Abstract
The anti-oxidant properties of vitamin C and of phenolic compounds of citrus fruits are well established. However, the evaluation of the anti-inflammatory and antithrombotic potential of both vitamin C and of the more amphiphilic and lipophilic components of citrus fruits needs further attention. In this study, the anti-inflammatory and antithrombotic properties of vitamin C and of freshly squeezed juices and their lipid bioactives from the Navalina and Sanguine orange varieties and the Clementine variety of mandarins, as well as from their remaining by-products, were evaluated against the inflammatory and thrombotic pathways of the platelet-activating factor (PAF) and thrombin in platelets, as well as against PAF-biosynthesis in leukocytes. The non-oxidized juices of these citrus fruits and a vitamin C supplement showed stronger anti-PAF and antithrombin effects than their oxidized versions through their general anti-oxidant effect in platelets. The total lipids (TLs) and the HPLC-derived fractions of phenolic compounds and of polar lipid bioactives from both juices and their peels’ by-products showed a more specific stronger inhibitory effect against the inflammatory and thrombotic pathways of PAF and thrombin in platelets, while these bioactives strongly inhibited also the specific enzyme activities of the main biosynthetic enzymes of PAF in leukocytes. The stronger bioactivity of the dietary bioactives found in the juices of these citrus fruits against specific biochemical pathways of inflammation and thrombosis seems to act with synergy with the anti-oxidant potential of their vitamin C content, which further supports the notion that these juices are functional foods with anti-inflammatory protective health benefits. In addition, the presence of these dietary bioactive phenolic compounds and polar lipid bioactives in the remaining peels’ wastes further enhance the valorization of such food industry by-products as potential sources of anti-inflammatory bioactives to be used as ingredients for novel functional products.
Keywords:
orange; sanguine; clementine; anti-inflammatory; antithrombotic; PAF; thrombin; platelets; leukocytes; PAF-synthesis; vitamin C 1. Introduction
Inflammation and thrombosis are important physiological procedures against tissue damage and insults from external agents. However, unresolved and chronic inflammatory and thrombotic manifestations are associated with several chronic disorders, including cardiovascular disorders (CVD) and cancer [1,2]. Platelet activation and aggregation and specific inflammatory and thrombotic mediators, such as the platelet-activating factor (PAF), and thrombin, which activate platelets and other important inflammatory cells, play a crucial role in the onset and development of such chronic disorders [1,2,3]. Moreover, during chronic inflammatory manifestations, the PAF-related inflammatory pathways and cytokines are induced by the observed increased levels of PAF due to a continuous cycle of PAF-synthesis activation in several cells, including cells of endothelium and leukocytes, associated with subsequent thrombo-inflammatory procedures taking place usually at the development of a dysfunctional endothelium [1,2,3].
Nevertheless, a great number of important epidemiological studies, such as the Lyon Diet Heart Study, the Nurses’ Health Study (NHS), the European Prospective Investigation into Cancer and Nutrition (EPIC) study, and several other studies, along with updated outcomes from randomized trials have highlighted the health benefits associated with adherence to healthy dietary patterns, such as the Mediterranean diet, which are rich in foods with strong anti-inflammatory dietary bioactives, anti-oxidants, and vitamins, with strong preventative and protective benefits against inflammation and associated chronic disorders [1,2,4,5,6,7].
Citrus fruits are among the fruits included in such diets with several proposed health benefits. The main benefits of citrus fruits were initially attributed to their richness in vitamin C content and their strong anti-oxidant potential. Healthy diets such as the Mediterranean diet are abundant in citrus fruits juices on a daily basis, which seems to provide the main dietary source for natural vitamin C. Apart from the classic anti-oxidant potency of vitamin C against free radicals, it should also be stressed out that its anti-oxidant capacity can be extended against the situation where oxidative stress is linked with inflammation [8]. However, several studies have highlighted that apart from vitamin C, other citrus phytochemicals and bioactives seem to contribute to their health benefits through several effects and not only their anti-oxidant potency. For example, even though the inhibition of cancer cell proliferation by extracts of berries, fruits, and citrus juices has been correlated with their levels in carotenoids and vitamin C; however, the same inhibition of cell proliferation could not be found by the ascorbate standard alone, which suggested that a synergistic effect of vitamin C with other substances of these fruits exist, such as anthocyanins, flavonoids such as hesperidin, hesperetin, and naringenin, and other compounds that have anti-cancer activity and that may act synergistically with both the fruits’ flavonoids and vitamin C [9,10]. This synergism may be due to the fact that the compounds are exerting their inhibitory effects by different mechanisms and not only by a general anti-oxidant effect.
For instance, dietary supplementation with vitamin C prevents the formation and accumulation of oxidized phospholipids that mimic PAF, which are also called PAF-like lipids and are usually produced during oxidative stress (inflammatory phospholipid oxidation products), such as that imposed by cigarette smoking, and thus vitamin C can prevent in this way the cigarette smoke-induced leukocyte adhesion to the vascular wall and formation of leukocyte-platelet aggregates and smoking-related CVD [8]. Similarly, phospholipid oxidation products with PAF-receptor agonistic inflammatory properties are also produced during oxidative inflammatory responses that take place in chronic disorders, such as renal ischemia–reperfusion and renal disorders in general, as well as during UVB irradiation, while anti-oxidant treatment with vitamin C inhibited both ROS and PAF-R agonistic activity produced by the oxidative inflammatory response or by UVB irradiation, and thus ameliorated the consequences of these inflammatory manifestations too [11,12].
Hesperetin, a dietary bioactive of citrus fruits such as oranges, is another candidate that may benefit the cardiovascular system since, apart from its classic anti-oxidant capacity, hesperetin also possesses strong anti-platelet effects against collagen and arachidonic-acid-induced platelet aggregation, with little effects however on thrombin- or U46619-, a Τhromboxane A2 (TXA2) mimic, mediated platelet aggregation [13]. The ability of citrus hesperetin to selectively inhibit collagen- and arachidonic acid-mediated signal transduction through inhibition of Phospholipase C-gamma2 (PLCγ) phosphorylation and cyclooxygenase-1 (COX-1) activity seems to contribute to the beneficial effects of grapefruits and oranges on the cardiovascular system [13]. Other similar citrus dietary bioactives, such as hesperidin and naringin, have also been found to inhibit human platelet aggregation too, at least partly through the inhibition of PLCγ and Protein kinase B (Akt)-associated signaling, leading to a decrease in Τhromboxane B2 (TXB2) formation, adenosine 5′ diphosphate (ADP) release and granule secretion [14]. Citrus extracts with strong anti-oxidant activity and high content in phenolics also showed strong anti-platelet effects against collagen-induced aggregation of platelets [15]. On the other hand, 10 mL of citrus (orange/grapefruit) juice/kg had no significant effect on platelet activity induced by collagen in dogs and monkeys (Macaca fasciularis) [16].
As far as we know, there is not any study so far evaluating the anti-inflammatory and antithrombotic potency of citrus fruits lipid extracts and of their amphiphilic and lipophilic compounds against the PAF and Thrombin-associated pathways of platelets’ activation, as well as against the inflammatory synthesis of PAF. Furthermore, little is known about the anti-platelet and anti-inflammatory potency of lipid extracts and of amphiphilic and lipophilic compounds from the wastes remaining after squeezing citrus fruits to produce juices. Citrus fruits’ peels can be exploited for extricating essential oils and flavonoids associated with anti-oxidant activity through various extraction processes [17,18,19,20,21]. Some essential oils and terpenes such as orange-derived limonenes, sesquiterpenes, and sesquiterpenoids have shown strong anti-inflammatory, anti-platelet, and antitumor potential [22,23]; however, more research is needed to fully evaluate the citrus industry wastes as sustainable sources of natural amphiphilic and lipophilic lipid bioactives.
