Abstract
In this study, peach pomace (PP) moisture reduction using conventional oven-drying was investigated by implementing the Box-Behnken experimental design, considering two major process variables, time—t and temperature—T. The purpose was the optimisation of the process to obtain PP extracts as rich as possible in total carotenoids (TCn). It was shown that effective moisture removal up to a final level of approximately 24%, could be achieved after 8.27 h (496 min) at 70 °C. Under these optimised drying conditions, the maximum carotenoid yield was 84.57 ± 8.56 μg CtE g−1 dm. This yield was by almost 63% lower than that achieved using fresh (non-dried) samples. Temperatures higher than 70 °C were demonstrated to be even more detrimental in this regard, yet from the model built, it was made clear that prolonged drying time may bring about a more pronounced negative effect on the total carotenoid yield. The drop in total carotenoid content of PP as a result of drying was accompanied by a significant decline in the antiradical activity of PP extracts.
1. Introduction
Food production and consumption inevitably generate a vast quantity of processing waste biomass, a large part of which is attributed to fruit waste. These residues have been identified as a major environmental concern since the fraction of discarded materials originating from fruit processing industries is exceptionally high [1]. Food by-products are rich in valuable organic substances, such as sugars, organic acids, dietary fiber, but also bioactive compounds, such as polyphenols and carotenoids. Their appealing composition, along with the growing interest for natural ingredients as alternatives to synthetic substances, has defined fruit processing wastes as an economically attractive source for the production of high value-added compounds, which may be used in food, pharmaceutics and cosmetics [2].
Carotenoids are the red, orange and yellow pigments found in numerous plant tissues and aside from pigmentation, carotenoids are also important for plants, where they are thought to function as antioxidants, protecting tissues from damage caused by light and oxygen [3]. Several carotenoids commonly found in foods are considered to play significant roles associated with maintaining bodily functions and preventing various diseases. β-Carotene, lycopene, lutein and zeaxanthin are some of the most common carotenoids considered to have health benefits, including decreased cancer risk.
Drying is the first step in handling perishable food residues, by reducing water content through heating. Moisture reduction greatly contributes to the preservation and avoidance of decomposition, but also reduces weight and size, thus facilitating transportation and storage. Furthermore, dried tissues become more brittle, which permits facile pulverisation that is highly essential for an extraction process. On the other hand, proper drying should ensure the highest possible retention of precious phytochemicals, which are the extraction target molecules. Since heating may profoundly affect thermolabile components, severe damage brought about by uncontrolled drying could negatively impact the compounds to be recovered and their bioactivities.
Peach pomace (PP), the pressing residue originating from the juicing process of peaches, is a solid waste that might be a bioresource of carotenoids, which could be used as lipophilic functional food additives (antioxidants) and pigments. Yet, to the best of the authors’ knowledge, PP has never been investigated in this regard. As a material rich in moisture, PP is highly perishable, and for this reason, drying is an indispensable step towards its stabilisation against enzymic and microbial deterioration. Studies on PP carotenoids and the effect of drying on carotenoid fate are particularly scarce. On such a basis, this investigation was undertaken with the objective to optimise the conditions for effective PP oven-drying, with the aim to maximise moisture removal with minimal carotenoid losses.
2. Materials and Methods
2.1. Chemicals and Reagents
2,2-Diphenyl-1-picrylhydrazyl radical (DPPH) was from Sigma (Darmstadt, Germany). Acetone, ethanol and hexane were from Merck (Darmstadt, Germany).
2.2. Peach Pomace (PP)
The peach pressing residue (peach pomace) generated from apricot processing was kindly provided by KRONOS S.A. (Prefecture of Pella, Greece). The waste material was collected immediately after peach pressing for juice production and placed in 5-kg tin cans. Upon receipt, PP was stored at 4 °C until used.
2.3. Determination of Moisture Content
A lot of 30 g PP was spread over an aluminium tray to form a layer of approximate thickness of 5 mm. The tray was placed in an oven (Binder BD56, Bohemia, NY, USA), at 100 °C for 48 h and moisture content was determined by weighting the residue after drying, as follows:
The terms dm and fm correspond to dry and fresh sample mass (g).
