A Lack of “Environmental Earth Data” at the Microhabitat Scale Impacts Efforts to Control Invasive Arthropods That Vector Pathogens
Abstract
:1. Introduction
2. Linking Environmental Earth Data and IAVPs
3. Gathering Environmental Data for SDMs
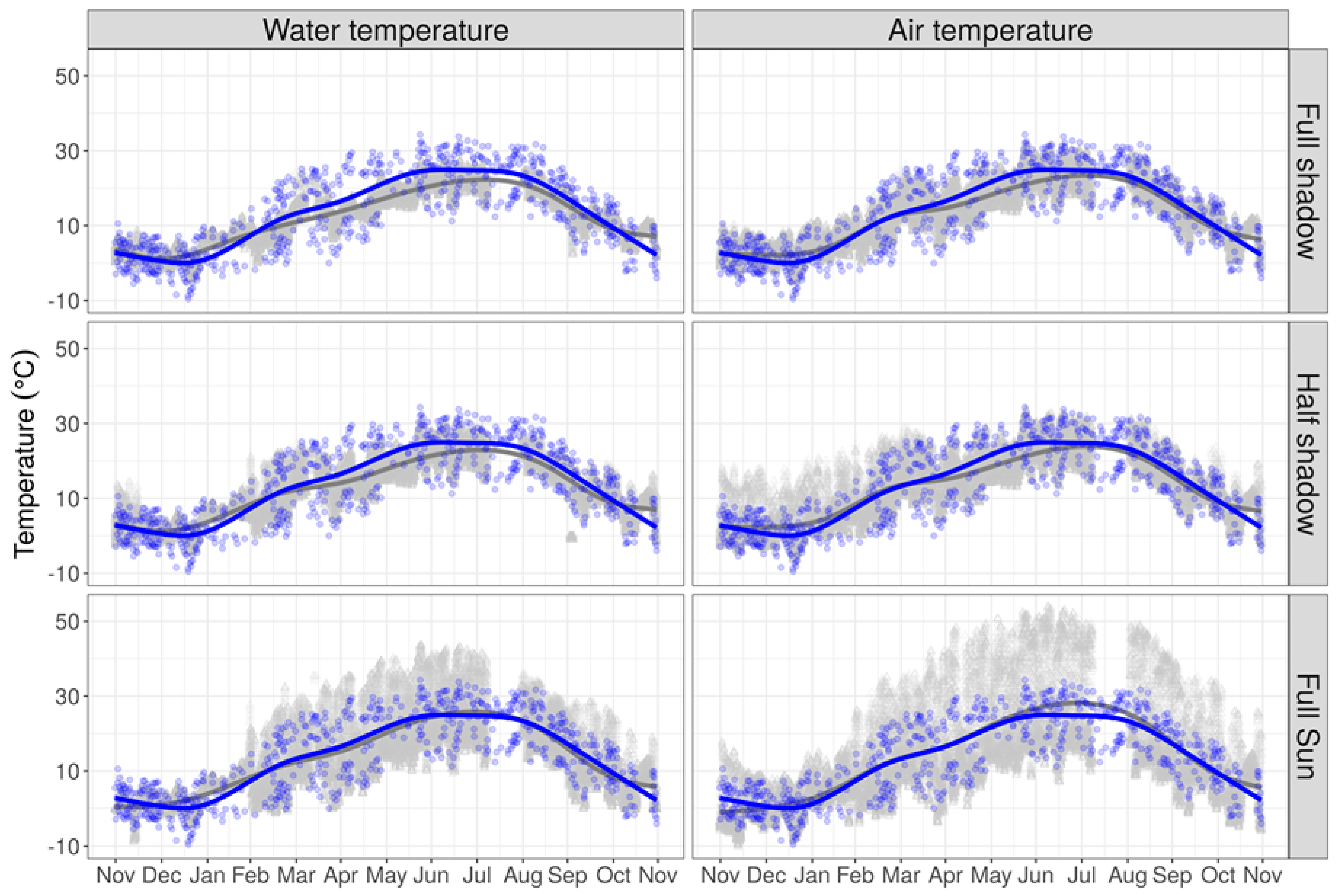
3.1. Bio-Physical Variables
3.2. Climatic Variables
4. Issues Faced When Using Environmental Data in IAVP Models
5. Attempting to Overcome the Lack of Microhabitat Data
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Dukes, J.S.; Mooney, H.A. Does global change increase the success of biological invaders? Trends Ecol. Evol. 1999, 14, 135–139. [Google Scholar] [CrossRef]
- Pyšek, P.; Richardson, D.M. Invasive species, environmental change and management, and health. Annu. Rev. Environ. Resour. 2010, 35, 25–55. [Google Scholar] [CrossRef]
- Charles, H.; Dukes, J.S. Impacts of Invasive Species on Ecosystem Services. In Biological Invasions; Ecological Studies, Nentwig, W., Eds.; Springer: Berlin/Heidelberg, Germany, 2007; pp. 217–237. ISBN 978-3-540-36920-2. [Google Scholar] [Green Version]
- U.S. Fish and Wildlife Services. The Cost of Invasive Species. 2012. Available online: https://www.fws.gov/verobeach/pythonpdf/costofinvasivesfactsheet.pdf (accessed on 20 February 2019).
- Baly, A.; Toledo, M.E.; Lambert, I.; Benítez, E.; Rodriguez, K.; Rodriguez, E.; Vanlerberghe, V.; der Stuyft, P.V.; Baly, A.; Toledo, M.E.; et al. Cost of intensive routine control and incremental cost of insecticide-treated curtain deployment in a setting with low Aedes aegypti infestation. Rev. Soc. Bras. Med. Trop. 2016, 49, 418–424. [Google Scholar] [CrossRef]
- Oreska, M.P.J.; Aldridge, D.C. Estimating the financial costs of freshwater invasive species in Great Britain: A standardized approach to invasive species costing. Biol. Invasions 2011, 13, 305–319. [Google Scholar] [CrossRef]
- Grard, G.; Caron, M.; Mombo, I.M.; Nkoghe, D.; Mboui Ondo, S.; Jiolle, D.; Fontenille, D.; Paupy, C.; Leroy, E.M. Zika virus in Gabon (central Africa)—2007: A new threat from Aedes albopictus? PLoS Negl. Trop. Dis. 2014, 8, e2681. [Google Scholar] [CrossRef]
- Rainey, T.; Occi, J.L.; Robbins, R.G.; Egizi, A. Discovery of Haemaphysalis longicornis (Ixodida: Ixodidae) parasitizing a sheep in New Jersey, United States. J. Med. Entomol. 2018, 55, 757–759. [Google Scholar] [CrossRef]
- Robles, N.J.C.; Han, H.J.; Park, S.-J.; Choi, Y.K. Epidemiology of severe fever and thrombocytopenia syndrome virus infection and the need for therapeutics for the prevention. Clin. Exp. Vaccine Res. 2018, 7, 43–50. [Google Scholar] [CrossRef] [Green Version]