In the present study, the anti-inflammatory and antithrombotic potency of lipid bioactives extracted and separated from fresh juices produced by squeezing oranges of the Navalina and Sanguine varieties and the Mediterranean mandarins that are also called clementines, as well as from their remaining by-products (peels’ wastes), were assessed for the first time against platelet activation induced by the well-established inflammatory and thrombotic mediators, PAF and thrombin, as well as against PAF-synthesis in leukocytes, in order to evaluate the contribution of amphiphilic and lipophilic lipid bioactives in the anti-inflammatory benefits observed by citrus fruits’ consumption, as well as for studying the potential valorization of the lipid bioactives present in citrus fruits by-products (peels’ wastes).
2. Materials and Methods
2.1. Samples Preparation and Lipids’ Extraction
Freshly squeezed juices were produced from Navalina and Sanguine orange (Citrus sinensis) varieties as well as from Mediterranean mandarines-clementines (Citrus reticulata) that were cultivated in Argolida, Greece, by using a Citrus Juicer Kenwood JE290A with a stainless steel filter. Total lipids (TLs) from all juices and their remaining wastes (squeezed peels) were extracted according to the Bligh and Dyer extraction method [24], as previously described [25,26]. Briefly, all samples were dissolved and homogenized in a monophasic mixture of solvents, chloroform/methanol/water in a 1:2:0.8 (v/v/v) ratio. The extraction of lipids from juices was conducted just after their production without adding any water into this mixture since the water content of the juices directly served as the 0.8 ratio of the water volume of this monophasic mixture. In contrast, water was added to the mixture of solvents with a 0.8 ratio for extracting the peels, which were firstly homogenized in such a monophasic system of solvents by using appropriate stainless steel blender.
The homogenized samples of both the juices and the peels were then filtrated under vacuum conditions by pumping in a Buchner-based filtering device with a 110 mm Whatman filter paper (Whatman, Maidstone, UK), as previously described [25,26]. Then, the filtrates from the homogenized juices and peels were transferred to separatory funnels, where appropriate volumes of water and chloroform were added in order for a new ratio of solvents to be achieved, containing a chloroform/methanol/water system with a ratio of 1:1:0.9 (v/v/v), in which after thorough shaking phase separation was achieved. The extracted total lipids (TLs) are present in the lower chloroform phases after some time in which phase balance was achieved. These organic phases from each extraction process of each sample were finally collected in separate round-bottom flasks, and the solvents were evaporated from the samples using flash rotary evaporation. Then, the remaining lipid extracts in the glass of the flasks were re-dissolved in small volumes of a chloroform/methanol solution, at a ratio of 1:1 (v/v), by which they were transferred into small pre-weighed glass vials, where all the remaining solvents were further evaporated under a stream of nitrogen. The obtained TL extracts were weighed and immediately used for further analysis and testing after being again re-dissolved either in 1 mL of the same chloroform/methanol solution of 1:1 (v/v) ratio for the HPLC analysis or in a saline-based bovine serum albumin (BSA) solution for the platelet aggregometry assays and the enzymatic assays in the leukocytes homogenates. The concentrations of these TLs were expressed as mg of extracted TLs per ml of the solution in which they were re-dissolved in each case. When the weighted TLs were not used immediately, they were stored dried in nitrogen stream at −20 °C until further analysis was needed.
2.2. Determination of Vitamin C
Vitamin C content in the fresh and oxidized juices and dissolved supplement was determined using a direct iodometric titration, as previously described [27]. Aliquots of 25 mL of fresh juices were placed in a 250 mL erlenmeyer flask, where 2 mL of starch indicator was added. Then, 0.01 N iodine solutions were used to titrate the samples. These iodine solutions were previously standardized with sodium thiosulfate in the matrix of potassium iodide. The sodium thiosulfate solution was standardized before use with a primary standard solution of potassium iodate in acidic environment. A blank titration was performed prior to titration of each sample (n = 3). Each ml of 0.01 N iodine is equivalent to 0.8806 mg ascorbic acid. Results were calculated as mg of L-ascorbic acid per 100 mL of juice. Each sample was prepared and analyzed in duplicate.
2.3. HPLC Analysis and Separation of Bioactive Lipid Molecules from Orange, Sanguine and Clementine Juices and Peels
The TL extracts obtained from the extractions of Navalina and Sanguine orange juices, as well as from Clementines’ juices and their peels, were further separated into several amphiphilic and lipophilic subclasses/fractions by a novel lipid-separation HPLC method of analysis, which separates several amphiphilic and lipophilic compounds from a lipid extract in a one-step procedure on a semi-preparative reversed-phase column Luna 5u C8(2) 100A of Phenomenex, with the use of a mobile phase being a gradient distribution of acetonitril (ACN) and distilled water (W), at room temperature (20 °C), as previously described [28,29,30,31]. The analysis was performed in a Hewlett-Packard series 1100 (Avondale, PA, USA), equipped with a G1314A HP UV spectrophotometer.
2.4. Biological Assays on Washed Rabbit Platelets
The inhibitory effects (antagonistic effects) against PAF and thrombin biological activities on platelets and/or the PAF-like activity for activating platelets (agonistic effects) of the controls and of the re-dissolved in BSA (2.5 mg BSA/mL saline; Sigma, St. Louis, MO, USA) TLs from Navalina and Sanguine orange juices, as well as from Clementines’ juices and their peels, and each of the HPLC lipid fraction obtained as described above, were evaluated by biological assays based on PAF/Thrombin-induced aggregation of washed rabbit platelets (WRP), as previously described [28,29,30]. Commercially purchased effervescent tablets of 135 mg vitamin C (Cebion®), also containing Sucrose, Saccharin sodium, Sodium hydrogen carbonate, Yellow orange S (E110), Citric acid anhydrous, Adipic acid, Orange flavor, were dissolved in saline just before and after 4 h of testing to evaluate the effects of oxidation on the anti-platelet properties of vitamin C.
The organic solvents of aliquots of a standard PAF solution (Sigma, St. Louis, MO, USA) in glass tubes were evaporated under a stream of nitrogen and re-dissolved in BSA (2.5 mg BSA/mL saline; Sigma, St. Louis, MO, USA) to obtain several concentrations (from 2–3 × 10−8 to 2–3 × 10−11 mol/L) prior testing. Ginkgolide B (BN 52021; Sigma, St. Louis, MO, USA) was used as a positive inhibitory control since it is a well-established specific PAF-antagonist for the PAF receptor and thus inhibits specifically the binding of PAF on its receptor and thus PAF activity. Standard active thrombin (Sigma, St. Louis, MO, USA) was dissolved in saline prior testing.
The ability of each selected sample to cause inhibition of PAF/Thrombin-induced aggregation of platelets was studied by adding various concentrations of each sample into WRP suspensions present in specific aggregometer cuvettes in a Chrono-Log aggregometer (model 400) (Havertown, PA, USA) coupled to a Chrono-Log recorder (Havertown) at 37 °C with constant stirring at 1200 min−1, 1–3 min prior to adding each of the inflammatory and thrombotic platelet agonist, PAF or thrombin.
