Innovative “Soft” Maceration Techniques in Red Grape Fermentation
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials, Fermentation, and Maceration Protocol
2.2. Chemical Analysis
2.3. Statistical Analysis
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hussain, M.; Cholette, S.; Castaldi, R.M. An Analysis of Globalization Forces in the Wine Industry. J. Glob. Mark. 2008, 21, 33–47. [Google Scholar] [CrossRef]
- Koes, R.E.; Quattrocchio, F.; Mol, J.N.M. The flavonoid biosynthetic pathway in plants: Function and evolution. BioEssays 1994, 16, 123–132. [Google Scholar] [CrossRef]
- Quattrocchio, F.; Lepiniec, L.; Grotewold, E. The Science of Flavonoids; Springer: Berlin/Heidelberg, Germany, 2006. [Google Scholar] [CrossRef]
- Şener, H. Effect of Temperature and Duration of Maceration on Colour and Sensory Properties of Red Wine: A Review. S. Afr. J. Enol. Vitic. 2018, 39, 227–234. [Google Scholar] [CrossRef]
- Aleixandre-Tudo, J.L.; Buica, A.; Nieuwoudt, H.; Aleixandre, J.L.; Du Toit, W. Spectrophotometric Analysis of Phenolic Compounds in Grapes and Wines. J. Agric. Food Chem. 2017, 65, 4009–4026. [Google Scholar] [CrossRef] [PubMed]
- Casassa, L.F.; Harbertson, J.F. Extraction, evolution, and sensory impact of phenolic compounds during red wine maceration. Annu. Rev. Food Sci. Technol. 2014, 5, 83–109. [Google Scholar] [CrossRef]
- Burns, J.; Gardner, P.T.; Matthews, D.; Duthie, G.G.; Lean, M.E.J.; Crozier, A. Extraction of Phenolics and Changes in Antioxidant Activity of Red Wines during Vinification. J. Agric. Food Chem. 2001, 49, 5797–5808. [Google Scholar] [CrossRef] [PubMed]
- Renouf, V.; Perello, M.C.; Strehaiano, P.; Lonvaud-Funel, A. Global survey of the microbial ecosystem during alcoholic fermentation in winemaking. J. Int. Des Sci. De La Vigne Et Du Vin 2006, 40, 101–116. [Google Scholar] [CrossRef]
