Study of High Power Ultrasound for Oak Wood Barrel Regeneration: Impact on Wood Properties and Sanitation Effect
Abstract
1. Introduction
2. Materials and Methods
2.1. Barrel Treatment
2.1.1. High Power Ultrasound (HPU)
2.1.2. Aqueous Steam Treatment
2.2. Lab Experimental Setup and Operating Conditions
2.3. Oak Wood Characterization
2.3.1. Determination of Specific Surface
2.3.2. Oxygen Desorption Staves
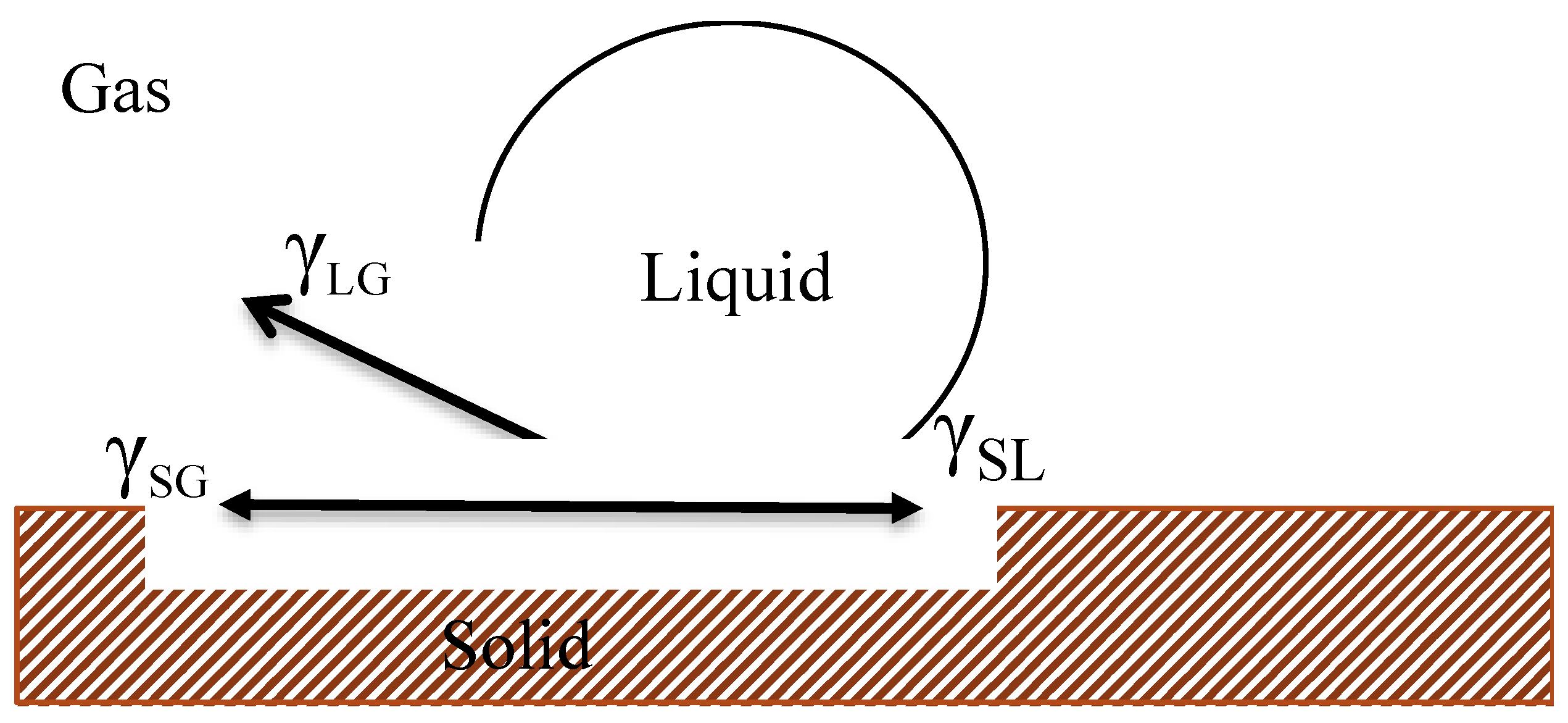
2.3.3. Contact Angle Measurement
2.4. Impact on Microorganisms
2.5. Statistical Analyses
3. Results and Discussion
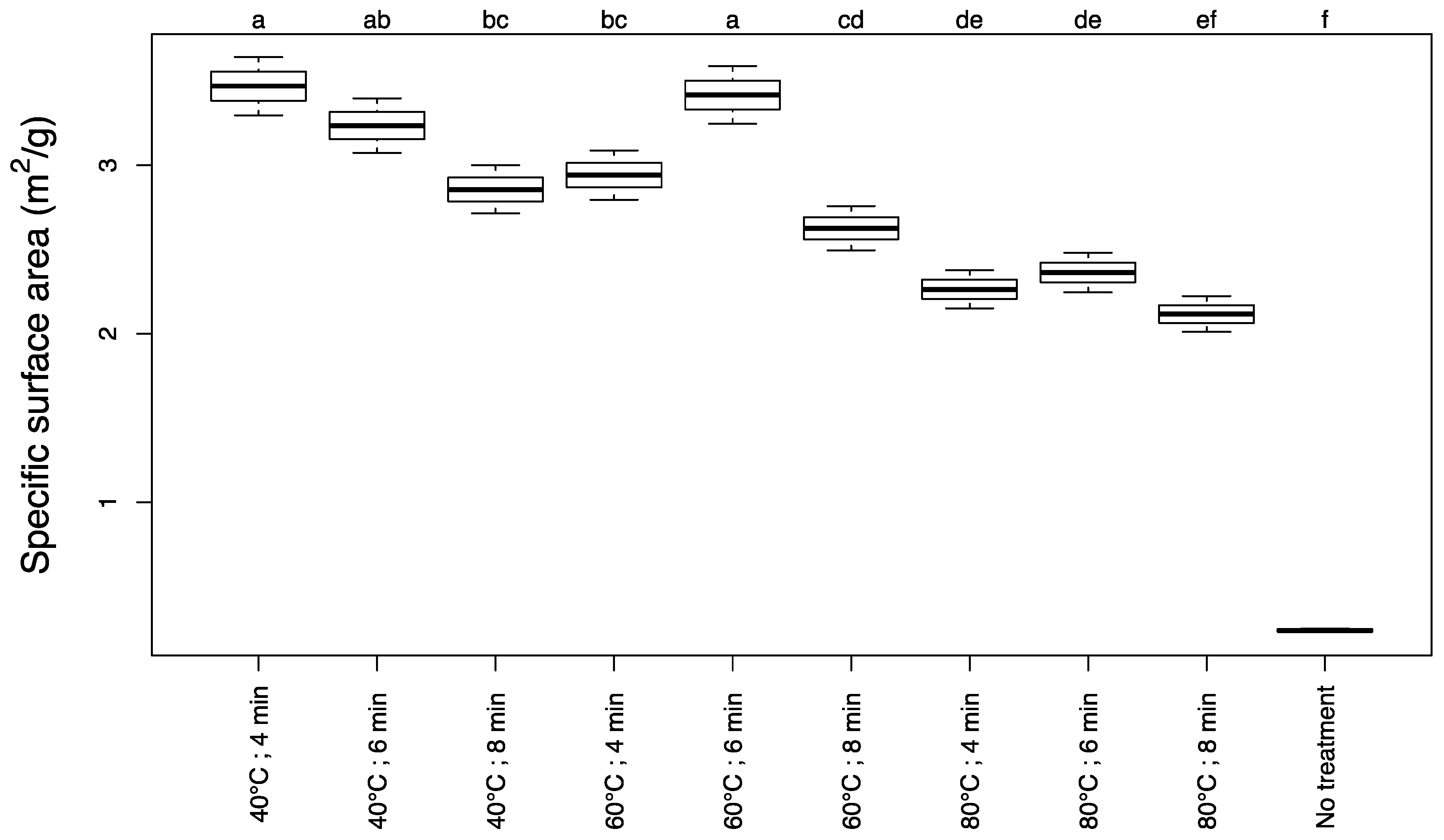
3.1. Specific Surface Area
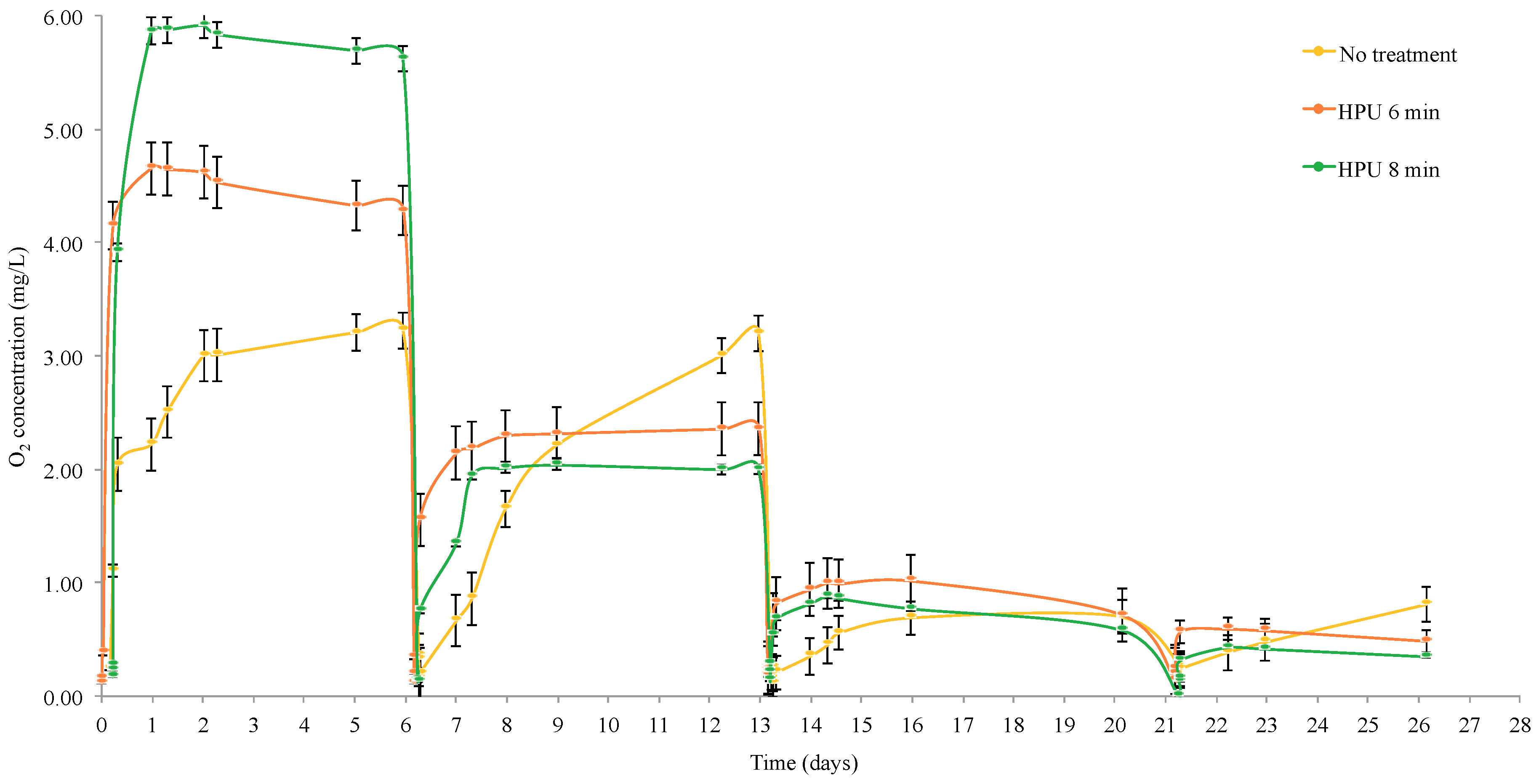
3.2. Oxygen Desorption of Staves
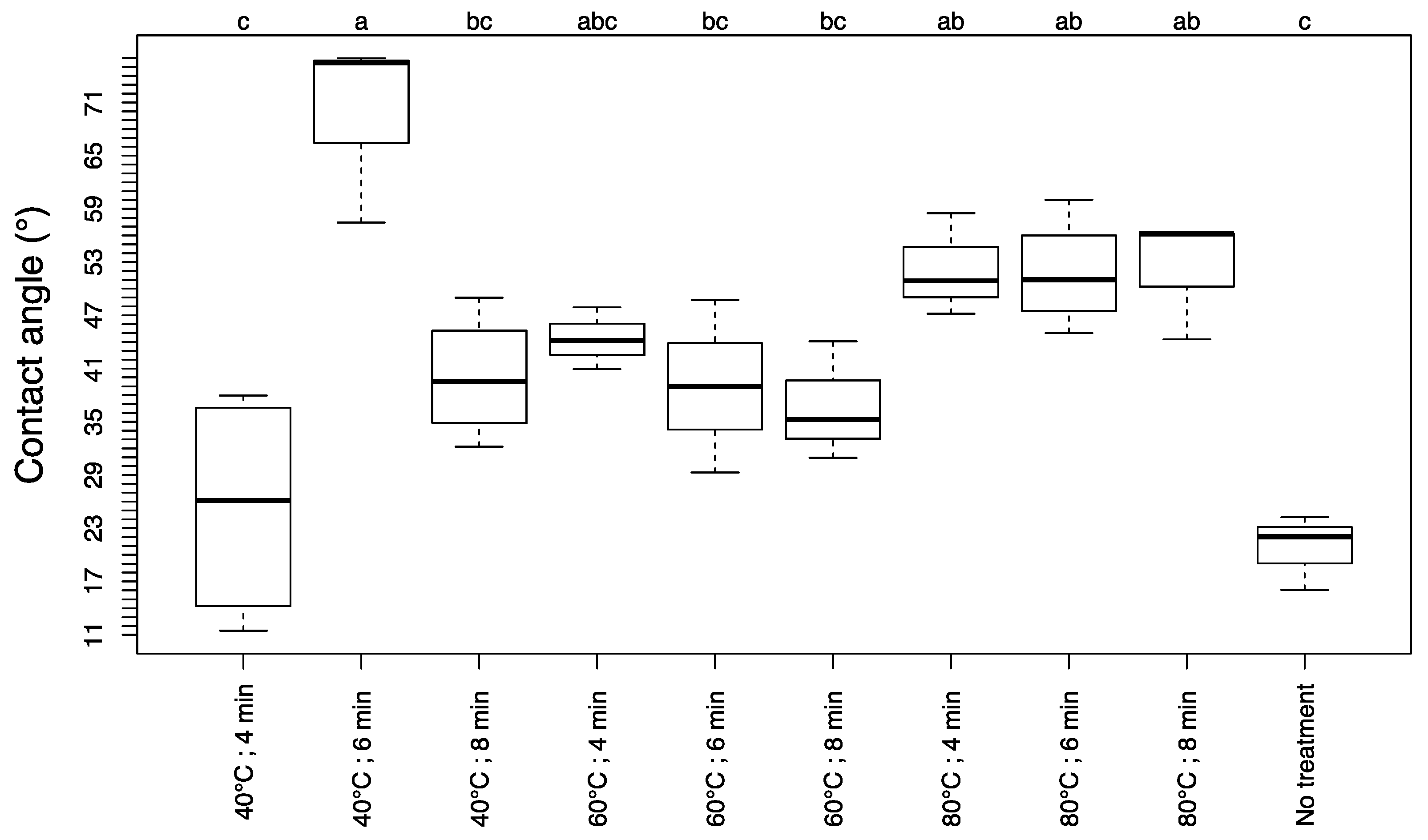
3.3. Contact Angle
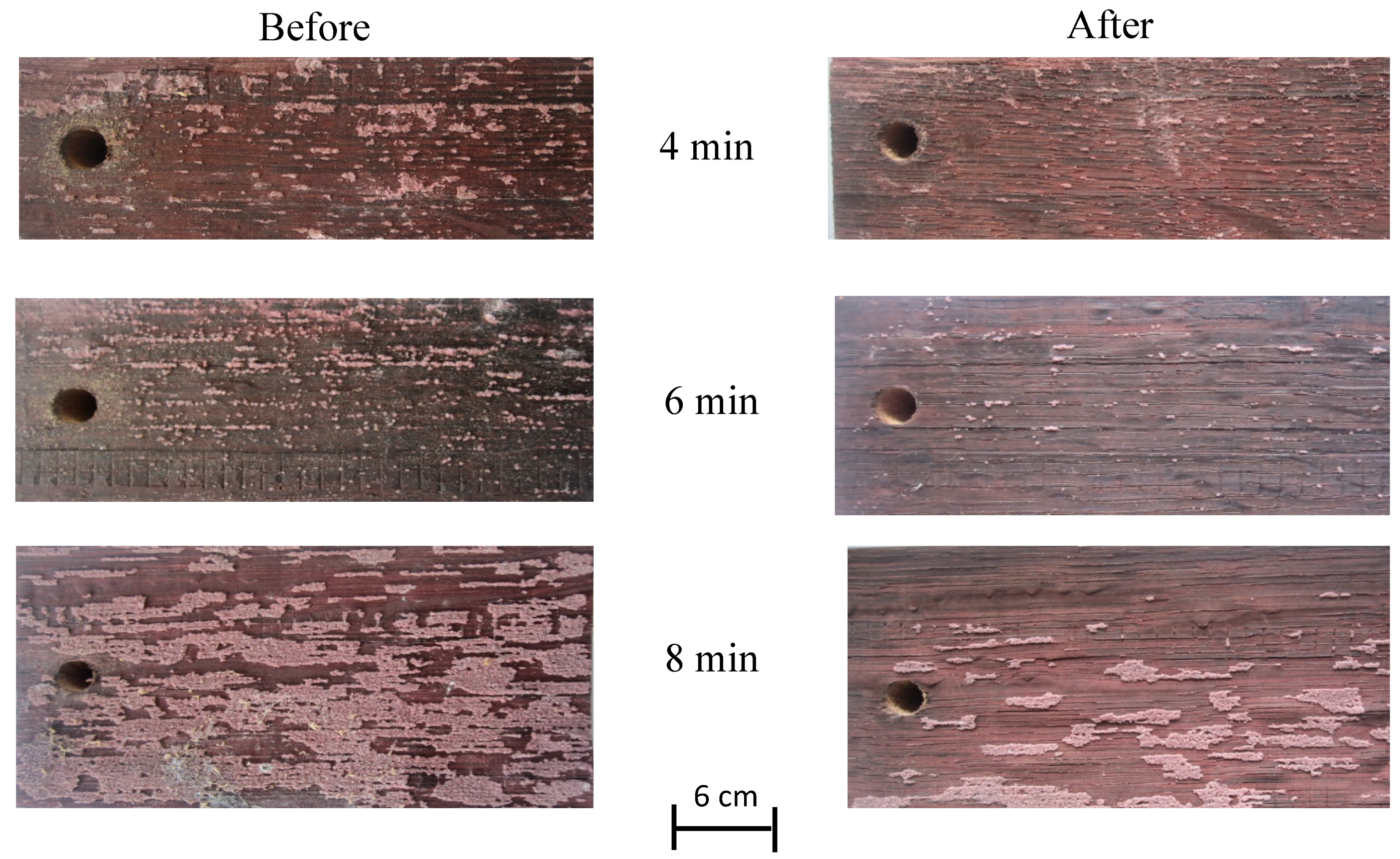