- Sanders, C.J.; Mellor, P.S.; Wilson, A.J. Invasive arthropods. Rev. Sci. Tech. 2010, 29, 273–286. [Google Scholar] [CrossRef]
- Simberloff, D. How much information on population biology is needed to manage introduced species? Conserv. Biol. 2003, 17, 83–92. [Google Scholar] [CrossRef]
- Malanson, G.P.; Walsh, S.J. A Geographical Approach to Optimization of Response to Invasive Species. In Science and Conservation in the Galapagos Islands: Frameworks & Perspectives; Social and Ecological Interactions in the Galapagos Islands, Walsh, S.J., Mena, C.F., Eds.; Springer: New York, NY, USA, 2013; pp. 199–215. ISBN 978-1-4614-5794-7. [Google Scholar]
- Carter, G.A.; Lucas, K.L.; Blossom, G.A.; Lassitter, C.L.; Holiday, D.M.; Mooneyhan, D.S.; Fastring, D.R.; Holcombe, T.R.; Griffith, J.A. Remote sensing and mapping of tamarisk along the Colorado River, USA: A comparative use of summer-acquired hyperion, Thematic Mapper and QuickBird Data. Remote Sens. 2009, 1, 318–329. [Google Scholar] [CrossRef]
- Rocchini, D.; Boyd, D.S.; Féret, J.-B.; Foody, G.M.; He, K.S.; Lausch, A.; Nagendra, H.; Wegmann, M.; Pettorelli, N. Satellite remote sensing to monitor species diversity: Potential and pitfalls. Remote Sens. Ecol. Conserv. 2015, 2, 25–36. [Google Scholar] [CrossRef]
- Eklundh, L.; Johansson, T.; Solberg, S. Mapping insect defoliation in Scots pine with MODIS time-series data. Remote Sens. Environ. 2009, 113, 1566–1573. [Google Scholar] [CrossRef]
- Jepsen, J.U.; Hagen, S.B.; Høgda, K.A.; Ims, R.A.; Karlsen, S.R.; Tømmervik, H.; Yoccoz, N.G. Monitoring the spatio-temporal dynamics of geometrid moth outbreaks in birch forest using MODIS-NDVI data. Remote Sens. Environ. 2009, 113, 1939–1947. [Google Scholar] [CrossRef]
- GrrlScientist. Massive Swarm of Ladybugs Detected by California Weather Radar. Forbes. 2019. Available online: https://www.forbes.com/sites/grrlscientist/2019/06/10/160-square-mile-swarm-of-ladybugs-detected-by-california-weather-radar/#54ac52054d64 (accessed on 7 August 2019).
- Nansen, C. The potential and prospects of proximal remote sensing of arthropod pests. Pest Manag. Sci. 2016, 72, 653–659. [Google Scholar] [CrossRef]
- Rey, B.; Aleixos, N.; Cubero, S.; Blasco, J. Xf-Rovim. A field robot to detect olive trees infected by Xylella fastidiosa using proximal sensing. Remote Sens. 2019, 11, 221. [Google Scholar] [CrossRef]
- Dunn, A.M.; Hatcher, M.J. Parasites and biological invasions: Parallels, interactions, and control. Trends Parasitol. 2015, 31, 189–199. [Google Scholar] [CrossRef]
- Elith, J.; Graham, C.H. Do they? How do they? WHY do they differ? On finding reasons for differing performances of species distribution models. Ecography 2009, 32, 66–77. [Google Scholar] [CrossRef]
