1. Introduction
Black tea is widely consumed throughout the day in Turkish society, where offering this beverage is considered an indication of hospitality. Indeed, Turkey ranks third in the world for tea consumption, consuming 228,000 tons of black tea according to the Food and Agriculture Organization of the United Nations [
1]. Nonetheless, drinking black tea has been associated with the staining of dental enamel across all regions of the country.
The ideal steeping time for tea is thought to be 5 min and, once steeped, this beverage should be consumed within 30 min. However, in Turkish society, the consumption time after steeping often exceeds this recommended time, and tea is consumed continuously from a teapot placed over low heat on the stove for a whole day. As a consequence, this tea often exhibits an increase in tannins and polyphenol-rich substances, the concentration of which can vary depending on steeping time [
2]. Of these substances, tannins are thought to be responsible for the staining of teeth [
3]. Tannins generally bind to and precipitate proteins [
4,
5]. Various proteins in human saliva also bind to tannins [
6,
7].
Tooth staining can also be caused by chlorhexidine, which is frequently prescribed as a mouthwash to treat infections of the oropharyngeal mucous membrane. Chlorhexidine is absorbed by the pellicle and persists on oral cavity surfaces [
8]. Depending on the application frequency and dosage, chlorhexidine stains oral tissues [
9,
10,
11]. Several studies have investigated the dental staining caused by frequently consumed beverages, such as tea and coffee [
12,
13,
14,
15]. The coloration caused by these drinks is increased when chlorhexidine mouthwash is used [
13,
14,
15]. This scenario is commonly observed in our Faculty of Dentistry clinics of Ankara University, when chlorhexidine mouthwashes are added to home care, and even short-term dark coloration can be observed on both the tongue and teeth (unpublished clinical observations).
Interestingly, the addition of milk causes the chelation of tannins (phenolic hydoxyl group) in the tea by the milk proteins [
16,
17,
18], suggesting that drinking tea with milk could decrease the severity of enamel staining. While previous studies have evaluated whether adding milk to tea reduces antioxidant activity [
19,
20,
21,
22,
23,
24], the effect of milk on dental staining was not evaluated. In addition, a literature search found no studies concerning the extent of enamel staining associated with the consumption of milk tea among individuals who drink large amounts of this beverage. One piece of research [
25] studied the stain-reducing properties of the component of milk on extracted human teeth and determined that casein was responsible for preventing the tea-induced staining of teeth. Given that the tannins in tea would bind to milk proteins (rather than saliva proteins) before entering the oral environment, we hypothesized that enamel staining would decrease if milk was added to tea before drinking. One more query was how varying tannin concentrations, due to the different tea preparation methods, would affect enamel staining. We also wanted to evaluate the effect of chlorhexidine in the same environment. Consequently, the aim of the present study was to evaluate color changes in the enamel surface of human teeth upon exposure to various preparations of Turkish and imported black tea in the presence or absence of milk and/or chlorhexidine. With this aim in mind, we wanted to determine whether milk reduced the staining caused by tea.
2. Materials and Methods
Four young healthy dental professionals voluntarily participated in the saliva collection. All subjects gave their informed consent for inclusion before they participated in the study. The study was conducted in accordance with the Declaration of Helsinki, and the protocol was approved by the Ethics Committee of the Ankara University Faculty of Dentistry (B.30.2.ANK.0.21.63.00/824–02/9–8/61).
2.1. Whole Saliva Sample Collection
The samples were typically collected after a 2 h fast conducted between 13:00 and 15:00 in the afternoon, as the salivary protein content is expected to peak under these conditions. Samples were stored at −80 °C until required for the experiments.
2.2. Selection of the Teeth
A total of 36 extracted human maxillary first incisors with a planar labial surface were selected from the pool of teeth, which were mostly extracted due to advanced periodontal disease in the Department of Oral Surgery of our faculty. The teeth were similar in size and had no enamel fractures.