3.4. Sanitation Effect on Spoilage Microorganism B. Bruxellensis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cerdán, T.G.; Ancín-Azpilicueta, C. Effect of oak barrel type on the volatile composition of wine: Storage time optimization. LTW Food Sci. Technol. 2006, 39, 199–205. [Google Scholar] [CrossRef]
- Ortega-Heras, M.; González-Sanjosé, M.L.; González-Huerta, C. Consideration of the influence of aging process, type of wine and oenological classic parameters on the levels of wood volatile compounds present in red wines. Food Chem. 2007, 103, 1434–1448. [Google Scholar] [CrossRef]
- Chira, K.; Teissedre, P.-L. Chemical and sensory evaluation of wine matured in oak barrel: Effect of oak species involved and toasting process. Eur. Food Res. Technol. 2014, 240, 533–547. [Google Scholar] [CrossRef]
- González-Centeno, M.R.; Chira, K.; Teissedre, P.-L. Comparison between Malolactic Fermentation Container and Barrel Toasting Effects on Phenolic, Volatile, and Sensory Profiles of Red Wines. J. Agric. Food Chem. 2017, 65, 3320–3329. [Google Scholar] [CrossRef] [PubMed]
- Malfeito-Ferreira, M.; Laureano, P.; Barata, A.; Antuono, I.D.; Stender, H.; Loureiro, V. Effect of different barrique sanitation procedures on yeasts isolated from the inner layers of Wood. In Proceedings of the ASEV 55th Annual Meeting, San Diego, CA, USA, 29–30 June 2004. [Google Scholar]
- Cibrario, A. Diversité Génétique et Phénotypique de L’espèce Brettanomyces bruxellensis: Influence sur son Potentiel D’altération des Vins Rouges. Ph.D. Thesis, Université de Bordeaux, Bordeaux, France, 2017. [Google Scholar]
