A Digital Approach to Evaluate the Effect of Berry Cell Death on Pinot Noir Wines’ Quality Traits and Sensory Profiles Using Non-Destructive Near-Infrared Spectroscopy
Abstract
1. Introduction
2. Materials and Methods
2.1. Vineyard and Berry Sampling Description
2.2. Living and Dead Mesocarp Tissue Assessment—Berries
2.2.1. Berry Analysis
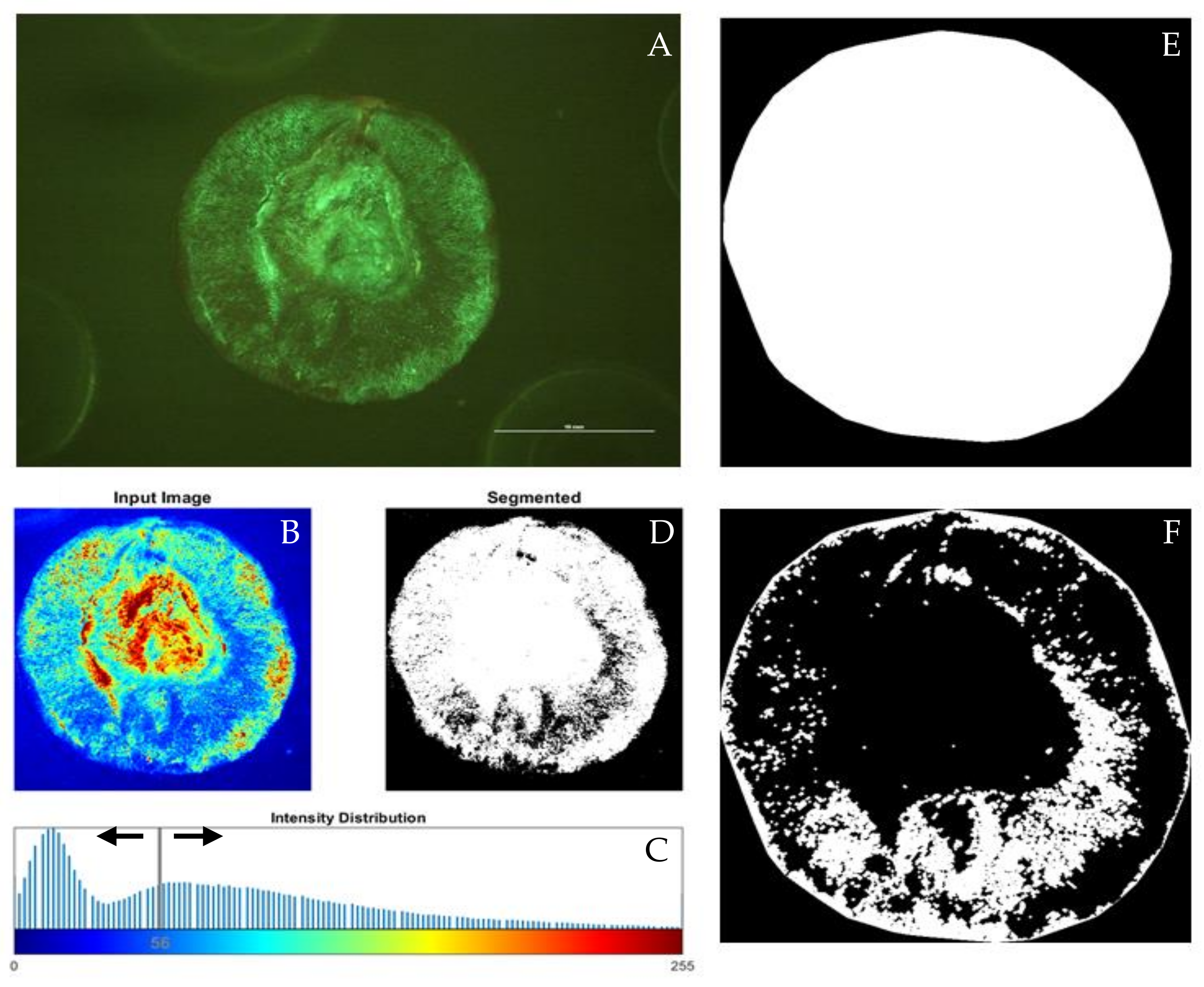
2.2.2. Analysis of Berry Tissue Vitality MATLAB® Code Improvement
2.3. Near-Infrared Spectroscopy—Berries
2.4. Descriptive Sensory Evaluation—Wines
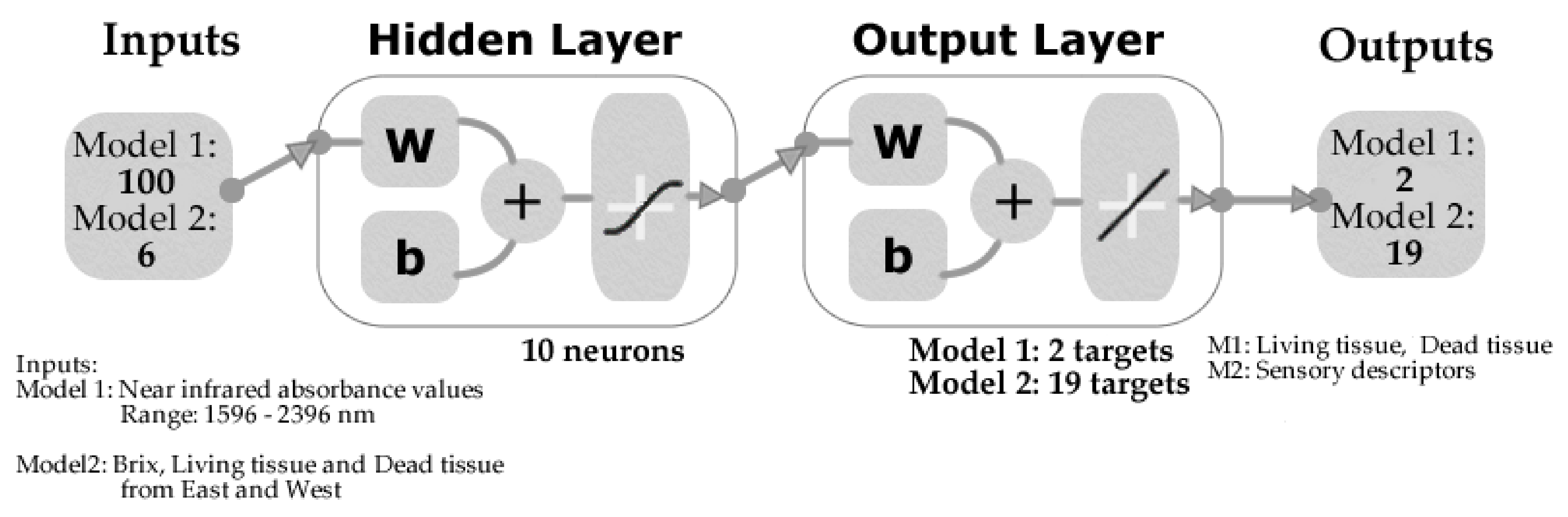
2.5. Statistical Analysis and Machine Learning Modeling
3. Results
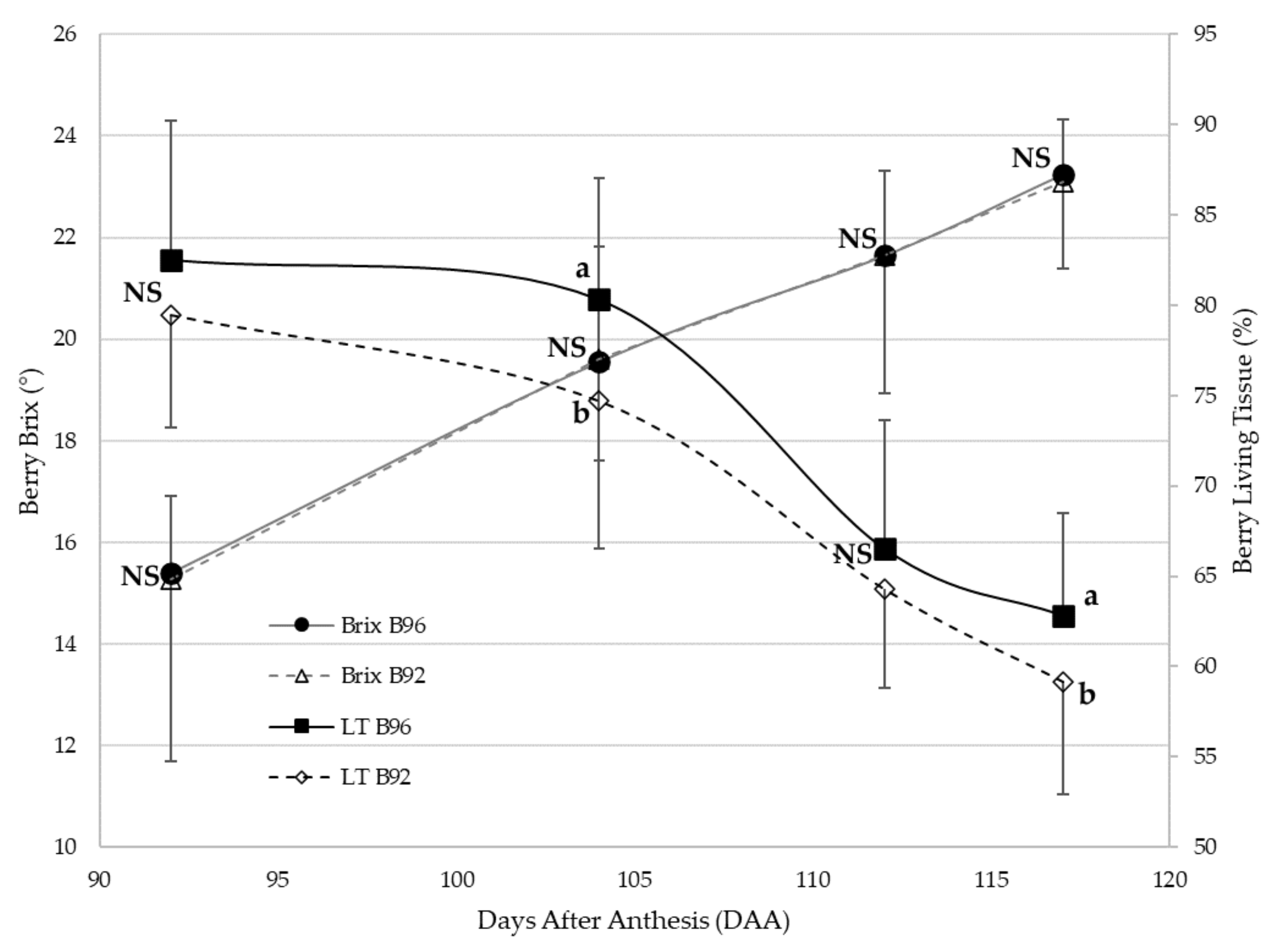
3.1. LT and Brix Patterns through the Season
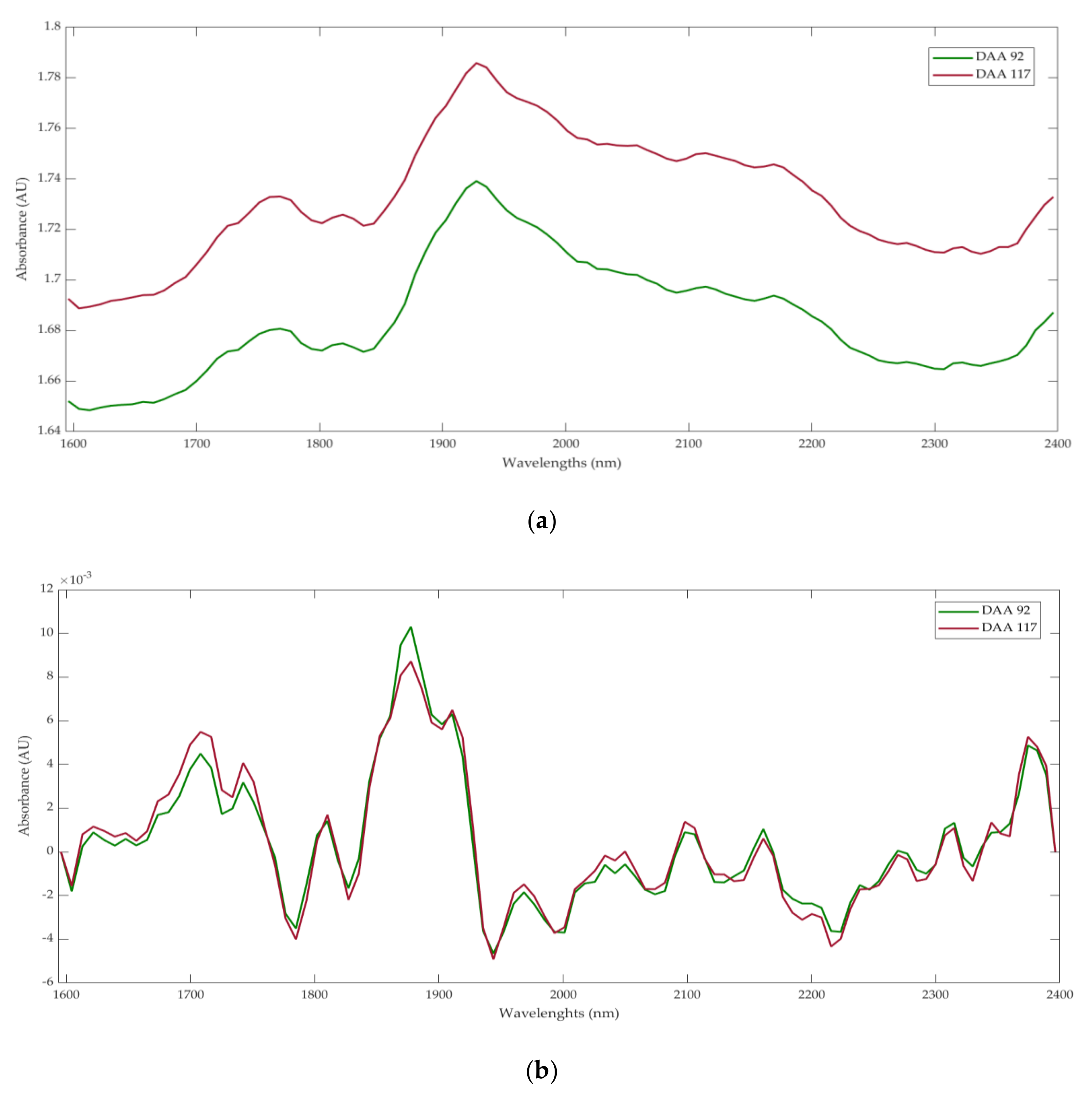
3.2. Near-Infrared Spectroscopy
3.3. Descriptive Sensory Evaluation
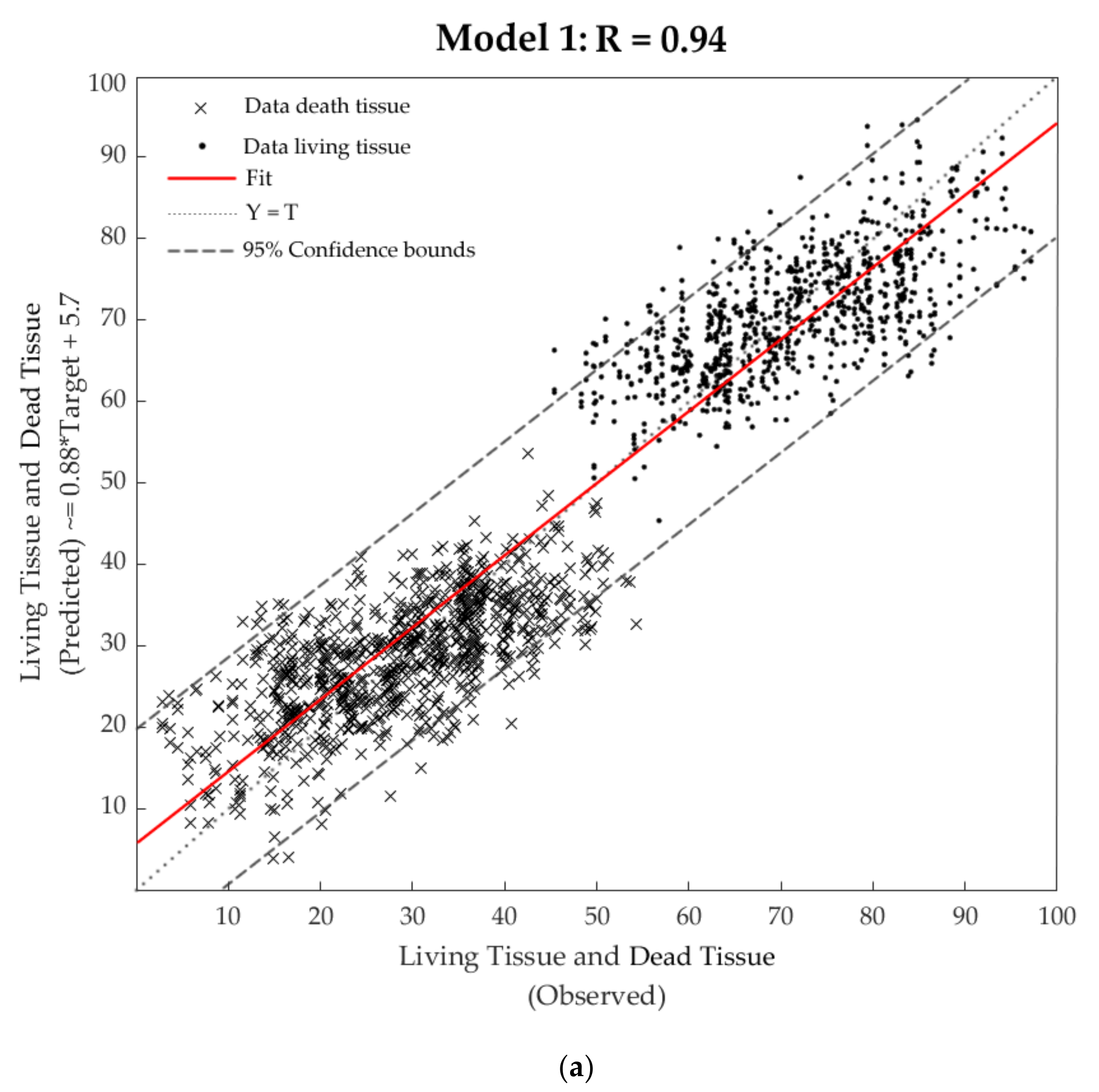
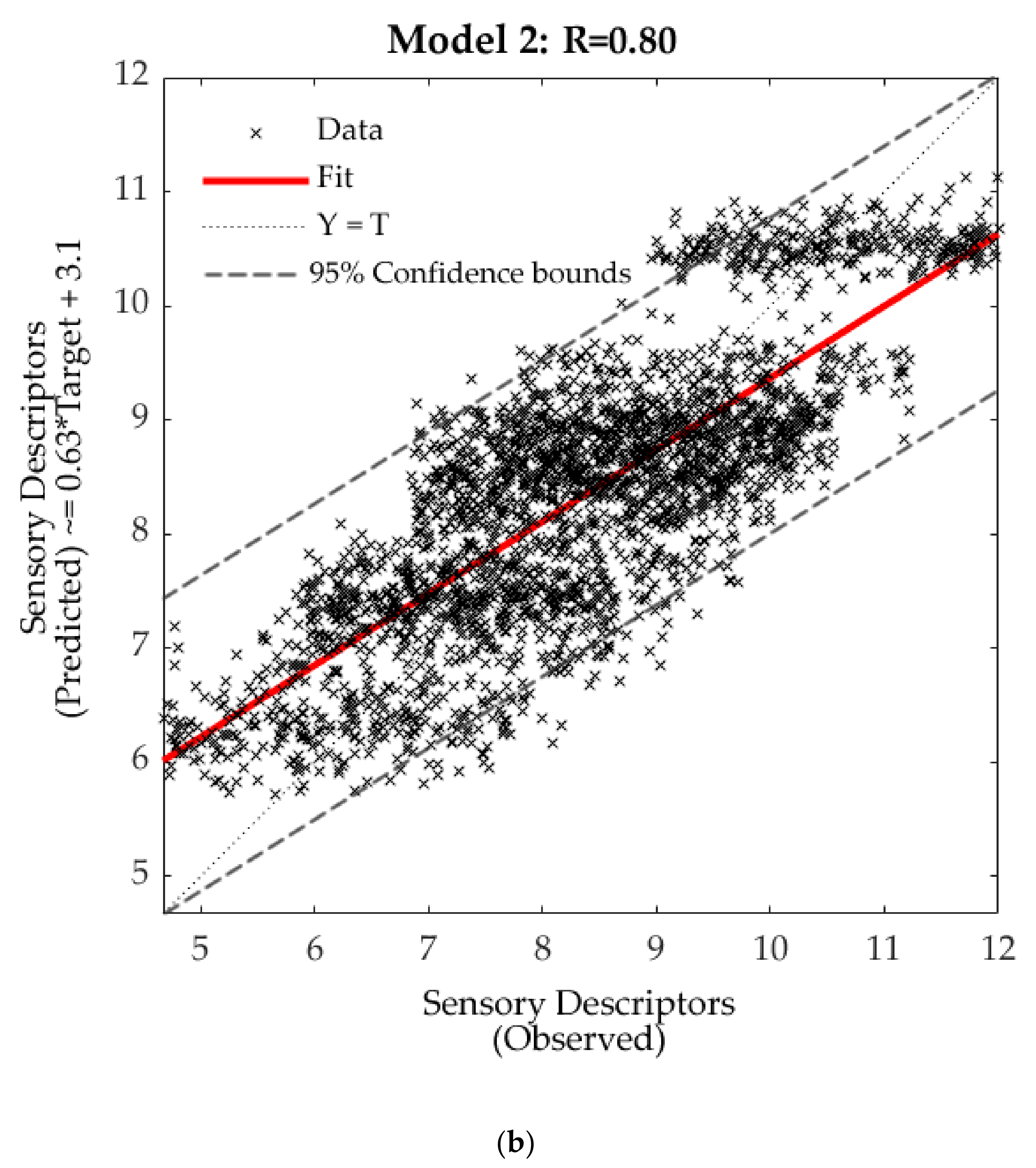
3.4. Machine Learning Models
4. Discussion
4.1. Berry LT and Brix Dynamics
4.2. Wine Sensory Profiles
4.3. Machine Learning Modeling for LT and Sensory Descriptors
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Fuentes, S.; Sullivan, W.; Tilbrook, J.; Tyerman, S. A novel analysis of grapevine berry tissue demonstrates a variety-dependent correlation between tissue vitality and berry shrivel. Aust. J. Grape Wine Res. 2010, 16, 327–336. [Google Scholar] [CrossRef]
- Tilbrook, J.; Tyerman, S.D. Cell death in grape berries: Varietal differences linked to xylem pressure and berry weight loss. Funct. Plant Biol. 2008, 35, 173–184. [Google Scholar] [CrossRef]
