Wine Polyphenols and Neurodegenerative Diseases: An Update on the Molecular Mechanisms Underpinning Their Protective Effects
Abstract
1. Introduction
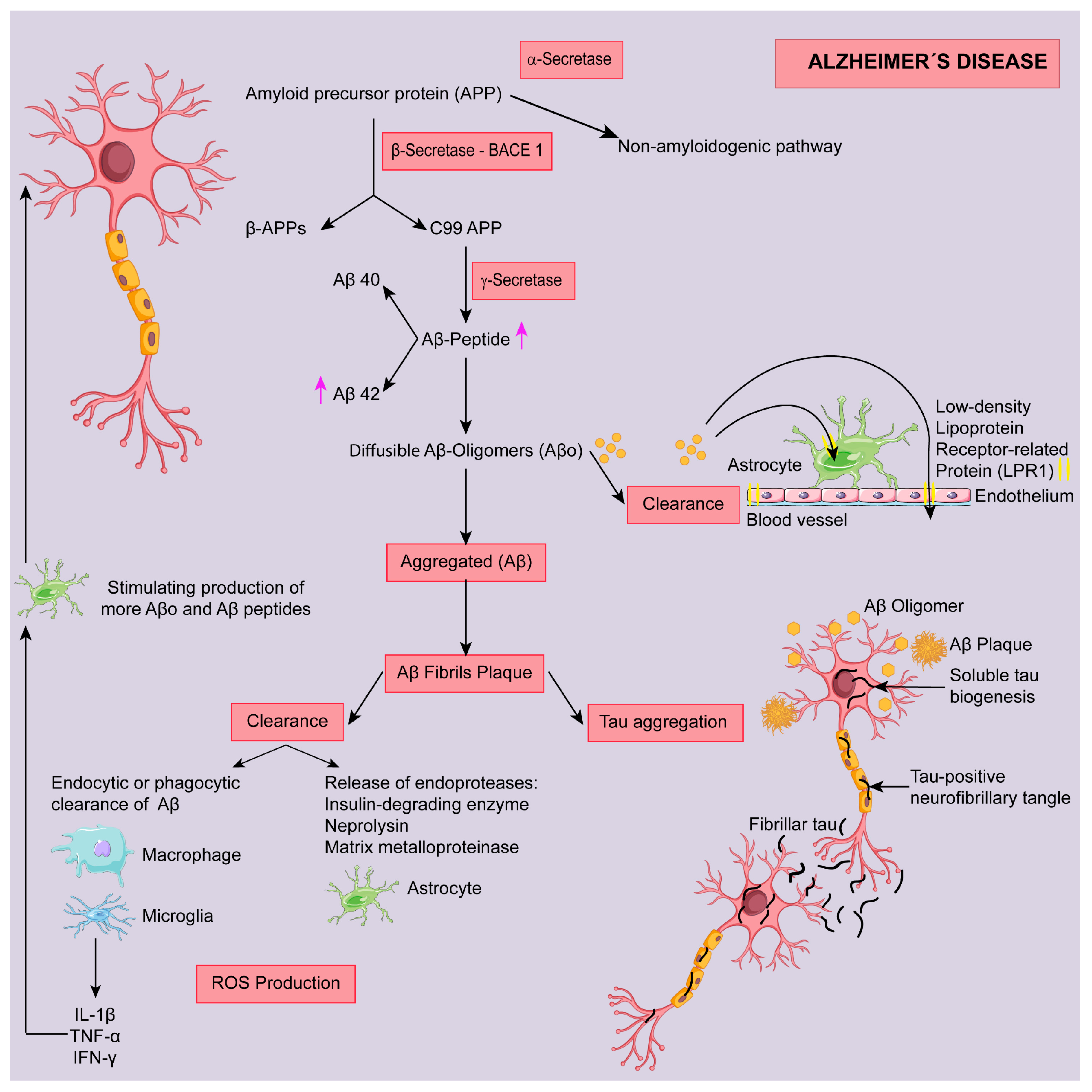
2. Alzheimer Disease (AD)
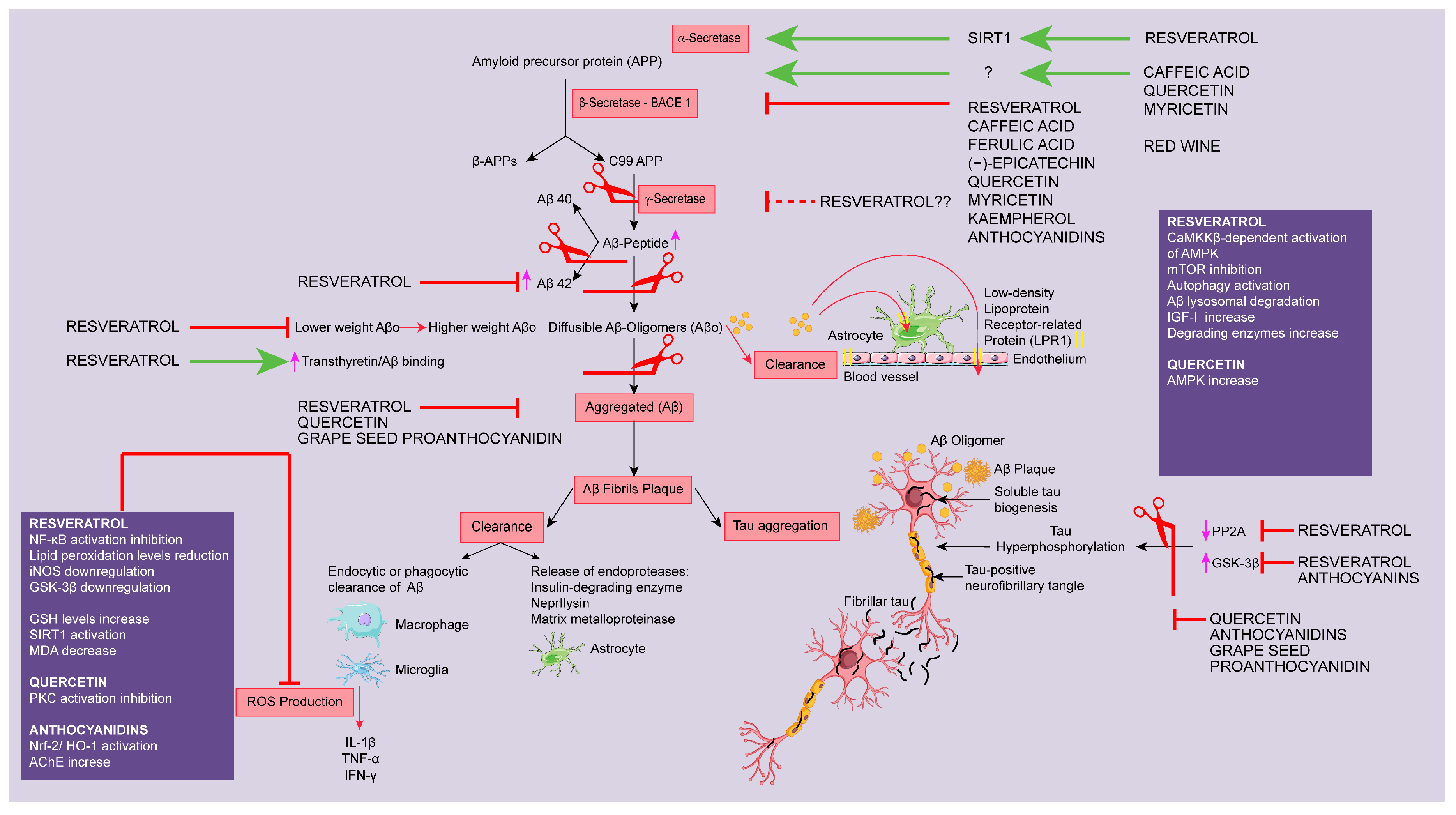
2.1. Targeting Aβ Protein
2.1.1. Modulation of Secretase Enzymes
α-Secretase
β-Secretase
γ-Secretase
2.1.2. Targeting Amyloid Clearance
2.1.3. Targeting Amyloid Aggregation
2.2. Targeting Tau Protein
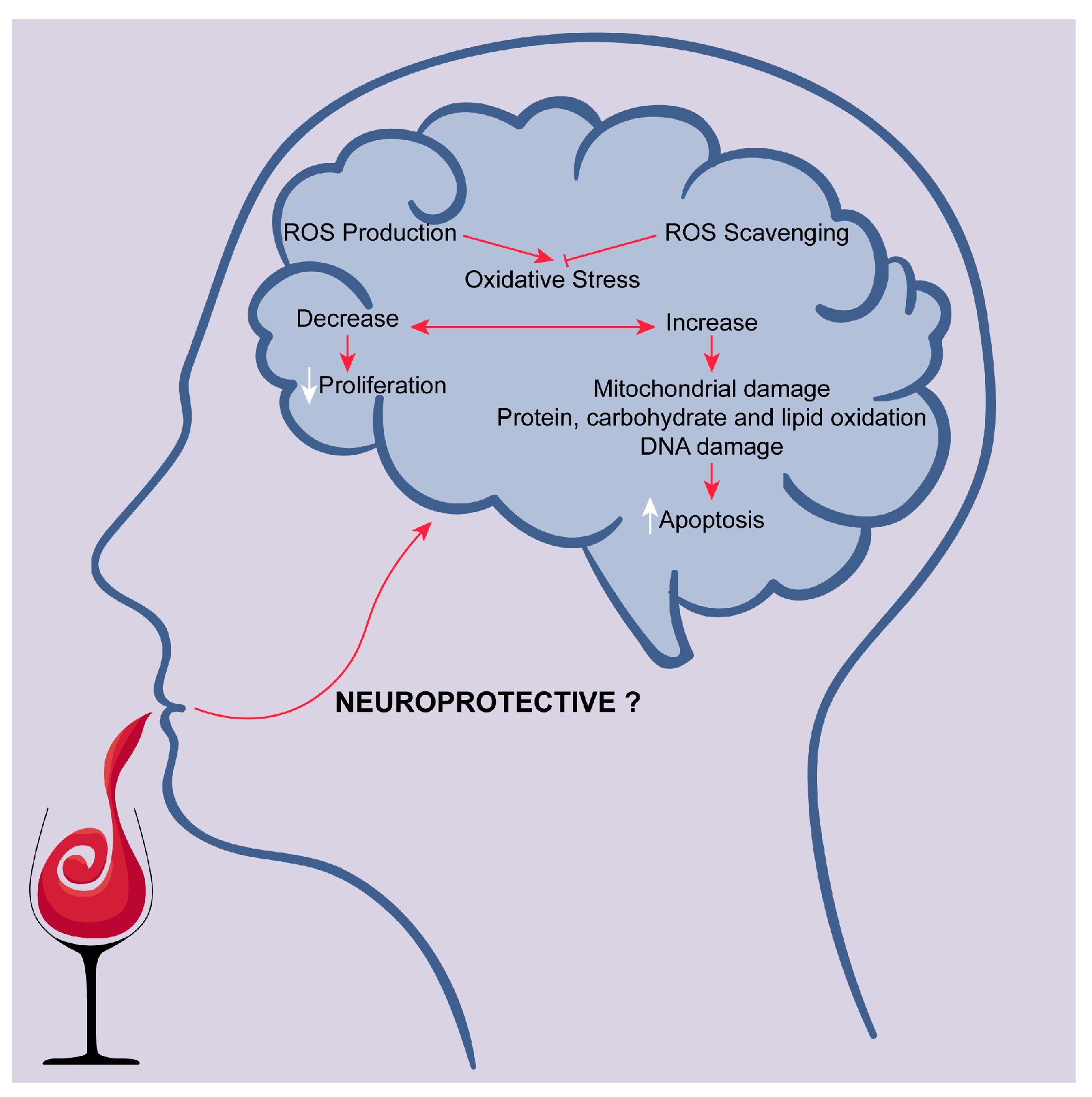
2.3. Targeting Oxidative Stress
3. Parkinson Disease (PD)
3.1. Targeting Dopaminergic Neurons Cytotoxicity
3.2. Targeting Oxidative Stress
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Prince, M.; Bryce, R.; Albanese, E.; Wimo, A.; Ribeiro, W.; Ferri, C.P. The global prevalence of dementia: A systematic review and metaanalysis. Alzheimer’s Dement. 2013, 9, 63–75. [Google Scholar] [CrossRef] [PubMed]
- Nussbaum, R.L.; Ellis, C.E. Alzheimer’s disease and Parkinson’s disease. N. Engl. J. Med. 2003, 348, 1356–1364. [Google Scholar] [CrossRef] [PubMed]
- Vauzour, D.; Vafeiadou, K.; Rodriguez-Mateos, A.; Rendeiro, C.; Spencer, J.P. The neuroprotective potential of flavonoids: A multiplicity of effects. Genes Nutr. 2008, 3, 115–126. [Google Scholar] [CrossRef] [PubMed]
- Letenneur, L. Risk of dementia and alcohol and wine consumption: A review of recent results. Biol. Res. 2004, 37, 189–193. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Wang, H.; Wan, Y.; Tan, C.; Li, J.; Tan, L.; Yu, J.T. Alcohol consumption and dementia risk: A dose-response meta-analysis of prospective studies. Eur. J. Epidemiol. 2017, 32, 31–42. [Google Scholar] [CrossRef] [PubMed]
- Lemeshow, S.; Letenneur, L.; Dartigues, J.F.; Lafont, S.; Orgogozo, J.M.; Commenges, D. Illustration of analysis taking into account complex survey considerations: The association between wine consumption and dementia in the PAQUID study. Am. J. Epidemiol. 1998, 148, 298–306. [Google Scholar] [CrossRef] [PubMed]
- Orgogozo, J.M.; Dartigues, J.F.; Lafont, S.; Letenneur, L.; Commenges, D.; Salamon, R.; Renaud, S.; Breteler, M.B. Wine consumption and dementia in the elderly: A prospective community study in the bordeaux area. Rev. Neurol. (Paris) 1997, 153, 185–192. [Google Scholar] [PubMed]
- Costanzo, S.; Di Castelnuovo, A.; Donati, M.B.; Iacoviello, L.; de Gaetano, G. Cardiovascular and Overall Mortality Risk in Relation to Alcohol Consumption in Patients With Cardiovascular Disease. Circulation 2010, 121, 1951–1959. [Google Scholar] [CrossRef] [PubMed]
- Klatsky, A.L.; Friedman, G.D.; Armstrong, M.A.; Kipp, H. Wine, liquor, beer, and mortality. Am. J. Epidemiol. 2003, 158, 585–595. [Google Scholar] [CrossRef] [PubMed]
- Rehm, J.T.; Bondy, S.J.; Sempos, C.T.; Vuong, C.V. Alcohol consumption and coronary heart disease morbidity and mortality. Am. J. Epidemiol. 1997, 146, 495–501. [Google Scholar] [CrossRef] [PubMed]
- Bianchini, F.; Vainio, H. Wine and resveratrol: Mechanisms of cancer prevention? Eur. J. Cancer Prev. 2003, 12, 417–425. [Google Scholar] [CrossRef] [PubMed]
- Kunzmann, A.T.; Coleman, H.G.; Huang, W.Y.; Berndt, S.I. The association of lifetime alcohol use with mortality and cancer risk in older adults: A cohort study. PLoS Med. 2018, 15, e1002585. [Google Scholar] [CrossRef] [PubMed]
- Napoli, R.; Cozzolino, D.; Guardasole, V.; Angelini, V.; Zarra, E.; Matarazzo, M.; Cittadini, A.; Sacca, L.; Torella, R. Red wine consumption improves insulin resistance but not endothelial function in type 2 diabetic patients. Metabolism 2005, 54, 306–313. [Google Scholar] [CrossRef] [PubMed]
- Sluik, D.; Jankovic, N.; Hughes, M.; O’Doherty, M.G.; Schottker, B.; Drygas, W.; Rolandsson, O.; Mannisto, S.; Ordonez-Mena, J.M.; Ferrieres, J.; et al. Alcoholic beverage preference and diabetes incidence across Europe: The Consortium on Health and Ageing Network of Cohorts in Europe and the United States (CHANCES) project. Eur. J. Clin. Nutr. 2017, 71, 659–668. [Google Scholar] [CrossRef] [PubMed]
- Prata-Sena, M.; Castro-Carvalho, B.M.; Nunes, S.; Amaral, B.; Silva, P. The terroir of port wine: Two hundred and sixty years of history. Food Chem. 2018, 257, 388–398. [Google Scholar] [CrossRef] [PubMed]
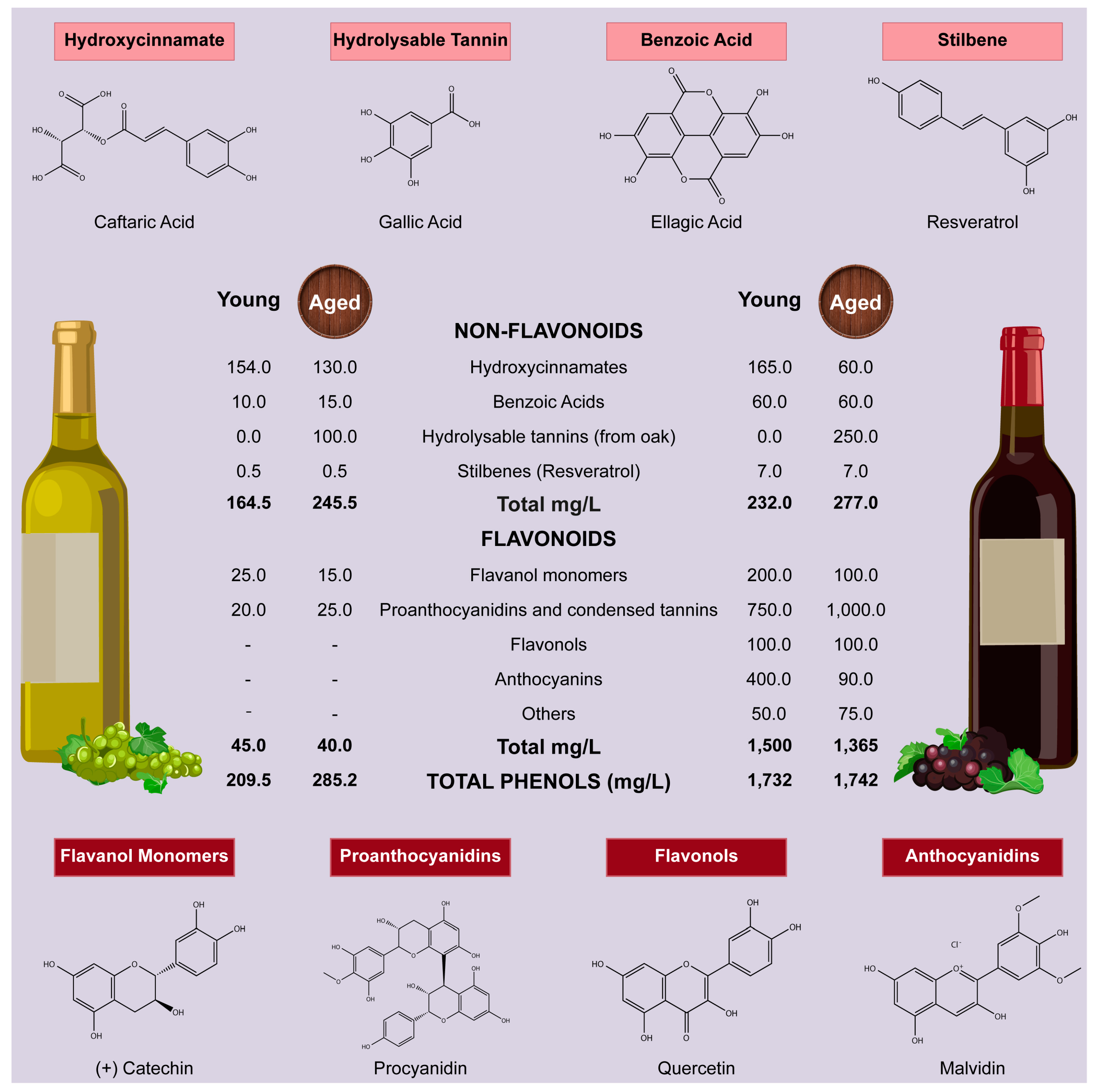
- Waterhouse, A.L. Wine phenolics. Ann. N. Y. Acad. Sci. 2002, 957, 21–36. [Google Scholar] [CrossRef] [PubMed]
- Flanagan, E.; Muller, M.; Hornberger, M.; Vauzour, D. Impact of flavonoids on cellular and molecular mechanisms underlying age-related cognitive decline and neurodegeneration. Curr. Nutr. Rep. 2018, 7, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Caruana, M.; Cauchi, R.; Vassallo, N. Putative role of red wine polyphenols against brain pathology in Alzheimer’s and Parkinson’s disease. Front. Nutr. 2016, 3, 31. [Google Scholar] [CrossRef] [PubMed]
- Selkoe, D.J.; Hardy, J. The amyloid hypothesis of Alzheimer’s disease at 25 years. EMBO Mol. Med. 2016, 8, 595–608. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Barres, B.A. Microglia and macrophages in brain homeostasis and disease. Nat. Rev. Immunol. 2018, 18, 225–242. [Google Scholar] [CrossRef] [PubMed]
