Why Oxidation Should Be Still More Feared in NABLABs: Fate of Polyphenols and Bitter Compounds
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Beer Samples
2.3. Standard Analyses
2.4. Solid-Phase Extraction of Beer Flavan-3-ols
2.5. RP-HPLC-ESI(-)-MS/MS Analyses of Flavan-3-os
2.6. Analyses of Bitter Compounds by RP-HPLC-UV
3. Results and Discussion
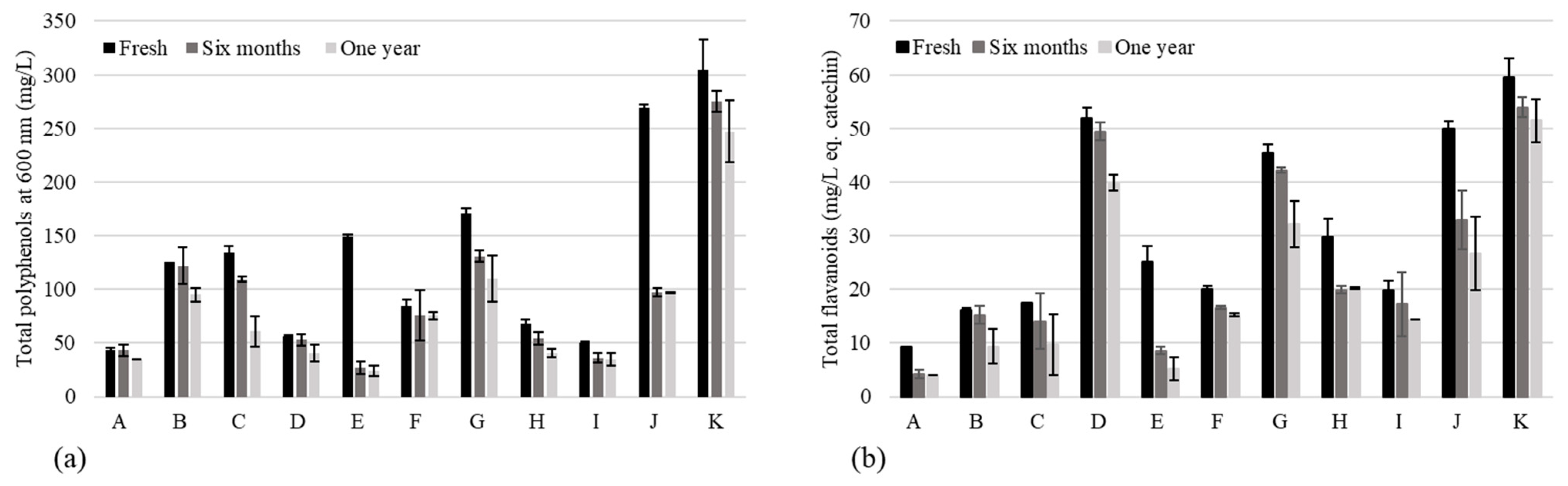
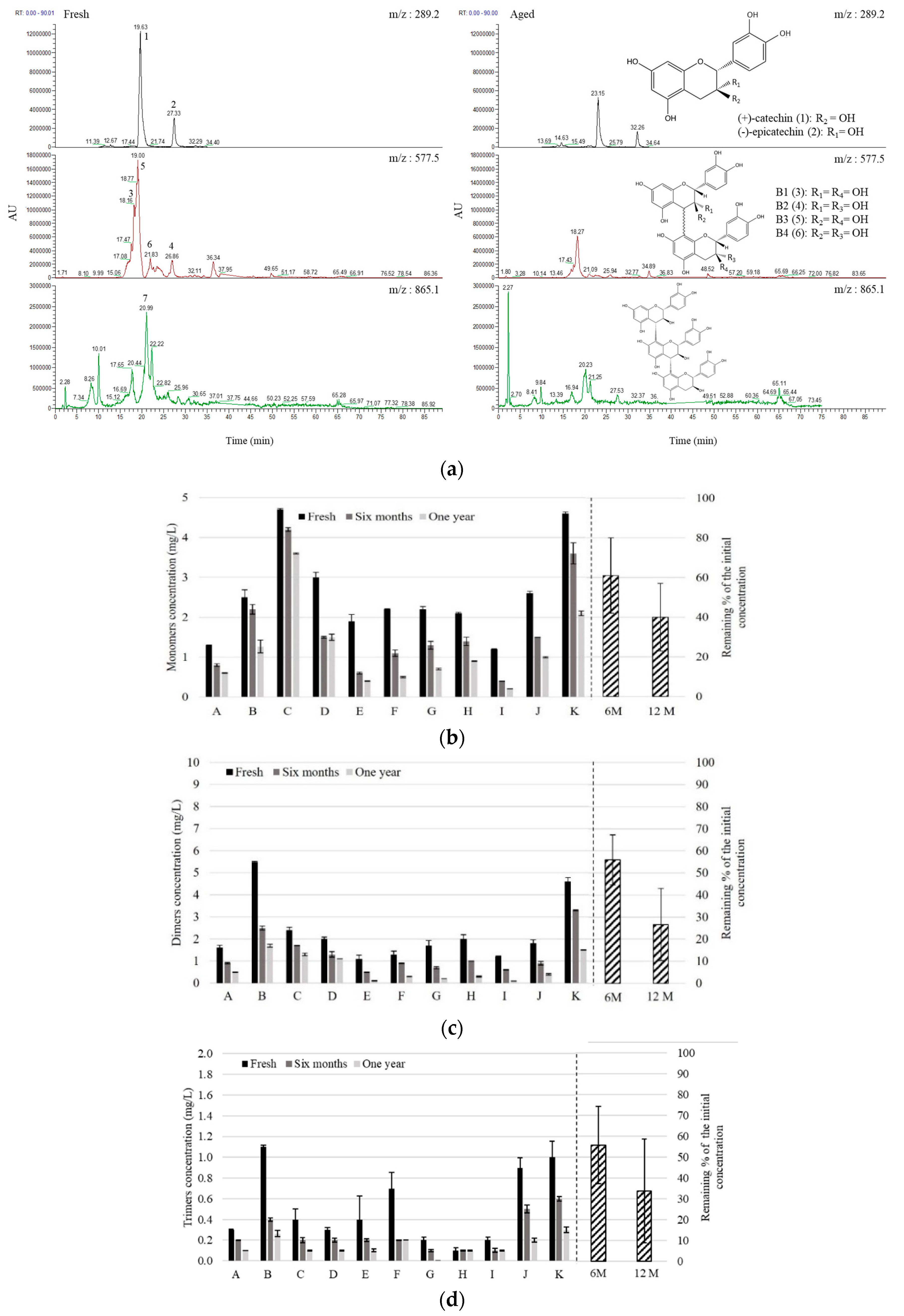
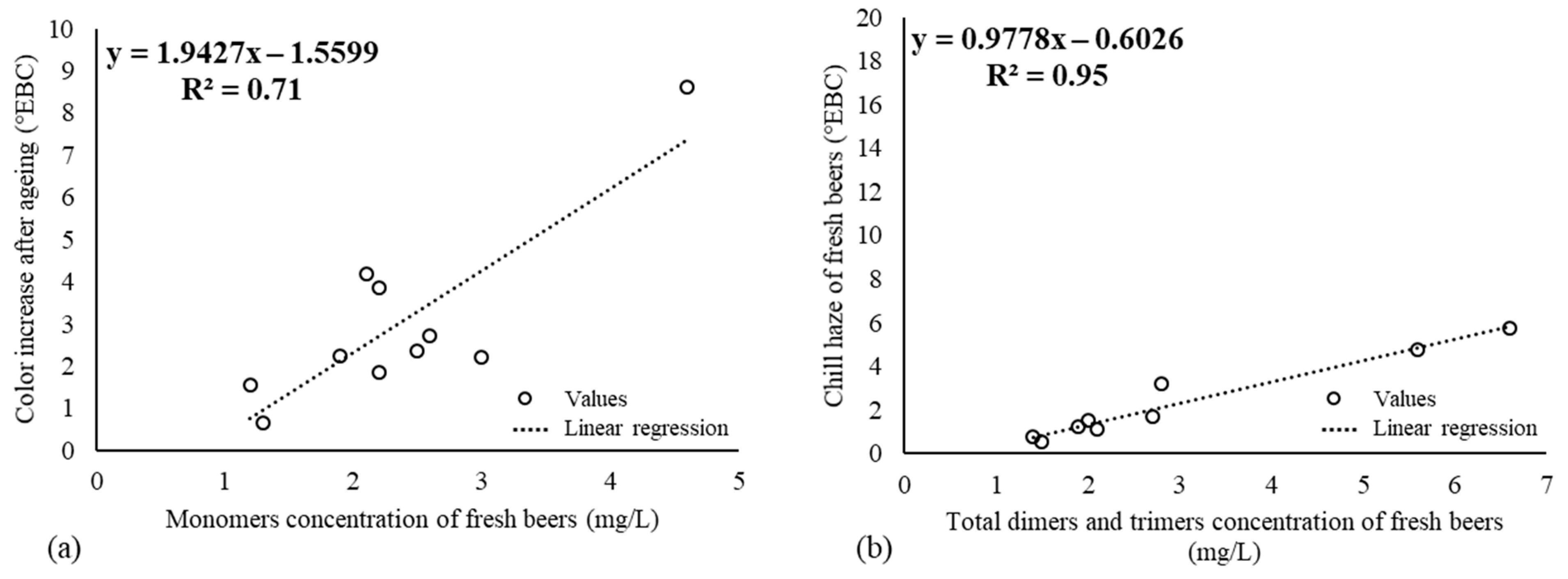
3.1. Chill Haze, Color Stability, Polyphenols in Fresh NABLABs, and Their Fate through Aging
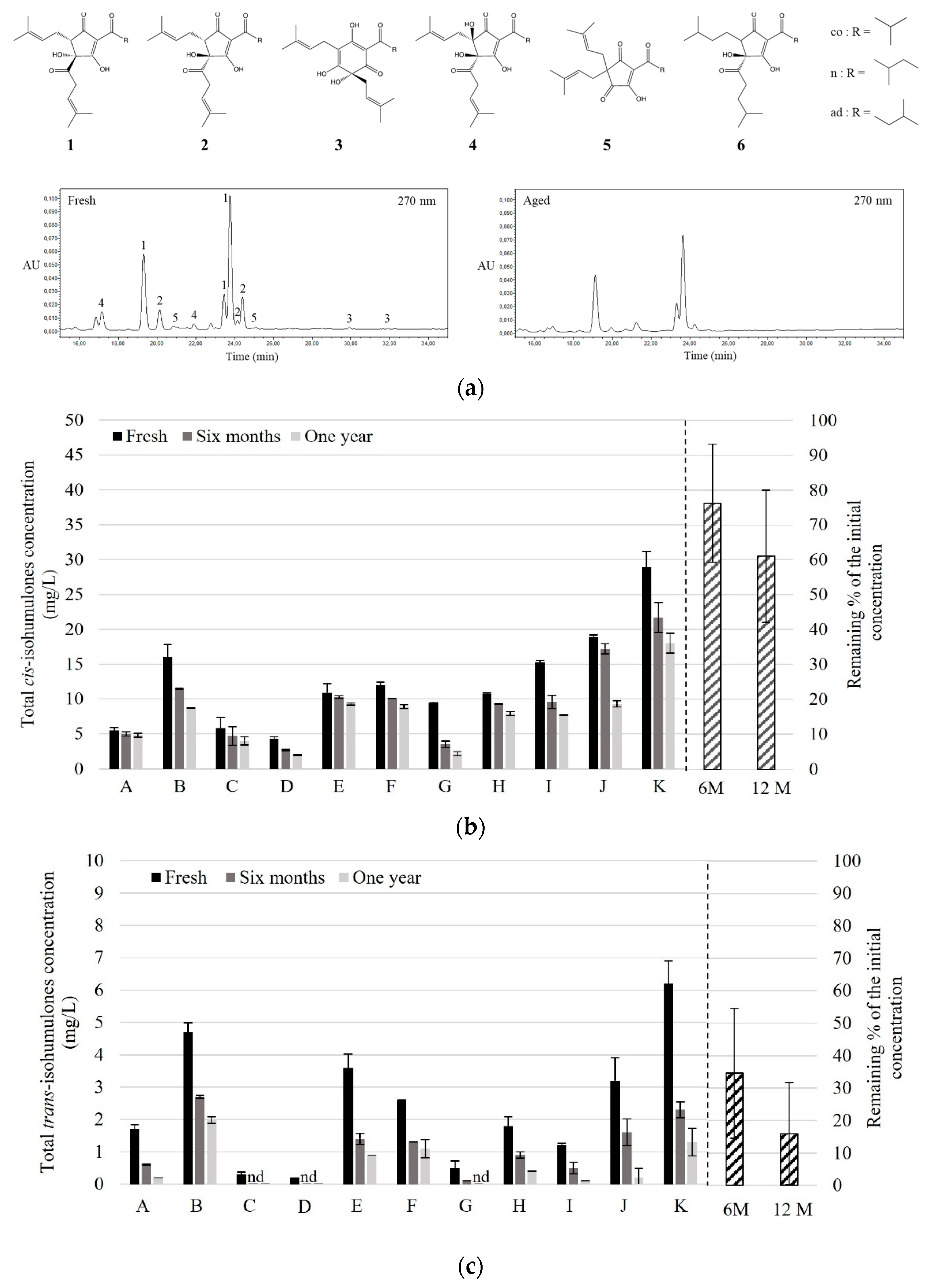
3.2. Bitter Compounds in Fresh NABLABs and Their Fate through Aging
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Brányik, T.; Silva, D.P.; Baszczyňski, M.; Lehnert, R.; Almeida, E.; Silva, J.B. A review of methods of low alcohol and alcohol-free beer production. J. Food Eng. 2012, 108, 493–506. [Google Scholar] [CrossRef]
- Sohrabvandi, S.; Mousavi, S.M.; Razavi, S.H.; Mortazavian, A.M.; Rezaei, K. Alcohol-free Beer: Methods of Production, Sensorial Defects, and Healthful Effects. Food Rev. Int. 2010, 26, 335–352. [Google Scholar] [CrossRef]
- Muller, C.; Neves, L.E.; Gomes, L.; Guimarães, M.; Ghesti, G. Processes for alcohol-free beer production: A review. Food Sci. Technol. 2019, 40, 273–281. [Google Scholar] [CrossRef]
- Perpète, P.; Collin, S. State of the art in low-alcohol beer production. Cerevisia 1999, 1, 27–33. [Google Scholar]
- Müller, M.; Bellut, K.; Tippmann, J.; Becker, T. Physical Methods for Dealcoholization of Beverages Matrices and their Impact on Quality Attributes. ChemBioEng. Rev. 2017, 4, 310–326. [Google Scholar] [CrossRef]
- Perpète, P.; Collin, S. Contribution of 3-Methylthiopropionaldehyde to the Worty Flavor of Alcohol-Free Beers. J. Agric. Food Chem. 1999, 47, 2374–2378. [Google Scholar] [CrossRef]