- Xiao, Z.; Rogiers, S.; Sadras, V.; Tyerman, S. Hypoxia in the grape berry linked to mesocarp cell death: The role of seed respiration and lenticels on the berry pedicel. bioRxiv 2017, 209890. [Google Scholar] [CrossRef]
- Clarke, S.J.; Rogiers, S.Y. The role of fruit exposure in the late season decline of grape berry mesocarp cell vitality. Plant Physiol. Biochem. 2019, 135, 69–76. [Google Scholar] [CrossRef]
- Krasnow, M.; Matthews, M.; Shackel, K. Evidence for substantial maintenance of membrane integrity and cell viability in normally developing grape (vitis vinifera l.) berries throughout development. J. Exp. Bot. 2008, 59, 849–859. [Google Scholar] [CrossRef] [PubMed]
- Bonada, M.; Sadras, V.O.; Fuentes, S. Effect of elevated temperature on the onset and rate of mesocarp cell death in berries of shiraz and chardonnay and its relationship with berry shrivel. Aust. J. Grape Wine Res. 2013, 19, 87–94. [Google Scholar] [CrossRef]
- Bonada, M.; Sadras, V.; Moran, M.; Fuentes, S. Elevated temperature and water stress accelerate mesocarp cell death and shrivelling, and decouple sensory traits in shiraz berries. Irrig. Sci. 2013, 31, 1317–1331. [Google Scholar] [CrossRef]
- Bondada, B.; Keller, M. Morphoanatomical symptomatology and osmotic behavior of grape berry shrivel. J. Am. Soc. Hortic. Sci. 2012, 137, 20–30. [Google Scholar] [CrossRef]