2.3. Preparation of the Teeth
All root canals of the teeth were prepared to prevent any internal staining from pulp remnants. For this purpose, the apical one-third of the roots was cut using diamond burs. The pulp was removed, and root canals were prepared using K and H files up to #40. Distilled water and sodium hypochlorite solution were used to irrigate the root canals. The tips of the root canals were then sealed with a bonding agent (Solo Bond; VOCO GmbH, Cuxhaven, Germany) and a composite resin (Clearfil Majesty Estethic; Kuraray Dental). Existing stains on the teeth were removed using rotating rubber prophy cups and paste for 5 s each. The prepared teeth were stored in distilled water at room temperature until required for the experiments.
A colorimeter (CR-321 Chromascop Minolta, Osaka, Japan) was used to evaluate the degree of staining in our study. The working principle of the device is based on sending light to the samples three times to obtain a color measurement, which is given as the average of three measurements. This method reduced potential error in the measurements and increased reliability.
2.4. Preparation of the Stabilizer Plate and Acrylic Resin Blocks for Colorimetry
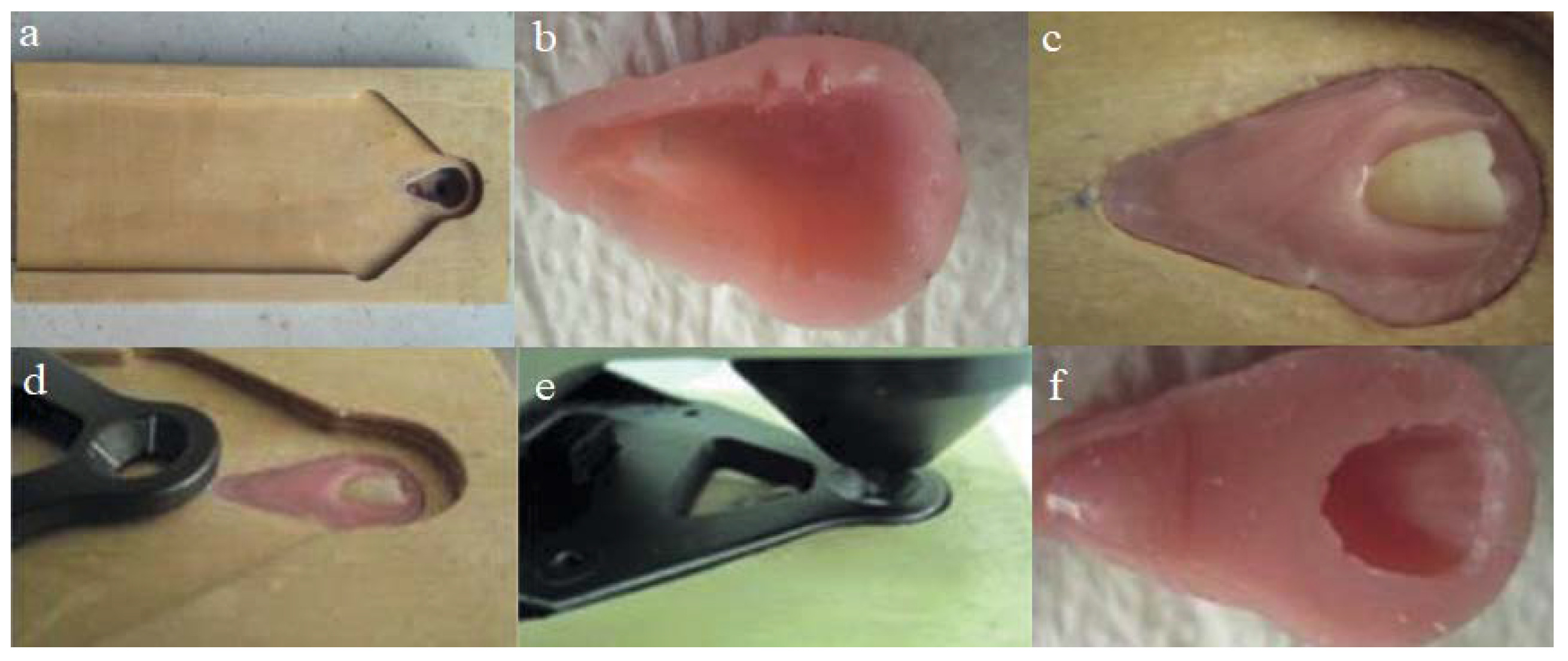
To standardize the measurements while using the colorimeter, we prepared a stable wooden plate that ensured the teeth were always placed in the same alignment. A socket for the acrylic block was carved on the wooden plate (
Figure 1a).