- Perpète, P.; Collin, S. Influence of beer ethanol content on the wort flavour perception. Food Chem. 2000, 71, 379–385. [Google Scholar] [CrossRef]
- Perpète, P.; Collin, S. Evidence of Strecker Aldehyde Excretion by Yeast in Cold Contact Fermentations. J. Agric. Food Chem. 2000, 48, 2384–2386. [Google Scholar] [CrossRef]
- Piornos, J.A.; Balagiannis, D.P.; Methven, L.; Koussissi, E.; Brouwer, E.; Parker, J.K. Elucidating the odor-active aroma compounds in alcohol-free beer and their contribution to the worty flavor. J. Agric. Food Chem. 2020, 68, 10088–10096. [Google Scholar] [CrossRef]
- Piornos, J.A.; Delgado, A.; de La Burgade, R.C.J.; Methven, L.; Balagiannis, D.P.; Koussissi, E.; Brouwer, E.; Parker, J.K. Orthonasal and retronasal detection thresholds of 26 aroma compounds in a model alcohol-free beer: Effect of threshold calculation method. Int. Food Res. J. 2019, 123, 317–326. [Google Scholar] [CrossRef]
- Simon, M.; Vuylsteke, G.; Collin, S. Flavor defects of fresh and aged NABLABs: New challenges against oxidation. J. Am. Soc. Brew. Chem. 2022, Submitted. [Google Scholar]
- Vanderhaegen, B.; Neven, H.; Verachtert, H.; Derdelinckx, G. The chemistry of beer aging—A critical review. Food Chem. 2006, 95, 357–381. [Google Scholar] [CrossRef]
- Andersen, M.L.; Skibsted, L.H. Electron spin resonance spin trapping identification of radical formed during aerobic forced aging of beer. J. Agric. Food Chem. 1998, 46, 1272–1275. [Google Scholar] [CrossRef]
- Andersen, M.L.; Outtrup, H.; Skibsted, L.H. Potential Antioxidants in Beer Assessed by ESR Spin Trapping. J. Agric. Food Chem. 2000, 48, 3106–3111. [Google Scholar] [CrossRef]
- Kaneda, H.; Kano, Y.; Osawa, T.; Kawakishi, S.; Kamada, K. The Role of Free Radicals in Beer Oxidation. J. Am. Soc. Brew. Chem. 1989, 47, 49–53. [Google Scholar] [CrossRef]
- Bamforth, C.W.; Muller, R.E.; Walker, M.D. Oxygen and Oxygen Radicals in Malting and Brewing: A Review. J. Am. Soc. Brew. Chem. 1993, 51, 79–88. [Google Scholar] [CrossRef]