- Congdon, E.E.; Sigurdsson, E.M. Tau-targeting therapies for Alzheimer disease. Nat. Rev. Neurol. 2018. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.T. Oxidative stress and mitochondrial dysfunction-linked neurodegenerative disorders. Neurol. Res. 2017, 39, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Salim, S. Oxidative stress and the central nervous system. J. Pharmacol. Exp. Ther. 2017, 360, 201–205. [Google Scholar] [CrossRef] [PubMed]
- Pchitskaya, E.; Popugaeva, E.; Bezprozvanny, I. Calcium signaling and molecular mechanisms underlying neurodegenerative diseases. Cell. Calcium. 2018, 70, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Oswald, M.C.W.; Garnham, N.; Sweeney, S.T.; Landgraf, M. Regulation of neuronal development and function by ros. FEBS Lett. 2018, 592, 679–691. [Google Scholar] [CrossRef] [PubMed]
- Kawahara, M.; Kato-Negishi, M.; Tanaka, K. Cross talk between neurometals and amyloidogenic proteins at the synapse and the pathogenesis of neurodegenerative diseases. Metallomics Integr. Biometal Sci. 2017, 9, 619–633. [Google Scholar] [CrossRef] [PubMed]
- Galimberti, D.; Ghezzi, L.; Scarpini, E. Immunotherapy against amyloid pathology in Alzheimer’s disease. J. Neurol. Sci. 2013, 333, 50–54. [Google Scholar] [CrossRef] [PubMed]
- Lichtenthaler, S.F. Alpha-secretase in Alzheimer’s disease: Molecular identity, regulation and therapeutic potential. J. Neurochem. 2011, 116, 10–21. [Google Scholar] [CrossRef] [PubMed]
- Tanzi, R.E.; Bertram, L. Twenty years of the Alzheimer’s disease amyloid hypothesis: A genetic perspective. Cell. 2005, 120, 545–555. [Google Scholar] [CrossRef] [PubMed]
- Sweeney, M.D.; Sagare, A.P.; Zlokovic, B.V. Blood-brain barrier breakdown in Alzheimer disease and other neurodegenerative disorders. Nature Rev. Neurol. 2018, 14, 133–150. [Google Scholar] [CrossRef] [PubMed]
- Oh, W.Y.; Shahidi, F. Lipophilization of resveratrol and effects on antioxidant activities. J. Agric. Food. Chem. 2017, 65, 8617–8625. [Google Scholar] [CrossRef] [PubMed]
- Porquet, D.; Casadesus, G.; Bayod, S.; Vicente, A.; Canudas, A.M.; Vilaplana, J.; Pelegri, C.; Sanfeliu, C.; Camins, A.; Pallas, M.; et al. Dietary resveratrol prevents Alzheimer’s markers and increases life span in samp8. Age (Dordrecht, Netherlands) 2013, 35, 1851–1865. [Google Scholar] [CrossRef] [PubMed]
- Corpas, R.; Grinan-Ferre, C.; Rodriguez-Farre, E.; Pallas, M.; Sanfeliu, C. Resveratrol induces brain resilience against Alzheimer neurodegeneration through proteostasis enhancement. Mol. Neurobiol. 2018. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Ho, L.; Zhao, Z.; Seror, I.; Humala, N.; Dickstein, D.L.; Thiyagarajan, M.; Percival, S.S.; Talcott, S.T.; Pasinetti, G.M. Moderate consumption of cabernet sauvignon attenuates abeta neuropathology in a mouse model of Alzheimer’s disease. FASEB J. Off. Pub. Fed. Am. Soc. Exp. Biol. 2006, 20, 2313–2320. [Google Scholar]
- Martin-Aragon, S.; Jimenez-Aliaga, K.L.; Benedi, J.; Bermejo-Bescos, P. Neurohormetic responses of quercetin and rutin in a cell line over-expressing the amyloid precursor protein (appswe cells). Phytomed. Inter. J. Phytother. Phytopharmacol. 2016, 23, 1285–1294. [Google Scholar] [CrossRef] [PubMed]
- Shimmyo, Y.; Kihara, T.; Akaike, A.; Niidome, T.; Sugimoto, H. Multifunction of myricetin on a beta: Neuroprotection via a conformational change of a beta and reduction of a beta via the interference of secretases. J. Neurosci. Res. 2008, 86, 368–377. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Jin, M.; Pi, R.; Zhang, J.; Chen, M.; Ouyang, Y.; Liu, A.; Chao, X.; Liu, P.; Liu, J.; et al. Protective effects of caffeic acid and caffeic acid phenethyl ester against acrolein-induced neurotoxicity in ht22 mouse hippocampal cells. Neurosci. Lett. 2013, 535, 146–151. [Google Scholar] [CrossRef] [PubMed]
- Mori, T.; Koyama, N.; Tan, J.; Segawa, T.; Maeda, M.; Town, T. Combination therapy with octyl gallate and ferulic acid improves cognition and neurodegeneration in a transgenic mouse model of Alzheimer’s disease. J. Biol. Chem. 2017, 292, 11310–11325. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.H.; Yoo, M.Y.; Choi, C.W.; Cha, M.R.; Yon, G.H.; Kwon, D.Y.; Kim, Y.S.; Park, W.K.; Ryu, S.Y. A new specific bace-1 inhibitor from the stembark extract of vitis vinifera. Planta Med. 2009, 75, 537–540. [Google Scholar] [CrossRef] [PubMed]
- Choi, C.W.; Choi, Y.H.; Cha, M.R.; Kim, Y.S.; Yon, G.H.; Hong, K.S.; Park, W.K.; Kim, Y.H.; Ryu, S.Y. In vitro bace-1 inhibitory activity of resveratrol oligomers from the seed extract of paeonia lactiflora. Planta Med. 2011, 77, 374–376. [Google Scholar] [CrossRef] [PubMed]
- Martinez, A.; Zahran, M.; Gomez, M.; Cooper, C.; Guevara, J.; Ekengard, E.; Nordlander, E.; Alcendor, R.; Hambleton, S. Novel multi-target compounds in the quest for new chemotherapies against Alzheimer’s disease: An experimental and theoretical study. Bioorg. Med. Chem. 2018. [Google Scholar] [CrossRef] [PubMed]
- Seino, S.; Kimoto, T.; Yoshida, H.; Tanji, K.; Matsumiya, T.; Hayakari, R.; Seya, K.; Kawaguchi, S.; Tsuruga, K.; Tanaka, H.; et al. Gnetin c, a resveratrol dimer, reduces amyloid-beta 1-42 (abeta42) production and ameliorates abeta42-lowered cell viability in cultured sh-sy5y human neuroblastoma cells. Biomed. Res. (Tokyo, Japan) 2018, 39, 105–115. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Lin, T.; Gao, Y.; Xu, J.; Jiang, C.; Wang, G.; Bu, G.; Xu, H.; Chen, H.; Zhang, Y.W. The resveratrol trimer miyabenol c inhibits beta-secretase activity and beta-amyloid generation. PLoS ONE 2015, 10, e0115973. [Google Scholar]