- Keller, M.; Shrestha, P.M.; Hall, G.E.; Bondada, B.R.; Davenport, J.R. Arrested sugar accumulation and altered organic acid metabolism in grape berries affected by berry shrivel syndrome. Am. J. Enol. Vit. 2016, 67, 398–406. [Google Scholar] [CrossRef]
- Tilbrook, J.; Tyerman, S.D. Hydraulic connection of grape berries to the vine: Varietal differences in water conductance into and out of berries, and potential for backflow. Funct. Plant Biol. 2009, 36, 541–550. [Google Scholar] [CrossRef]
- Griesser, M.; Martinez, S.C.; Weidinger, M.L.; Kandler, W.; Forneck, A. Challenging the potassium deficiency hypothesis for induction of the ripening disorder berry shrivel in grapevine. Sci. Hortic. 2017, 216, 141–147. [Google Scholar] [CrossRef]
- Rogiers, S.Y.; Holzapfel, B.P. The plasticity of berry shrivelling in ‘shiraz’: A vineyard survey. Vitis-J. Grapevine Res. 2015, 54, 1–8. [Google Scholar]
- Krasnow, M.; Matthews, M.; Smith, R.; Benz, J.; Weber, E.; Shackel, K. Distinctive symptoms differentiate four common types of berry shrivel disorder in grape. Calif. Agric. 2010, 64, 155–159. [Google Scholar] [CrossRef]
- Caravia Bayer, L. Heat Wave Mitigation Strategies for Wine Grape Production and Measures of the Impact of Heat on Berry Ripening and Wine Composition; University of Adelaide, School of Agriculture, Food and Wine: Adelaide, SA, Australia, 2016. [Google Scholar]
- Tyerman, S.D.; Fuentes, S.; Collins, C.; Bastian, S. Is the Shiraz berry the biggest loser? Grapegrow. Winemak. 2012, 583, 42–44. [Google Scholar]