More specifically, 250 µL of WRP suspensions were transferred to an aggregometer cuvette, at 37 °C, with a continuous stirring at 1200 rpm and were always calibrated prior to testing with a blank containing 250 μL of saline buffer. By adding PAF at approximately 2–3 × 10−11 M final concentration or active thrombin at approximately 0.001–0.04 IU/mL in the aggregometer cuvette, the maximum-reversible (or the minimum-irreversible) PAF-induced/thrombin-induced platelet aggregation was determined as the 100% aggregation, which was also used as the baseline of inhibition (0% inhibition). In order to assess the ability of each sample (the dissolved vitamin C supplement, the freshly squeezed juices, the oxidized supplement and juices, the lipid extracts of the citrus fruits juices and of their peels’ wastes, as well as their HPLC-derived fractions) to inhibit PAF/Thrombin induced aggregation of platelets, each WRP suspension was pre-incubated- in the presence of a variety of concentrations of the tested sample, and after 1–3 min of this incubation the appropriate amount of PAF/thrombin (needed for maximum reversible platelet aggregation) was added in each cuvette, and the inhibition of platelet aggregation was calculated. Within a range of 20–80% inhibition against PAF/Thrombin-induced aggregation of platelets, a linear curve to the concentrations of each sample with inhibitory potency was deduced. From this curve, the amount (μg) of the sample that led to 50% of inhibition of platelet aggregation induced by PAF or thrombin in WRP suspensions of 0.250 mL was calculated as the 50% inhibitory concentration value (half maximal inhibitory concentration; IC50 value) for each sample (Figure 1). The resulting IC50 values were expressed as a mean value of the mass (μg) of lipid or vitamin C in the aggregometer cuvette ± standard deviation (SD). Biological assays were performed several times (n > 6) to ensure the validity of results. It should also be stressed that the lower the IC50 value for a sample, the stronger its inhibitory effect against the PAF and/or thrombin pathway.
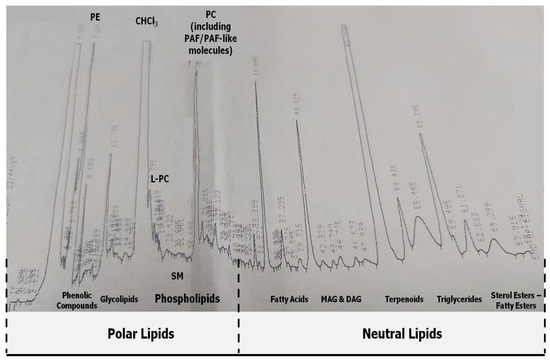
Figure 1.
A representative chromatograph of the HPLC-separation of the TL extracts from fresh squeezed juices of Navalina and Sanguine orange varieties and of Clementine mandarins, as well as from their remaining by-products (peels’ wastes). The more polar phenolic compounds from all these TL extracts are eluted first (at 2–8 min) followed by the amphiphilic polar lipids (PL), such as glycolipids (at 8–14 min) and L-PC, SM and PC (at 14–30 min). The elution of the more Neutral lipids (NL) starts after 30 min, with essential citrus oils being the first to be eluted (30–40 min) followed by fatty acids, mono-/di/alcylglycerols (MAG and DAG), triglycerides and fatty/sterol esters. Radioactive 3H-PAF and standard semi-synthetic cold PAF are usually eluted at RT of 23–28 min respectively, while PC-molecules with PAF-like activity are eluted at similar RT (23–30 min), depending on the length of the carbon chains of their fatty acids. Elution times of standard phospholipids subclasses are also shown (PC: Phosphatidylcholine, L-PC: Lyso- Phosphatidylcholine, SM: Sphingomyelin).
Some samples were able to induce platelet aggregation when assessed in much higher amounts than those of their IC50 values. The aggregatory (agonistic) effect of such samples on platelets was also studied in WRP suspensions in aggregometer cuvettes, as previously described [28,29,30]. Briefly, various concentrations of each sample were added to the aggregometer cuvette, and the aggregation of platelets induced by the sample was measured in a similar way to the aggregation of platelets induced by PAF/Thrombin. A linear relationship was found between the concentration of the sample (usually higher than its IC50 value) that induces platelet aggregation within the range of 20 to 80% of the maximum-reversible aggregation of platelets. The amount in mg of the sample needed to induce 50% of platelet aggregation is defined as the EC50 value (half maximal effective concentration). The lower the EC50 value, the stronger its agonistic effect on platelet aggregation. In desensitization (a) and cross desensitization (b) experiments, platelets were activated by the addition of the test sample (a) or PAF (b) to the platelet suspension at a concentration that caused reversible aggregation. Stimulation with the test sample (a) or PAF (b) with the same or reversed order was performed immediately after complete disaggregation in each case, as previously described [28,29,30].
2.5. Enzymatic Assays of PAF-CPT and Lyso-PAF-AT in Rabbit Leukocytes
2.5.1. Isolation of Rabbit Leukocytes
In each case, the isolation of rabbit plasma, leukocytes, and platelets from rabbit blood was performed as previously described [32]. Briefly, an amount of 9.0 mL of blood from healthy New Zealand white rabbits was obtained from each rabbit in tubes containing 1.0 mL of an anticoagulant solution of 0.085 M sodium citrate/0.065 M citric acid. Rabbit leukocytes were isolated from the erythrocytes in dextran solution, while any contaminating erythrocytes that may have migrated in the sediment pellet of leukocytes were lysed by adding a lysis buffer, and the lysed solution was discarded as the supernatant of centrifugation. The remaining leukocyte pellets were washed with saline and re-suspended in Tris buffer. The re-suspended pellet of leukocytes was sonicated and then centrifuged at 500 g for 10 min at 4 C. Total protein was determined in the supernatant that contains leukocyte homogenates, according to the method of Bradford [33], with BSA as the protein standard.
2.5.2. DTT-Insensitive PAF-Cholinephosphotransferase (PAF-CPT) Activity Assays
The assay was performed in the presence or the absence (control) of vitamin C and of the TL extracts of the juices from Navalina and Sanguine oranges and from Clementines, as well as of their peels wastes and the most bioactive HPLC-derived lipid fractions of each sample, as previously described [34]. Briefly, the reaction was carried out at 37 °C for 20 min in a final volume of 200 μL containing 100 mM Tris-HCl (pH 8.0), 15 mM dithiothreitol, 0.5 mM EDTA (Sigma, St. Louis, MO, USA), 20 mM MgCl2 (Sigma, St. Louis, MO, USA), 1 mg/mL BSA, leukocytes homogenates 0.1 mg in total protein, as well as the substrates 100 μM 1-O-alkyl-2-sn-acetylglycerol (AAG) dissolved in 2 μL of absolute ethanol and 100 μM CDP-Choline, in the absence or the presence of several concentrations (mg/mL) of the aforementioned citrus lipid bioactives. Both substrates were added to the assay just 30 s after the addition of the leukocytes homogenates, which was the time of initiation of the enzyme reaction. Apart from AAG, which was purchased from BIOMOL International LP (Exeter, UK), all the other aforementioned reagents and substrates for this assay were purchased from Sigma (St. Louis, MO, USA).
The assay was stopped after 20 min of its initiation by adding 0.5 mL of cold methanol (2% in acetic acid). The lipid products were extracted into chloroform according to the method of Bligh and Dyer [24]. The lipid products were further separated by thin-layer chromatography, and the PAF fractions were scrapped off and again extracted by the Bligh–Dyer method, while after evaporation of the solvents under a stream of nitrogen, the produced PAF was dissolved in BSA and determined by the washed rabbit platelet aggregation assay. Specific enzyme activities of PAF-CPT are expressed as nmol of produced PAF/min/mg of leukocytes homogenates protein present in each assay. The inhibitory effect of each bioactive (vitamin C, TL extracts, and lipid bioactives of HPLC-derived fractions) are expressed as IC50-values (half maximal inhibitory concentration) measured as μg of the bioactive needed for inhibiting 50% the specific enzyme activity of PAF-CPT of platelet leukocytes.
