1. Introduction
Food waste production covers the whole food life cycle from agricultural and industrial production and processing, to retail and domestic consumptions. In developed countries, 42% of food waste is produced during domestic consumption, while 39% is from the food manufacturing industry, 14% is from the food services sector and 5% is from the retail and distribution sectors [
1]. Nowadays, industrial ecology concepts have been evaluated as a leading principle of eco-innovation that targets the
zero waste economy, where waste is used as a raw material for new products and applications. Large quantities of waste generated by food industries cause serious problems both economically and environmentally, as well as resulting in a great loss of high-added value compounds. Moreover, most of these residues have reusable potential in other production systems.
The wastes of fruit and vegetable processes are the most important resources of various types of antioxidants and dietary fibers. The reason for this is that the corresponding byproducts are made from soft tissue that is rich in both components, allowing simultaneous extraction into two separate streams [
2]. Citrus is the largest fruit crop in the world [
3,
4], with a global production of approximately 115 million tons per year [
5]. Since their water content is less than half the total weight of the fruits, their main byproducts are their peels after processing [
6]. The concerned wastes are traditionally evaluated for animal feed, pectin and fuel production [
5,
7]. Recent studies evaluating these wastes have suggested that some fruit or vegetable byproducts may be natural antioxidant sources. Orange peel has been used for the recovery of phenolic materials, flavonoids, essential oils and carotenoids [
5,
8,
9]. Karsheva et al. obtained extracts from mandarin peels, and examined the level of biophenols in their extract content [
6]. Singanusong et al. also found the antioxidant capacity of mandarin peels by various methods, and analyzed their total biophenolic substances [
10]. Ateş et al. extracted phenolic antioxidants by various advanced separation methods (microwave-assisted extraction and supercritical-CO
2 extraction), using mandarin peel as a raw material [
11]. Lemon peel was also used as raw material for pectin and flavonoid production [
3,
4]. Li et al. extracted lemon peels by enzyme-assisted extraction to get bioactive ingredients [
12]. Furthermore, Guimarães et al. compared the water content of the lemon peel in terms of antioxidants, and observed a higher level (about eight times) of phenolic material in lemon peels compared to that of water [
13]. Moreover, the same research group compared grapefruit juice and peels with respect to phenolic material. They observed that the peel had an approximately six times higher biophenol level compared to that of grapefruit water. Li et al. recycled grapefruit peels for biologically active materials by means of enzyme-assisted extraction [
12].
On the other hand, olive trees are one of the most important fruit trees in Mediterranean countries, covering eight million hectares, which corresponds to about 98% of the world crop. This output demonstrates the economic and social importance of this crop [
14]. Olive leaves, which are byproducts of this crop, also represent 10% of the total weight of the harvested olives, but this residue remains agricultural waste if it is not assessed [
15]. Since olive leaf is a rich source of bioactive substances that have been proven many times to possess health effects, a wide variety of studies have been carried out in this regard [
16]. Şahin et al. obtained extract rich in biofenol and flavonoid by using ultrasound-assisted extraction [
17]. Xynos et al. [
18] and Putnik et al. [
19] applied pressurized liquid extraction as an environmentally friendly technology to extract olive leaf for its biophenol substances. Şahin et al. utilized solvent-free microwave extraction to attain olive leaf extract [
20]. Mourtzinos et al. [
21] and Athanasiadis et al. [
22] obtained a rich extract of antioxidants using environmentally friendly and novel solvents, respectively. Khemakhem et al. applied novel separation methods (microfiltration, ultrafiltration and nanofiltration) to acquire extract with a high level of oleuropein (the main ingredient of the olive leaf) [
23]. To our knowledge, the number of studies of citrus peels in terms of polyphenol level is quite inadequate, although there have been many studies in which olive leaf has been examined for its natural antioxidants. For this reason, evaluation of these resources, and comparison of the related wastes, is carried out in this study. On the other hand, the concerned waste samples have been extracted with homogenizer-assisted extraction (HAE), which is an extremely simple system with extremely minimal time and investment cost requirements. As the rotary blade spins at a very high speed, the texture is rapidly reduced in size by a combination of excessive shear, cavitation and scissor-like mechanical shear in the narrow gap between the rotor and the stator. Since most rotor stator homogenizers have an open configuration, the product is recirculated repeatedly. Depending on the processing speed and hardness of the tissue sample, the desired results are usually obtained in 15–120 s. The goal of the present study is to optimize the conditions of HAE for obtaining the selected raw materials, depending on their phenolic content, and to identify the richest waste byproduct with respect to bioactive properties.