- Xiao, Z.; Liao, S.; Rogiers, S.; Sadras, V.O.; Tyerman, S. Effect of water stress and elevated temperature on hypoxia and cell death in the mesocarp of Shiraz berries. Aust. J. Grape Wine Res. 2018, 24, 487–497. [Google Scholar] [CrossRef]
- Pilati, S.; Brazzale, D.; Guella, G.; Milli, A.; Ruberti, C.; Biasioli, F.; Zottini, M.; Moser, C. The onset of grapevine berry ripening is characterized by ROS accumulation and lipoxygenase-mediated membrane peroxidation in the skin. BMC Plant Biol. 2014, 14, 87. [Google Scholar] [CrossRef]
- Hardie, W.J.; O’Brien, T.P.; Jaudzems, V.G. Morphology, anatomy and development of the pericarp after anthesis in grape, Vitis vinifera L. Aust. J. Grape Wine Res. 1996, 2, 97–142. [Google Scholar] [CrossRef]
- Bonada, M. The impact of Water Deficit and High Temperature on Berry Biophysical Traits and Berry and Wine Chemical and Sensory Traits. Ph.D. Thesis, University of Adelaide, School of Agriculture, Food and Wine, Adelaide, SA, Australia, 2014. [Google Scholar]
- Gonzalez Viejo, C.; Torrico, D.D.; Dunshea, F.R.; Fuentes, S. Emerging technologies based on artificial intelligence to assess the quality and consumer preference of beverages. Beverages 2019, 5, 62. [Google Scholar] [CrossRef]
- Fuentes, S.; Gonzalez Viejo, C.; Wang, X.; Torrico, D.D. Aroma and quality assessment for vertical vintages using machine learning modelling based on weather and management information. In Proceedings of the 21st Giesco, Thessaloniki, Greece, 23–28 June 2019; pp. 23–28. [Google Scholar]