- de Cooman, L.; Aerts, G.; Overmeire, H.; de Keukeleire, D. Alterations of the profiles of iso-α-acids during beer ageing, marked instability of trans-iso-α-acids and implications for beer bitterness consistency in relation to tetrahydroiso-α-acids. J. Inst. Brew. 2000, 106, 169–178. [Google Scholar] [CrossRef]
- Silva Ferreira, C.; Collin, S. Fate of bitter compounds through dry-hopped beer aging. Why cis-humulinones should be as feared as trans-isohumulones? J. Am. Soc. Brew. Chem. 2020, 78, 103–113. [Google Scholar] [CrossRef]
- McMurrough, I.; Madigan, D.; Kelly, R.J.; Smyth, M.R. The Role of Flavanoid Polyphenols in Beer Stability. J. Am. Soc. Brew. Chem. 1996, 54, 141–148. [Google Scholar] [CrossRef]
- Aron, P.M.; Shellhammer, T.H. A Discussion of Polyphenols in Beer Physical and Flavour Stability. J. Inst. Brew. 2010, 116, 369–380. [Google Scholar] [CrossRef]
- Silva Ferreira, C.; Simon, M.; Collin, S. Why Catechin and Epicatechin from Early Hopping Impact the Color of Aged Dry-Hopped Beers while Flavan-3-ol Oligomers from Late and Dry Hopping Increase Colloidal Instability. J. Am. Soc. Brew. Chem. 2022, 1–10. [Google Scholar] [CrossRef]
- Buggey, L.A. A review of polyphenolic antioxidants in hops, brewing and beer. Brew. Int. 2001, 4, 21–25. [Google Scholar]
- Plumb, G.W.; de Pascual-Teresa, S.; Santos-Buelga, C.; Cheynier, V.; Williamson, G. Antioxidant properties of catechins and proanthocyanidins: Effect of polymerisation, galloylation and glycosylation. Free Radic. Res. 1998, 29, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Counet, C.; Collin, S. Effect of Number of Flavanol Units on the Antioxidant Activity of Procyanidin Fractions Isolated from Chocolate. J. Agric. Food Chem. 2003, 51, 6816–6822. [Google Scholar] [CrossRef] [PubMed]
- Callemien, D.; Collin, S. Involvement of Flavanoids in Beer Color Instability during Storage. J. Agric. Food Chem. 2007, 55, 9066–9073. [Google Scholar] [CrossRef] [PubMed]