- Mazza, G.F.; Francis, J. Anthocyanins in Grapes and Grape Products. Crit. Rev. Food Sci. Nutr. 1995, 35, 341–371. [Google Scholar] [CrossRef]
- Bosso, A.; Guaita, M.; Panero, L.; Borsa, D.; Follis, R. Influence of Two Winemaking Techniques on Polyphenolic Composition and Color of Wines. Am. J. Enol. Vitic. 2009, 60, 379–385. [Google Scholar]
- Canals, R.; Llaudy, M.C.; Valls, J.; Canals, J.M.; Zamora, F. Influence of ethanol concentration on the extraction of color and phenolic compounds from the skin and seeds of tempranillo grapes at different stages of ripening. J. Agric. Food Chem. 2005, 53, 4019–4025. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, A.; Santini, G.; Piombino, P.; Pittari, E.; Sanmartin, C.; Moio, L.; Modesti, M.; Bellincontro, A.; Mencarelli, F. Nitrogen maceration of wine grape: An alternative and sustainable technique to carbonic maceration. Food Chem. 2022, 134138. [Google Scholar] [CrossRef]
- Yacco, R.S.; Watrelot, A.A.; Kennedy, J.A. Red Wine Tannin Structure-Activity Relationships during Fermentation and Maceration. J. Agric. Food Chem. 2016, 64, 860–869. [Google Scholar] [CrossRef] [PubMed]
- Sacchi, K.L.; Bisson, L.F.; Adams, D.O. A Review of the Effect of Winemaking Techniques on Phenolic Extraction in Red Wines. Am. J. Enol. Vitic. 2005, 56, 197–206. [Google Scholar]
- He, F.; Liang, N.N.; Mu, L.; Pan, Q.H.; Wang, J.; Reeves, M.J.; Duan, C.Q. Anthocyanins and their variation in red wines II. Anthocyanin derived pigments and their color evolution. Molecules 2012, 17, 1483–1519. [Google Scholar] [CrossRef]
- Smith, P.A.; Mcrae, J.M.; Bindon, K.A. Impact of winemaking practices on the concentration and composition of tannins in red wine. Aust. J. Grape Wine Res. 2015, 21, 601–614. [Google Scholar] [CrossRef]