2.4. Drying Process Optimisation with Response Surface Methodology
For all drying experiments, the equipment and methodology used are as described in Section 2.3. The purpose of the optimisation process was to minimise moisture content in PP, while retaining the maximum content of total carotenoids (TCn). In this framework, two basic factors (independent variables) were considered; time, t (termed as X1) and temperature, T (termed as X2). The percentage moisture content and the yield in total carotenoids (YTCn) were the responses. Drying time and temperature ranges were selected based on preliminary examinations and published data [4]. The mode of experiment chosen was a central composite on-face design, with 5 central points, and both independent variables X1 and X2 were coded between −1 (lower limit) and +1 (upper limit) (Table 1), using the equation:

Table 1.
Experimental values and coded levels of the central composite on-face experimental design deployed.
The terms assigned as xi and Xi correspond to the dimensionless and the actual value of the independent variable i, X0 is the actual value of the independent variable i at the central point, and ΔXi the step change of Xi corresponding to a unit variation of the dimensionless value. The experimental data obtained were analysed using analysis of variance (ANOVA), to evaluate the significance of the model. Three-dimensional graphs were constructed using the fitted model.
2.5. Carotenoid Extraction
Dried PP was initially ground in a domestic blender. Carotenoid extraction was carried out as previously described [5], with some minor modifications. The extraction solvent was composed of 50% hexane, 25% acetone and 25% ethanol (v/v/v). An amount of 0.5 g dried and ground material was placed in a 25-mL screw-cap, glass vial, covered with aluminium foil, and 10 mL of extraction solvent was added. The mixture was stirred on a magnetic stirrer at 300 rpm, for 30 min at room temperature (22 ± 2 °C) and then 1.5 mL of cold distilled water was added. The mixture was stirred for another 5 min and allowed to stand for a further 5 min to ensure phase separation.
2.6. Total Carotenoid Determination
Following phase separation, a suitable volume of the organic phase was diluted 1:20 with acetone and transferred into a 1-cm quartz cell. The absorbance was recorded at 450 nm, using a Shimadzu UV-1700 PharmaSpec spectrophotometer (Shimadzu, Kyoto, Japan), and the total carotenoid concentration (CTCn) was determined as follows [6]:
where A is the absorbance at 450 nm, FD the dilution factor, A1% = 2500 and C1% = 10,000 μg mL−1. Extraction yield in TCn (YTCn), expressed as β-carotene equivalents (CtE), was then calculated.
where V is the hexane phase volume (in mL) and dm the dry PP mass used for the extraction (in g).
2.7. Antiradical Activity (AAR) Determination
A stoichiometric methodology was employed [7], using DPPH as the radical probe. Each extract was diluted 1:10 with methanol before analysis, and then 0.025 mL of the diluted sample was mixed with 0.975 mL DPPH (100 μM in methanol) and incubated at ambient temperature. The absorbance at 515 nm was read at t = 0 min (A515(i)) and at t = 30 min (A515(f)). The AAR of the extract was determined using the equation
where ΔA = A515(i) − A515(f), ε (DPPH) = 11,126 × 106 μM−1 cm−1, C = CTCn × 0.025 × dilution (1/10), YTCn the extraction yield (μg g−1) in TCn of each extract assayed, and l the path length (1 cm). AAR was given as μmol DPPH g−1 dm.
2.8. Statistics
Drying experiments were performed at least in duplicate and determinations at least in triplicate. Values reported are means ± standard deviation. The design of experiment and related statistics (ANOVA) were carried out using JMP™ Pro 13 (SAS, Buckinghamshire, UK), at least at a 95% significance level. 3D plots were created using SigmaPlot™ 12.5 (Systat Software Inc., San Jose, CA, USA).
3. Results and Discussion
3.1. Drying Process Optimisation
Since there is a lack of data on PP drying available in the literature, observations on apricot pomace were used to draw some basic relevant information. Previous investigations showed that effective air-drying of apricot may be achieved at temperatures ranging from 40 to 70 °C and a time period of 300 to 500 min [4]. Desired moisture levels could be attained by regulating the levels of t and T since the drying rate is higher at higher temperatures [8]. On this ground, the experimental setup included a T range from 40 to 70 °C and a resident t from 3 to 9 h (180−540 min). The scope was the building of a model that would allow for PP moisture estimation by switching both independent variables (t, T) simultaneously.