- Fuentes, S.; Chacon, G.; Torrico, D.D.; Zarate, A.; Gonzalez Viejo, C. Spatial variability of aroma profiles of cocoa trees obtained through computer vision and machine learning modelling: A cover photography and high spatial remote sensing application. Sensors 2019, 19, 3054. [Google Scholar] [CrossRef]
- Fuentes, S.; Hernández-Montes, E.; Escalona, J.M.; Bota, J.; Gonzalez Viejo, C.; Poblete-Echeverría, C.; Tongson, E.; Medrano, H. Automated grapevine cultivar classification and water stress assessment based on machine learning using leaf morpho-colorimetry, fractal dimension and near-infrared spectroscopy. Comput. Electron. Agric. 2018, 151, 311–318. [Google Scholar] [CrossRef]
- Fuentes, S.; Tongson, E.J.; De Bei, R.; Gonzalez Viejo, C.; Ristic, R.; Tyerman, S.; Wilkinson, K. Non-invasive tools to detect smoke contamination in grapevine canopies, berries and wine: A remote sensing and machine learning modeling approach. Sensors 2019, 19, 3335. [Google Scholar] [CrossRef] [PubMed]
- Fuentes, S.; Tongson, E.; Torrico, D.D.; Gonzalez Viejo, C. Modeling pinot noir aroma profiles based on weather and water management information using machine learning algorithms: A vertical vintage analysis using artificial intelligence. Foods 2020, 9, 33. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez Viejo, C.; Fuentes, S.; Torrico, D.; Howell, K.; Dunshea, F.R. Assessment of beer quality based on foamability and chemical composition using computer vision algorithms, near infrared spectroscopy and machine learning algorithms. J. Sci. Food Agric. 2018, 98, 618–627. [Google Scholar] [CrossRef] [PubMed]