- Cox, C.J.; Choudhry, F.; Peacey, E.; Perkinton, M.S.; Richardson, J.C.; Howlett, D.R.; Lichtenthaler, S.F.; Francis, P.T.; Williams, R.J. Dietary (-)-epicatechin as a potent inhibitor of betagamma-secretase amyloid precursor protein processing. Neurobiol. Aging 2015, 36, 178–187. [Google Scholar] [CrossRef] [PubMed]
- Shimmyo, Y.; Kihara, T.; Akaike, A.; Niidome, T.; Sugimoto, H. Flavonols and flavones as bace-1 inhibitors: Structure-activity relationship in cell-free, cell-based and in silico studies reveal novel pharmacophore features. Biochim. Biophys. Acta 2008, 1780, 819–825. [Google Scholar] [CrossRef] [PubMed]
- De Simone, A.; Mancini, F.; Real Fernandez, F.; Rovero, P.; Bertucci, C.; Andrisano, V. Surface plasmon resonance, fluorescence, and circular dichroism studies for the characterization of the binding of bace-1 inhibitors. Anal. Bioanal.Chem. 2013, 405, 827–835. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, S.; Kumar, S.; Basu, S. Conformational transition in the substrate binding domain of beta-secretase exploited by nma and its implication in inhibitor recognition: Bace1-myricetin a case study. Neurochem. Inter. 2011, 58, 914–923. [Google Scholar] [CrossRef] [PubMed]
- Jimenez-Aliaga, K.; Bermejo-Bescos, P.; Benedi, J.; Martin-Aragon, S. Quercetin and rutin exhibit antiamyloidogenic and fibril-disaggregating effects in vitro and potent antioxidant activity in appswe cells. Life Sci. 2011, 89, 939–945. [Google Scholar] [CrossRef] [PubMed]
- Amin, F.U.; Shah, S.A.; Badshah, H.; Khan, M.; Kim, M.O. Anthocyanins encapsulated by plga@peg nanoparticles potentially improved its free radical scavenging capabilities via p38/jnk pathway against aβ(1–42)-induced oxidative stress. J. Nanobiotechnol. 2017, 15, 12. [Google Scholar] [CrossRef] [PubMed]
- Shih, P.H.; Wu, C.H.; Yeh, C.T.; Yen, G.C. Protective effects of anthocyanins against amyloid beta-peptide-induced damage in neuro-2a cells. J. Agric. Food Chem. 2011, 59, 1683–1689. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Wu, D.M.; Zheng, Y.L.; Hu, B.; Zhang, Z.F.; Shan, Q.; Zheng, Z.H.; Liu, C.M.; Wang, Y.J. Quercetin activates amp-activated protein kinase by reducing pp2c expression protecting old mouse brain against high cholesterol-induced neurotoxicity. J. Pathol. 2010, 222, 199–212. [Google Scholar] [CrossRef] [PubMed]
- Huebbe, P.; Wagner, A.E.; Boesch-Saadatmandi, C.; Sellmer, F.; Wolffram, S.; Rimbach, G. Effect of dietary quercetin on brain quercetin levels and the expression of antioxidant and Alzheimer’s disease relevant genes in mice. Pharmacol. Res. 2010, 61, 242–246. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Rui, Y.; Qin, L.; Xu, J.; Han, S.; Yuan, L.; Yin, X.; Wan, Z. Vitamin d combined with resveratrol prevents cognitive decline in samp8 mice. Curr. Alzheimer Res. 2017, 14, 820–833. [Google Scholar] [CrossRef] [PubMed]
- Eom, T.K.; Ryu, B.; Lee, J.K.; Byun, H.G.; Park, S.J.; Kim, S.K. Beta-secretase inhibitory activity of phenolic acid conjugated chitooligosaccharides. J. Enzyme Inhib. Med. Chem. 2013, 28, 214–217. [Google Scholar] [CrossRef] [PubMed]
- Mori, T.; Koyama, N.; Guillot-Sestier, M.V.; Tan, J.; Town, T. Ferulic acid is a nutraceutical beta-secretase modulator that improves behavioral impairment and Alzheimer-like pathology in transgenic mice. PLoS ONE 2013, 8, e55774. [Google Scholar] [CrossRef] [PubMed]
- Choi, B.; Kim, S.; Jang, B.-G.; Kim, M.-J. Piceatannol, a natural analogue of resveratrol, effectively reduces beta-amyloid levels via activation of alpha-secretase and matrix metalloproteinase-9. J. Funct. Foods 2016, 23, 124–134. [Google Scholar] [CrossRef]
- Ohta, K.; Mizuno, A.; Ueda, M.; Li, S.; Suzuki, Y.; Hida, Y.; Hayakawa-Yano, Y.; Itoh, M.; Ohta, E.; Kobori, M.; et al. Autophagy impairment stimulates ps1 expression and gamma-secretase activity. Autophagy 2010, 6, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Marambaud, P.; Zhao, H.; Davies, P. Resveratrol promotes clearance of Alzheimer’s disease amyloid-beta peptides. J. Biol. Chem. 2005, 280, 37377–37382. [Google Scholar] [CrossRef] [PubMed]
- Vingtdeux, V.; Giliberto, L.; Zhao, H.; Chandakkar, P.; Wu, Q.; Simon, J.E.; Janle, E.M.; Lobo, J.; Ferruzzi, M.G.; Davies, P.; et al. Amp-activated protein kinase signaling activation by resveratrol modulates amyloid-β peptide metabolism. J. Biol. Chem. 2010, 285, 9100–9113. [Google Scholar] [CrossRef] [PubMed]
- Frater, J.; Lie, D.; Bartlett, P.; McGrath, J.J. Insulin-like growth factor 1 (igf-1) as a marker of cognitive decline in normal ageing: A review. Ageing Res. Rev. 2018, 42, 14–27. [Google Scholar] [CrossRef] [PubMed]
- Harada, N.; Zhao, J.; Kurihara, H.; Nakagata, N.; Okajima, K. Resveratrol improves cognitive function in mice by increasing production of insulin-like growth factor-i in the hippocampus. J. Nutr. Biochem. 2011, 22, 1150–1159. [Google Scholar] [CrossRef] [PubMed]
- Deng, H.; Mi, M.T. Resveratrol attenuates abeta25-35 caused neurotoxicity by inducing autophagy through the tyrrs-parp1-sirt1 signaling pathway. Neurochem. Res. 2016, 41, 2367–2379. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.M.; Li, S.Q.; Wu, W.L.; Zhu, X.Y.; Wang, Y.; Yuan, H.Y. Effects of long-term treatment with quercetin on cognition and mitochondrial function in a mouse model of Alzheimer’s disease. Neurochem. Res. 2014, 39, 1533–1543. [Google Scholar] [CrossRef] [PubMed]