This socket was lined with an isolation material, filled with an acrylic resin, and allowed to polymerize. The acrylic block was then carved, and a few retention grooves were prepared (
Figure 1b). To stabilize the teeth, an acrylic socket was filled with soft acrylic denture lining material (Ufi Gel P-VOCO GmbH, Cuxhaven, Germany), and the tooth was immersed in the acrylic block (
Figure 1c). The measuring part of the colorimeter was placed on the middle third of the tooth crown, and complete polymerization was allowed to occur (
Figure 1d,e). When polymerization was complete, the tooth was carefully removed (
Figure 1f). The entire procedure was repeated, and specific acrylic blocks were prepared and numbered for each tooth. The prepared teeth were stored in distilled water until required for the experiments.
2.5. Measurement of Tooth Color
We used the measuring module of L* for lightness, and opponent color axes a* for redness–greenness versus b* for yellowness–blueness in this study, and calibrated the colorimeter before the measurements (L* = 93.05; a* = −4.84; b* = 6.95). Calibration was performed according to the manufacturer’s manual using the calibration plate in the same environment. Measurements were performed using daylight bulbs (Sylvania Activa 172, Erlangen, Germany). Following calibration, measurements were performed at baseline (∆E
1) and repeated after the experiments (∆E
2). Data were recorded as L*, a*, b* and total color difference was calculated using the formula [
26] ΔE = [(ΔL*)
2 + (Δa*)
2 + (Δb*)
2]
½. 2.6. Tea Preparation
We made four main tea preparations, as described below:
A 2 g tea bag that was made in Turkey (Çaykur, Rize, Turkey) was added to 100 mL of boiling hot distilled water in a glass beaker and left to infuse for 5 min.
Two grams of tea leaves from Turkey (Çaykur, Rize, Turkey) were allowed to steep for 30 min in 100 mL of distilled water in a teapot over a teakettle filled with boiling water.
An imported (Twinings, London, UK) 2 g tea bag was added to 100 mL of boiling hot water in a glass beaker and left to infuse for 5 min.
Two grams of imported tea leaves (Twinings, London, UK) were allowed to steep for 30 min in 100 mL of distilled water in a teapot over a teakettle filled with boiling water.
Then, previously collected saliva samples were thawed and used in further tea preparations. A total of 18 different combinations of tea solutions with or without milk and/or chlorhexidine were prepared (presented in the
supplementary section). Pasteurized skim milk was used to prepare the milk tea, and chlorhexidine gluconate (0.02%) mouthwash (Corsodyl, GlaxoSmithKline, Brentford, UK) was used to prepare some of the tea preparations. The study was designed as CHX was used as a mouthwash right after drinking milk tea in real life.
2.7. Study Design
To minimize the trial error and prevent bias in the study, we used a replicated Latin square experimental design (presented in the
supplementary section). The 36 teeth were divided into two groups of 18 teeth. Each group was then exposed to 18 different solutions. Each tooth in the group was exposed to all 18 solutions on one occasion per solution.
The prepared solutions were pipetted into separate glass tubes containing the teeth and incubated in a water bath shaker at 50 rpm, 50 °C for 24 h. The teeth were then removed and washed with distilled water, and measurements of color change were performed. Following colorimetric analysis, the stains on the teeth were removed using rotating rubber prophy cups and paste for 5 s each, and the teeth were used for the next experiment.
