD3 | 48,680 | 36,216 | 36,024 | 415 | 51.43 | 99.93 | 99.47 | 74 |
| CAD4 | 43,186 | 32,376 | 31,971 | 411 | 52.16 | 99.93 | 99.5 | 74.03 |
| CAD5 | 64,590 | 48,332 | 47,062 | 415 | 51.65 | 99.94 | 99.51 | 72.86 |
| CAD6 | 62,329 | 48,171 | 45,133 | 417 | 50.25 | 99.93 | 99.48 | 72.41 |
| CAD7 | 58,846 | 45,378 | 45,056 | 415 | 51.38 | 99.94 | 99.52 | 76.57 |
| CON1 | 62,327 | 45,367 | 41,099 | 412 | 52.2 | 99.93 | 99.5 | 65.94 |
| CON2 | 57,390 | 42,638 | 42,318 | 412 | 52.15 | 99.94 | 99.52 | 73.74 |
| CON3 | 58,440 | 44,221 | 41,845 | 415 | 51.33 | 99.93 | 99.5 | 71.6 |
| CON4 | 58,091 | 43,488 | 39,230 | 416 | 51.95 | 99.93 | 99.48 | 67.53 |
| CON5 | 58,144 | 44,068 | 40,895 | 414 | 51.33 | 99.94 | 99.53 | 70.33 |
| CON6 | 53,779 | 39,464 | 39,239 | 413 | 51.61 | 99.93 | 99.45 | 72.96 |
| CON7 | 64,423 | 47,898 | 45,013 | 413 | 51.89 | 99.93 | 99.49 | 69.87 |
| Sample | Phylum | Class | Order | Family | Genus |
|---|---|---|---|---|---|
| CAD1 | 6 | 9 | 27 | 52 | 102 |
| CAD2 | 6 | 10 | 28 | 52 | 105 |
| CAD3 | 6 | 11 | 28 | 54 | 110 |
| CAD4 | 6 | 10 | 28 | 53 | 103 |
| CAD5 | 6 | 10 | 28 | 55 | 110 |
| CAD6 | 6 | 10 | 29 | 55 | 108 |
| CAD7 | 6 | 10 | 27 | 51 | 104 |
| CON1 | 7 | 12 | 29 | 52 | 111 |
| CON2 | 7 | 12 | 29 | 51 | 106 |
| CON3 | 7 | 12 | 29 | 49 | 105 |
| CON4 | 7 | 12 | 28 | 48 | 105 |
| CON5 | 7 | 12 | 29 | 51 | 107 |
| CON6 | 7 | 12 | 29 | 48 | 103 |
| CON7 | 7 | 12 | 29 | 53 | 113 |
| Total | 7 | 12 | 31 | 61 | 129 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Mi, J.; Yang, K.; Wang, L. Environmental Cadmium Exposure Perturbs Gut Microbial Dysbiosis in Ducks. Vet. Sci. 2023, 10, 649. https://doi.org/10.3390/vetsci10110649
Wang X, Mi J, Yang K, Wang L. Environmental Cadmium Exposure Perturbs Gut Microbial Dysbiosis in Ducks. Veterinary Sciences. 2023; 10(11):649. https://doi.org/10.3390/vetsci10110649
Chicago/Turabian StyleWang, Xuefei, Junxian Mi, Kun Yang, and Lian Wang. 2023. "Environmental Cadmium Exposure Perturbs Gut Microbial Dysbiosis in Ducks" Veterinary Sciences 10, no. 11: 649. https://doi.org/10.3390/vetsci10110649
APA StyleWang, X., Mi, J., Yang, K., & Wang, L. (2023). Environmental Cadmium Exposure Perturbs Gut Microbial Dysbiosis in Ducks. Veterinary Sciences, 10(11), 649. https://doi.org/10.3390/vetsci10110649