2. Materials and Methods
2.1. Plant and Chemical Materials
Olive leaf samples were supplied from Özgün Olive, Olive Oil Co in the Aegean part of Turkey (Ayvalik, Balikesir). Citrus fruits were provided by the Bati Akdeniz Agricultural Research Institute (BATEM) in Antalya, Turkey. The samples were dried at ambient conditions. The dried leaves and peels were ground by a grinder (Moulinex Super Blender Grinder, LM209041, Paris, France), and screened through a 22-mesh sieve.
Ethanol (>99.5%) and methanol (>99.8%) were from Merck (Darmstadt, Germany), while sodium carbonate, Folin–Ciocalteu, 2,2-diphenyl-1-picrylhydrazyl (DPPH•), 2,9-dimethyl-1,10-phenanthroline (neocuproine), 6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid (trolox), gallic acid, oleuropein, naringin, formic acid and acetonitrile were from Sigma-Aldrich (St. Louis, MO, USA).
2.2. Homogenizer-Assisted Extraction
Residue samples were extracted three times by an ethanol-water solution (
v/
v) of different concentrations by means of an IKA T25 (ULTRA-TURRAX, Staufen, Germany) brand homogenizer. The homogenization was adjusted to several conditions (
Table 1). Before analysis, extracts were filtered through a syringe filter (0.45 μm) and kept in dark at −20 °C.
2.3. Spectrophotometric Analyzes
Total biophenolic content (TBC) determination of the extracts was carried out spectrophotometrically (PG Instruments, T60/Leicestershire, Leicester, England) depending on the Folin–Ciocalteu method at a wavelength of 765 nm [
24]. The findings were expressed as a gallic acid equivalent on a dried base (mg-GAE/g-DW). Scavenging activity of the ABTS radical was measured following the procedure of Re et al. with slight modifications [
25]. The wavelength was selected as 734 nm. Inhibition of ABTS was given as mg trolox equivalent antioxidant activity on a dried base (mg-TEAC/g-DW). Free-radical scavenging activity against the DPPH radical was also achieved by following the report of Yu et al. [
26] with some modifications [
27]. The wavelength was selected as 517 nm. Inhibition of DPPH was given as mg-TEAC/g-DW. Moreover, cupric ion reducing antioxidant capacity (CUPRAC) assay was applied to measure the antioxidant activity of the residues [
28]. Maximum absorbance was observed at 450 nm. The antioxidant activity of the samples was also stated as mg-TEAC/g-DW.
2.4. Chromatographic Analysis
Individual phenolic quantification was performed by high-performance liquid chromatography (HPLC). The main ingredients of the selected wastes have been investigated in the literature. After determination of the prominent compounds of the samples, the relevant compounds were provided as standards. Then, standard solutions were prepared in several concentrations to draw a calibration curve. After measuring the absorbance of the samples, the concentrations were determined using the calibration curve. Conditions of HPLC are given in
Table 2.