- Busse-Valverde, N.; Bautista-Ortín, A.B.; Gómez-Plaza, E.; Fernández-Fernández, J.I.; Gil-Muñoz, R. Influence of skin maceration time on the proanthocyanidin content of red wines. Eur. Food Res. Technol. 2012, 235, 1117–1123. [Google Scholar] [CrossRef]
- Renard, C.M.G.C.; Watrelot, A.A.; Le Bourvellec, C. Interactions between polyphenols and polysaccharides: Mechanisms and consequences in food processing and digestion. Trends Food Sci. Technol. 2017, 60, 43–51. [Google Scholar] [CrossRef]
- Watrelot, A.A.; Le Bourvellec, C.; Imberty, A.; Renard, C.M.G.C. Interactions between pectic compounds and procyanidins are influenced by methylation degree and chain length. Biomacromolecules 2013, 14, 709–718. [Google Scholar] [CrossRef] [PubMed]
- Bautista-Ortín, A.B.; Abdallah, R.B.; Castro-López, L.D.R.; Jiménez-Martínez, M.D.; Gómez-Plaza, E. Technological implications of modifying the extent of cell wall-proanthocyanidin interactions using enzymes. Int. J. Mol. Sci. 2016, 17, 123. [Google Scholar] [CrossRef]
- Castro-López, L.D.R.; Gómez-Plaza, E.; Ortega-Regules, A.; Lozada, D.; Bautista-Ortín, A.B. Role of cell wall deconstructing enzymes in the proanthocyanidin-cell wall adsorption-desorption phenomena. Food Chem. 2016, 196, 526–532. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Zietsman, A.J.J.; Vivier, M.A.; Moore, J.P. Deconstructing wine grape cell walls with enzymes during winemaking: New insights from glycan microarray technology. Molecules 2019, 24, 165. [Google Scholar] [CrossRef] [PubMed]
- Coletta, C.; Botondi, R.; Forniti, R.; Baccelloni, S.; Bellincontro, A.; Mencarelli, F. Alternating temperature in postharvest cooling treatment of ‘Fiano’ and ‘Falanghina’ grapes affects cell wall enzyme rate, berry softening and polyphenols. J. Sci. Food Agric. 2019, 99, 3142–3148. [Google Scholar] [CrossRef] [PubMed]