- Callemien, D.; Collin, S. Use of RP-HPLC-ESI(–)-MS/MS to Differentiate Various Proanthocyanidin Isomers in Lager Beer Extracts. J. Am. Soc. Brew. Chem. 2008, 66, 109–115. [Google Scholar] [CrossRef]
- Asano, K.; Ohtsu, K.; Shinagawa, K.; Hashimoto, N. Affinity of proanthocyanidins and their oxidation products for haze-forming proteins of beer and the formation of chill haze. Agric. Biol. Chem. 1984, 48, 1139–1146. [Google Scholar] [CrossRef]
- McGuinness, J.D.; Eastmond, R.; Laws, D.R.J.; Gardner, R.J. The Use of14c-Labelled Polyphenols to Study Haze Formation in Beer. J. Inst. Brew. 1975, 81, 287–292. [Google Scholar] [CrossRef]
- Gramshaw, W.J. Phenolic constituents of beer and brewing materials. IV. Further observations on anthocyanogens and catechins as haze precursors in beer. J. Inst. Brew. 1969, 75, 61–83. [Google Scholar] [CrossRef]
- Poupard, P.; Sanoner, P.; Baron, A.; Renard, C.M.G.C.; Guyot, S. Characterization of procyanidin B2 oxidation products in apple juice model solution and confirmation of their presence in apple juice by high-performance liquid chromatography couplet to electrospray ion trap mass spectrometry. J. Mass Spectrom. 2011, 46, 1186–1197. [Google Scholar] [CrossRef] [PubMed]
- Millet, M.; Poupard, P.; Guilois-Dubois, S.; Zanchi, D.; Guyot, S. Self-aggregation of oxidized procyanidins contributes to the formation of heat-reversible haze in apple-based liqueur wine. Food Chem. 2019, 276, 797–805. [Google Scholar] [CrossRef] [PubMed]
- Guyot, S.; Vercauteren, J.; Cheynier, V. Structural determination of colourless and yellow dimers resulting from (+)-catechin coupling catalysed by grape polyphenoloxidase. Phytochemistry 1996, 42, 1279–1288. [Google Scholar] [CrossRef]