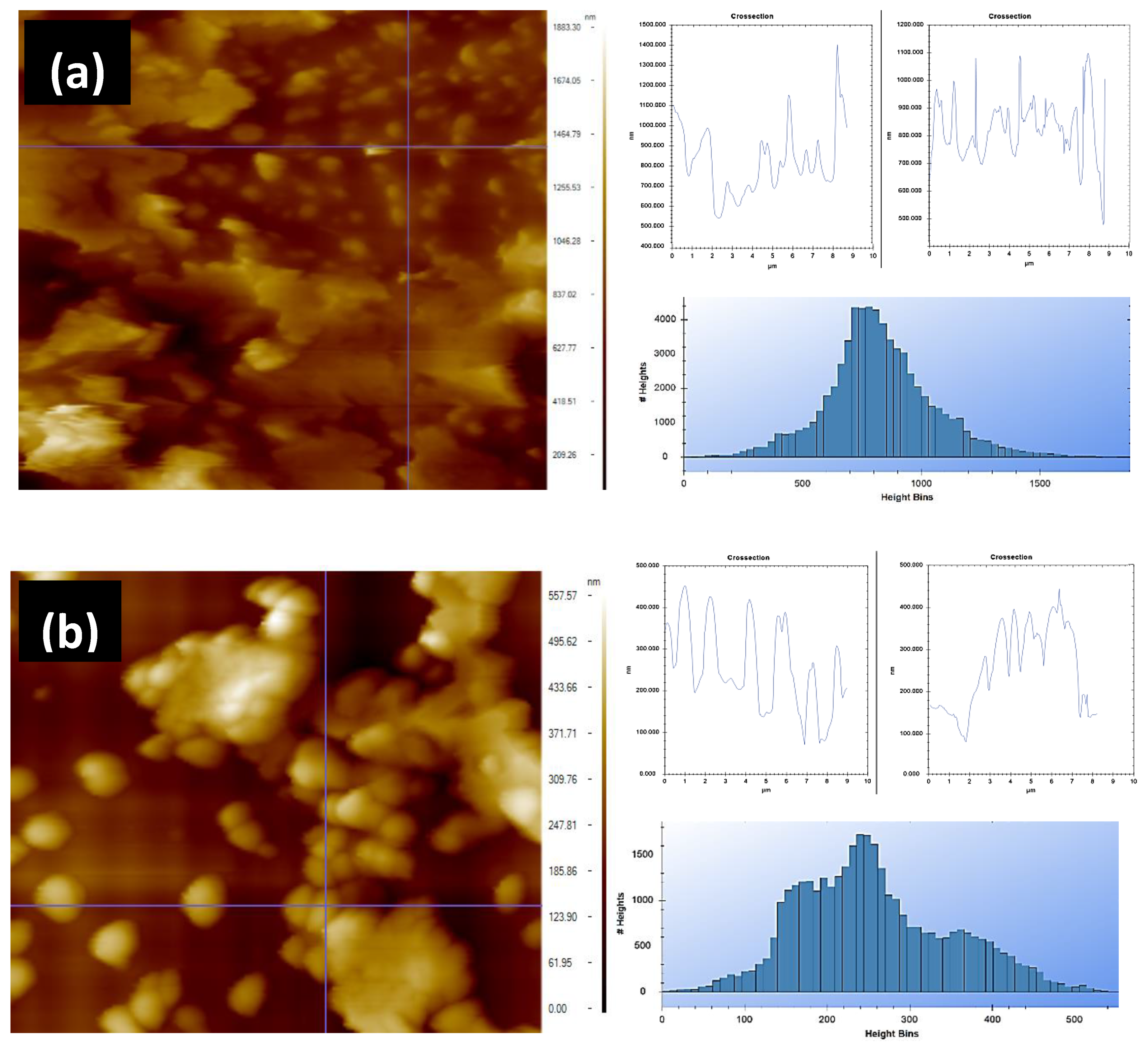
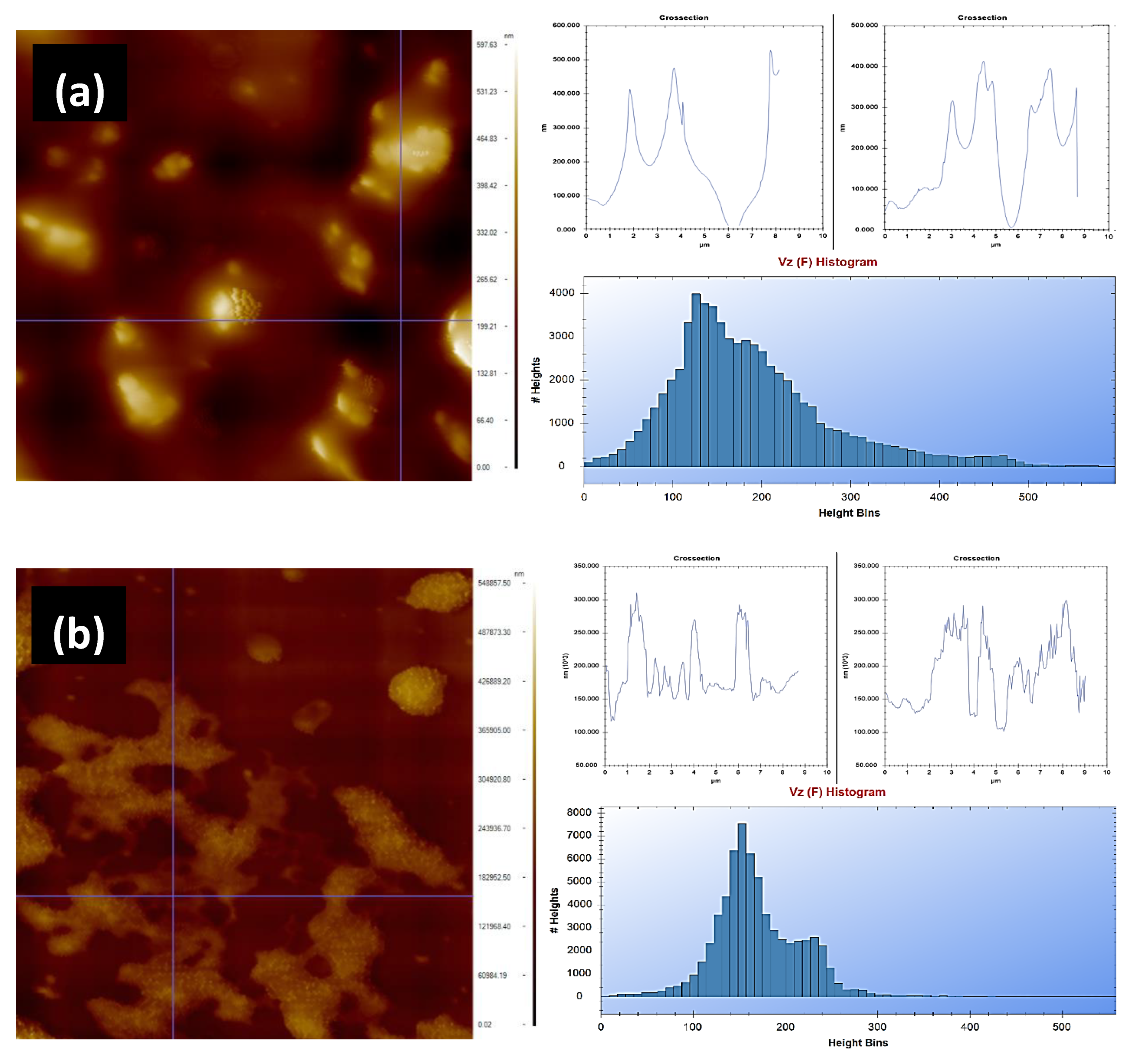
2.5. Atomic Force Microscopy
The nanostructural morphologies and height profiles of the lemon peels and olive leaves were examined with an atomic force microscopy (AFM) instrument, which was provided by Nanomagnetics Instruments. It was operated in tapping mode at room temperature using silicon probes coated with the aluminum (PPP–NCLR nanosensors). Samples were scanned before and after extraction processes utilizing a 10 µm/s scanning rate and a 256 × 256 pixel resolution. The statistical parameters were evaluated from AFM images using the image analysis software NMI Viewer 2.0.7.
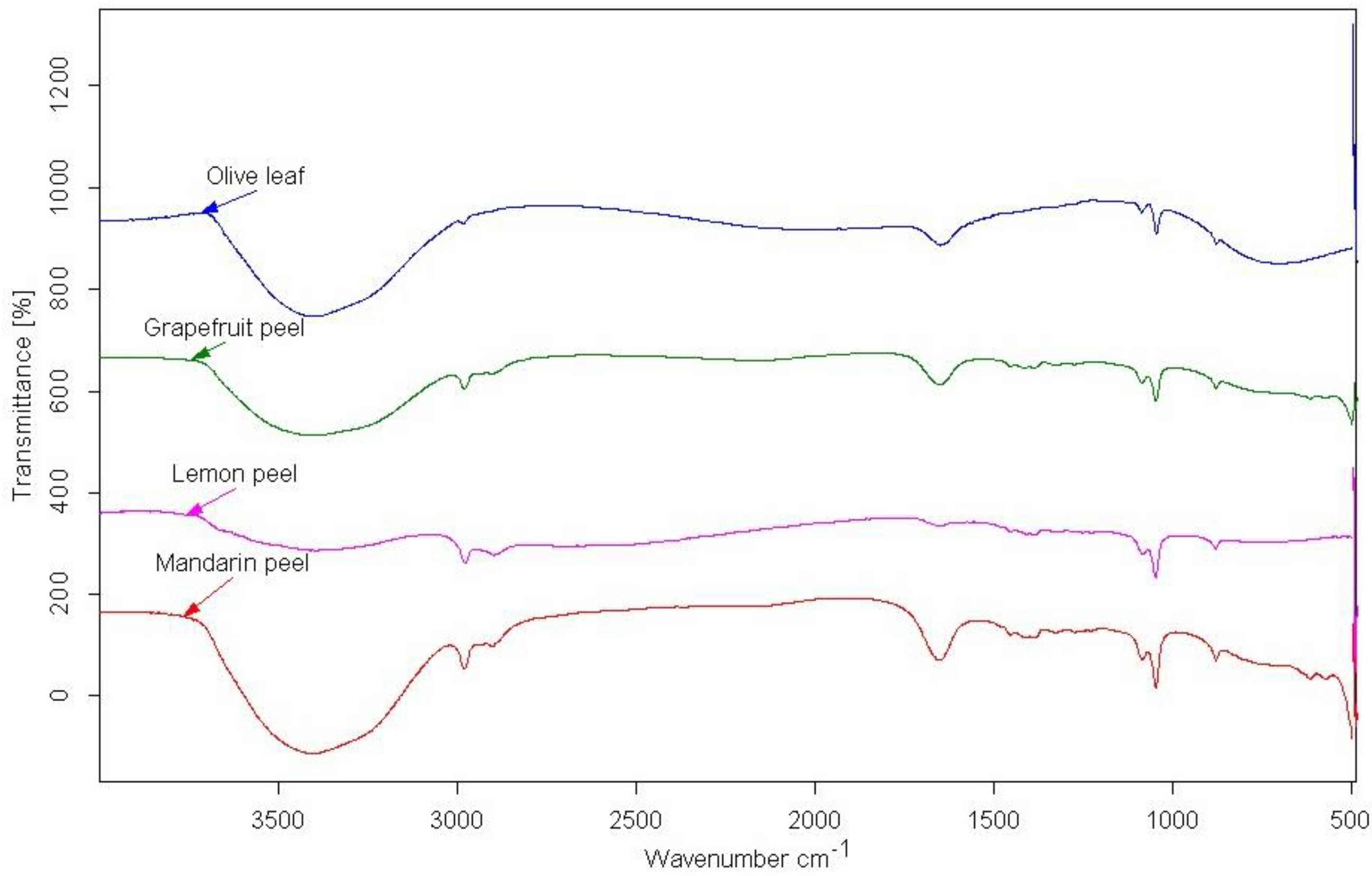
2.6. Fourier-Transform Infrared-Attenuated Total Reflectance