- Nel, A.P. Tannins and anthocyanins: From their origin to wine analysis—A review. S. Afr. J. Enol. Vitic. 2018, 39, 1–20. [Google Scholar] [CrossRef]
- Bai, B.; He, F.; Chen, F.; Reeves, M.J.; Li, J. Comparative study of phenolic compounds in Cabernet Sauvignon wines made in traditional and Ganimede fermenters. Food Chem. 2013, 141, 3984–3992. [Google Scholar] [CrossRef] [PubMed]
- Baiano, A.; Scrocco, C.; Sepielli, G.; Del Nobile, M.A. Wine Processing: A Critical Review of Physical, Chemical, and Sensory Implications of Innovative Vinification Procedures. Crit. Rev. Food Sci. Nutr. 2016, 56, 2391–2407. [Google Scholar] [CrossRef]
- Bianchi, A.; Taglieri, I.; Venturi, F.; Sanmartin, C.; Ferroni, G.; Macaluso, M.; Palla, F.; Flamini, G.; Zinnai, A. Technological Improvements on FML in the Chianti Classico Wine Production: Co-Inoculation or Sequential Inoculation ? Foods 2022, 11, 1011. [Google Scholar] [CrossRef]
- Glories, Y. La couleur des vins rouges. Mesure, origine et interprétation. Connaiss. De La Vigne Et Du Vin 1984, 18, 253–271. [Google Scholar] [CrossRef]
- De Santis, D.; Bellincontro, A.; Forniti, R.; Botondi, R. Time of postharvest ethylene treatments affects phenols, anthocyanins, and volatile compounds of cesanese red wine grape. Foods 2021, 10, 322. [Google Scholar] [CrossRef]
- Lerno, L.; Reichwager, M.; Ponangi, R.; Hearne, L.; Block, D.E.; Oberholster, A. Effects of cap and overall fermentation temperature on phenolic extraction in cabernet sauvignon fermentations. Am. J. Enol. Vitic. 2015, 66, 444–453. [Google Scholar] [CrossRef]