The model fitting evaluation was demonstrated by carrying out ANOVA (Figure 1, inset table), considering the correlation between the measured and predicted values (Table 2). The mathematical model, from which the non-significant terms were omitted, along with the square correlation coefficient (R2) and the and the p value for lack of fit were as follows:
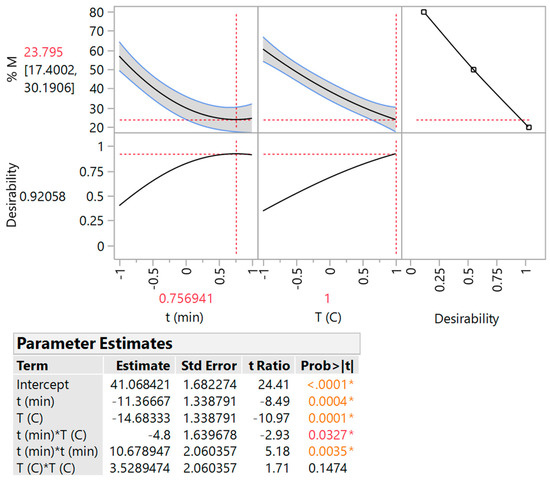
Figure 1.
Desirability function and response surface statistics associated with PP drying modelling. Asterisk denotes statistically significant terms.

Table 2.
Measured and predicted responses for all design points considered for the experimental design.
As can be observed, the model was highly significant, suggesting that the mathematical Equation (5) may be reliably used to predict moisture levels. The 3D plot derived by using the predicted moisture values (Figure 2) gives an at-a-glance picture of the effect of the independent variables (t, T) on the response (% moisture).
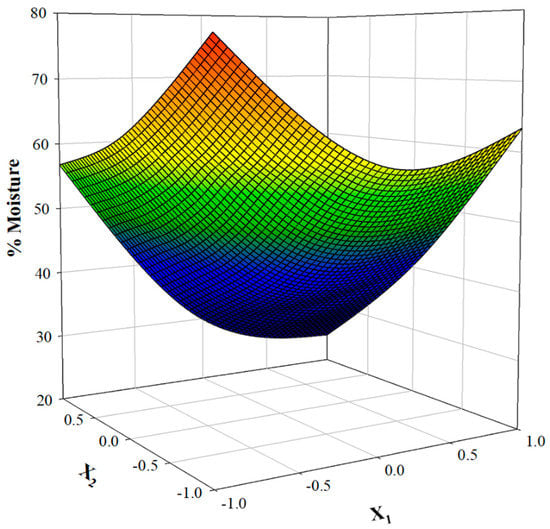
Figure 2.
3D Response surface plot illustrating the effect of the simultaneous variation of T and t on PP moisture levels.
Through the desirability function (Figure 1), the theoretical minimum moisture value was determined to be 23.8 ± 6.4%, achieved by setting t = 8.27 h and T = 70 °C. Model validation included the performance of PP drying under optimised conditions in triplicate and yielded an average moisture value of 24.3 ± 4.8%, which was practically equal to the minimum predicted one. Furthermore, to investigate the effect of higher temperatures on the moisture levels at the optimal t, drying was also repeated at 75, 80 and 85 °C, giving corresponding moisture of 9.0, 3.4 and 2.6%.
3.2. Effects on Carotenoid Content
The central issue in the process developed was the effect of drying on the recovery of total carotenoids. Previous studies have unequivocally demonstrated the detrimental effects of peach drying on the carotenoid content and profile [8,9], and therefore, the objective was the effective PP drying with minimal carotenoid losses. To investigate the simultaneous and combined effect of the independent variables t and T, a similar model to that developed for drying was implemented. In this case, too, the model was highly significant, and thus the derived mathematical Equation (6) given below could be used to make a credible prediction of carotenoid losses during drying, under various t and T combinations:
The visualised outcome was also given as a 3D plot (Figure 3). When the optimal drying conditions (t = 8.27 h and T = 70 °C) were used in Equation (6), the predicted YTCn was 84.57 ± 8.56 μg CtE g−1 dm. This value was confirmed by carrying out three runs under the same conditions, which gave YTCn of 86.42 ± 7.32 μg CtE g−1 dm. To obtain a picture regarding the magnitude of the effect of drying on the carotenoid content in PP, a fresh sample was also analysed for YTCn. As can be seen in Figure 4, drying under optimal conditions provoked a decrease in YTCn almost by 63%. Drying for the same period (8.25 h) at 75, 80 and 85 °C, gave corresponding YTCn reduction by 71.2%, 75.6% and 77.2%. This finding pointed strongly to a severe effect of temperature on APP carotenoids.