The chemical characterization of the extracts was made using a Bruker Alpha-T DRIFT spectrometer with a 528/D model through OPUS 6.5 software (Bruker Optics Inc., Coventry, UK).
2.7. Statistical Experimental Design
Box–Behnken design was applied into the selected HAE process as a three-level factorial design for the optimization of four process parameters (
Table 1). Since there are relatively many independent variables, Box–Behnken design was selected in order to be more economical in a more effective way [
29]. Furthermore, Box–Behnken design, along with response surface methodology (RSM) provides an evaluation of the effects of process parameters and their interactions with the relevant system. In this study, Design-Expert (Stat-Ease, Minneapolis, MN, USA) software version 10.0.4 was used.
The quadratic model of response is described the equation given below:
where β
0 is the constant, β
i is the linear and β
ii is the quadratic (i and j = 4) interaction coefficient.
Xi (i = 1–4) is the non-coded factor, while Y represents the dependent parameter, known as the response.
An analysis of variance (ANOVA) test is utilized to assess the model fitting, as well as to determine the interaction between the variables using the same software. A lack of fit test was further applied to the independent and dependent variables for verification of the model fitting.
2.8. Statistical Analysis
Analysis of variance (ANOVA) statistical testing was utilized through Tukey’s test of InStat software (GraphPad, San Diego, CA, USA) to analyze the means of three replicate outputs.