- Regitz, C.; Fitzenberger, E.; Mahn, F.L.; Dussling, L.M.; Wenzel, U. Resveratrol reduces amyloid-beta (abeta(1)(-)(4)(2))-induced paralysis through targeting proteostasis in an Alzheimer model of caenorhabditis elegans. Eur. J. Nutr. 2016, 55, 741–747. [Google Scholar] [CrossRef] [PubMed]
- Fu, Z.; Aucoin, D.; Ahmed, M.; Ziliox, M.; Van Nostrand, W.E.; Smith, S.O. Capping of abeta42 oligomers by small molecule inhibitors. Biochemistry 2014, 53, 7893–7903. [Google Scholar] [CrossRef] [PubMed]
- Ghobeh, M.; Ahmadian, S.; Meratan, A.A.; Ebrahim-Habibi, A.; Ghasemi, A.; Shafizadeh, M.; Nemat-Gorgani, M. Interaction of abeta(25-35) fibrillation products with mitochondria: Effect of small-molecule natural products. Biopolymers 2014, 102, 473–486. [Google Scholar] [CrossRef] [PubMed]
- Riviere, C.; Papastamoulis, Y.; Fortin, P.Y.; Delchier, N.; Andriamanarivo, S.; Waffo-Teguo, P.; Kapche, G.D.; Amira-Guebalia, H.; Delaunay, J.C.; Merillon, J.M.; et al. New stilbene dimers against amyloid fibril formation. Bioorg. Med. Chem. Lett. 2010, 20, 3441–3443. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Wang, X.P.; Yang, S.G.; Wang, Y.J.; Zhang, X.; Du, X.T.; Sun, X.X.; Zhao, M.; Huang, L.; Liu, R.T. Resveratrol inhibits beta-amyloid oligomeric cytotoxicity but does not prevent oligomer formation. Neurotoxicology 2009, 30, 986–995. [Google Scholar] [CrossRef] [PubMed]
- Ge, J.F.; Qiao, J.P.; Qi, C.C.; Wang, C.W.; Zhou, J.N. The binding of resveratrol to monomer and fibril amyloid beta. Neurochem. Inter. 2012, 61, 1192–1201. [Google Scholar] [CrossRef] [PubMed]
- Ladiwala, A.R.; Lin, J.C.; Bale, S.S.; Marcelino-Cruz, A.M.; Bhattacharya, M.; Dordick, J.S.; Tessier, P.M. Resveratrol selectively remodels soluble oligomers and fibrils of amyloid abeta into off-pathway conformers. J. Biol. Chem. 2010, 285, 24228–24237. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, C.A.; Saraiva, M.J.; Cardoso, I. Stability of the transthyretin molecule as a key factor in the interaction with a-beta peptide--relevance in Alzheimer’s disease. PLoS ONE 2012, 7, e45368. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Ho, L.; Zhao, W.; Ono, K.; Rosensweig, C.; Chen, L.; Humala, N.; Teplow, D.B.; Pasinetti, G.M. Grape-derived polyphenolics prevent abeta oligomerization and attenuate cognitive deterioration in a mouse model of Alzheimer’s disease. J. Neurosci. Off. J. Soc. Neurosci. 2008, 28, 6388–6392. [Google Scholar] [CrossRef] [PubMed]
- Espargaro, A.; Ginex, T.; Vadell, M.D.; Busquets, M.A.; Estelrich, J.; Munoz-Torrero, D.; Luque, F.J.; Sabate, R. Combined in vitro cell-based/in silico screening of naturally occurring flavonoids and phenolic compounds as potential anti-Alzheimer drugs. J. Nat. Prod. 2017, 80, 278–289. [Google Scholar] [CrossRef] [PubMed]
- Sabogal-Guaqueta, A.M.; Munoz-Manco, J.I.; Ramirez-Pineda, J.R.; Lamprea-Rodriguez, M.; Osorio, E.; Cardona-Gomez, G.P. The flavonoid quercetin ameliorates Alzheimer’s disease pathology and protects cognitive and emotional function in aged triple transgenic Alzheimer’s disease model mice. Neuropharmacology 2015, 93, 134–145. [Google Scholar] [CrossRef] [PubMed]
- Ho, L.; Ferruzzi, M.G.; Janle, E.M.; Wang, J.; Gong, B.; Chen, T.Y.; Lobo, J.; Cooper, B.; Wu, Q.L.; Talcott, S.T.; et al. Identification of brain-targeted bioactive dietary quercetin-3-o-glucuronide as a novel intervention for Alzheimer’s disease. FASEB J. Off. Pub. Fed. Am. Soc. Exp. Biol. 2013, 27, 769–781. [Google Scholar] [CrossRef] [PubMed]
- Lian, Q.; Nie, Y.; Zhang, X.; Tan, B.; Cao, H.; Chen, W.; Gao, W.; Chen, J.; Liang, Z.; Lai, H.; et al. Effects of grape seed proanthocyanidin on Alzheimer’s disease in vitro and in vivo. Exp. Ther. Med. 2016, 12, 1681–1692. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Li, Z.; Rizak, J.D.; Wu, S.; Wang, Z.; He, R.; Su, M.; Qin, D.; Wang, J.; Hu, X. Resveratrol attenuates formaldehyde induced hyperphosphorylation of tau protein and cytotoxicity in n2a cells. Front. Neurosci. 2016, 10, 598. [Google Scholar] [CrossRef] [PubMed]
- Jhang, K.A.; Park, J.S.; Kim, H.S.; Chong, Y.H. Resveratrol ameliorates tau hyperphosphorylation at ser396 site and oxidative damage in rat hippocampal slices exposed to vanadate: Implication of erk1/2 and gsk-3beta signaling cascades. J. Agric. Food. Chem. 2017, 65, 9626–9634. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.Y.; Luo, T.; Li, S.; Ting, O.Y.; He, F.; Xu, J.; Wang, H.Q. Quercetin inhibits okadaic acid-induced tau protein hyperphosphorylation through the ca2+calpainp25cdk5 pathway in ht22 cells. Int. J. Mol. Med. 2018, 41, 1138–1146. [Google Scholar] [PubMed]
- Ali, T.; Kim, M.J.; Rehman, S.U.; Ahmad, A.; Kim, M.O. Anthocyanin-loaded peg-gold nanoparticles enhanced the neuroprotection of anthocyanins in an abeta1-42 mouse model of Alzheimer’s disease. Mol. Neurobiol. 2017, 54, 6490–6506. [Google Scholar] [CrossRef] [PubMed]
- Sul, D.; Kim, H.S.; Lee, D.; Joo, S.S.; Hwang, K.W.; Park, S.Y. Protective effect of caffeic acid against beta-amyloid-induced neurotoxicity by the inhibition of calcium influx and tau phosphorylation. Life Sci. 2009, 84, 257–262. [Google Scholar] [CrossRef] [PubMed]