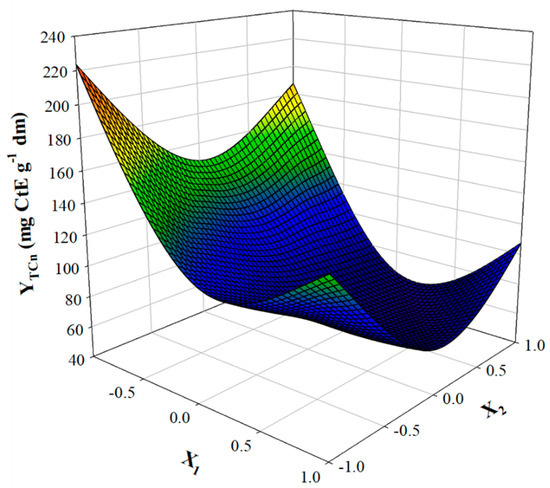
Figure 3.
3D Response surface plot illustrating the effect of the simultaneous variation of T and t on total carotenoid recovery yield from APP.
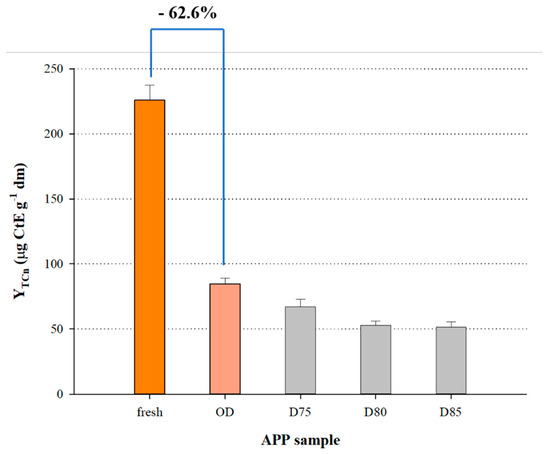
Figure 4.
Bar plot showing the effect of APP drying under optimised conditions on the yield of total carotenoid recovery, as compared with the non-dried APP and APP dried at elevated temperatures. Assignments: OD, optimal drying (t = 8.27 h and T = 70 °C). D75, D80 and D85 correspond to APP drying at 75, 80 and 85 °C, for t = 8.27 h).
In fact, reduction by 70% at 70 °C has been reported during apricot drying [10], but not all carotenoids displayed the same susceptibility to thermal degradation. For all-trans-β-carotene, the major apricot carotenoid, kinetic studies showed a significant dependence on temperature, exhibiting activation energy (Ea) of 91 kJ mol−1 [10]. Drying time was also significant in this regard, with the effect being more pronounced with microwave drying than hot-air drying. Data from other studies concerning the comparison between hot-air and microwave drying of apricots were in the same line [9]. In another investigation on apricot drying, it was shown that the longer the hot-air drying time, the higher the β-carotene losses and that treatments combining higher temperatures and shorter times were more favourable in retaining higher levels of β-carotene [8]. Similarly, it was demonstrated that for a given drying time, apricots retained higher β-carotene levels as the drying temperature increased [11]. Indeed, considering the factors of the independent variables in Equation (6), increases in t (X1) would cause higher YTCn reduction than increases in T (X2).
3.3. Effects on Antiradical Activity
The radical-scavenging activity of lipophilic plant extracts has been closely associated with their carotenoid content and composition [12]. Furthermore, several studies on pure carotenoids demonstrated their ability as effective radical scavengers [13,14]. On this basis, the estimation of the antiradical activity during APP drying may reflect compositional changes on carotenoids and vice versa.