2.5.3. Lyso-PAF-AT Activity Assays
The assay was performed in the presence and in the absence (control) of vitamin C and of TL extracts from Navalina and Sanguine oranges and from Clementines, as well as of their most bioactive HPLC-derived lipid fractions as previously described [35]. Briefly, the reaction was carried out at 37 °C for 30 min in a final volume of 200 μL containing 50 mM Tris-HCl (pH 7,4), 0.25 mg/mL BSA, 20 μM Lyso-PAF, and 200 μM acetyl-CoA and leukocytes homogenates of 0.25 mg in total protein, in the absence or the presence of several concentrations (mg/mL) of the aforementioned citrus lipid bioactives. The reaction was started by the addition of the leukocytes homogenate and was stopped after 30 min by adding 2% acetic acid methanol, and the extraction, purification, and determination of the produced PAF were carried out similarly to the aforementioned procedure in the PAF-CPT-assay.
Specific enzyme activities of Lyso-PAF-AT are expressed as nmol of produced PAF/min/mg of leukocytes homogenates protein present in each assay. The inhibitory effect of each bioactive (vitamin C, TL extracts, and lipid bioactives of HPLC-derived fractions) are expressed as IC50-values (half maximal inhibitory concentration) measured as μg of the bioactive needed for inhibiting 50% the specific enzyme activity of PAF-CPT of platelet leukocytes.
2.6. Statistical Analysis
The significant differences (p < 0.05) between the IC50-values against PAF and/or thrombin for each tested sample were defined by the one-way analysis of variance (ANOVA) test. The data were analyzed using a statistical software package (IBM-SPSS statistics 26 for Windows, SPSS Inc., Chicago, IL, USA).
3. Results and Discussion
3.1. Anti-Inflammatory and Antithrombotic Potency of TLs from Juices of Oranges and Mandarines and from Their by-Products in Platelets
In the present study, the anti-inflammatory and antithrombotic potency of dietary lipophilic and amphiphilic lipid bioactives extracted and separated from fresh juices produced by squeezing specific citrus fruits that are thoroughly used in the food industry, such as the Navalina and Sanguine orange varieties and the Mediterranean type of mandarins-clementines, as well as from their remaining peels’ wastes, against the pathways of platelet activation and aggregation induced by the well-established inflammatory and thrombotic mediators, PAF and thrombin, were assessed for the first time, and in comparison to the same effects studied for vitamin C. The potency of the antagonistic inhibition of the lipid bioactives from these samples against PAF/thrombin-activities, as well as the anti-platelet effect of vitamin C, were defined by their IC50-values (half maximal inhibitory concentration) measured in mass (μg) of lipid sample or vitamin C content needed for a 50% of inhibition of the PAF/Thrombin-induced aggregation of WRP by 50% in each case (Table 1). It should be noted here that the lower the IC50-value for a dietary bioactive against PAF/Thrombin, the stronger its antagonistic inhibitory effect against the inflammatory and thrombotic activities of these mediators.

Table 1.
In vitro bioactivities (antagonistic/inhibitory effects) of vitamin C and of the TL extracts from citrus fruits’ fresh squeezed juices and from their by-products (peels’ wastes), against PAF/Thrombin-induced platelet aggregation. Biological assays were performed several times to ensure reproducibility.
Ascorbic acid has multiple biochemical roles, with the primary ones being its role as the main water-soluble anti-oxidant in the human body, as well as its important role as a co-enzyme in the formation of collagen, the main protein of connective tissue, whose adequate synthesis is essential for proper resistance and strength of tendons, ligaments, skin, and other connective tissues [36]. Thus, vitamin C has a very important nutritional role, and the symptoms of its absence in the diet have been known since the time of the ancient Egyptians and Greeks. Subsequently, a deficiency in vitamin C leads to scurvy, whose symptoms are well established. In developed countries, this deficiency is only found in people suffering from alcoholism, cancer cachexia, drug dependence, or malabsorption. Since humans lack the enzyme machinery to produce vitamin C, they should receive it from their diet. Several foods of plant origin contain vitamin C, including the rich in vitamin C citrus fruits, such as the assessed in this study orange, sanguine, and clementine, while for the last decade, supplements of vitamin C are also a common source of ascorbic acid intake.
In the present study, a classic vitamin C supplement was assessed in rabbit platelets and showed a non-specific anti-platelet effect against the well-established inflammatory and thrombotic agonists, PAF and thrombin. Since ascorbic acid seems not to interact with the receptors of PAF and thrombin, while it possesses direct strong anti-oxidant properties by quenching superoxide radicals, it seems that the observed anti-platelet effects of vitamin C are derived from an indirect effect of vitamin C in the released superoxide radicals during activation of platelets by either PAF or thrombin [37]. During activation of platelets by such agonists, specific signaling takes place, in some of which the production of superoxide radicals is also involved, which in turn participate in propagating platelet activation and the thrombo-inflammatory response [37]. Administration of vitamin C has been found to actually exert an anti-oxidant effect that resulted in platelet reactive oxygen species (ROS) inhibition and ultimately reduced platelet activation [36]. Thus, it seems that an anti-oxidant treatment with vitamin C can inhibit both ROS and oxidized phospholipid PAF-R agonist activity, which are usually produced during an oxidative inflammatory response [8,11,12,37].
The results obtained in the present study for the vitamin C supplement in platelets further support this notion, especially when this supplement was dissolved in an uncapped and transparent bottle and was left there for 4 h in the presence of atmospheric oxygen and light exposure. In these conditions and after 4 h vitamin C is usually degraded due to oxidation, approximately half of its initial content, as also was previously observed [27]. Vitamin C degradation was also demonstrated by the reduced anti-platelet effects of the oxidized supplement assessed against both PAF and thrombin, as the IC50 values were increased almost twice for the oxidized supplements. Both the intact dissolved vitamin C supplement and the oxidized for 4 h dissolved vitamin C supplement showed a stronger anti-platelet effect against the PAF-associated pathway in comparison to that against the thrombin pathway, which further suggests that the PAF-induced platelet activation involves more ROS production and thus the presence of vitamin C is more active against this pathway.
Similar outcomes were observed for the tested fresh and oxidized juices from the Navalina and Sanguine orange varieties, as well as from clementines. More specifically, fresh juices from these citrus fruits showed stronger anti-PAF, and antithrombin effects than the oxidized ones since leaving these juices for 4 h in an uncapped and transparent glass vial provide the conditions for reduction of both vitamin C and its anti-platelet potency, as well as oxidation of other organic bioactives such as flavonoids. Moreover, the anti-inflammatory and antithrombotic potency of fresh juices against both the PAF and thrombin pathways were much stronger than the ones observed for the vitamin C supplements and again with higher specificity against the PAF pathway. This result further suggests that other bioactive compounds are present in these fresh juices, which can directly and/or indirectly interact with the thrombin and PAF pathways of platelet activation in synergy with the anti-ROS anti-oxidant effects of vitamin C on platelets.
Indeed, the TL extracts of the juices and the peels’ by-products, which contain their more amphiphilic and lipophilic substances, showed the strongest anti-inflammatory and antithrombotic potency against the PAF/Thrombin-induced activation of platelets. Since vitamin C is water soluble, this result further suggests the presence of other amphiphilic and lipophilic lipid bioactives in the TLs of juices and peels from oranges and clementines that seem to possess strong anti-inflammatory potency against PAF and antithrombotic capacity against thrombin. Again, the TLs of the juices from both Navalina and Sanguine orange varieties and from the clementines, as well as from their remaining by-products, had higher specificity against the PAF-pathway in comparison to their antithrombin effect since their IC50 values against PAF were at least twice lower than their antithrombin ones.