- Breniaux, M.; Dutilh, L.; Petrel, M.; Gontier, E.; Campbell-Sills, H.; Deleris-Bou, M.; Krieger, S.; Teissedre, P.L.; Jourdes, M.; Reguant, C.; et al. Adaptation of two groups of Oenococcus oeni strains to red and white wines: The role of acidity and phenolic compounds. J. Appl. Microbiol. 2018, 125, 1117–1127. [Google Scholar] [CrossRef] [PubMed]
- Chatonnet, P.; Dubourdieu, D.; Boidron, J.N.; Pons, M. The origin of ethylphenols in wines. J. Sci. Food Agric. 1992, 60, 165–178. [Google Scholar] [CrossRef]
- Coulon, J.; Perello, M.C.; Funel, A.L.; de Revel, G.; Renouf, V. Brettanomyces bruxellensis evolution and volatile phenols production in red wines during storage in bottles. J. Appl. Microbiol. 2010, 108, 1450–1458. [Google Scholar] [CrossRef]
- Yap, A.; Jiranek, V.; Grbin, P.; Barnes, M.; Bates, D. Studies on the application of high-power ultrasonics for barrel and plank cleaning and disinfection. Aust. N. Z. Wine Ind. J. 2007, 22, 96–104. [Google Scholar]
- Conterno, L.; Joseph, C.; Arvik, T.; Henick-Kling, T.; Bisson, L.F. Genetic and physiological characterization of Brettanomyces bruxellensis strains isolated from wines. Am. J. Enol. Viticult. 2006, 57, 139–147. [Google Scholar]
- Curtin, C.D.; Bellon, J.R.; Henschke, P.A.; Godden, P.W.; de Barros Lopes, M.A. Genetic diversity of Dekkera bruxellensis yeasts isolated from Australian wineries. FEMS Yeast Res. 2007, 7, 471–481. [Google Scholar] [CrossRef]
- Guzzon, R.; Widmann, G.; Malacarne, M.; Nardin, T.; Nicolini, G.; Larcher, R. Survey of the yeast population inside wine barrels and the effects of certain techniques in preventing microbiological spoilage. Eur. Food Res. Technol. 2011, 233, 285–291. [Google Scholar] [CrossRef]
- González-Arenzana, L.; Santamaría, P.; López, R.; Garijo, P.; Gutiérrez, A.R.; Garde-Cerdán, T.; López-Alfaro, I. Microwave technology as a new tool to improve microbiological control of oak barrels: A preliminary study. Food Control. 2013, 30, 536–539. [Google Scholar] [CrossRef]
- Poupault, P.; Richard, R. Bio-Adhésion des Levures du Genre Brettanomyces et Conséquences sur L’hygiène de la Barrique. Matevi-France.com 2006, 76, 1–5. Available online: http://www.matevi-france.com/fileadmin/user_upload/fichiers_matevi/Autres_materiels_pdf/Bio-adhesion_microbienne_et_hygiene_des_barriques_Matevi_2016.pdf (accessed on 5 December 2018).