Figure 5 shows the AAR of the fresh PP and the PP dried under optimised conditions, together with the AAR of samples dried at higher T. Drying at 70 °C caused a decrease in AAR by approximately 73%, while drying at T higher than 70 °C was even more destructive in this regard. This finding is in accordance with previous investigations [9], which showed that apricot air-drying at 60 °C reduced antioxidant activity, yet the lack of more extended bibliographic data put these results in ambiguity. On the other hand, since the decrease in YTCn was accompanied by a decrease in AAR, it could be argued that there might be a correlation between these two phenomena.
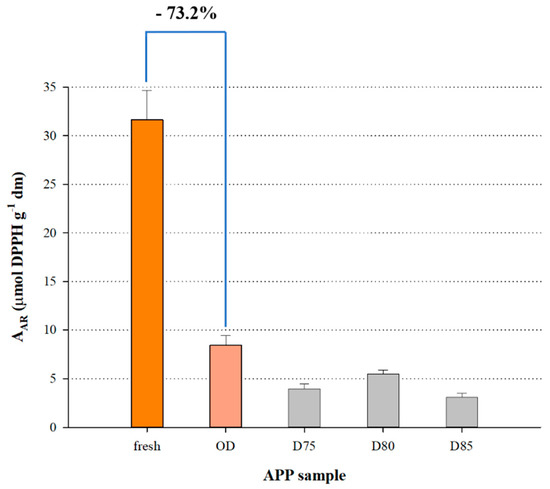
Figure 5.
Bar plot showing the effect of APP drying under optimised conditions on the AAR, as compared with the non-dried APP and APP dried at elevated temperatures. Assignments: OD, optimal drying (t = 8.27 h and T = 70 °C). D75, D80 and D85 correspond to APP drying at 75, 80 and 85 °C, for t = 8.27 h).
The theoretical basis to support this assumption might lie on the scavenging ability of β-carotene, which is the principal peach carotenoid [15,16]. β-Carotene is one of the most powerful radical scavengers compared with other carotenes and xanthophylls [17] and owed to its high content in lipophilic apricot extracts, it would normally be expected to exert the most significant radical-scavenging effect. Thus, its drastic decomposition during PP drying, as this could be implied by Figure 4, would entail a decrease in AAR. At this point, it should be emphasised that β-carotene is prone to thermal degradation, and hot-air drying may afford a decline by as high as 70% at 70 °C [10].
4. Conclusions
In this study, drying of PP was attempted by employing oven-drying, a low-cost and easy to perform drying technique. Drying process optimisation by implementing a Box-Behnken experimental design showed that using 70 °C as the maximum temperature, effective moisture removal up to an approximately final level of 24%, could be achieved after 8.27 h (496 min). Modelling of carotenoid losses during drying made it possible to estimate that, under optimised drying conditions, the maximum carotenoid yield was 84.57 ± 8.56 μg CtE g−1 dm. This yield, however, was almost 63% lower than that achieved using fresh (non-dried) samples. Temperatures higher than 70 °C were demonstrated to be even more detrimental in this regard, yet from the model built it was made clear that prolonged drying time may bring about a more pronounced negative effect on the total carotenoid yield. The drop in total carotenoid content of PP as a result of drying was accompanied by a significant decline in the antiradical activity of PP extracts. This fact was an indication of a correlation between the content of carotenoids and antioxidant activity of the extracts. It is concluded that, in a prospect of industrial valorisation of PP, drying process optimisation should be an indispensable element, since the application of empirical and inaccurate methodologies may provoke carotenoid losses that cannot be overlooked. In any case, carotenoid losses during drying are an inevitable compromise to effectively dry the otherwise susceptible PP and avoid enzymic and/or microbial spoilage.