- Viana, A.C.; Pimentel, T.C.; do Vale, R.B.; Clementino, L.S.; Januario Ferreira, E.T.; Magnani, M.; dos Santos Lima, M. American pale Ale craft beer: Influence of brewer’s yeast strains on the chemical composition and antioxidant capacity. LWT 2021, 152, 112317. [Google Scholar] [CrossRef]
- Intelmann, D.; Hofmann, T. On the Autoxidation of Bitter-Tasting Iso-α-acids in Beer. J. Agric. Food Chem. 2010, 58, 5059–5067. [Google Scholar] [CrossRef]
- Intelmann, D.; Haseleu, G.; Dunkel, A.; Lagemann, A.; Stephan, A.; Hofmann, T. Comprehensive Sensomics Analysis of Hop-Derived Bitter Compounds during Storage of Beer. J. Agric. Food Chem. 2011, 59, 1939–1953. [Google Scholar] [CrossRef]
- Cook, A.H.; Howard, G.A.; Slater, C.A. Chemistry of hop constituents: VIII*. Oxidation of humulone and cohumulone. J. Inst. Brew. 1955, 61, 321–325. [Google Scholar] [CrossRef]
- European Brewery Convention, Analytica-EBC; Fachverlag Hans Carls: Nürnberg, Germany, 2006.
- McMurrough, I.; Madigan, D.; Smyth, M.R. Semipreparative Chromatographic Procedure for the Isolation of Dimeric and Trimeric Proanthocyanidins from Barley. J. Agric. Food Chem. 1996, 44, 1731–1735. [Google Scholar] [CrossRef]
- Alonso Garcia, A.; Cancho Grande, B.; Simal Gándara, J. Development of a rapid method based on solid-phase extraction and liquid chromatography with ultraviolet absorbance detection for the determination of polyphenols in alcohol-free beers. J. Chromatogr. A. 2004, 1054, 175–180. [Google Scholar] [CrossRef]
- Callemien, D.; Guyot, S.; Collin, S. Use of thiolysis hyphenated to RP-HPLC-ESI(-)-MS/MS for the analysis of flavanoids in fresh lager beers. Food Chem. 2008, 110, 1012–1018. [Google Scholar] [CrossRef]
- Silva Ferreira, C.; de Chanvalon, E.T.; Bodart, E.; Collin, S. Why humulinones are key better constituents only after dry hopping: Comparison with other Belgian styles. J. Am. Soc. Brew. Chem. 2018, 76, 236–246. [Google Scholar]
- Rehberger, A.J.; Bradee, L.H. Hop oxidative transformations and control of beer bitterness. Tech. Q. Master Brew. Assoc. Am. 1975, 12, 1–8. [Google Scholar]