The experimental procedure was repeated 18 times. Teeth numbered from 1 to 18 in each of the two groups were shifted by one each time and exposed to different solutions in each experiment. Therefore, upon completion of the 18 tests, each tooth had been exposed to each solution only once.
2.8. Statistical Analysis
All the data obtained in the study were analyzed using the SPSS Statistics for Windows version 15.0 statistical analysis program (IBM Corp., Armonk, NY, USA). Descriptive statistics are presented as the mean ± standard deviation. The differences between the solutions and the solution groups were evaluated by one-way analysis of variance. The Tukey test was used for the post hoc identification of key variables in the analysis of variance. A value of p < 0.05 was considered statistically significant.
3. Results
3.1. Colorimetric Findings
Colorimetric measurements were performed before and after the experiments to reveal the intensity of color changes due to teeth’s exposure to the solutions. The ΔE, ΔL, Δa, and Δb values for the 18 solutions used in this study are presented in
Table 1.
The ΔE, ΔL, Δa, and Δb were obtained by subtracting the baseline measurements from the post-trial measurements. A negative ΔL value indicates a color change from white toward black, whereas a positive ΔL value indicates a color change from black toward white. A negative Δa value indicates a color change from red toward green, whereas a positive Δa value indicates a color change from green toward red. A negative Δb value indicates a shift toward blue, whereas a positive Δb value indicates a yellow color.
The total color changes (ΔE) and the color changes on the redness–greenness axis (Δa) data do not follow a specific trend depending on the solution used to treat the tooth surface (
Table 1). The color changes in the teeth caused by all types of tea were found to have negative ΔL values when only milk was added (
Table 1). By contrast, the color changes were caused by all types of tea, with the exception of the UK steeped tea + chlorhexidine solution, which had positive ΔL values when chlorhexidine was added in the absence of milk (
Table 1). The solutions prepared by adding only milk to the tea caused a color change in the enamel surface toward blue on the yellow–blue color scale (Δb). By contrast, in the solution groups in which chlorhexidine was added to the tea, the color change caused on teeth was in the yellow direction regardless of whether milk was added (
Table 1).
When the effect of chlorhexidine solution alone on tooth enamel was evaluated in terms of ΔL values, the color change occurred in the white direction, and the color changes were toward green and yellow for Δa, and Δb, respectively (
Table 1).
Taken together, these results suggest that when milk alone was added to tea, it mitigated the color change values of yellowness and changed them toward the blue direction. On the contrary, when chlorhexidine alone was in the environment, either alone or in milk, together with the tested tea, the color change trend was always toward yellow.
3.2. Comparison of the Color Changes on the Enamel Surface
Among the solutions used in this study, the color-changing effects of Turkish and UK tea with only milk added were compared with those where milk + chlorhexidine was added, and the paired comparison of those solutions revealed no statistically significant differences in the ΔE and Δa values (
Table 2). For the ΔL values, all the comparisons were statistically significant, and for Δb, we found statistically significant differences for all the groups except for the comparison between Turkish steeped tea with milk and Turkish steeped tea with milk and chlorhexidine (
Table 2).
The tea solutions, regardless of the country of origin of tea, were grouped as those containing tea only, tea + milk, tea + milk + chlorhexidine and tea + chlorhexidine, and the colorimetric findings are presented in
Table 3.
The color changes in the tea-only and tea + milk groups were from white toward black (
Table 3). The color change in the tea + milk + chlorhexidine and tea + chlorhexidine groups was from black toward white (
Table 3). Color changes in all groups were toward green in the Δa. Regarding Δb, the descriptive statistics indicate that the color change for tea + milk was toward blue on the yellow–blue scale, whereas this change was towards yellow for the tea-only, tea + milk + chlorhexidine and tea + chlorhexidine groups (
Table 3).
Further statistical comparisons of ΔE, ΔL, Δa and Δb are presented in
Table 4.