- Lerno, L.A.; Panprivech, S.; Ponangi, R.; Hearne, L.; Blair, T.; Oberholster, A.; Block, D.E. Effect of pump-over conditions on the extraction of phenolic compounds during cabernet sauvignon fermentation. Am. J. Enol. Vitic. 2018, 69, 295–301. [Google Scholar] [CrossRef]
- Quirós, M.; Rojas, V.; Gonzalez, R.; Morales, P. Selection of non-Saccharomyces yeast strains for reducing alcohol levels in wine by sugar respiration. Int. J. Food Microbiol. 2014, 181, 85–91. [Google Scholar] [CrossRef]
- Varela, C.; Cuijvers, K.; Van Den Heuvel, S.; Rullo, M.; Solomon, M.; Borneman, A.; Schmidt, S. Effect of aeration on yeast community structure and volatile composition in uninoculated chardonnay wines. Fermentation 2021, 7, 97. [Google Scholar] [CrossRef]
- Contreras, A.; Hidalgo, C.; Schmidt, S.; Henschke, P.A.; Curtin, C.; Varela, C. The application of non-Saccharomyces yeast in fermentations with limited aeration as a strategy for the production of wine with reduced alcohol content. Int. J. Food Microbiol. 2015, 205, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Ichikawa, M.; Ono, K.; Hisamoto, M.; Matsudo, T.; Okuda, T. Effect of cap management technique on the concentration of proanthocyanidins in Muscat bailey a wine. Food Sci. Technol. Res. 2012, 18, 201–207. [Google Scholar] [CrossRef]
- Bosso, A.; Panero, L.; Petrozziello, M.; Follis, R.; Motta, S.; Guaita, M. Influence of submerged-cap vinification on polyphenolic composition and volatile compounds of barbera wines. Am. J. Enol. Vitic. 2011, 62, 503–511. [Google Scholar] [CrossRef]
- Pérez-Serradilla, J.A.; de Castro, M.D.L. Role of lees in wine production: A review. Food Chem. 2008, 111, 447–456. [Google Scholar] [CrossRef]