| Biological Processes | Physical Processes | |||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Special Yeast | Mixed Fermentation | Cold Contact | Distillation | Membrane Filtration | ||||||||||||||||||
| Compound | A | B ▲ | C ▲ | D *▲ | E | F | G | H | I | J | K | |||||||||||
| Fresh | 12M | Fresh | 12M | Fresh | 12M | Fresh | 12M | Fresh | 12M | Fresh | 12M | Fresh | 12M | Fresh | 12M | Fresh | 12M | Fresh | 12M | Fresh | 12M | |
| (+)-Catechin | 1.0 + | 0.5 | 1.5 + | 0.8 | 3.5 + | 2.7 | 2.1 | 1.1 | 1.1 + | 0.3 | 1.4 | 0.4 | 1.6 | 0.6 | 1.8 | 0.7 | 0.9 + | 0.1 | 1.9 | 0.7 | 3.6 + | 1.8 |
| (50) | (51) | (77) | (52) | (27) | (29) | (37) | (39) | (11) | (37) | (50) | ||||||||||||
| (-)-Epicatechin | 0.3 + | 0.1 | 1.0 | 0.5 | 1.2 + | 0.9 | 0.9 + | 0.4 | 0.8 | 0.1 | 0.8 | 0.1 | 0.6 | 0.1 | 0.3 | 0.2 | 0.3 | 0.1 | 0.7 | 0.3 | 1.0 | 0.3 |
| (33) | (51) | (75) | (44) | (12) | (12) | (17) | (67) | (33) | (43) | (30) | ||||||||||||
| Total monomers | 1.3 + | 0.6 | 2.5 + | 1.3 | 4.7 + | 3.6 | 3.0 | 1.5 | 1.9 + | 0.4 | 2.2 + | 0.5 | 2.2 + | 0.7 | 2.1 + | 0.9 | 1.2 + | 0.2 | 2.6 + | 1.0 | 4.6 + | 2.1 |
| (46) | (51) | (77) | (50) | (21) | (23) | (32) | (43) | (17) | (38) | (46) | ||||||||||||
| Procyanidin B1 | 0.3 | 0.1 | 1.8 | 0.4 | 0.6 | 0.3 | 0.2 | 0.1 | 0.2 | nd | 0.2 | nd | 0.5 | 0.1 | 0.6 | 0.1 | 0.3 | nd | 0.4 | 0.1 | 0.9 | 0.4 |
| Procyanidin B2 | 0.1 | nd | 0.6 | 0.1 | 0.3 | nd | 0.3 | 0.1 | 0.1 | nd | 0.1 | nd | 0.1 | nd | 0.2 | nd | 0.1 | nd | 0.1 | nd | 0.4 | nd |
| Procyanidin B3 | 1.1 | 0.4 | 2.0 | 0.9 | 1.0 | 0.8 | 1.2 | 0.7 | 0.7 | 0.1 | 0.9 | 0.3 | 0.9 | 0.1 | 1.0 | 0.2 | 0.7 | 0.1 | 1.1 | 0.3 | 2.6 | 1.0 |
| Procyanidin B4 | 0.1 | nd | 1.1 | 0.3 | 0.5 | 0.2 | 0.4 | 0.2 | 0.1 | nd | 0.1 | nd | 0.2 | nd | 0.2 | nd | 0.1 | nd | 0.2 | nd | 0.7 | 0.1 |
| Total dimers ♦ | 1.6 + | 0.5 | 5.5+ | 1.7 | 2.4 + | 1.3 | 2.0+ | 1.1 | 1.1 | 0.1 | 1.3 | 0.3 | 1.7 | 0.2 | 2.0 + | 0.3 | 1.2+ | 0.1 | 1.8 + | 0.4 | 4.6 + | 1.5 |