- Pimenta, A.M.; Scafi, S.H.; Pasquini, C.; Raimundo, I.M., Jr.; Rohwedder, J.J.; Montenegro, M.D.; Araújo, A.N. Determination of hydrogen peroxide by near infrared spectroscopy. J. Near Infrared Spectrosc. 2003, 11, 49–53. [Google Scholar] [CrossRef]
- De Bei, R.; Cozzolino, D.; Sullivan, W.; Cynkar, W.; Fuentes, S.; Dambergs, R.; Pech, J.; Tyerman, S. Non-destructive measurement of grapevine water potential using near infrared spectroscopy. Aust. J. Grape Wine Res. 2011, 17, 62–71. [Google Scholar] [CrossRef]
- Fuentes, S.; Gonzalez Viejo, C.; Torrico, D.D.; Dunshea, F.R. Development of a biosensory computer application to assess physiological and emotional responses from sensory panelists. Sensors 2018, 18, 2958. [Google Scholar] [CrossRef]
- Xiao, Z.; Rogiers, S.Y.; Sadras, V.O.; Tyerman, S.D. Hypoxia in grape berries: The role of seed respiration and lenticels on the berry pedicel and the possible link to cell death. J. Exp. Bot. 2018, 69, 2071–2083. [Google Scholar] [CrossRef]
- Sadras, V.O.; Moran, M.A. Elevated temperature decouples anthocyanins and sugars in berries of Shiraz and cabernet franc. Aust. J. Grape Wine Res. 2012, 18, 115–122. [Google Scholar] [CrossRef]
- Zhang, P.; Barlow, S.; Krstic, M.; Herderich, M.; Fuentes, S.; Howell, K. Within-vineyard, within-vine, and within-bunch variability of the rotundone concentration in berries of vitis vinifera l. Cv. Shiraz. J. Agric. Food Chem. 2015, 63, 4276–4283. [Google Scholar] [CrossRef]