Moreover, the TL extracts of the wastes that remained after the squeezing for producing the juices from these citrus fruits showed the strongest anti-PAF potency and an antithrombin activity that was similar to that of the juices TL ones, suggesting that amphiphilic and lipophilic anti-inflammatory bioactives of these citrus fruits with anti-PAF activities remain in their by-products after processing. Thus, the by-products remaining after the processing of the citrus fruits for producing juices seem to be a sustainable source of lipophilic and amphiphilic bioactives with anti-inflammatory and antithrombotic potential to be used as ingredients for novel functional products and supplements.
From all the samples tested, only the TLs from the citrus fruits’ by-products also showed an agonistic effect on platelets since two orders of magnitude higher amounts of these TL extracts induced platelet aggregation. Desensitization and cross desensitization tests, in which platelets were activated and aggregated by the addition of either these TL extracts or by PAF, followed by an additional stimulation with either the TLs or PAF in each case that was performed immediately after complete disaggregation of the initially activated platelets, showed that the agonistic effect of these TL extracts takes place through the PAF-pathway. Thus, lipophilic PAF-like molecules seem to be present in the TL extracts of the citrus fruits’ by-products.
3.2. Anti-Inflammatory and Antithrombotic Potency of HPLC-Derived Bioactive Fractions from TLs of Juices of Oranges and Mandarines and from Their by-Products in Platelets
In order to evaluate the type, class, and subclass of the amphiphilic and lipophilic bioactives that are present in the TL extracts of the juices and the remaining peels’ wastes of these citrus fruits, an HPLC-based analysis was performed that allows the one-step separation of these TLs in several non-water soluble lipophilic classes and amphiphilic compounds, as previously described [28,29,30,31]. A characteristic chromatogram of such an analysis is presented in Figure 1. As is shown in this figure, The more polar phenolic compounds and glycolipids are eluted first in the conditions applied on the reverse phase C8 column, followed by the class of the amphiphilic phospholipid compounds (Figure 1). The more neutral lipophilic compounds, such as the citrus essential oils and terpenes, fatty acids, mono-/di-alcyloglycerols (MAG and DAG), triglycerides, lipid esters, and sterol esters were eluted afterward within this order (Figure 1).
From weighing the HPLC fractions obtained from such HPLC analysis of 1 mg of the TL extracts from all samples, very similar percentage values for each fraction were observed between the different juices and between the different peels’ waists, whereas some differences were observed between the percentage values for each fraction of juices when compared with those of its relative peels’ by-products. More specifically, approximately 85–95% of the TLs from the orange juices were polar amphiphilic compounds eluted in the fractions of Phenolic compounds, Glycolipids, L-PC, SM, and PC/PAF-like molecules, with the fractions of PC and phenolics being the most prevalent compounds (30–40% each), followed by glycolipids (10–20%) and L-PC and SM (5–10% each) from the more polar compounds. From the TLs, only the 5–15% were neutral lipids that were eluted mostly in fractions of triglycerides, terpenoids, MAG, DAG, and fatty acids. In contrast, in the peels’ wastes (by-products), the percentage of neutral lipids was increased up to 25–35% due to an increase in the most prevalent neutral compounds, terpenoids, and triglycerides fractions, while again, the percentage of the more polar lipid bioactives was higher, but not so much as the one observed in juices. Thus, the more polar compounds were the 65–75% of the TLs of the peels, which were mostly eluted at the PC and phenolics fractions (35–45% each), lesser in glycolipids (approx. 15%), and much lesser in L-PC and SM fractions (3–8%). Such relative % composition of the PL fractions within the PL extracts observed in the juices and the peels’ waists of these citrus fruits are similar to the ones previously observed in both other fruits such as apples [25,26]. However, more sophisticated lipidomic approaches, based on modern MALDI-TOF LC–MS, are needed for quantifying and structurally elucidating all these bioactive molecules present in each HPLC fraction from the TL extracts of the citrus fruits juices and their by-products
Each one of thus separated amphiphilic and lipophilic compounds and classes of molecules were further assessed for their ability to inhibit PAF-induced platelet aggregation, as well as for their potential agonistic ability to induce activation and aggregation of platelets through the PAF pathway in higher amounts. The anti-inflammatory potency and agonistic activities of these compounds against the PAF -induced aggregation of WRP were defined again by their IC50/EC50-values measured in mass (μg) of lipid sample (Table 2). The most bioactive fractions in all cases were those containing the more polar amphiphilic/lipophilic compounds, such as the phenolics compounds, glycolipids, and phosphatidylcholines (PC), while the more neutral lipophilic compounds such as triglycerides and fatty esters did not show any activity, apart from the neutral essential oils’ fractions that showed considerably strong anti-PAF activity, and especially the essential oils of the by-products of the citrus fruits. These results come in accordance with outcomes of other studies in lipophilic bioactives from other plant-derived sources, such as apple juice and apple pomace [25,26]. The overall anti-inflammatory and antithrombotic potency of the TL extracts for each case against the PAF-pathway seems to be derived from the co-presence of all these bioactives in the lipophilic extract of the TLs of these citrus fruits and of their by-products.

Table 2.
In vitro bioactivities (antagonistic/inhibitory effects and agonistic/aggregatory effects) of the most bioactive HPLC-derived amphiphilic and lipophilic fractions of TLs from fresh squeezed juices of Navalina and Sanguine orange varieties, of clementines and from their by-products (peels’ wastes), against PAF -induced platelet aggregation. Biological assays were performed several times to ensure reproducibility.
Citrus fruits and their juices are among the most common rich dietary sources of phenolic compounds and especially flavonoid bioactives. Dietary flavonoids such as hesperidin, hesperetin, and naringenin, which are the most abundant flavonoid component in orange and mandarin juices, may help to supplement the body not only with anti-oxidant defenses against free radicals but also with anti-inflammatory, anti-platelet, anti-allergenic, antimicrobial, antiviral, antiulcer and analgesic activities [36]. Such flavonoids are also present in high amounts in orange peel, which is the primary waste fraction in the production of orange juice. It has been proposed that supplementation with such flavonoids found in orange peel extracts may be beneficial only when the body is under severe oxidative stress and when supplementation is given over time and not as a single administration [38].
The anti-platelet effect of flavonoids found in citrus fruits and their peels, such as hesperetin, hesperidin, and naringin, has been previously reported against other pathways of platelet activation involving collagen, arachidonic acid, and the decrease in TXB2 formation, ADP release and granule secretion [13,14,15,19,38]. In addition, naringenin, the aglycon analog of naringin, along with quercetin, have both been shown to antagonistically inhibit the inflammatory PAF-pathway with an IC50 value similar to the ones for the fractions of phenolic compounds of oranges and mandarins-clementines, observed in the present study [39]. Thus, it seems that such anti-oxidant and anti-inflammatory protective flavonoids seem to be present in the bioactive fraction of phenolic compounds separated from citrus fruits’ juices and from their peels’ wastes with strong anti-platelet potency against the inflammatory mediator PAF. However, more studies are needed to further clarify the individual contribution of each type of phenolic compound in the overall anti-PAF effect of the TLs from oranges and mandarins and from their remaining by-products.