| (31) | (31) | (54) | (55) | (9) | (23) | (12) | (15) | (8) | (22) | (33) | ||||||||||||
| Procyanidin C2 | 0.2 | 0.5 | 0.3 | 0.2 | 0.2 | 0.4 | 0.1 | 0.1 | 0.1 | 0.5 | 0.5 | |||||||||||
| Total trimers ♦ | 0.3 + | 0.1 | 1.1 | 0.3 | 0.4 | 0.1 | 0.3 | 0.1 | 0.4 | 0.1 | 0.7 | 0.2 | 0.2 | nd | 0.1 | 0.1 | 0.2 | 0.1 | 0.9 + | 0.2 | 1.0 | 0.3 |
| (33) | (24) | (25) | (33) | (25) | (29) | (0) | (100) | (50) | (22) | (30) | ||||||||||||
| Biological Processes | Physical Processes | |||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Special Yeast | Mixed Fermentation | Cold Contact | Distillation | Membrane Filtration | ||||||||||||||||||
| Compound | A | B ▲ | C ▲ | D *▲ | E | F | G | H | I | J | K | |||||||||||
| Fresh | 12M | Fresh | 12M | Fresh | 12M | Fresh | 12M | Fresh | 12M | Fresh | 12M | Fresh | 12M | Fresh | 12M | Fresh | 12M | Fresh | 12M | Fresh | 12M | |
| Bitterness units (BU) | 12.8 | 9.2 | 30.7 | 15.4 | 11.0 | 5.5 | 6.8 | 3.6 | 19.2 | 13.7 | 15.2 | 8.7 | 10.2 | 4.0 | 12.6 | 9.6 | 19.9 | 8.4 | 20.6 | 10.8 | 37.3 | 22.1 |
| (72) | (50) | (50) | (53) | (72) | (57) | (39) | (76) | (42) | (52) | (59) | ||||||||||||
| cis-Isohumulones | 5.5 | 4.8 | 16.0 + | 8.7 | 5.8 | 4.0 | 4.3 | 2.0 | 10.9 | 9.3 | 12.0 + | 8.9 | 9.4 + | 2.2 | 10.7 + | 7.9 | 15.3 + | 7.7 | 18.9 + | 9.3 | 28.9 | 18.0 |
| (87) | (55) | (69) | (47) | (85) | (74) | (23) | (74) | (50) | (49) | (62) | ||||||||||||
| trans-Isohumulones | 1.7 + | 0.2 | 4.7 + | 2.0 | 0.3 | nd | 0.2 + | nd | 3.6 | 0.9 | 2.6 | 1.1 | 0.5 | nd | 1.8 | 0.4 | 1.2 + | 0.1 | 3.2 | 0.2 | 6.2 | 1.3 |
| (12) | (42) | (0) | (0) | (25) | (42) | (0) | (22) | (8) | (6) | (21) | ||||||||||||
| Total isohumulones | 7.2 | 5.0 | 20.7 + | 10.7 | 6.1 | 4.0 | 4.5 | 2.0 | 14.5 | 10.2 | 14.6 + | 10.0 | 9.9 + | 2.2 | 12.5 + | 8.3 | 16.5 + | 7.8 | 22.1 + | 9.5 | 35.1 | 19.3 |