- Draczynska-Lusiak, B.; Chen, Y.M.; Sun, A.Y. Oxidized lipoproteins activate nf-kappab binding activity and apoptosis in pc12 cells. Neuroreport 1998, 9, 527–532. [Google Scholar] [CrossRef] [PubMed]
- Dasgupta, B.; Milbrandt, J. Resveratrol stimulates amp kinase activity in neurons. Proc. Natl. Acad. Sci. USA 2007, 104, 7217–7222. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Feng, L.; Xing, Y.; Wang, Y.; Du, L.; Xu, C.; Cao, J.; Wang, Q.; Fan, S.; Liu, Q.; et al. Radioprotective and antioxidant effect of resveratrol in hippocampus by activating sirt1. Int. J. Mol. Sci. 2014, 15, 5928–5939. [Google Scholar] [CrossRef] [PubMed]
- Khan, R.S.; Fonseca-Kelly, Z.; Callinan, C.; Zuo, L.; Sachdeva, M.M.; Shindler, K.S. Sirt1 activating compounds reduce oxidative stress and prevent cell death in neuronal cells. Front. Cell. Neurosci. 2012, 6, 63. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Nguyen, M.D.; Dobbin, M.M.; Fischer, A.; Sananbenesi, F.; Rodgers, J.T.; Delalle, I.; Baur, J.A.; Sui, G.; Armour, S.M.; et al. Sirt1 deacetylase protects against neurodegeneration in models for Alzheimer’s disease and amyotrophic lateral sclerosis. EMBO J. 2007, 26, 3169–3179. [Google Scholar] [CrossRef] [PubMed]
- Karuppagounder, S.S.; Pinto, J.T.; Xu, H.; Chen, H.L.; Beal, M.F.; Gibson, G.E. Dietary supplementation with resveratrol reduces plaque pathology in a transgenic model of Alzheimer’s disease. Neurochem. Inter. 2009, 54, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Rege, S.D.; Geetha, T.; Broderick, T.L.; Babu, J.R. Resveratrol protects beta amyloid-induced oxidative damage and memory associated proteins in h19-7 hippocampal neuronal cells. Curr. Alzheimer Res. 2015, 12, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.C.; Lu, K.T.; Wo, Y.Y.; Wu, Y.J.; Yang, Y.L. Resveratrol protects rats from abeta-induced neurotoxicity by the reduction of inos expression and lipid peroxidation. PLoS ONE 2011, 6, e29102. [Google Scholar] [CrossRef] [PubMed]
- Sakata, Y.; Zhuang, H.; Kwansa, H.; Koehler, R.C.; Dore, S. Resveratrol protects against experimental stroke: Putative neuroprotective role of heme oxygenase 1. Exp. Neurol. 2010, 224, 325–329. [Google Scholar] [CrossRef] [PubMed]
- Savaskan, E.; Olivieri, G.; Meier, F.; Seifritz, E.; Wirz-Justice, A.; Muller-Spahn, F. Red wine ingredient resveratrol protects from beta-amyloid neurotoxicity. Gerontology 2003, 49, 380–383. [Google Scholar] [CrossRef] [PubMed]
- Kwon, K.J.; Kim, H.J.; Shin, C.Y.; Han, S.H. Melatonin potentiates the neuroprotective properties of resveratrol against beta-amyloid-induced neurodegeneration by modulating amp-activated protein kinase pathways. J. Clin. Neurol. 2010, 6, 127–137. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.S.; Zhang, Z.S.; Yang, B.; He, W. Resveratrol attenuates oxidative damage and ameliorates cognitive impairment in the brain of senescence-accelerated mice. Life Sci. 2012, 91, 872–877. [Google Scholar] [CrossRef] [PubMed]
- Bastianetto, S.; Zheng, W.H.; Quirion, R. Neuroprotective abilities of resveratrol and other red wine constituents against nitric oxide-related toxicity in cultured hippocampal neurons. Br. J. Pharmacol. 2000, 131, 711–720. [Google Scholar] [CrossRef] [PubMed]
- Rishitha, N.; Muthuraman, A. Therapeutic evaluation of solid lipid nanoparticle of quercetin in pentylenetetrazole induced cognitive impairment of zebrafish. Life Sci. 2018, 199, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.M.; Kim, B.C.; Cho, Y.H.; Choi, K.H.; Chang, J.; Park, M.S.; Kim, M.K.; Cho, K.H.; Kim, J.K. Effects of flavonoid compounds on beta-amyloid-peptide-induced neuronal death in cultured mouse cortical neurons. Chonnam Med. J. 2014, 50, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Pacheco, S.M.; Soares, M.S.P.; Gutierres, J.M.; Gerzson, M.F.B.; Carvalho, F.B.; Azambuja, J.H.; Schetinger, M.R.C.; Stefanello, F.M.; Spanevello, R.M. Anthocyanins as a potential pharmacological agent to manage memory deficit, oxidative stress and alterations in ion pump activity induced by experimental sporadic dementia of Alzheimer’s type. J. Nut. Biochem. 2018, 56, 193–204. [Google Scholar] [CrossRef] [PubMed]
- Poewe, W.; Seppi, K.; Tanner, C.M.; Halliday, G.M.; Brundin, P.; Volkmann, J.; Schrag, A.E.; Lang, A.E. Parkinson disease. Nat. Rev. Dis Primers 2017, 3, 17013. [Google Scholar] [CrossRef] [PubMed]
- Pfeiffer, R.F. Non-motor symptoms in Parkinson’s disease. Parkinsonism Relat. Disorders 2016, 22 Suppl 1, S119–S122. [Google Scholar] [CrossRef] [PubMed]
- Spillantini, M.G.; Schmidt, M.L.; Lee, V.M.; Trojanowski, J.Q.; Jakes, R.; Goedert, M. Alpha-synuclein in lewy bodies. Nature 1997, 388, 839–840. [Google Scholar] [CrossRef] [PubMed]
- Luth, E.S.; Bartels, T.; Dettmer, U.; Kim, N.C.; Selkoe, D.J. Purification of alpha-synuclein from human brain reveals an instability of endogenous multimers as the protein approaches purity. Biochemistry 2015, 54, 279–292. [Google Scholar] [CrossRef] [PubMed]
- Blesa, J.; Trigo-Damas, I.; Quiroga-Varela, A.; Jackson-Lewis, V.R. Oxidative stress and Parkinson’s disease. Front. Neuroanat. 2015, 9, 91. [Google Scholar] [CrossRef] [PubMed]