- Mawson, R.; Knoerzer, K. A brief history of the application of ultrasonics in food processing. In Proceedings of the 19th International Congress on Acoustics, Madrid, Spain, 2–7 September 2007. [Google Scholar]
- Patist, A.; Bates, D. Ultrasonic innovations in the food industry: From the laboratory to commercial production. Innov. Food Sci. Emerg. Technol. 2008, 9, 147–154. [Google Scholar] [CrossRef]
- McClements, D.J. Advances in the application of ultrasound in food analysis and processing. Trends Food Sci. Technol. 1995, 6, 293–299. [Google Scholar] [CrossRef]
- Leighton, T. Ultrasound in Food Processing; Springer Science & Business Media: Berlin, Germany, 1998. [Google Scholar]
- Villamiel, M.; de Jong, P. Influence of high-intensity ultrasound and heat treatment in continuous flow on fat, proteins, and native enzymes of milk. J. Agric. Food Chem. 2000, 48, 472–478. [Google Scholar] [CrossRef]
- Maisonhaute, E.; Prado, C.; White, P.; Compton, R.G. Surface acoustic cavitation understood via nanosecond electrochemistry. Part III: Shear stress in ultrasonic cleaning. Ultrason. Sonochem. 2002, 9, 297–303. [Google Scholar] [CrossRef]
- Krefting, D.; Mettin, R.; Lauterborn, W. High-speed observation of acoustic cavitation erosion in multibubble systems. Ultrason. Sonochem. 2004, 11, 119–123. [Google Scholar] [CrossRef]
- Leighton, T. What is ultrasound? Prog. Biophys. Mol. Biol. 2007, 93, 3–83. [Google Scholar] [CrossRef]
- Piyasena, P.; Mohareb, E.; McKellar, R. Inactivation of microbes using ultrasound: A review. Int. J. Food Microbiol. 2003, 87, 207–216. [Google Scholar] [CrossRef]
- Jiranek, V.; Grbin, P.; Yap, A.; Barnes, M.; Bates, D. High power ultrasonics as a novel tool offering new opportunities for managing wine microbiology. Biotechnol. Lett. 2008, 30, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Guerrero, S.; López-Malo, A.; Alzamora, S.M. Effect of ultrasound on the survival of Saccharomyces cerevisiae: Influence of temperature, pH and amplitude. Innov. Food Sci. Emerg. Technol. 2001, 2, 31–39. [Google Scholar] [CrossRef]
- Furuta, M.; Yamaguchi, M.; Tsukamoto, T.; Yim, B.; Stavarache, C.E.; Hashiba, K.; Maeda, Y. Inactivation of Escherichia coli by ultrasonic irradiation. Ultrason. Sonochem. 2004, 11, 57–60. [Google Scholar] [CrossRef]
- Tsukamoto, I.; Yim, B.; Stavarache, C.E.; Furuta, M.; Hashiba, K.; Maeda, Y. Inactivation of Saccharomyces cerevisiae by ultrasonic irradiation. Ultrason. Sonochem. 2004, 11, 61–65. [Google Scholar] [CrossRef]
- Borthwick, K.A.J.; Coakley, W.T.; McDonnell, M.B.; Nowotny, H.; Benes, E.; Gröschl, M. Development of a novel compact sonicator for cell disruption. J. Microbiol. Methods 2005, 60, 207–216. [Google Scholar] [CrossRef]
- López-Malo, A.; Palou, E.; Jiménez-Fernández, M.; Alzamora, S.M.; Guerrero, S. Multifactorial fungal inactivation combining thermosonication and antimicrobials. J. Food Eng. 2005, 67, 87–93. [Google Scholar] [CrossRef]
- Yap, A.; Schmid, F.; Jiranek, V.; Grbin, P.; Bates, D. Inactivation of Brettanomyces/Dekkera in wine barrels by high power ultrasound. Aust. N. Z. Wine Ind. J. 2008, 23, 32–40. [Google Scholar]
- Porter, G.W.; Lewis, A.; Barnes, M.; Williams, R. Evaluation of high power ultrasound porous cleaning efficacy in American oak wine barrels using X-ray tomography. Innov. Food Sci. Emerg. Technol. 2011, 12, 509–514. [Google Scholar] [CrossRef]
- Hromádková, Z.; Ebringerová, A. Ultrasonic extraction of plant materials––investigation of hemicellulose release from buckwheat hulls. Ultrason. Sonochem. 2003, 10, 127–133. [Google Scholar] [CrossRef]