- Zhang, P.; Howell, K.; Krstic, M.; Herderich, M.; Barlow, E.W.; Fuentes, S. Environmental factors and seasonality affect the concentration of rotundone in vitis vinifera l. Cv. Shiraz wine. PLoS ONE 2015, 10, e0133137. [Google Scholar] [CrossRef]
- Moskowitz, H.R.; Muñoz, A.M.; Gacula, M.C., Jr. Viewpoints and Controversies in Sensory Science and Consumer Product Testing; Food & Nutrition Press, Inc.: Trumbull, CT, USA, 2008. [Google Scholar]
- FAO. Sensory assessment of fish quality. In Torry Advisory; Torry Research Station, MAFF: Aberdeen, UK, 2001; Volume 91. [Google Scholar]
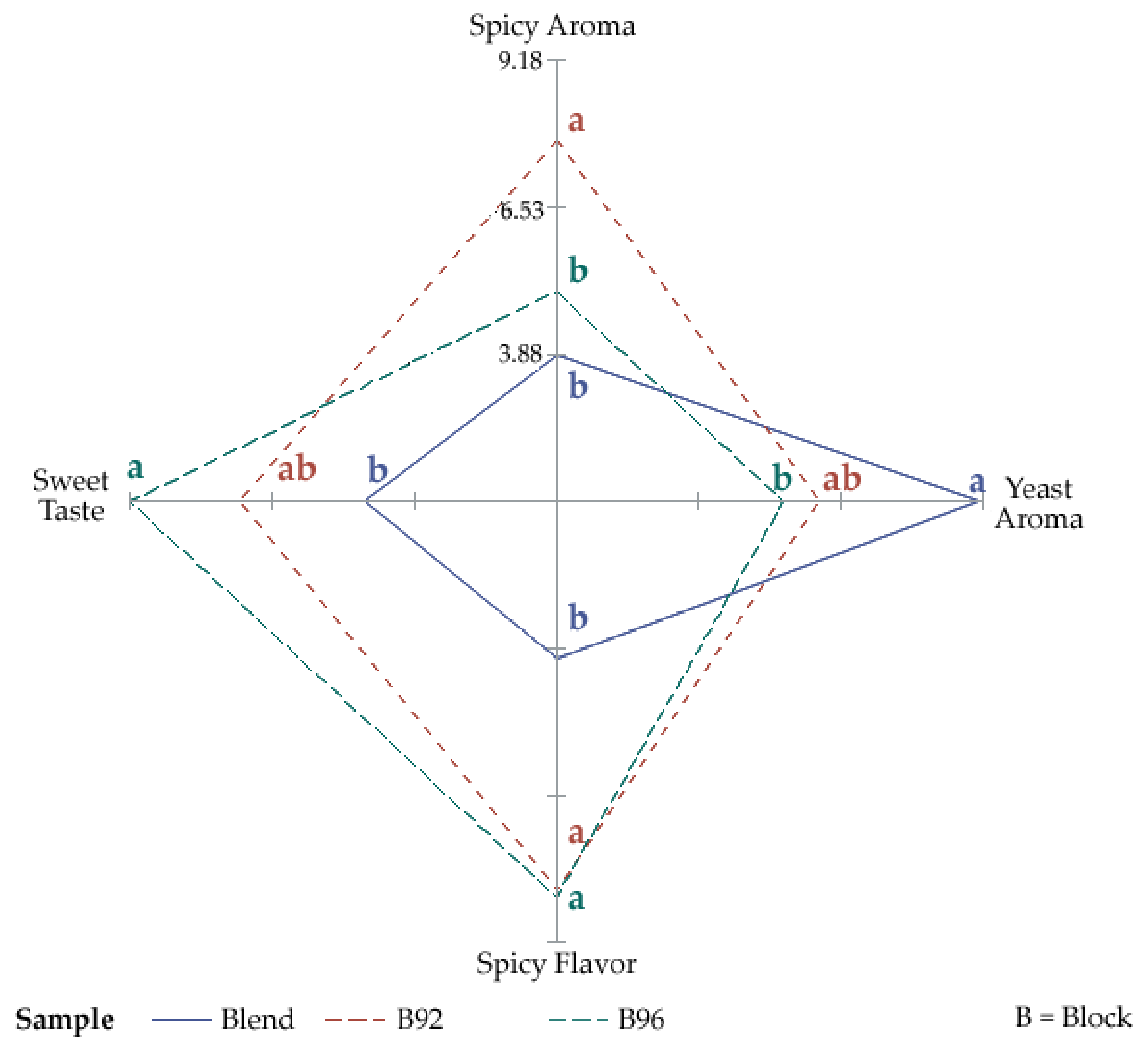
| Descriptor | Abbreviations |
|---|---|
| Color intensity | CInt |
| Red fruits aroma | ARF |
| Black fruits aroma | ABF |
| Yeast aroma | AYeast |
| Spicy aroma | ASpicy |
| Floral aroma | AFloral |
| Oak aroma | AOak |
| Sweet aroma | ASweet |
| Sweet taste | TSweet |
| Acidic taste | TAcidic |
| Bitter taste | TBitter |
| Oak flavor | FOak |
| Herbs flavor | FHerbs |
| Red fruits flavor | FRF |
| Black fruits flavor | FBF |
| Spicy flavor | FSpicy |
| Body | Body |
| Astringency | Astringency |
| Warming mouthfeel | MWarm |
| Stage | Samples | Observations (Samples x Targets) | R | Performance (MSE) | Slope |
|---|---|---|---|---|---|
| Model 1—Living and dead tissue | |||||
| Training | 600 | 1200 | 0.95 | 56 | 0.88 |
| Validation | 129 | 258 | 0.93 | 71 | 0.92 |
| Testing | 129 | 258 | 0.92 | 81 | 0.85 |
| Overall | 858 | 1716 | 0.94 | - | 0.88 |