- Shabani, F.; Kumar, L. Should species distribution models use only native or exotic records of existence or both? Ecol. Inform. 2015, 29, 57–65. [Google Scholar] [CrossRef]
- Gutierrez, A.P.; Ponti, L.; Dalton, D.T. Analysis of the invasiveness of spotted wing Drosophila (Drosophila suzukii) in North America, Europe, and the Mediterranean Basin. Biol. Invasions 2016, 18, 3647–3663. [Google Scholar] [CrossRef]
- Proestos, Y.; Christophides, G.K.; Ergüler, K.; Tanarhte, M.; Waldock, J.; Lelieveld, J. Present and future projections of habitat suitability of the Asian tiger mosquito, a vector of viral pathogens, from global climate simulation. Philos. Trans. R. Soc. B 2015, 370, 20130554. [Google Scholar] [CrossRef] [Green Version]
- Lobo, J.M.; Jiménez-Valverde, A.; Hortal, J. The uncertain nature of absences and their importance in species distribution modelling. Ecography 2010, 33, 103–114. [Google Scholar] [CrossRef]
- Cord, A.F.; Meentemeyer, R.K.; Leitão, P.J.; Václavík, T. Modelling species distributions with remote sensing data: Bridging disciplinary perspectives. J. Biogeogr. 2013, 40, 2226–2227. [Google Scholar] [CrossRef]
- Hay, S.I. An overview of remote sensing and geodesy for epidemiology and public health application. Adv. Parasitol. 2000, 47, 1–35. [Google Scholar] [Green Version]
- Kyba, C.C.M.; Garz, S.; Kuechly, H.; De Miguel, A.S.; Zamorano, J.; Fischer, J.; Hölker, F. High-resolution imagery of Earth at night: New sources, opportunities and challenges. Remote Sens. 2015, 7, 1–23. [Google Scholar] [CrossRef]
- Elvidge, C.; Baugh, K.; Hobson, V.; Kihn, E.; Kroehl, H.; Davis, E.; Cocero, D. Satellite inventory of human settlements using nocturnal radiation emissions: A contribution for the global toolchest. Glob. Chang. Biol. 1997, 3, 387–395. [Google Scholar] [CrossRef]
- Marcantonio, M.; Rizzoli, A.; Metz, M.; Rosà, R.; Marini, G.; Chadwick, E.; Neteler, M. Identifying the environmental conditions favouring West Nile virus outbreaks in Europe. PLoS ONE 2015, 10, e0121158. [Google Scholar] [CrossRef]
- Pareeth, S.; Salmaso, N.; Adrian, R.; Neteler, M. Homogenised daily lake surface water temperature data generated from multiple satellite sensors: A long-term case study of a large sub-Alpine lake. Sci. Rep. 2016, 6, 31251. [Google Scholar] [CrossRef] [Green Version]
- Metz, M.; Andreo, V.; Neteler, M. A new fully gap-free time series of land surface temperature from MODIS LST data. Remote Sens. 2017, 9, 1333. [Google Scholar] [CrossRef]
- European Environment Agency Copernicus Land Monitoring Service—Corine Land Cover. Available online: https://www.eea.europa.eu/data-and-maps/data/copernicus-land-monitoring-service-corine (accessed on 20 August 2019).