- Medina-Plaza, C.; Beaver, J.W.; Lerno, L.; Dokoozlian, N.; Ponangi, R.; Blair, T.; Block, D.E.; Oberholster, A. Impact of temperature, ethanol and cell wall material composition on cell wall-anthocyanin interactions. Molecules 2019, 24, 8–11. [Google Scholar] [CrossRef]
- Merrell, C.P.; Larsen, R.C.; Harbertson, J.F. Effects of berry maturity and wine alcohol on phenolic content during winemaking and aging. Am. J. Enol. Vitic. 2017, 69, 1–11. [Google Scholar] [CrossRef]
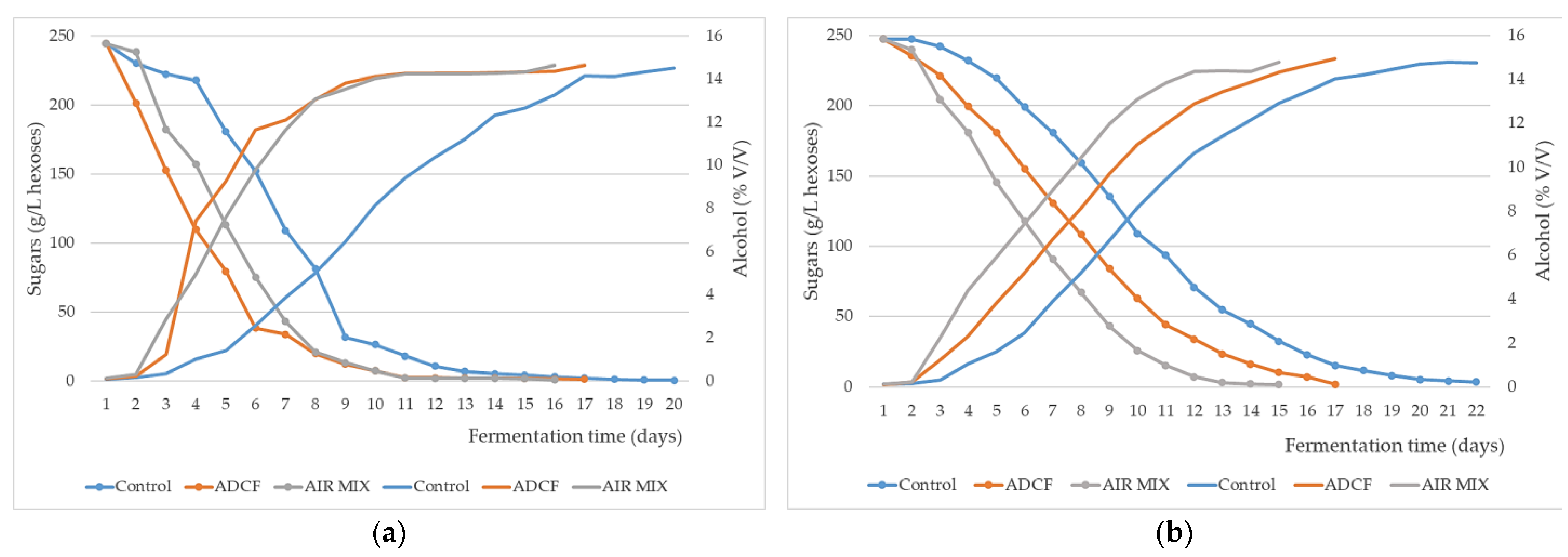
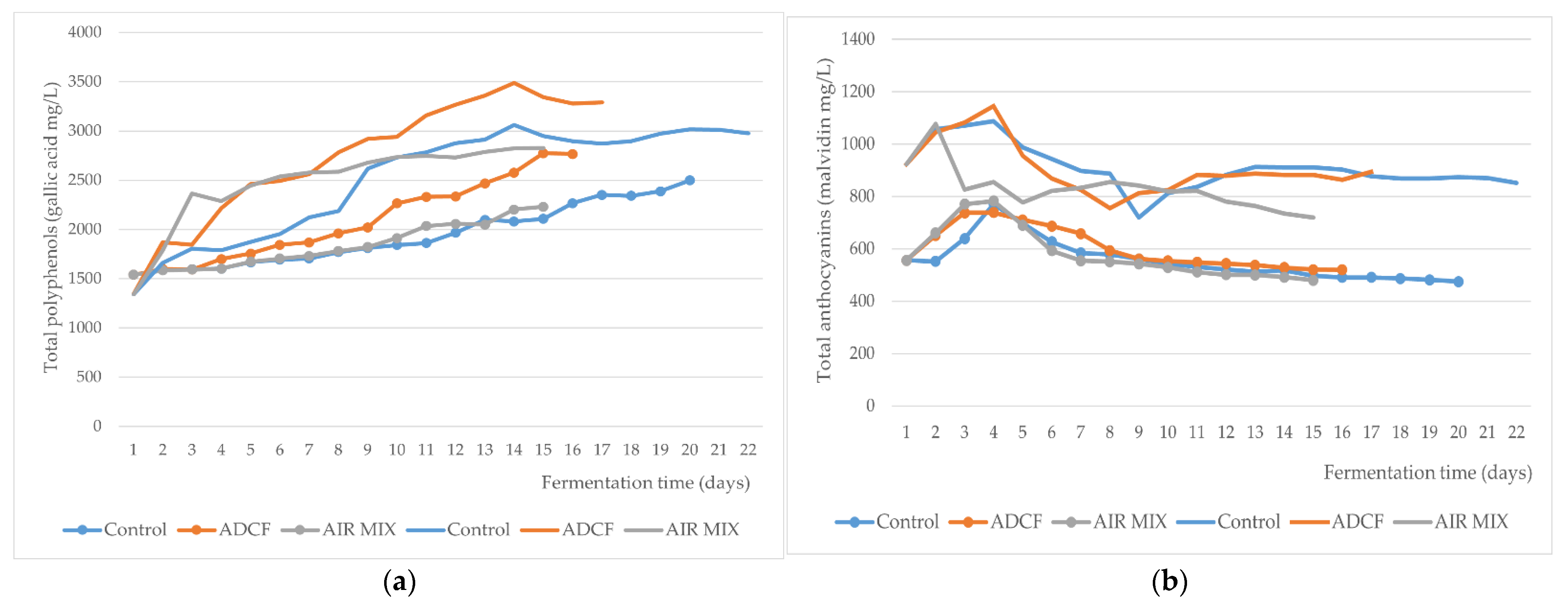
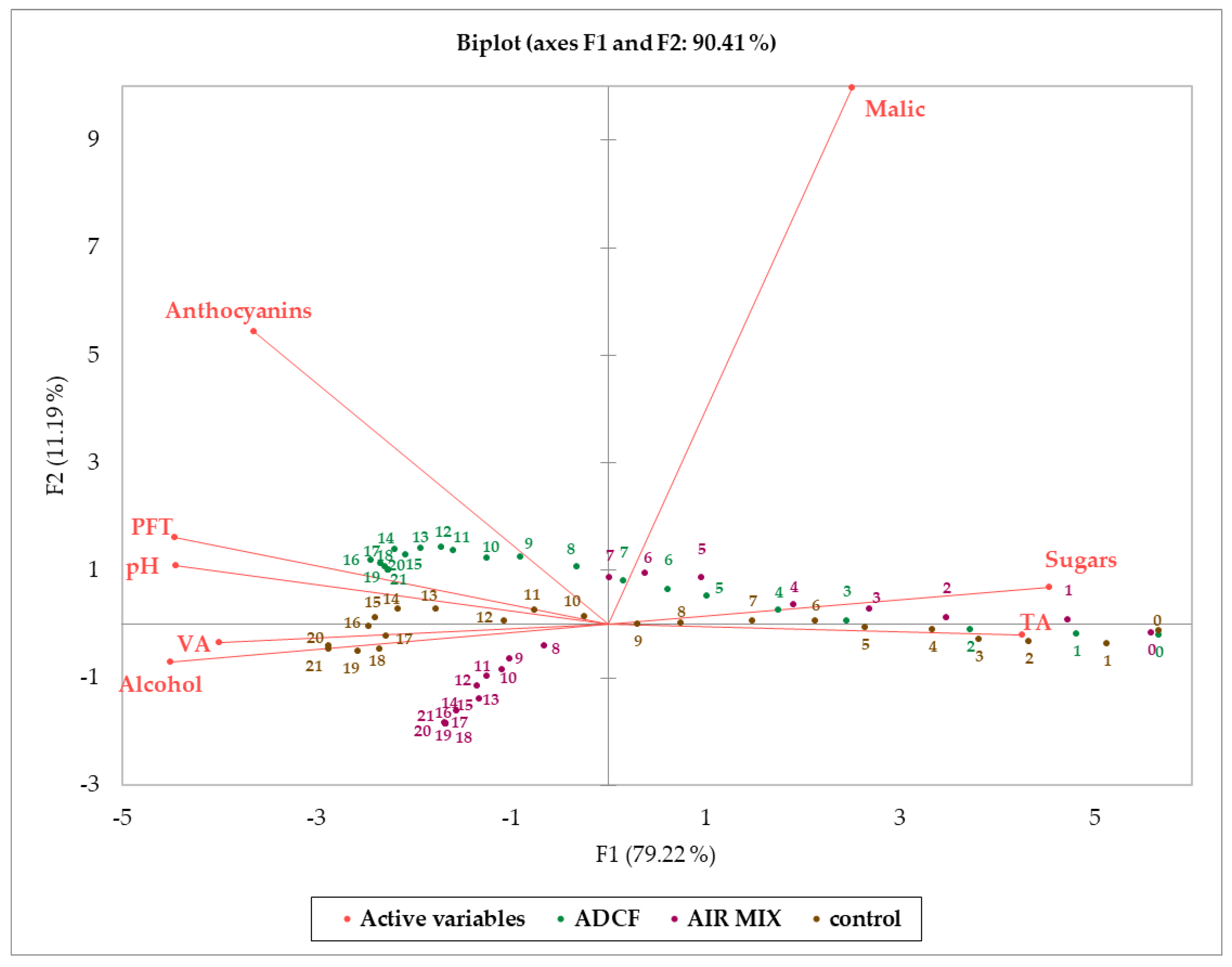
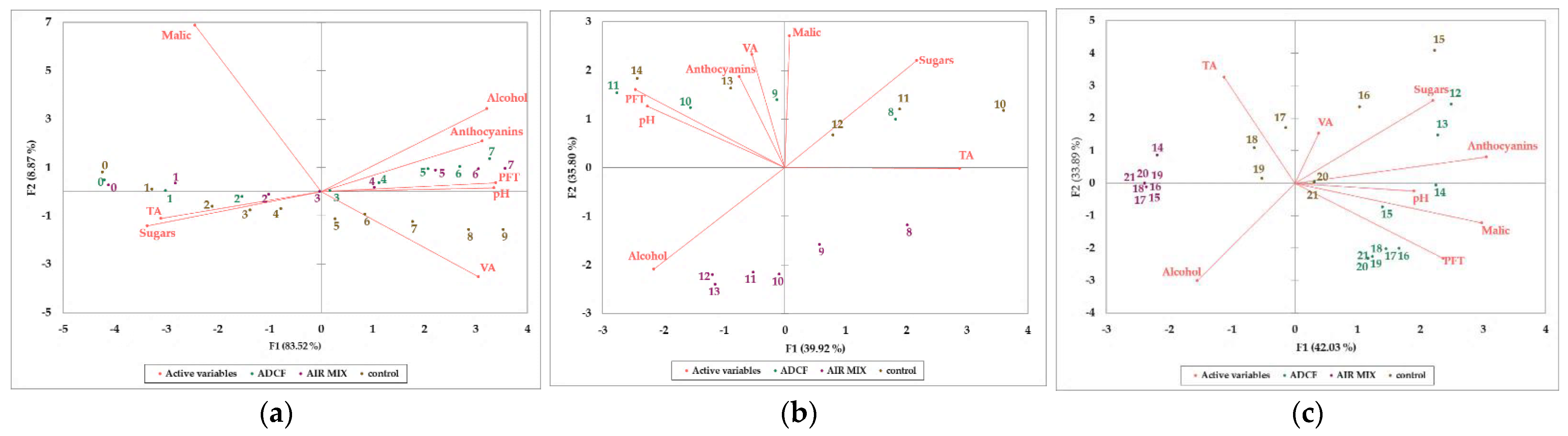
| Parameters | Unit | Grape at Harvest 2020 | Control | ADCF | AIR MIX |
|---|---|---|---|---|---|
| Alcohol | % V/V | n.d. | 14.77 ± 0.23 a | 14.64 ± 0.14 a | 14.62 ± 0.17 a |
| Sugars | g/L hexoses | 244.5 ± 4.1 | 0.35 ± 0.13 b | 1.03 ± 0.11 a | 0.78 ± 0.19 a |
| pH | 3.47 ± 0.09 | 3.84 ± 0.05 a | 3.79 ± 0.03 a | 3.81 ± 0.05 a | |
| Titratable acidity | g/L tartaric acid | 6.14 ± 0.17 | 5.72 ± 0.07 a | 5.36 ± 0.05 b | 5.31 ± 0.07 b |
| Volatile acidity | g/L acetic acid | n.d. | 0.51 ± 0.02 a | 0.27 ± 0.01 c | 0.33 ± 0.02 b |
| Malic acid | g/L | 1.34 ± 0.11 | 0.92 ± 0.06 b | 1.20 ± 0.09 a | 0.90 ± 0.06 b |
| Lactic acid | g/L | n.d. | 0.25 ± 0.09 a | 0.18 ± 0.05 a | 0.24 ± 0.07 a |
| Tartaric acid | g/L | 4.71 ± 0.19 | 3.63 ± 0.13 a | 3.47 ± 0.21 a | 3.51 ± 0.18 a |
| Citric acid | g/L | 0.17 ± 0.06 | 0.14 ± 0.03 a | 0.13 ± 0.02 a | 0.16 ± 0.02 a |
| Total extract | g/L | 271.3 ± 5.1 | 35.9 ± 1.9 a | 33.3 ± 2.4 ab | 31.0 ± 1.7 b |
| Ash | g/L | 2.02 ± 0.11 | 1.71 ± 0.05 a | 1.82 ± 0.08 a | 1.82 ± 0.04 a |
| YAN | mg/L | 202 ± 9 | 63 ± 2 c | 91 ± 3 a | 85 ± 2 b |
| Total anthocyanins | mg/L malvidin | 745 ± 23 | 475 ± 16 b | 520 ± 15 a | 480 ± 19 b |
| Total polyphenols | mg/L gallic acid | 3140 ± 123 | 2388 ± 103 b | 2773 ± 92 a | 2300 ± 128 b |