4. Conclusions
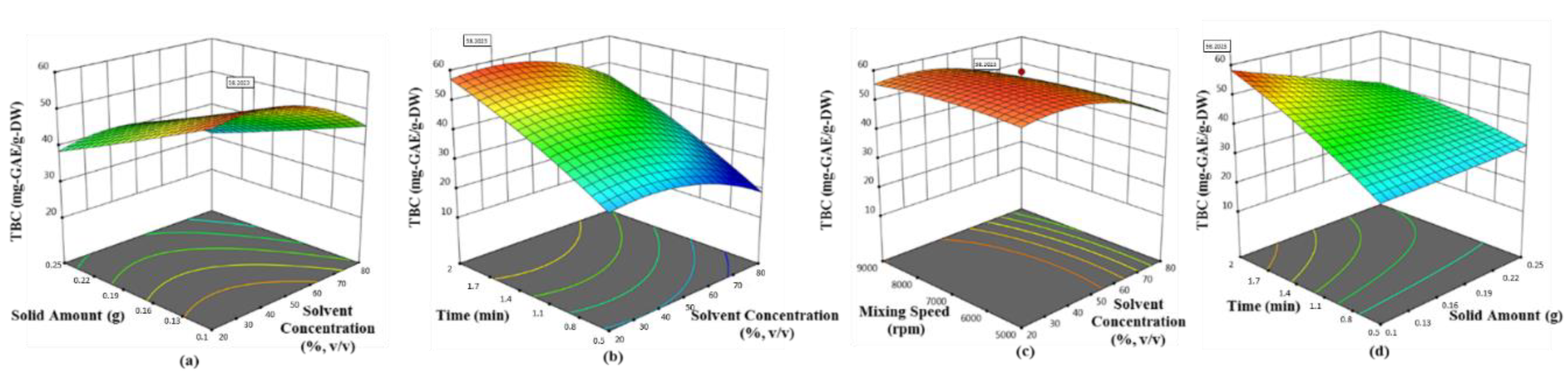
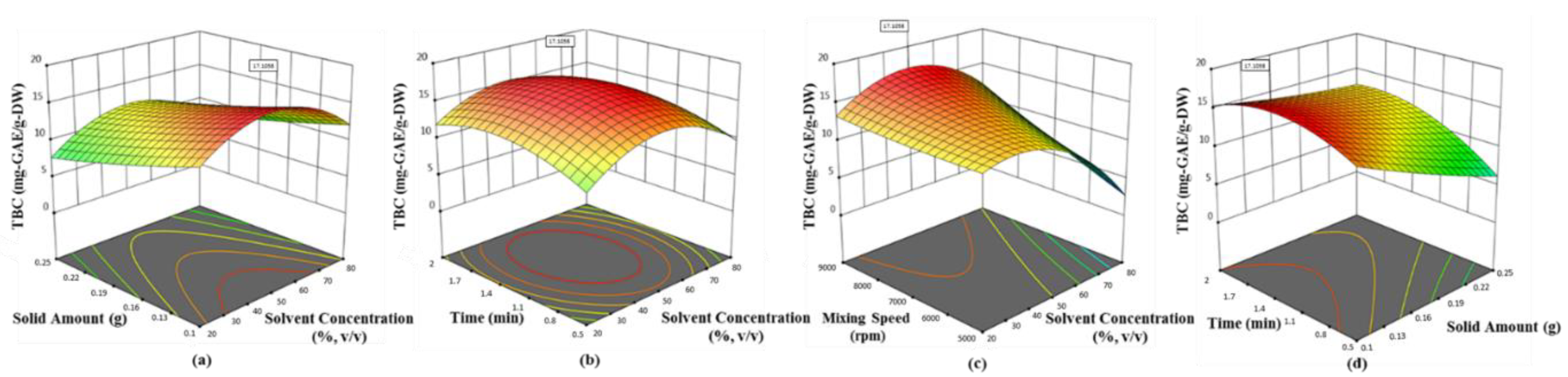
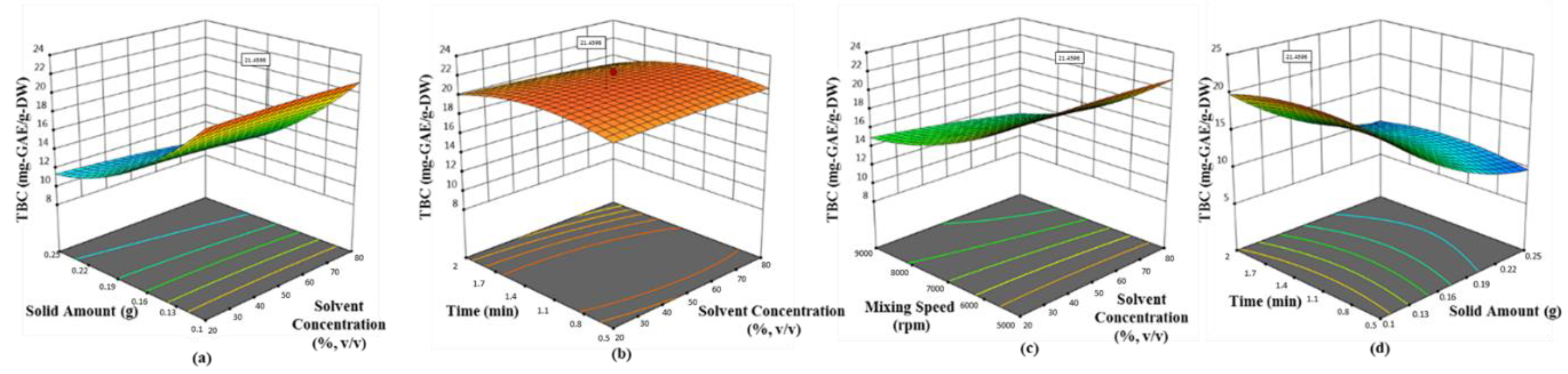
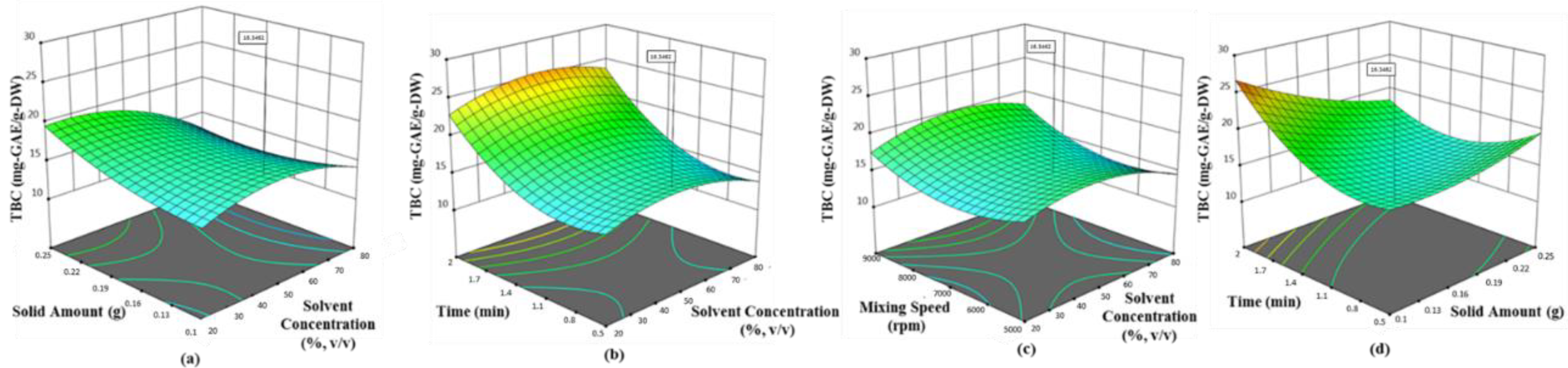
Olive leaf was found to possess the highest phenolic ingredients (58.62 mg-GAE/g-DW with 0.1 g sample, 42.5% ethanol at 6522.2 rpm for 2 min), followed by mandarin peel (27.79 mg-GAE/g-DW with 0.1 g sample, 34.24% ethanol at 8772 rpm for 1.99 min), grapefruit peel (21.12 mg-GAE/g-DW with 0.1 g sample, 42.33% ethanol at 5000 rpm for 1.125 min) and lemon peel (16.89 mg-GAE/g-DW with 0.1 g sample, 33.62% ethanol at 5007 rpm for 1.282 min). The quadratic models proposed by Box–Behnken design were found satisfactory, depending on the statistical results (p < 0.0001 and R2 > 0.89). The convincing correlation coefficients (>0.94) between the phenolic ingredients and antioxidant activity tested by each assay proved that polyphenols in the selected waste products were the most contributing substances of bioactive properties. Hereby, the present findings will be guidance for science researchers, consumers and commercial entities (cosmetic, pharmaceutical and food industries) with a green process developed for the extraction of the most popular Mediterranean crops. On the other hand, additional studies are necessary to determine whether there is a link between possible prominent compounds in the extracts related to health benefits. In short, the need for newly developed natural additives is very clear, but the most important issue is to be careful that the product is reliable.