- Land Monitoring Service CORINE Land Cover—Copernicus Land Monitoring Service. Available online: https://land.copernicus.eu/pan-european/corine-land-cover (accessed on 20 August 2019).
- Yang, L.; Jin, S.; Danielson, P.; Homer, C.; Gass, L.; Bender, S.M.; Case, A.; Costello, C.; Dewitz, J.; Fry, J.; et al. A new generation of the United States National Land Cover Database: Requirements, research priorities, design, and implementation strategies. ISPRS J. Photogramm. Remote Sens. 2018, 146, 108–123. [Google Scholar] [CrossRef]
- Friedl, M.A.; Sulla-Menashe, D.; Tan, B.; Schneider, A.; Ramankutty, N.; Sibley, A.; Huang, X. MODIS Collection 5 global land cover: Algorithm refinements and characterization of new datasets. Remote Sens. Environ. 2010, 114, 168–182. [Google Scholar] [CrossRef]
- Fick, S.E.; Hijmans, R.J. WorldClim 2: New 1-km spatial resolution climate surfaces for global land areas. Int. J. Climatol. 2017, 37, 4302–4315. [Google Scholar] [CrossRef]
- Hijmans, R.J.; Cameron, S.E.; Parra, J.L.; Jones, P.G.; Jarvis, A. Very high resolution interpolated climate surfaces for global land areas. Int. J. Climatol. 2005, 25, 1965–1978. [Google Scholar] [CrossRef]
- Jarnevich, C.S.; Reynolds, L.V. Challenges of predicting the potential distribution of a slow-spreading invader: A habitat suitability map for an invasive riparian tree. Biol. Invasions 2011, 13, 153–163. [Google Scholar] [CrossRef]
- Kreakie, B.J.; Fan, Y.; Keitt, T.H. Enhanced migratory waterfowl distribution modeling by inclusion of depth to water table data. PLoS ONE 2012, 7, e30142. [Google Scholar] [CrossRef]
- Pascoe, E.L.; Marcantonio, M.; Caminade, C.; Foley, J.E. Modeling potential habitat for Amblyomma tick species in California. Insects 2019, 10, 201. [Google Scholar] [CrossRef]
- Cornes, R.C.; van der Schrier, G.; van den Besselaar, E.J.M.; Jones, P.D. An ensemble version of the E-OBS temperature and precipitation data sets. J. Geophys. Res. Atmos. 2018, 123, 9391–9409. [Google Scholar] [CrossRef]
- Bedia, J.; Herrera, S.; Gutiérrez, J.M. Dangers of using global bioclimatic datasets for ecological niche modeling. Limitations for future climate projections. Glob. Planet. Chang. 2013, 107, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Hofstra, N.; Haylock, M.; New, M.; Jones, P.; Frei, C. Comparison of six methods for the interpolation of daily, European climate data. J. Geophys. Res. Atmos. 2008, 113, D21. [Google Scholar] [CrossRef]
- Center for Diseases Control and Prevention (CDC). ESTIMATED Potential Range of Aedes aegypti and Aedes albopictus in the United States, 2017; CDC: Atlanta, GA, USA, 2018.