Author Contributions
A.A. and E.B. carried out experimental work. S.L. and D.P.M. conceived the idea, organized the experimental procedure, handled the data and wrote the paper.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
Nomenclature
| AAR | antiradical activity (μmol DPPH g−1 dm) |
| CTCn | total carotenoid concentration (μg mL−1) |
| dm | dry mass (g) |
| t | time (min) |
| T | temperature (°C) |
| YTCn | yield in total carotenoids (μg CtE g−1 dm) |
References
- Schieber, A. Side streams of plant food processing as a source of valuable compounds: Selected examples. Annu. Rev. Food Sci. Technol. 2017, 8, 97–112. [Google Scholar] [CrossRef] [PubMed]
- Barbulova, A.; Colucci, G.; Apone, F. New trends in cosmetics: By-products of plant origin and their potential use as cosmetic active ingredients. Cosmetics 2015, 2, 82–92. [Google Scholar] [CrossRef]
- Boon, C.S.; McClements, D.J.; Weiss, J.; Decker, E.A. Factors influencing the chemical stability of carotenoids in foods. Crit. Rev. Food Sci. Nutr. 2010, 50, 515–532. [Google Scholar] [CrossRef] [PubMed]
- Kayran, S.; Doymaz, İ. Determination of drying kinetics and physicochemical characterization of apricot pomace in hot-air dryer. J. Therm. Anal. Calorim. 2017, 130, 1163–1170. [Google Scholar] [CrossRef]
- Perkins-Veazie, P.; Collins, J.K.; Pair, S.D.; Roberts, W. Lycopene content differs among red-fleshed watermelon cultivars. J. Sci. Food Agric. 2001, 81, 983–987. [Google Scholar] [CrossRef]
- Scott, K.J. Detection and measurement of carotenoids by UV/VIS spectrophotometry. Curr. Prot. Food Anal. Chem. 2001. [Google Scholar] [CrossRef]
- Shi, H.; Niki, E. Stoichiometric and kinetic studies on Ginkgo biloba extract and related antioxidants. Lipids 1998, 33, 365. [Google Scholar] [CrossRef] [PubMed]
- Karabulut, I.; Topcu, A.; Duran, A.; Turan, S.; Ozturk, B. Effect of hot air drying and sun drying on color values and β-carotene content of apricot (Prunus armenica L.). LWT-Food Sci. Technol. 2007, 40, 753–758. [Google Scholar] [CrossRef]
- Albanese, D.; Cinquanta, L.; Cuccurullo, G.; Di Matteo, M. Effects of microwave and hot-air drying methods on colour, β-carotene and radical scavenging activity of apricots. Int. J. Food Sci. Technol. 2013, 48, 1327–1333. [Google Scholar] [CrossRef]
- Fratianni, A.; Niro, S.; Messia, M.C.; Cinquanta, L.; Panfili, G.; Albanese, D.; Di Matteo, M. Kinetics of carotenoids degradation and furosine formation in dried apricots (Prunus armeniaca L.). Food Res. Int. 2017, 99, 862–867. [Google Scholar] [CrossRef] [PubMed]
- Ihns, R.; Diamante, L.M.; Savage, G.P.; Vanhanen, L. Effect of temperature on the drying characteristics, colour, antioxidant and beta-carotene contents of two apricot varieties. Int. J. Food Sci. Technol. 2011, 46, 275–283. [Google Scholar] [CrossRef]
- Rodriguez-Amaya, D.B. Quantitative analysis, in vitro assessment of bioavailability and antioxidant activity of food carotenoids—A review. J. Food Compos. Anal. 2010, 23, 726–740. [Google Scholar] [CrossRef]
- Skibsted, L.H. Carotenoids in antioxidant networks. Colorants or radical scavengers. J. Agric. Food Chem. 2012, 60, 2409–2417. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, D.; Freitas, M.; Silva, A.M.; Carvalho, F.; Fernandes, E. Antioxidant and pro-oxidant activities of carotenoids and their oxidation products. Food Chem. Toxicol. 2018, 120, 681–699. [Google Scholar] [CrossRef] [PubMed]
- Campbell, O.E.; Padilla-Zakour, O.I. Phenolic and carotenoid composition of canned peaches (Prunus persica) and apricots (Prunus armeniaca) as affected by variety and peeling. Food Res. Int. 2013, 54, 448–455. [Google Scholar] [CrossRef]
- Giuffrida, D.; Torre, G.; Dugo, P.; Dugo, G. Determination of the carotenoid profile in peach fruits, juice and jam. Fruits 2013, 68, 39–44. [Google Scholar] [CrossRef][Green Version]
- Mortensen, A.; Skibsted, L.H. Importance of carotenoid structure in radical-scavenging reactions. J. Agric. Food Chem. 1997, 45, 2970–2977. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).