Statistically significant differences in total color difference (ΔE) values were noted between the tea-only groups and tea + milk groups as well as tea + chlorhexidine groups (
p < 0.001 for both comparisons;
Table 4).
Regarding ΔL values, statistically significant differences were noted between the tea-only groups and tea + milk-, tea + milk + chlorhexidine- and tea + chlorhexidine-containing groups (
p < 0.001,
p < 0.001,
p < 0.007, respectively;
Table 4). A statistically significant difference in ΔL values was also observed (
p < 0.001) between tea + milk and tea + milk + chlorhexidine groups. No statistically significant differences were observed between the tea + chlorhexidine and tea + milk + chlorhexidine groups (
p > 0.05). When these two groups were compared, the value for the tea + milk group was greater than that for the tea-only group (
p < 0.001;
Table 4). Compared with the tea-only group, the difference was significant for both of these groups (
p < 0.001 and
p < 0.007, respectively).
No statistically significant difference in Δa was observed (
Table 4).
Between-group comparisons of Δb among revealed statistically significant differences between the tea (0.1879) group and the tea + milk (−0.7210) and tea + chlorhexidine (0.8224) groups (
p < 0.001 for both comparisons;
Table 4). Comparisons of the tea + milk + chlorhexidine (0.2449) group with the tea + milk (−0.7210) and tea + chlorhexidine (0.8224) groups also revealed statistically significant differences (
p < 0.001 for both comparisons). However, no statistically significant difference was found for the tea + milk + chlorhexidine (0.2449) group versus the tea-only (0.1879) group (
Table 4).
These detailed results show that tea increases Δb readings; when chlorhexidine is included in tea, the Δb readings further increased. When milk is included in tea, Δb readings decreased to minus values, and when chlorhexidine is included in milk tea, milk was still effective in decreasing Δb readings but with a lower strength.
No statistically significant differences were found between the distilled water only and the other 17 solutions to which the teeth were exposed. The distilled water group exhibited a color change in the white direction on the black–white scale. This finding might indicate that the color could be lightened in the subsequent 18 experimental procedures. When the tooth was subjected to another solution in the previous test procedure, it was then immersed in distilled water in the following procedure.
4. Discussion
When tea was used together with chlorhexidine, a substantial color change was observed in our study. Milk reduced the staining caused by tea and neutralized some of the yellow staining caused by chlorhexidine. The effect of milk tea with chlorhexidine on staining was not previously investigated, and this is the first study to address this question.
All the chlorhexidine solutions might have been expected to cause black staining on the basis of clinical observations of darkening of the teeth and tongue among individuals who use chlorhexidine-containing mouthwashes. However, this darkening was not observed, indicating that the chlorhexidine stains on teeth are not black.
While previous studies have described teeth staining by chlorhexidine [
11,
27,
28], they did not investigate the color scale of the staining. Thus, our study is the first to report that chlorhexidine causes color changes in the white and yellow directions on the color scale. In those reports, the researchers used spectrophotometry, light microscopic and color television images or ellipsometry to analyze the staining. We used a colorimeter, which made it possible to analyze the staining in three dimensions. In the previous studies, the tea type or preparation of the tea was not considered, but in our study, we wanted to see the difference in staining effect caused by different types of tea and different types of tea preparations; furthermore, we wanted to find a solution to the staining caused by tea and mouth rinse by adding milk to the solutions that we tested.
In general, tea causes a yellow coloration. When milk was added to tea, the yellow coloring was inhibited, and the coloration became blue. Turkish infusion tea + milk, Turkish steeped tea + milk, UK infusion tea + milk and UK steeped tea + milk were associated with color change to blue. All tea-only applications (Turkish infusion tea, Turkish steeped tea, UK infusion tea and UK steeped tea) and their chlorhexidine-added versions were associated with a color change to yellow. The color change values in the Turkish infusion tea + chlorhexidine and Turkish steeped tea + chlorhexidine groups were greater (1.1931 ± 1.5464 and 1.0236 ± 1.4807, respectively) than the other groups and in the yellow direction.