| (69) | (52) | (66) | (44) | (70) | (68) | (22) | (66) | (47) | (43) | (55) | ||||||||||||
| cis/trans | 3 | 3 | nc | nc | 3 | 5 | nc | 6 | nc | 6 | 5 | |||||||||||
| Tetra | 4.0 | 3.1 | 2.1 | 1.1 | 2.0 + | 1.5 | nd | nd | nd | nd | nd | nd | nd | nd | 3.2 | 1.8 | 3.2 + | 2.0 | 3.1 | 1.6 | nd | nd |
| (77) | (52) | (75) | (100) | (100) | (100) | (100) | (56) | (62) | (52) | (100) | ||||||||||||
| Humulones | nd | nd | 0.5 | nd | 0.2 | nd | nd | nd | nd | nd | 1.5 + | nd | nd | nd | nd | nd | nd | nd | nd | nd | 0.9 | nd |
| (100) | (0) | (0) | (100) | (100) | (0) | (100) | (100) | (100) | (100) | (0) | ||||||||||||
| cis-Humulinones | 0.3 + | 0.1 | 7.0 + | 5.3 | 2.8 + | 0.3 | 2.7 | 2.0 | 3.8 + | 0.8 | 0.4 | 0.2 | 0.4 | 0.1 | 0.4 | 0.1 | 0.2 | nd | 0.9 | 0.5 | 1.6 | 0.2 |
| (33) | (76) | (11) | (74) | (21) | (50) | (25) | (25) | (0) | (56) | (12) | ||||||||||||
| Hulupones | 0.3 | 0.2 | 1.5 | 1.0 | 1.2 + | 0.3 | 0.8 | 0.6 | 1.7 | 1.2 | nd | nd | nd | nd | nd | nd | 0.5 | 0.2 | 0.2 | 0.1 | 1.0 | 0.4 |
| (67) | (69) | (25) | (75) | (71) | (100) | (100) | (100) | (40) | (50) | (40) | ||||||||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Simon, M.; Collin, S. Why Oxidation Should Be Still More Feared in NABLABs: Fate of Polyphenols and Bitter Compounds. Beverages 2022, 8, 61. https://doi.org/10.3390/beverages8040061
Simon M, Collin S. Why Oxidation Should Be Still More Feared in NABLABs: Fate of Polyphenols and Bitter Compounds. Beverages. 2022; 8(4):61. https://doi.org/10.3390/beverages8040061
Chicago/Turabian StyleSimon, Margaux, and Sonia Collin. 2022. "Why Oxidation Should Be Still More Feared in NABLABs: Fate of Polyphenols and Bitter Compounds" Beverages 8, no. 4: 61. https://doi.org/10.3390/beverages8040061
APA StyleSimon, M., & Collin, S. (2022). Why Oxidation Should Be Still More Feared in NABLABs: Fate of Polyphenols and Bitter Compounds. Beverages, 8(4), 61. https://doi.org/10.3390/beverages8040061