| Model 2—Sensory descriptors | |||||
| Training | 100 | 1900 | 0.81 | 0.76 | 0.64 |
| Validation | 22 | 418 | 0.81 | 0.83 | 0.61 |
| Testing | 22 | 418 | 0.76 | 0.99 | 0.59 |
| Overall | 144 | 2736 | 0.80 | - | 0.63 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fuentes, S.; Tongson, E.; Chen, J.; Gonzalez Viejo, C. A Digital Approach to Evaluate the Effect of Berry Cell Death on Pinot Noir Wines’ Quality Traits and Sensory Profiles Using Non-Destructive Near-Infrared Spectroscopy. Beverages 2020, 6, 39. https://doi.org/10.3390/beverages6020039
Fuentes S, Tongson E, Chen J, Gonzalez Viejo C. A Digital Approach to Evaluate the Effect of Berry Cell Death on Pinot Noir Wines’ Quality Traits and Sensory Profiles Using Non-Destructive Near-Infrared Spectroscopy. Beverages. 2020; 6(2):39. https://doi.org/10.3390/beverages6020039
Chicago/Turabian StyleFuentes, Sigfredo, Eden Tongson, Juesheng Chen, and Claudia Gonzalez Viejo. 2020. "A Digital Approach to Evaluate the Effect of Berry Cell Death on Pinot Noir Wines’ Quality Traits and Sensory Profiles Using Non-Destructive Near-Infrared Spectroscopy" Beverages 6, no. 2: 39. https://doi.org/10.3390/beverages6020039
APA StyleFuentes, S., Tongson, E., Chen, J., & Gonzalez Viejo, C. (2020). A Digital Approach to Evaluate the Effect of Berry Cell Death on Pinot Noir Wines’ Quality Traits and Sensory Profiles Using Non-Destructive Near-Infrared Spectroscopy. Beverages, 6(2), 39. https://doi.org/10.3390/beverages6020039