- Nagatsu, T.; Sawada, M. Molecular mechanism of the relation of monoamine oxidase b and its inhibitors to Parkinson’s disease: Possible implications of glial cells. J. Neural Transm. Suppl. 2006, 53–65. [Google Scholar]
- Subramaniam, S.R.; Chesselet, M.F. Mitochondrial dysfunction and oxidative stress in Parkinson’s disease. Pro. Neurobiol. 2013, 106–107, 17–32. [Google Scholar] [CrossRef] [PubMed]
- Keane, P.C.; Kurzawa, M.; Blain, P.G.; Morris, C.M. Mitochondrial dysfunction in Parkinson’s disease. Parkinson’s Dis. 2011, 2011, 716871. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Liu, Y.; Zhou, J. Neuroinflammation in Parkinson’s disease and its potential as therapeutic target. Transl. Neurodegener. 2015, 4, 19. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.K.; Kang, S.J.; Poncz, M.; Song, K.J.; Park, K.S. Resveratrol protects sh-sy5y neuroblastoma cells from apoptosis induced by dopamine. Exp. Mol. Med. 2007, 39, 376–384. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.M.; Ahmad, A.; Ishrat, T.; Khan, M.B.; Hoda, M.N.; Khuwaja, G.; Raza, S.S.; Khan, A.; Javed, H.; Vaibhav, K.; et al. Resveratrol attenuates 6-hydroxydopamine-induced oxidative damage and dopamine depletion in rat model of Parkinson’s disease. Brain Res. 2010, 1328, 139–151. [Google Scholar] [CrossRef] [PubMed]
- Lim, M.L.; Mercer, L.D.; Nagley, P.; Beart, P.M. Rotenone and mpp+ preferentially redistribute apoptosis-inducing factor in apoptotic dopamine neurons. Neuroreport 2007, 18, 307–312. [Google Scholar] [CrossRef] [PubMed]
- Okawara, M.; Katsuki, H.; Kurimoto, E.; Shibata, H.; Kume, T.; Akaike, A. Resveratrol protects dopaminergic neurons in midbrain slice culture from multiple insults. Biochem. Pharmacol. 2007, 73, 550–560. [Google Scholar] [CrossRef] [PubMed]
- Zeng, W.; Zhang, W.; Lu, F.; Gao, L.; Gao, G. Resveratrol attenuates mpp(+)-induced mitochondrial dysfunction and cell apoptosis via akt/gsk-3beta pathway in sn4741 cells. Neurosci. Lett. 2017, 637, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Fan, W.; Wang, H.; Bao, L.; Li, G.; Li, T.; Song, S.; Li, H.; Hao, J.; Sun, J. Resveratrol protects pc12 cell against 6-ohda damage via cxcr4 signaling pathway. Evidence-Based Complementary Alter. Med. eCAM 2015, 2015, 730121. [Google Scholar] [CrossRef] [PubMed]
- Sallusto, F.; Baggiolini, M. Chemokines and leukocyte traffic. Nature Immunol. 2008, 9, 949–952. [Google Scholar] [CrossRef] [PubMed]
- Shimoji, M.; Pagan, F.; Healton, E.B.; Mocchetti, I. Cxcr4 and cxcl12 expression is increased in the nigro-striatal system of Parkinson’s disease. Neurotoxicity Res. 2009, 16, 318–328. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.K.; Chen, S.D.; Chuang, Y.C.; Lin, H.Y.; Huang, C.R.; Chuang, J.H.; Wang, P.W.; Huang, S.T.; Tiao, M.M.; Chen, J.B.; et al. Resveratrol partially prevents rotenone-induced neurotoxicity in dopaminergic sh-sy5y cells through induction of heme oxygenase-1 dependent autophagy. Int. J. Mol. Sci. 2014, 15, 1625–1646. [Google Scholar] [CrossRef] [PubMed]
- Zou, X.D.; Guo, S.Q.; Hu, Z.W.; Li, W.L. Nampt protects against 6-hydroxydopamine-induced neurotoxicity in pc12 cells through modulating sirt1 activity. Mol. Med. Rep. 2016, 13, 4058–4064. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Dong, X.; Liu, Z.; Zhu, S.; Liu, H.; Fan, W.; Hu, Y.; Hu, T.; Yu, Y.; Li, Y.; et al. Resveratrol suppresses rotenone-induced neurotoxicity through activation of sirt1/akt1 signaling pathway. Anatomical Record 2018, 301, 1115–1125. [Google Scholar] [CrossRef] [PubMed]
- Mudo, G.; Makela, J.; Di Liberto, V.; Tselykh, T.V.; Olivieri, M.; Piepponen, P.; Eriksson, O.; Malkia, A.; Bonomo, A.; Kairisalo, M.; et al. Transgenic expression and activation of pgc-1alpha protect dopaminergic neurons in the mptp mouse model of Parkinson’s disease. Cell. Mol. Life Sci. CMLS 2012, 69, 1153–1165. [Google Scholar] [CrossRef] [PubMed]
- Ferretta, A.; Gaballo, A.; Tanzarella, P.; Piccoli, C.; Capitanio, N.; Nico, B.; Annese, T.; Di Paola, M.; Dell’aquila, C.; De Mari, M.; et al. Effect of resveratrol on mitochondrial function: Implications in Parkin-associated familiar Parkinson’s disease. Biochim. Biophys. Acta 2014, 1842, 902–915. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Li, X.; Zhu, J.X.; Xie, W.; Le, W.; Fan, Z.; Jankovic, J.; Pan, T. Resveratrol-activated ampk/sirt1/autophagy in cellular models of Parkinson’s disease. Neuro-Signals 2011, 19, 163–174. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.J.; Dong, S.Y.; Cui, X.X.; Feng, Y.; Liu, T.; Yin, M.; Kuo, S.H.; Tan, E.K.; Zhao, W.J.; Wu, Y.C. Resveratrol alleviates mptp-induced motor impairments and pathological changes by autophagic degradation of alpha-synuclein via sirt1-deacetylated lc3. Mol. Nutr. Food Res. 2016, 60, 2161–2175. [Google Scholar] [CrossRef] [PubMed]
- Ay, M.; Luo, J.; Langley, M.; Jin, H.; Anantharam, V.; Kanthasamy, A.; Kanthasamy, A.G. Molecular mechanisms underlying protective effects of quercetin against mitochondrial dysfunction and progressive dopaminergic neurodegeneration in cell culture and mitopark transgenic mouse models of Parkinson’s disease. J. Neurochem. 2017, 141, 766–782. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Han, S.; Fink, A.L. Oxidized quercetin inhibits alpha-synuclein fibrillization. Biochim. Biophys. Acta 2013, 1830, 2872–2881. [Google Scholar] [CrossRef] [PubMed]