- Rose, R.I. Pesticides and public health: Integrated methods of mosquito management. Emerg. Infect. Dis. 2001, 7, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Born, W.; Rauschmayer, F.; Bräuer, I. Economic evaluation of biological invasions—A survey. Ecol. Econ. 2005, 55, 321–336. [Google Scholar] [CrossRef]
- Paaijmans, K.P.; Imbahale, S.S.; Thomas, M.B.; Takken, W. Relevant microclimate for determining the development rate of malaria mosquitoes and possible implications of climate change. Malar. J. 2010, 9, 196. [Google Scholar] [CrossRef] [PubMed]
- Schulze, T.L.; Jordan, R.A.; Hung, R.W. Effects of microscale habitat physiognomy on the focal distribution of Ixodes scapularis and Amblyomma americanum (Acari: Ixodidae) nymphs. Environ. Entomol. 2002, 31, 1085–1090. [Google Scholar] [CrossRef]
- Beck-Johnson, L.M.; Nelson, W.A.; Paaijmans, K.P.; Read, A.F.; Thomas, M.B.; Bjørnstad, O.N. The importance of temperature fluctuations in understanding mosquito population dynamics and malaria risk. R. Soc. Open Sci. 2017, 4, 160969. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gray, J.S.; Estrada-Peña, A.; Vial, L. Ecology of nidicolous ticks. In Biology of Ticks; Oxford University Press: Oxford, UK, 2014; Volume 2, pp. 39–60. [Google Scholar]
- Burks, C.S.; Stewart, R.L.; Needham, G.R.; Lee, R.E. The role of direct chilling injury and inoculative freezing in cold tolerance of Amblyomma americanum, Dermacentor variabilis and Ixodes scapularis. Physiol. Entomol. 1996, 21, 44–50. [Google Scholar] [CrossRef]
- Yoder, J.A.; Tank, J.L. Similarity in critical transition temperature for ticks adapted for different environments: Studies on the water balance of unfed ixodid larvae. Int. J. Acarol. 2006, 32, 323–329. [Google Scholar] [CrossRef]
- Allingham, P.G. Effect of temperature on late immature stages of Culicoides brevitarsis (Diptera: Ceratopogonidae). J. Med. Entomol. 1991, 28, 878–881. [Google Scholar] [CrossRef] [PubMed]
- Zellweger, F.; De Frenne, P.; Lenoir, J.; Rocchini, D.; Coomes, D. Advances in microclimate ecology arising from remote sensing. Trends Ecol. Evol. 2019, 34, 327–341. [Google Scholar] [CrossRef] [PubMed]
- Chabot-Couture, G.; Nigmatulina, K.; Eckhoff, P. An environmental data set for vector-borne disease modeling and epidemiology. PLoS ONE 2014, 9, e94741. [Google Scholar] [CrossRef] [PubMed]
- Vancutsem, C.; Ceccato, P.; Dinku, T.; Connor, S.J. Evaluation of MODIS land surface temperature data to estimate air temperature in different ecosystems over Africa. Remote Sens. Environ. 2010, 114, 449–465. [Google Scholar] [CrossRef]
- Vallorani, R.; Angelini, P.; Bellini, R.; Carrieri, M.; Crisci, A.; Zeo, S.M.; Messeri, G.; Venturelli, C. Temperature characterization of different urban microhabitats of Aedes albopictus (Diptera Culicidae) in Central–Northern Italy. Environ. Entomol. 2015, 44, 1182–1192. [Google Scholar] [CrossRef]
- Becker, N. Mosquitoes and Their Control; Springer: Berlin/Heidelberg, Germany; London, UK, 2010; ISBN 978-3-540-92874-4. [Google Scholar]