Apart from the phenolic fractions, the polar lipid fractions of glycolipids and PC phospholipids also showed strong anti-PAF potency in both the juices and the peels. This result comes in agreement with previously reported results in juices from other fruits (apple juices) and of their by-products (apple pomace) [25,26] and further support the notion that fruits that are thoroughly used in healthy diets such as the Mediterranean Diet, such as the studied citrus fruits, contain bioactive PL (glycolipids and PC) with strong anti-inflammatory specificity against the PAF-pathway, which contribute to the health benefits of such diets. Moreover, the peels’ PC-fraction showed also a platelet agonistic effect through the PAF/PAF-receptor pathway, as shown by the positive desensitization and cross-desensitization tests, in much higher amounts than those needed for PAF inhibition since the EC50 value for the agonistic effect of this fraction was at least one order of magnitudes higher than its IC50 value for its antagonistic effect against PAF. This seems to be related to the presence of PC molecules with PAF-like structure and activity in the TLs of citrus peels, which beneficially influence the PAF pathway due to structural homology with PAF and specific binding to PAF-receptor.
More specifically, such PAF-like molecules that are eluted in the PC fraction of the citrus peels TLs, which in low amounts inhibit PAF antagonistically and in higher amounts show an agonistic effect on PAF-pathway, through affecting the PAF/PAF-R signaling, are considered as potent specific PAF-inhibitors, because they disallow the far more active PAF-molecule to act through its PAF-R related inflammatory pathways. Nevertheless, such agonistic effect of inducing platelet reactivity by the presence of such molecules in orange peels may also be associated with induced by the citrus peels hypersensitivity and allergic reactions. Thus, more studies are needed to evaluate the safety of valorizing such lipid bioactives from citrus by-products such as orange and mandarine peels.
Another bioactive fraction with strong anti-PAF properties was the peels’ TL fraction of terpenoids. Citrus fruits’ peel oils are rich in terpenoids and especially in monoterpenes such as limonoids and limonenes [36], which have previously been reported that possess strong anti-oxidant properties and activities anti-platelet effects [38]. Terpenoids from other sources, such as the ginkgolides from the Ginkgo biloba tree, are classic PAF-antagonists, which may explain why the terpenoids’ fraction of citrus fruits peels, but also those of citrus fruits juices, also have strong anti-PAF potential. Nevertheless, more studies are needed in order to reveal the exact citrus-derived terpenoid molecules involved in these effects, as well as the potential synergy of citrus carotenoids that are co-eluted in this lipid fraction of citrus fruits.
3.3. Anti-Inflammatory Effects of TLs and HPLC-Derived Bioactive Fractions from Juices of Oranges and Mandarines and from Their by-Products against PAF-Synthesis in Leukocytes
The TLs and the fractions of phenolic compounds and of polar lipids (glycolipids and PC molecules) from all samples strongly inhibited also the main regulatory enzymes of PAF-biosynthesis in leukocytes, PAF-CPT and Lyso-PAF-AT, while the more neutral lipids, terpenoids and the supplement of vitamin C did not show such an effect (Table 3). The de novo biochemical pathway of PAF-synthesis was initially thought to be mainly responsible for the production of the basal levels of PAF in cells, with PAF-CPT being its main regulatory enzyme, while the remodeling pathway, with Lyso-PAF-AT being its main regulatory enzyme, is mainly responsible for an acute increase in PAF-synthesis in case of need, such as during the initiation and propagation of inflammatory stimuli, in both acute and chronic inflammatory responses [2,3,34].

Table 3.
In vitro bioactivities (inhibitory effects) of the TL extracts from fresh squeezed juices of Navalina and Sanguine oranges and clementines and their by-products (peels’ wastes), as well as of their most bioactive HPLC-derived lipid fractions, against the specific activities of the basic biosynthetic enzymes of PAF (PAF-CPT and Lyso-PAF-AT) in rabbit leukocytes. Enzyme assays were performed several times to ensure reproducibility.
Nevertheless, the latest findings have pointed out that PAF-synthesis in chronic inflammatory conditions is also increased by the induction of the de novo biochemical pathway, as increased PAF-CPT activity has also been observed in chronic inflammatory conditions [2,3]. In such conditions, increased vascular permeability of inflammation is accounted for by the formation/release of mediators, such as lipids derived from ether phospholipids such as PAF, as well as from arachidonate (eicosanoids: prostaglandins, thromboxane A2 or leukotrienes). Acute and chronic inflammation can be controlled by inhibitors of mediator synthesis or by antagonists, as well as at the level of the reactivity of vascular endothelium or leukocytes. This modulation can be obtained with several bioactives that can inhibit both PAF actions and reduce its synthesis.
Thus, the ability of the phenolic compounds and of the glycolipids and PC molecules of the TLs from orange and mandarine juices and of their remaining by-products to strongly inhibit both the biosynthetic biochemical routes of PAF-synthesis in leukocytes further support their anti-inflammatory potential, since the reduction of PAF-synthesis results in reduced released PAF-levels from leukocytes to blood, and thus amelioration of the PAF-associated inflammatory response. The polar lipids, glycolipids, and PC of the TLs of these citrus fruits and of their remaining by-products seem to inhibit both these enzymes due to structural resemblance to the substrates of these enzymes and comes in accordance with a similar effect observed in similar bioactive polar lipids from other sources, such as fish [34,40,41] and olive pomace [34,42]. Interestingly phenolic compounds have strongly inhibited both these enzymes without having a chemical resemblance to the enzymes’ substrates, suggesting that their inhibitory effect is taking place through the presence of other allosteric cites for their binding on these enzymes, which also comes in accordance with previous results observed for such phenolics such as resveratrol [34].
Overall, the inhibitory effect of the TL extracts from the Navalina and Sanguine oranges and clementines, as well as from their by-products (peels’ wastes), against the basic biosynthetic enzymes of PAF (PAF-CPT and Lyso-PAF-AT) in leukocytes, seems to be the accumulating synergy of their bioactive compounds in the fractions of phenolics and polar lipid bioactives being present in these TL extracts. The non-detected effect of the anti-oxidant vitamin C in these inflammatory pathways of PAF-synthesis further indicates that the anti-inflammatory potency of orange and mandarin juices and peels is associated with these phenolic compounds and polar lipid bioactives being present in these citrus fruits’ products and by-products.
4. Conclusions
In general, the anti-oxidant properties of vitamin C have been proposed to decrease markers of thrombosis, such as tissue plasminogen activator and von Willebrand factor, directly in high-risk patients with cardiovascular disease and diabetes and to reduce indirectly the thrombo-inflammatory effects of oxidative stress, which is a strong trigger for the non-enzymatically synthesis of PAF and of PAF-like oxidized phospholipid derivatives that activate the PAF receptor and its associated inflammatory signaling.
Within the present study, it was shown for the first time that in juices of navalina and Sanguine oranges and Clementine mandarins, as well as in their remaining by-products (peels’ wastes), apart from the anti-oxidant capacity of their vitamin C content that showed anti-platelet effects, there are also present other dietary bioactives, such as bioactive phenolic compounds and polar lipids bioactives, which exhibit strong anti-inflammatory and antithrombotic potency against the PAF and thrombin pathways in platelets, with higher specificity against the inflammatory effects of PAF. In addition, these dietary citrus bioactives were able to potently inhibit the enzymatic PAF-biosynthesis in leukocytes too, and thus reduce the inflammatory signaling associated with increased PAF synthesis and enzymatically induced increased PAF levels. On the other hand, an antithrombotic effect was also observed in a vitamin C supplement, while oxidation of both this supplement and the juices of these citrus fruits also showed an effect on their antithrombotic potency.