| Color intensity | n.m. | 0.77 ± 0.09 b | 0.99 ± 0.05 a | 0.81 ± 0.07 b | |
| Tonality | n.m. | 0.82 ± 0.07 b | 0.98 ± 0.06 a | 0.88 ± 0.04 b |
| Parameters | Unit | Grape at Harvest 2021 | Control | ADCF | AIR MIX |
|---|---|---|---|---|---|
| Alcohol | % V/V | n.d. | 14.76 ± 0.21 a | 14.90 ± 0.24 a | 14.78 ± 0.26 a |
| Sugars | g/L hexoses | 247.5 ± 3.2 | 3.73 ± 0.14 a | 2.05 ± 0.11 b | 1.80 ± 0.19 b |
| pH | 3.38 ± 0.07 | 3.55 ± 0.07 b | 3.68 ± 0.03 a | 3.64 ± 0.05 a | |
| Titratable acidity | g/L tartaric acid | 7.10 ± 0.23 | 7.02 ± 0.09 a | 6.42 ± 0.05 b | 6.49 ± 0.07 b |
| Volatile acidity | g/L acetic acid | n.d. | 0.31 ± 0.03 a | 0.19 ± 0.01 c | 0.24 ± 0.02 b |
| Malic acid | g/L | 1.87 ± 0.13 | 0.95 ± 0.02 b | 1.14 ± 0.09 a | 1.20 ± 0.06 a |
| Lactic acid | g/L | n.d. | 0.21 ± 0.05 a | 0.19 ± 0.05 a | 0.12 ± 0.07 a |
| Tartaric acid | g/L | 5.82 ± 0.23 | 5.63 ± 0.06 a | 5.47 ± 0.02 b | 5.51 ± 0.02 b |
| Citric acid | g/L | 0.22 ± 0.08 | 0.20 ± 0.06 a | 0.23 ± 0.02 a | 0.21 ± 0.02 a |
| Total extract | g/L | 279.4 ± 3.2 | 35.3 ± 2.8 a | 35.2 ± 1.7 a | 33.2 ± 2.9 a |
| Ash | g/L | 2.25 ± 0.08 | 2.11 ± 0.04 a | 2.02 ± 0.08 ab | 1.97 ± 0.04 b |
| YAN | mg/L | 253 ± 5 | 73 ± 5 b | 87 ± 3 a | 80 ± 6 ab |
| Total anthocyanins | mg/L malvidin | 1083 ± 15 | 851 ± 31 a | 894 ± 22 a | 770 ± 27 b |
| Total polyphenols | mg/L gallic acid | 3540 ± 42 | 2977 ± 106 b | 3291 ± 132 a | 2918 ± 90 b |
| Color intensity | n.m. | 1.02 ± 0.03 b | 1.24 ± 0.04 a | 0.91 ± 0.05 c | |
| Tonality | n.m. | 0.93 ± 0.03 b | 1.07 ± 0.07 a | 0.90 ± 0.08 b |
| 2020 | 2021 | |||||
|---|---|---|---|---|---|---|
| Compound | Control | ADCF | AIR MIX | Control | ADCF | AIR MIX |
| Malvidin | 200.98 ± 7.28 ab | 220.33 ± 8.23 a | 189.77 ± 10.28 b | 463.43 ± 23.13 a | 481.56 ± 11.19 a | 421.84 ± 16.19 b |