- Marcantonio, M. Environmental Modelling and Spatial Ecology with Focus on Invasive Aedes Mosquitoes and Emergent Mosquito-Borne Pathogens. Ph.D. Thesis, Technische Universität Berlin, Berlin, Germany, 2017. [Google Scholar]
- Yee, D.A. Thirty years of Aedes albopictus (Diptera: Culicidae) in America: An introduction to current perspectives and future challenges. J. Med. Entomol. 2016, 53, 989–991. [Google Scholar] [CrossRef]
- Hoogstraal, H. A Preliminary, Annotated list of ticks (Ixodoidea) of the Anglo-Egyptian Sudan. J. Parasitol. 1954, 40, 304. [Google Scholar] [CrossRef]
- Vial, L. Biological and ecological characteristics of soft ticks (Ixodida: Argasidae) and their impact for predicting tick and associated disease distribution. Parasite 2009, 16, 191–202. [Google Scholar] [CrossRef] [PubMed]
- Nagendra, H.; Rocchini, D. High resolution satellite imagery for tropical biodiversity studies: The devil is in the detail. Biodivers. Conserv. 2008, 17, 3431. [Google Scholar] [CrossRef]
- McFeeters, S.K. Using the normalized difference water index (NDWI) within a geographic information system to detect swimming pools for mosquito abatement: A practical approach. Remote Sens. 2013, 5, 3544–3561. [Google Scholar] [CrossRef]
- O’Connor, B.; Secades, C.; Penner, J.; Sonnenschein, R.; Skidmore, A.; Burgess, N.D.; Hutton, J.M. Earth observation as a tool for tracking progress towards the Aichi Biodiversity Targets. Remote Sens. Ecol. Conserv. 2015, 1, 19–28. [Google Scholar] [CrossRef]
- Schmeller, D.S.; Weatherdon, L.V.; Loyau, A.; Bondeau, A.; Brotons, L.; Brummitt, N.; Geijzendorffer, I.R.; Haase, P.; Kuemmerlen, M.; Martin, C.S.; et al. A suite of essential biodiversity variables for detecting critical biodiversity change. Biol. Rev. 2018, 93, 55–71. [Google Scholar] [CrossRef] [PubMed]
- Vihervaara, P.; Auvinen, A.-P.; Mononen, L.; Törmä, M.; Ahlroth, P.; Anttila, S.; Böttcher, K.; Forsius, M.; Heino, J.; Heliölä, J.; et al. How Essential Biodiversity Variables and remote sensing can help national biodiversity monitoring. Glob. Ecol. Conserv. 2017, 10, 43–59. [Google Scholar] [CrossRef]
- Jetz, W.; McGeoch, M.A.; Guralnick, R.; Ferrier, S.; Beck, J.; Costello, M.J.; Fernandez, M.; Geller, G.N.; Keil, P.; Merow, C.; et al. Essential biodiversity variables for mapping and monitoring species populations. Nat. Ecol. Evol. 2019, 3, 539. [Google Scholar] [CrossRef] [PubMed]
- Bridge, T.; Beaman, R.; Done, T.; Webster, J. Predicting the location and spatial extent of submerged coral reef habitat in the Great Barrier Reef World Heritage Area, Australia. PLoS ONE 2012, 7, e48203. [Google Scholar] [CrossRef] [PubMed]
- Jueterbock, A.; Tyberghein, L.; Verbruggen, H.; Coyer, J.A.; Olsen, J.L.; Hoarau, G. Climate change impact on seaweed meadow distribution in the North Atlantic rocky intertidal. Ecol. Evol. 2013, 3, 1356–1373. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quillfeldt, P.; Masello, J.F.; Navarro, J.; Phillips, R.A. Year-round distribution suggests spatial segregation of two small petrel species in the South Atlantic. J. Biogeogr. 2013, 40, 430–441. [Google Scholar] [CrossRef]