Overall, the presence of such dietary anti-inflammatory and antithrombotic bioactives in citrus juices further supports the functional benefits of these products in comparison with vitamin C supplements that luck such bioactive nutrients, especially in conditions where their anti-inflammatory capacity is continuously needed, such as atherosclerosis and CVD, cancer autoimmune disorders, renal diseases, neurodegenerative disorders, and persistent infections. Nevertheless, more studies are needed in such conditions to fully unveil the anti-inflammatory benefits of citrus juices. In addition, the presence of such bioactive dietary phenolic compounds and polar lipid bioactives in the remaining by-products—peels’ wastes of both oranges and mandarins—have strong anti-inflammatory protection against the biochemical pathways of PAF and thrombin and against the inflammatory increase in PAF levels, by inhibiting PAF-synthesis, further support the valorization of such by-products of the food industry as potential sustainable sources of added value anti-inflammatory ingredients for the production of novel functional foods and supplements.
Funding
This research received no external funding.
Data Availability Statement
Not applicable.
Acknowledgments
The author would like to thank the Greek State Scholarships Foundation and the Department of Chemistry of the National and Kapodistrian University of Athens in Greece, and especially the former Director of its Biochemistry and Food Chemistry Lab, Constantinos A. Demopoulos, as well as the Department of Biological Sciences, of the University of Limerick (UL), in Ireland, and especially the current and former Deans of the Faculty of Science and Engineering of UL, Sean Arkins and Edmund Magner, respectively, and Audrey Tierney and Phil Jakeman and all my other colleagues in UL for their continuous support.
Conflicts of Interest
The author declares no conflict of interest.
References
- Tsoupras, A.; Lordan, R.; Zabetakis, I. Inflammation and cardiovascular diseases. In The Impact of Nutrition and Statins on Cardiovascular Diseases; Zabetakis, I., Lordan, R., Tsoupras, A., Eds.; Academic Press: Cambridge, MA, USA, 2019; Chapter 3; pp. 53–117. [Google Scholar]
- Tsoupras, A.; Lordan, R.; Zabetakis, I. Inflammation, not Cholesterol, Is a Cause of Chronic Disease. Nutrients 2018, 10, 604. [Google Scholar] [CrossRef] [Green Version]
- Tsoupras, A.B.; Iatrou, C.; Frangia, C.; Demopoulos, C.A. The implication of platelet activating factor in cancer growth and metastasis: Potent beneficial role of PAF-inhibitors and antioxidants. Infect. Disord.-Drug Targets 2009, 4, 390–399. [Google Scholar] [CrossRef] [PubMed]
- De Lorgeril, M.; Salen, P.; Martin, J.L.; Monjaud, I.; Delaye, J.; Mamelle, N. Mediterranean Diet, Traditional Risk Factors and the Rate of Cardiovascular Complications after Myocardial Infarction: Final report of the Lyon Diet Heart Study. Circulation 1999, 99, 779–785. [Google Scholar] [CrossRef] [PubMed]
- Hoevenaar-Blom, M.P.; Nooyens, A.C.; Kromhout, D.; Spijkerman, A.M.; Beulens, J.W.; Van Der Schouw, Y.T.; Bueno-De-Mesquita, B.; Verschuren, W.M. Mediterranean style diet and 12-year incidence of cardiovascular diseases: The EPIC-NL cohort study. PLoS ONE 2012, 7, e45458. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fung, T.T.; Willett, W.C.; Stampfer, M.J.; Manson, J.E.; Hu, F.B. Dietary patterns and the risk of coronary heart disease in women. Arch. Intern. Med. 2001, 161, 1857–1862. [Google Scholar] [CrossRef] [Green Version]
- Tierney, A.; Lordan, R.; Tsoupras, A.; Zabetakis, I. Diet and cardiovascular disease: The Mediterranean diet. In The Impact of Nutrition and Statins on Cardiovascular Diseases; Zabetakis, I., Lordan, R., Tsoupras, A., Eds.; Academic Press: Cambridge, MA, USA, 2019; Chapter 8; pp. 267–288. [Google Scholar]
- Lehr, H.A.; Weyrich, A.S.; Saetzler, R.K.; Jurek, A.; Arfors, K.E.; Zimmerman, G.A.; Prescott, S.M.; McIntyre, T.M. Vitamin C blocks inflammatory platelet-activating factor mimetics created by cigarette smoking. J. Clin. Investig. 1997, 99, 2358–2364. [Google Scholar] [CrossRef]
- Olsson, M.E.; Gustavsson, K.E.; Andersson, S.; Nilsson, A.; Duan, R.D. Inhibition of cancer cell proliferation in vitro by fruit and berry extracts and correlations with antioxidant levels. J. Agric. Food Chem. 2004, 52, 7264–7271. [Google Scholar] [CrossRef]
- Guthrie, N.; Carroll, K.K. Inhibition of mammary cancer by citrus flavonoids. Adv. Exp. Med. Biol. 1998, 439, 227–236. [Google Scholar] [CrossRef]
- Lloberas, N.; Torras, J.; Herrero-Fresneda, I.; Cruzado, J.M.; Riera, M.; Hurtado, I.; Grinyó, J.M. Postischemic renal oxidative stress induces inflammatory response through PAF and oxidized phospholipids. Prevention by antioxidant treatment. FASEB J. 2002, 16, 908–910. [Google Scholar] [CrossRef]
- Yao, Y.; Harrison, K.A.; Al-Hassani, M.; Murphy, R.C.; Rezania, S.; Konger, R.L.; Travers, J.B. Platelet-Activating Factor Receptor Agonists Mediate Xeroderma Pigmentosum A Photosensitivity. J. Biol. Chem. 2012, 287, 9311–9321. [Google Scholar] [CrossRef] [Green Version]
- Jin, Y.R.; Han, X.H.; Zhang, Y.H.; Lee, J.J.; Lim, Y.; Chung, J.H.; Yun, Y.P. Antiplatelet activity of hesperetin, a bioflavonoid, is mainly mediated by inhibition of PLC-gamma2 phosphorylation and cyclooxygenase-1 activity. Atherosclerosis 2007, 194, 144–152. [Google Scholar] [CrossRef]
- Kim, T.H.; Kim, H.M.; Park, S.W.; Jung, Y.S. Inhibitory effects of yuzu and its components on human platelet aggregation. Biomol. Ther. 2015, 23, 149–155. [Google Scholar] [CrossRef] [Green Version]
- Assefa, A.D.; Ko, E.Y.; Moon, S.H.; Keum, Y.-S. Antioxidant and antiplatelet activities of flavonoid-rich fractions of three citrus fruits from Korea. Biotech 2016, 6, 109. [Google Scholar] [CrossRef] [Green Version]
- Osman, H.E.; Maalej, N.; Shanmuganayagam, D.; Folts, J.D. Grape Juice but Not Orange or Grapefruit Juice Inhibits Platelet Activity in Dogs and Monkeys (Macaca fasciularis). J. Nutr. 1998, 128, 2307–2312. [Google Scholar] [CrossRef] [Green Version]
- Bhattacharya, S. Cultivation of essential oils. In Essential Oils in Food Preservation, Flavor and Safety; Preedy, V.R., Ed.; Academic Press: Cambridge, MA, USA, 2016; Chapter 3; pp. 19–29. ISBN 9780124166417. [Google Scholar] [CrossRef]