| Malvidin acetate | 132.25 ± 6.15 b | 154.61 ± 8.12 a | 141.26 ± 8.12 b | 202.47 ± 10.17 a | 222.48 ± 13.18 a | 171.32 ± 9.12 b |
| Malvidin coumarate | 58.68 ± 6.18 ab | 62.24 ± 4.19 a | 57.98 ± 1.14 b | 62.45 ± 1.05 b | 68.78 ± 2.08 a | 56.64 ± 2.06 c |
| Equipment | Control | ADCF | AIR MIX |
|---|---|---|---|
| Pump-over pump (min) | 1280 | 1220 | 0 |
| Irroration pump (min) | 27 | 0 | 0 |
| Racking pump (min) | 27 | 17 | 10 |
| Cooling equipment (min) | 560 | 460 | 118 |
| Gas compressor (min) | 0 | 0 | 175 |
| Total | 1894 | 1697 | 303 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pettinelli, S.; Pardini, L.; De Angeli, G.; Bianchi, A.; Najar, B.; Cerreta, R.; Bellincontro, A.; Floridia, G.; Mencarelli, F. Innovative “Soft” Maceration Techniques in Red Grape Fermentation. Beverages 2022, 8, 62. https://doi.org/10.3390/beverages8040062
Pettinelli S, Pardini L, De Angeli G, Bianchi A, Najar B, Cerreta R, Bellincontro A, Floridia G, Mencarelli F. Innovative “Soft” Maceration Techniques in Red Grape Fermentation. Beverages. 2022; 8(4):62. https://doi.org/10.3390/beverages8040062
Chicago/Turabian StylePettinelli, Stefano, Luca Pardini, Giorgio De Angeli, Alessandro Bianchi, Basma Najar, Raffaele Cerreta, Andrea Bellincontro, Giuseppe Floridia, and Fabio Mencarelli. 2022. "Innovative “Soft” Maceration Techniques in Red Grape Fermentation" Beverages 8, no. 4: 62. https://doi.org/10.3390/beverages8040062
APA StylePettinelli, S., Pardini, L., De Angeli, G., Bianchi, A., Najar, B., Cerreta, R., Bellincontro, A., Floridia, G., & Mencarelli, F. (2022). Innovative “Soft” Maceration Techniques in Red Grape Fermentation. Beverages, 8(4), 62. https://doi.org/10.3390/beverages8040062