- Tyberghein, L.; Verbruggen, H.; Pauly, K.; Troupin, C.; Mineur, F.; De Clerck, O. Bio-ORACLE: A global environmental dataset for marine species distribution modelling: Bio-ORACLE marine environmental data rasters. Glob. Ecol. Biogeogr. 2012, 21, 272–281. [Google Scholar] [CrossRef]
- Guden, R.M.E.; Vafeiadou, A.M.; De Meester, N.; Derycke, S.; Moens, T. Relative Abundance Data of 4 Cryptic Lineages of the Nematode Litoditis Marina in a Saltmarsh Habitat in the Western-Scheldt Estuary; The Flanders Marine Institute: Integrated Marine Information System: London, UK, 2018. [Google Scholar]
- Díaz-Delgado, R.; Ónodi, G.; Kröel-Dulay, G.; Kertész, M. Enhancement of ecological field experimental research by means of UAV multispectral sensing. Drones 2019, 3, 7. [Google Scholar] [CrossRef]
- Harvey, M.C.; Rowland, J.V.; Luketina, K.M. Drone with thermal infrared camera provides high resolution georeferenced imagery of the Waikite geothermal area, New Zealand. J. Volcanol. Geotherm. Res. 2016, 325, 61–69. [Google Scholar] [CrossRef]
- Afán, I.; Máñez, M.; Díaz-Delgado, R. Drone monitoring of breeding waterbird populations: The case of the Glossy ibis. Drones 2018, 2, 42. [Google Scholar] [CrossRef]
- Bonnin, N.; Van Andel, A.C.; Kerby, J.T.; Piel, A.K.; Pintea, L.; Wich, S.A. Assessment of chimpanzee nest detectability in drone-acquired images. Drones 2018, 2, 17. [Google Scholar] [CrossRef]
- Wilkening, J.L.; Ray, C.; Beever, E.A.; Brussard, P.F. Modeling contemporary range retraction in Great Basin pikas (Ochotona princeps) using data on microclimate and microhabitat. Quat. Int. 2011, 235, 77–88. [Google Scholar] [CrossRef]
- Beever, E.A.; Ray, C.; Mote, P.W.; Wilkening, J.L. Testing alternative models of climate-mediated extirpations. Ecol. Appl. 2010, 20, 164–178. [Google Scholar] [CrossRef]
- Bryant, S.R.; Thomas, C.D.; Bale, J.S. The influence of thermal ecology on the distribution of three nymphalid butterflies. J. Appl. Ecol. 2002, 39, 43–55. [Google Scholar] [CrossRef]
- Muller, C.L.; Chapman, L.; Johnston, S.; Kidd, C.; Illingworth, S.; Foody, G.; Overeem, A.; Leigh, R.R. Crowdsourcing for climate and atmospheric sciences: Current status and future potential. Int. J. Climatol. 2015, 35, 3185–3203. [Google Scholar] [CrossRef]
- Thomas, S.M.; Obermayr, U.; Fischer, D.; Kreyling, J.; Beierkuhnlein, C. Low-temperature threshold for egg survival of a post-diapause and non-diapause European aedine strain, Aedes albopictus (Diptera: Culicidae). Parasites Vectors 2012, 5, 100. [Google Scholar] [CrossRef] [PubMed]
- Watt, J.H.; van den Berg, S. Chapter 15. Semi-Controlled Environments: Field Research. In Research Methods for Communication Science; Allyn & Bacon: Boston, MA, USA, 2002; pp. 227–241. [Google Scholar]
- Asare, E.O.; Tompkins, A.M.; Amekudzi, L.K.; Ermert, V. A breeding site model for regional, dynamical malaria simulations evaluated using in situ temporary ponds observations. Geospat. Health 2016, 11, 390. [Google Scholar] [CrossRef] [PubMed]
| Mission/Sensor | Type of Sensing | Environmental Variables for IAVPs | Spatial Resolution | Temporal Grain and Extent | Extent |