- Sharmila, V.G.; Kavitha, S.; Obulisamy, P.K.; Rajesh Banu, J. Production of fine chemicals from food wastes. In Food Waste to Valuable Resources; Rajesh Banu, J., Kumar, G., Gunasekaran, M., Kavitha, S., Eds.; Academic Press: Cambridge, MA, USA, 2020; Chapter 8; pp. 163–188. ISBN 9780128183533. [Google Scholar] [CrossRef]
- Williams, C.A. Specialized dietary supplements. In Equine Applied and Clinical Nutrition; Raymond, J., Geor, P., Harris, A., Coenen, M., Saunders, W.B., Eds.; Elsevier: Edinburgh, UK, 2013; Chapter 19; pp. 351–366. ISBN 9780702034220. [Google Scholar] [CrossRef]
- Ladaniya, M.S. (Ed.) Fruit biochemistry. In Citrus Fruit; Academic Press: Cambridge, MA, USA, 2008; Chapter 6; pp. 125–190. ISBN 9780123741301. [Google Scholar] [CrossRef]
- Khan, N.; Monagas, M.; Urpi-sarda, M.; Llorach, R.; Andres-Lacueva, C. Contribution of bioactive foods and their emerging role in immunomodulation, inflammation, and arthritis. In Preedy, Bioactive Food as Dietary Interventions for Arthritis and Related Inflammatory Diseases; Watson, R.R., Victor, R., Eds.; Academic Press: Cambridge, MA, USA, 2013; Chapter 4; pp. 43–65. ISBN 9780123971562. [Google Scholar] [CrossRef]
- Moharam, B.A.; Jantan, I.; Ahmad, F.B.; Jalil, J. Antiplatelet Aggregation and Platelet Activating Factor (PAF) Receptor Antagonistic Activities of the Essential Oils of Five Goniothalamus Species. Molecules 2010, 15, 5124–5138. [Google Scholar] [CrossRef] [Green Version]
- Kuttan, G.; Pratheeshkumar, P.; Manu, K.A.; Kuttan, R. Inhibition of tumor progression by naturally occurring terpenoids. Pharm. Biol. 2011, 49, 995–1007. [Google Scholar] [CrossRef]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef]
- Tsoupras, A.; Donal, M.; Pleskach, H.; Durkin, M.; Traas, C.; Zabetakis, I. Beneficial Anti-Platelet and Anti-Inflammatory Properties of Irish Apple Juice and Cider Bioactives. Foods 2021, 10, 412. [Google Scholar] [CrossRef]
- Tsoupras, A.; Moran, D.; Byrne, T.; Ryan, J.; Barrett, L.; Traas, C.; Zabetakis, I. Anti-Inflammatory and Anti-Platelet Properties of Lipid Bioactives from Apple Cider By-Products. Molecules 2021, 26, 2869. [Google Scholar] [CrossRef]
- Sapei, L.; Hwa, L. Study on the Kinetics of Vitamin C Degradation in Fresh Strawberry Juices. Procedia Chem. 2014, 9, 62–68. [Google Scholar] [CrossRef] [Green Version]
- Tsoupras, A.; Kouvelis, V.N.; Pappas, K.M.; Demopoulos, C.A.; Typas, M.A. Anti-inflammatory and anti-thrombotic properties of lipid bioactives from the entomopathogenic fungus Beauveria bassiana. Prostaglandins Other Lipid Mediat. 2022, 158, 106606. [Google Scholar] [CrossRef]
- Koukouraki, P.; Tsoupras, A.; Sotiroudis, G.; Demopoulos, C.A.; Sotiroudis, T.G. Antithrombotic properties of Spirulina extracts against platelet-activating factor and thrombin. Food Biosci. 2020, 37, 100686. [Google Scholar] [CrossRef]
- Tsoupras, A.B.; Demopoulos, C.A.; Pappas, K.M. Platelet-activating factor detection, metabolism, and inhibitors in the ethanologenic bacterium Zymomonas mobilis. Eur. J. Lipid Sci. Technol. 2012, 114, 123–133. [Google Scholar] [CrossRef]
- Tsoupras, A.; Pappas, K.M.; Sotiroudis, T.G.; Demopoulos, C.A. One-step separation system of bio-functional lipid compounds from natural sources. MethodsX 2021, 8, 101380. [Google Scholar] [CrossRef]
- Tsoupras, A.B.; Chini, M.; Mangafas, N.; Tsogas, N.; Stamatakis, G.; Tsantila, N.; Fragopoulou, E.; Antonopoulou, S.; Gargalianos, P.; Demopoulos, C.A.; et al. Platelet-activating factor and its basic metabolic enzymes in blood of naive HIV-infected patients. Angiology 2012, 63, 343–352. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Tsoupras, A.B.; Fragopoulou, E.; Nomikos, T.; Iatrou, C.; Antonopoulou, S.; Demopoulos, C.A. Characterization of the de novo biosynthetic enzyme of platelet activating factor, DDT-insensitive cholinephosphotransferase, of human mesangial cells. Mediat. Inflamm. 2007, 2007, 27683. [Google Scholar] [CrossRef] [Green Version]
- Tsoupras, A.B.; Chini, M.; Tsogas, N.; Fragopoulou, E.; Nomikos, T.; Lioni, A.; Mangafas, N.; Demopoulos, C.A.; Antonopoulou, S.; Lazanas, M.C. Anti-platelet-activating factor effects of highly active antiretroviral therapy (HAART): A new insight in the drug therapy of HIV infection? AIDS Res. Hum. Retrovir. 2008, 24, 1079–1086. [Google Scholar] [CrossRef] [PubMed]
- Martí, N.; Mena, P.; Cánovas, J.A.; Micol, V.; Saura, D. Vitamin C and the role of citrus juices as functional food. Nat. Prod. Commun. 2009, 4, 677–700. [Google Scholar] [CrossRef] [Green Version]
- Violi, F.; Pignatelli, P.; Basili, S. Nutrition, supplements and vitamins in platelet function and bleeding. Circulation 2010, 121, 1033–1044. [Google Scholar] [CrossRef]
- Tamer, F.; Tullemans, B.M.E.; Kuijpers, M.J.E.; Claushuis, T.A.M.; Heemskerk, J.W.M. Nutrition Phytochemicals Affecting Platelet Signaling and Responsiveness: Implications for Thrombosis and Hemostasis. Thromb. Haemost. 2021. [Google Scholar] [CrossRef] [PubMed]
- Ling, S.K.; Pisar, M.M.; Man, S. Platelet-activating factor (PAF) receptor binding antagonist activity of the methanol extracts and isolated flavonoids from Chromolaena odorata (L.) King and Robinson. Biol. Pharm. Bull. 2007, 30, 1150–1152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsoupras, A.; Brummell, C.; Kealy, C.; Vitkaitis, K.; Redfern, S.; Zabetakis, I. Cardio-Protective Properties and Health Benefits of Fish Lipid Bioactives; The Effects of Thermal Processing. Mar. Drugs 2022, 20, 187. [Google Scholar] [CrossRef] [PubMed]
- Nasopoulou, C.; Tsoupras, A.B.; Karantonis, H.C.; Demopoulos, C.A.; Zabetakis, I. Fish polar lipids retard atherosclerosis in rabbits by down-regulating PAF biosynthesis and up-regulating PAF catabolism. Lipids Health Dis. 2011, 10, 213. [Google Scholar] [CrossRef] [Green Version]
- Tsoupras, A.B.; Fragopoulou, E.; Iatrou, C.; Demopoulos, C.A. In Vitro Protective Effects of Olive Pomace Polar Lipids towards Platelet Activating Factor Metabolism in Human Renal Cells. Curr. Top. Nutraceutical Res. 2011, 9, 105–110. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).