- Fazili, N.A.; Naeem, A. Anti-fibrillation potency of caffeic acid against an antidepressant induced fibrillogenesis of human alpha-synuclein: Implications for Parkinson’s disease. Biochimie 2015, 108, 178–185. [Google Scholar] [CrossRef] [PubMed]
- Ardah, M.T.; Paleologou, K.E.; Lv, G.; Abul Khair, S.B.; Kazim, A.S.; Minhas, S.T.; Al-Tel, T.H.; Al-Hayani, A.A.; Haque, M.E.; Eliezer, D.; et al. Structure activity relationship of phenolic acid inhibitors of alpha-synuclein fibril formation and toxicity. Front. Aging Neurosci. 2014, 6, 197. [Google Scholar] [CrossRef] [PubMed]
- Jimenez-Del-Rio, M.; Guzman-Martinez, C.; Velez-Pardo, C. The effects of polyphenols on survival and locomotor activity in drosophila melanogaster exposed to iron and paraquat. Neurochem. Res. 2010, 35, 227–238. [Google Scholar] [CrossRef] [PubMed]
- Askar, M.; Hussein, A.M.; Al-Basiony, S.; Meseha, R.; Metias, E.; Salama, M.; Antar, A.; El Sayed, A. Effects of exercise and ferulic acid on alpha synuclein and neuroprotective heat shock protein 70 in an experimental model of Parkinsonism disease. CNS Neurol. Disorders Drug Targets 2018. [Google Scholar] [CrossRef] [PubMed]
- Vidoni, C.; Secomandi, E.; Castiglioni, A.; Melone, M.A.B.; Isidoro, C. Resveratrol protects neuronal-like cells expressing mutant huntingtin from dopamine toxicity by rescuing atg4-mediated autophagosome formation. Neurochem. Int. 2018, 117, 174–187. [Google Scholar] [CrossRef] [PubMed]
- Long, J.; Gao, H.; Sun, L.; Liu, J.; Zhao-Wilson, X. Grape extract protects mitochondria from oxidative damage and improves locomotor dysfunction and extends lifespan in a drosophila Parkinson’s disease model. Rejuvenation Res. 2009, 12, 321–331. [Google Scholar] [CrossRef] [PubMed]
- Jin, F.; Wu, Q.; Lu, Y.F.; Gong, Q.H.; Shi, J.S. Neuroprotective effect of resveratrol on 6-ohda-induced Parkinson’s disease in rats. Eur. J. Pharmacol. 2008, 600, 78–82. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Shi, J.S.; Zhou, H.; Wilson, B.; Hong, J.S.; Gao, H.M. Resveratrol protects dopamine neurons against lipopolysaccharide-induced neurotoxicity through its anti-inflammatory actions. Mol. Pharmacol. 2010, 78, 466–477. [Google Scholar] [CrossRef] [PubMed]
- Gaballah, H.H.; Zakaria, S.S.; Elbatsh, M.M.; Tahoon, N.M. Modulatory effects of resveratrol on endoplasmic reticulum stress-associated apoptosis and oxido-inflammatory markers in a rat model of rotenone-induced Parkinson’s disease. Chem. Biol. Interact. 2016, 251, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Blanchet, J.; Longpre, F.; Bureau, G.; Morissette, M.; DiPaolo, T.; Bronchti, G.; Martinoli, M.G. Resveratrol, a red wine polyphenol, protects dopaminergic neurons in mptp-treated mice. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2008, 32, 1243–1250. [Google Scholar] [CrossRef] [PubMed]
- Lu, K.T.; Ko, M.C.; Chen, B.Y.; Huang, J.C.; Hsieh, C.W.; Lee, M.C.; Chiou, R.Y.; Wung, B.S.; Peng, C.H.; Yang, Y.L. Neuroprotective effects of resveratrol on mptp-induced neuron loss mediated by free radical scavenging. J. Agric. Food Chem. 2008, 56, 6910–6913. [Google Scholar] [CrossRef] [PubMed]
- Anandhan, A.; Tamilselvam, K.; Vijayraja, D.; Ashokkumar, N.; Rajasankar, S.; Manivasagam, T. Resveratrol attenuates oxidative stress and improves behaviour in 1 -methyl-4-phenyl-1,2,3,6-tetrahydropyridine (mptp) challenged mice. Annals Neurosci. 2010, 17, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Kaariainen, T.M.; Piltonen, M.; Ossola, B.; Kekki, H.; Lehtonen, S.; Nenonen, T.; Lecklin, A.; Raasmaja, A.; Mannisto, P.T. Lack of robust protective effect of quercetin in two types of 6-hydroxydopamine-induced Parkinsonian models in rats and dopaminergic cell cultures. Brain Res. 2008, 1203, 149–159. [Google Scholar] [CrossRef] [PubMed]
- Ossola, B.; Kaarainen, T.M.; Raasmaja, A.; Mannisto, P.T. Time-dependent protective and harmful effects of quercetin on 6-ohda-induced toxicity in neuronal sh-sy5y cells. Toxicology 2008, 250, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Vauzour, D.; Ravaioli, G.; Vafeiadou, K.; Rodriguez-Mateos, A.; Angeloni, C.; Spencer, J.P. Peroxynitrite induced formation of the neurotoxins 5-s-cysteinyl-dopamine and dhbt-1: Implications for Parkinson’s disease and protection by polyphenols. Arch. Biochem. Biophys. 2008, 476, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Mukai, R.; Kawabata, K.; Otsuka, S.; Ishisaka, A.; Kawai, Y.; Ji, Z.S.; Tsuboi, H.; Terao, J. Effect of quercetin and its glucuronide metabolite upon 6-hydroxydopamine-induced oxidative damage in neuro-2a cells. Free Radical Rres. 2012, 46, 1019–1028. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.; McGeer, E.G.; McGeer, P.L. Quercetin, not caffeine, is a major neuroprotective component in coffee. Neurobiol. Aging 2016, 46, 113–123. [Google Scholar] [CrossRef] [PubMed]
- Ahn, T.B.; Jeon, B.S. The role of quercetin on the survival of neuron-like pc12 cells and the expression of alpha-synuclein. Neural Regener. Res. 2015, 10, 1113–1119. [Google Scholar]
- Zbarsky, V.; Datla, K.P.; Parkar, S.; Rai, D.K.; Aruoma, O.I.; Dexter, D.T. Neuroprotective properties of the natural phenolic antioxidants curcumin and naringenin but not quercetin and fisetin in a 6-ohda model of Parkinson’s disease. Free Radical Res. 2005, 39, 1119–1125. [Google Scholar] [CrossRef] [PubMed]