| NASA-MODIS | Multi-satellite | NDVI, NDWI, LST, Land cover | 0.25–1 km | 4-times/day [2001–present] | Global |
| NASA-USGS Landsat series | Multi-satellite | NDVI, NDWI, imagery | 30 m | 16 d [1972–present] | Global |
| ESA SENTINEL missions | Multi-satellite | NDVI, NDWI, LST, imagery | 10–300 m | 3–10 d [2015–present] | Global |
| NOAA VIIRS | Multi-satellite | NDVI, NDWI, LST, imagery, human settlements | 375–750 m | 1 d–monthly [2015–present] | Global |
| Global Precipitation Measurement Mission (GPMM) | Multi-satellite | Precipitation | 11 km* | 2–3 h [2015–present] | Global (65S-65N) |
| Tropical Rainfall Measuring Mission (TRMM) | Multi-satellite | Rainfall | 28 km* | 3 h–7 d [1998–2015) | Tropical and sub-tropical regions |
| USDA-NAIP | Airborne | NDVI, imagery | 60 cm– 1 m | “Snapshot” every 3 years [2003–present] | Mainland USA (variable coverage) |
| Dataset Name | Ancillary Data | Environmental Variables for IAVPs | Spatial Resolution | Temporal Grain and Extent | Extent |
| WordClim | Weather station | 2 m air temperature and precipitation | 1 km* | LTA 1950–2000 | Global |
| MODIS Land Cover Type/Dynamics | Satellite | Land cover | 0.5–1 km | Yearly/twice a year [2001–present) | Global |
| Copernicus Land Cover | Multi-satellite (SPOT, PROBA-V, Sentinel-2) | Land cover | 100 m | Multi-year [2015–present) | Global |
| USGS Land Cover maps | Satellite (Landsat) and geospatial ancillary datasets | Land cover/impervious surface | 30 m | Multi-year [2001–present] | Continental US |
| CORINE Land Cover maps | Multi-satellite (Landsat, SPOT, IRS, RapidEye, Sentinel-2) | Land cover | 100 m | Multi-year [1990–present] | Extended EU |
| PRISM Climate data | Weather station | Air temperature, precipitation, vapor pressure, day length | 0.8–4 km | Daily [1895–**) | Continental US |
| Daily Surface weather and climatological summaries (DAYMET) | Weather station | Air temperature, precipitation, vapor pressure, day length | 1 km | Daily [1980–present calendar year] | North America, Puerto Rico and Hawaii |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pascoe, E.L.; Pareeth, S.; Rocchini, D.; Marcantonio, M. A Lack of “Environmental Earth Data” at the Microhabitat Scale Impacts Efforts to Control Invasive Arthropods That Vector Pathogens. Data 2019, 4, 133. https://doi.org/10.3390/data4040133
Pascoe EL, Pareeth S, Rocchini D, Marcantonio M. A Lack of “Environmental Earth Data” at the Microhabitat Scale Impacts Efforts to Control Invasive Arthropods That Vector Pathogens. Data. 2019; 4(4):133. https://doi.org/10.3390/data4040133
Chicago/Turabian StylePascoe, Emily L., Sajid Pareeth, Duccio Rocchini, and Matteo Marcantonio. 2019. "A Lack of “Environmental Earth Data” at the Microhabitat Scale Impacts Efforts to Control Invasive Arthropods That Vector Pathogens" Data 4, no. 4: 133. https://doi.org/10.3390/data4040133
APA StylePascoe, E. L., Pareeth, S., Rocchini, D., & Marcantonio, M. (2019). A Lack of “Environmental Earth Data” at the Microhabitat Scale Impacts Efforts to Control Invasive Arthropods That Vector Pathogens. Data, 4(4), 133. https://doi.org/10.3390/data4040133