- Mason, T.; Paniwnyk, L.; Lorimer, J.P. The uses of ultrasound in food technology. Ultrason. Sonochem. 1996, 3, S253–S260. [Google Scholar] [CrossRef]
- Sališová, M.; Toma, Š.; Mason, T.J. Comparison of conventional and ultrasonically assisted extractions of pharmaceutically active compounds from Salvia officinalis. Ultrason. Sonochem. 1997, 4, 131–134. [Google Scholar] [CrossRef]
- Qiu, Y. Phénomènes de Transfert D’oxygène à Travers la Barrique. Ph.D. Thesis, Université de Bordeaux, Bordeaux, France, 2015. [Google Scholar]
- Gindl, M.; Sinn, G.; Gindl, W.; Reiterer, A.; Tschegg, S. A comparison of different methods to calculate the surface free energy of wood using contact angle measurements. Colloids Surf. A: Physicochem. Eng. Aspects 2001, 181, 279–287. [Google Scholar] [CrossRef]
- He, Z.; Wang, Z.; Zhao, Z.; Yi, S.; Mu, J.; Wang, X. Influence of ultrasound pretreatment on wood physiochemical structure. Ultrason. Sonochem. 2017, 34, 136–141. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.; Lacampagne, S.; Mirabel, M.; Peuchot, M.M.; Ghidossi, R. Oxygen desorption and oxygen transfer through oak staves and oak stave gaps: An innovative permeameter. OENO ONE 2018, 52, 1–14. [Google Scholar] [CrossRef]
- Young, R.A. Wettability of Wood Pulp Fibers: Applicability of Methodology. Wood Fiber Sci. 2007, 8, 120–128. [Google Scholar]


| Treatment Temperature (°C) | Treatment Time (min) | Liquid Used for Treatment | HPU Power (kW) |
|---|---|---|---|
| 40 | 4 | water | 3.8 |
| 6 | |||
| 8 | |||
| 60 | 4 | ||
| 6 | |||
| 8 | |||
| 80 | 4 | ||
| 6 | |||
| 8 | |||
| No treatment | |||
| Type of Treatment | Total Oxygen Concentration (mg/L) |
|---|---|
| No treatment | 5.57 ± 1.05 a |
| HPU 60 °C 6 min | 8.11 ± 1.15 b |
| HPU 60 °C 8 min | 8.63 ± 0.43 b |
| Stave Age (year) | Type of Treatment | Sampling Depth (mm) | B. bruxellensis Population before Treatment (log CFU/g) | B. bruxellensis Population Post Treatment (log CFU/g) |
|---|---|---|---|---|
| 1 | HPU 6 min 60 °C | 0–2 | 7.73 ± 0.02 | <DL |
| 2–5 | 5.89 ± 0.04 | <DL | ||
| 5–9 | 4.23 ± 0.01 | <DL | ||
| Steam 10 min 110 °C | 0–2 | 7.79 ± 0.05 | <DL | |
| 2–5 | 5.71 ± 0.03 | 4.92 ± 0.04 | ||
| 5–9 | 4.63 ± 0.02 | 4.59 ± 0.03 | ||
| 2 | HPU 6 min 60 °C | 0–2 | 8.11 ± 0.04 | <DL |
| 2–5 | 6.08 ± 0.02 | <DL | ||
| 5–9 | 5.61 ± 0.01 | <DL | ||
| Steam 10 min 110 °C | 0–2 | 7.91 ± 0.05 | <DL | |
| 2–5 | 6.82 ± 0.03 | 5.96 ± 0.02 | ||
| 5–9 | 5.71 ± 0.02 | 5.63 ± 0.03 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Breniaux, M.; Renault, P.; Meunier, F.; Ghidossi, R. Study of High Power Ultrasound for Oak Wood Barrel Regeneration: Impact on Wood Properties and Sanitation Effect. Beverages 2019, 5, 10. https://doi.org/10.3390/beverages5010010
Breniaux M, Renault P, Meunier F, Ghidossi R. Study of High Power Ultrasound for Oak Wood Barrel Regeneration: Impact on Wood Properties and Sanitation Effect. Beverages. 2019; 5(1):10. https://doi.org/10.3390/beverages5010010
Chicago/Turabian StyleBreniaux, Marion, Philippe Renault, Fabrice Meunier, and Rémy Ghidossi. 2019. "Study of High Power Ultrasound for Oak Wood Barrel Regeneration: Impact on Wood Properties and Sanitation Effect" Beverages 5, no. 1: 10. https://doi.org/10.3390/beverages5010010
APA StyleBreniaux, M., Renault, P., Meunier, F., & Ghidossi, R. (2019). Study of High Power Ultrasound for Oak Wood Barrel Regeneration: Impact on Wood Properties and Sanitation Effect. Beverages, 5(1), 10. https://doi.org/10.3390/beverages5010010