- Haleagrahara, N.; Siew, C.J.; Mitra, N.K.; Kumari, M. Neuroprotective effect of bioflavonoid quercetin in 6-hydroxydopamine-induced oxidative stress biomarkers in the rat striatum. Neurosci. Lett. 2011, 500, 139–143. [Google Scholar] [CrossRef] [PubMed]
- Karuppagounder, S.S.; Madathil, S.K.; Pandey, M.; Haobam, R.; Rajamma, U.; Mohanakumar, K.P. Quercetin up-regulates mitochondrial complex-i activity to protect against programmed cell death in rotenone model of Parkinson’s disease in rats. Neuroscience 2013, 236, 136–148. [Google Scholar] [CrossRef] [PubMed]
- El-Horany, H.E.; El-Latif, R.N.; ElBatsh, M.M.; Emam, M.N. Ameliorative effect of quercetin on neurochemical and behavioral deficits in rotenone rat model of Parkinson’s disease: Modulating autophagy (quercetin on experimental Parkinson’s disease). J. Biochem. Mol. Toxicol. 2016, 30, 360–369. [Google Scholar] [CrossRef] [PubMed]
- Haleagrahara, N.; Siew, C.J.; Ponnusamy, K. Effect of quercetin and desferrioxamine on 6-hydroxydopamine (6-ohda) induced neurotoxicity in striatum of rats. J. Toxicol. Sci. 2013, 38, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Denny Joseph, K.M.; Muralidhara. Combined oral supplementation of fish oil and quercetin enhances neuroprotection in a chronic rotenone rat model: Relevance to Parkinson’s disease. Neurochem. Res. 2015, 40, 894–905. [Google Scholar] [CrossRef] [PubMed]
- Gomez del Rio, M.A.; Sanchez-Reus, M.I.; Iglesias, I.; Pozo, M.A.; Garcia-Arencibia, M.; Fernandez-Ruiz, J.; Garcia-Garcia, L.; Delgado, M.; Benedi, J. Neuroprotective properties of standardized extracts of hypericum perforatum on rotenone model of Parkinson’s disease. CNS Neurol. Disorders Drug Targets 2013, 12, 665–679. [Google Scholar] [CrossRef]
- Singh, S.; Jamwal, S.; Kumar, P. Neuroprotective potential of quercetin in combination with piperine against 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced neurotoxicity. Neural Regener. Res. 2017, 12, 1137–1144. [Google Scholar]
- Vauzour, D.; Vafeiadou, K.; Corona, G.; Pollard, S.E.; Tzounis, X.; Spencer, J.P. Champagne wine polyphenols protect primary cortical neurons against peroxynitrite-induced injury. J. Agric. Food Chem. 2007, 55, 2854–2860. [Google Scholar] [CrossRef] [PubMed]
- Vauzour, D.; Corona, G.; Spencer, J.P. Caffeic acid, tyrosol and p-coumaric acid are potent inhibitors of 5-s-cysteinyl-dopamine induced neurotoxicity. Arch. Biochem. Biophys. 2010, 501, 106–111. [Google Scholar] [CrossRef] [PubMed]
- Mansouri, M.T.; Farbood, Y.; Sameri, M.J.; Sarkaki, A.; Naghizadeh, B.; Rafeirad, M. Neuroprotective effects of oral gallic acid against oxidative stress induced by 6-hydroxydopamine in rats. Food Chem. 2013, 138, 1028–1033. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, M.D.; Souza, C.M.; Menezes, A.P.; Carmo, M.R.; Fonteles, A.A.; Gurgel, J.P.; Lima, F.A.; Viana, G.S.; Andrade, G.M. Catechin attenuates behavioral neurotoxicity induced by 6-ohda in rats. Pharmacol. Biochem. Behav. 2013, 110, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Pu, X.P. Neuroprotective effect of kaempferol against a 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced mouse model of Parkinson’s disease. Biol. Pharm. Bull. 2011, 34, 1291–1296. [Google Scholar] [CrossRef] [PubMed]
- Espeland, M.A.; Gu, L.; Masaki, K.H.; Langer, R.D.; Coker, L.H.; Stefanick, M.L.; Ockene, J.; Rapp, S.R. Association between reported alcohol intake and cognition: Results from the women’s health initiative memory study. Am. J. Epidemiol. 2005, 161, 228–238. [Google Scholar] [CrossRef] [PubMed]
- Stampfer, M.J.; Kang, J.H.; Chen, J.; Cherry, R.; Grodstein, F. Effects of moderate alcohol consumption on cognitive function in women. N. Engl. J. Med. 2005, 352, 245–253. [Google Scholar] [CrossRef] [PubMed]
- Ganguli, M.; Vander Bilt, J.; Saxton, J.A.; Shen, C.; Dodge, H.H. Alcohol consumption and cognitive function in late life: A longitudinal community study. Neurology 2005, 65, 1210–1217. [Google Scholar] [CrossRef] [PubMed]
- Mehlig, K.; Skoog, I.; Guo, X.; Schutze, M.; Gustafson, D.; Waern, M.; Ostling, S.; Bjorkelund, C.; Lissner, L. Alcoholic beverages and incidence of dementia: 34-year follow-up of the prospective population study of women in goteborg. Am. J. Epidemiol. 2008, 167, 684–691. [Google Scholar] [CrossRef] [PubMed]

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, P.; Vauzour, D. Wine Polyphenols and Neurodegenerative Diseases: An Update on the Molecular Mechanisms Underpinning Their Protective Effects. Beverages 2018, 4, 96. https://doi.org/10.3390/beverages4040096
Silva P, Vauzour D. Wine Polyphenols and Neurodegenerative Diseases: An Update on the Molecular Mechanisms Underpinning Their Protective Effects. Beverages. 2018; 4(4):96. https://doi.org/10.3390/beverages4040096
Chicago/Turabian StyleSilva, Paula, and David Vauzour. 2018. "Wine Polyphenols and Neurodegenerative Diseases: An Update on the Molecular Mechanisms Underpinning Their Protective Effects" Beverages 4, no. 4: 96. https://doi.org/10.3390/beverages4040096
APA StyleSilva, P., & Vauzour, D. (2018). Wine Polyphenols and Neurodegenerative Diseases: An Update on the Molecular Mechanisms Underpinning Their Protective Effects. Beverages, 4(4), 96. https://doi.org/10.3390/beverages4040096