However, when chlorhexidine was added, regardless of whether the solution contained milk, the coloration tended toward yellow again.
Optical whiteners and/or polishers—used in textiles, detergents, cosmetics and paper products—reflect visible blue light by absorbing invisible ultraviolet and violet light. Consequently, these agents increase the blue effect and reduce the yellow effect, thereby creating an optical illusion of whitening that makes the color appear brighter to the eye. Optical whiteners are reported to penetrate the products with the blue color they contain and neutralize the yellow color tone in the product, thus making the product appear whiter [
29].
In our present study, the staining on teeth exposed to the solutions obtained by adding milk to tea occurred in the blue direction on the yellow–blue scale. Given that yellow and blue are on the same scale, the yellow color ratio decreases as the blue color ratio increases. This effect makes teeth appear clinically whiter; however, this effect is only an optical whiteness. Since our data suggest that some solutions, especially those containing milk, can cause optical illusions, to determine whether these findings are reflected clinically, further in vivo studies could involve treating the teeth with milk and observing the color changes in the dental surfaces before and after using numerical scales or a standard tooth color scale, such as the vita scale. The results of such a study may lead to further studies on the product development of new oral optical whiteners/brighteners containing milk and no other harmful ingredients, such as abrasives or chemical bleaches.
Furthermore, the whitening effect of milk needs to be investigated when it is used in conjunction with other beverages that cause tooth staining, such as coffee.
In the present study, we searched for the darkening effect of chlorhexidine when used together with tea and the brightening effect of milk in that condition. There is a need to show the brightening effect of milk on chlorhexidine staining when it is used alone without the additive effect of tea.
In our study, the total color change that occurred in the tea + chlorhexidine group (2.63631 ± 3.87976) was greater compared to that in the tea-only group (1.47102 ± 1.223), which is consistent with previous reports [
11,
13,
14].
The neutralizing blue effect of milk is strongest for tea alone and weakest in the presence of chlorhexidine. Paired comparisons revealed the same level of statistical significance as in the three comparisons for Δb (p < 0.05). However, the value for tea + chlorhexidine was the highest, which was followed by tea + milk (with minus value) and tea + milk + chlorhexidine. The lowest value was obtained for the tea-only group. This finding reveals how yellowness and blueness changes are neutralized according to the concentration of the agents used. The mechanism underpinning the optical illusion caused by the addition of milk is unknown; however, two potential factors might be considered. First, milk neutralizes the yellow staining caused by tea by providing a blue coloration. Second, the ability of milk proteins such as casein to bind to tannins could prevent tea from staining tooth enamel yellow. Further studies are required to discriminate between these two possibilities.
5. Conclusions
In conclusion, the hypothesis of our study is based on the whiteness effect of blue tones, which is formed in tea solutions to which milk is added. The whitening effect of milk was also seen with chlorhexidine, and this can be tested for other stain-forming beverages such as coffee, coke and wine in future studies. Here, we did not consider any order in which the agents should be applied to the tooth surface, and we applied our solutions by mixing the components at the start. It is possible that patients are recommended to consume tea with some milk added, especially if they use mouthwashes. However, there are other beverages (e.g., wine and coke) where the addition of milk is not preferable; instead, it can be used before or after. Additionally, the question of how mouthwash users can benefit from the whitening effect of milk can be considered a topic of the future studies. Although our study responds to a number of questions about the properties of dental staining by tea and chlorhexidine and reversing it by milk, it offers new questions and different perspectives, especially regarding the role of milk in tooth enamel staining. It can be helpful to find whether the order of exposure to milk can change the degree of staining so that it can be used before or after consuming any cold beverage. Can milk be used in some innovated brightening products? Thus, our results suggest that further studies may be performed to examine the structure of milk proteins and the compound resulting from the interaction with tea in more detail.
 ± SD
± SD ± SD
± SD ± SD
± SD ± SD
± SD .
. .
.





