Design of a Coffee Alternative by Brewing Roasted Seeds from Baobab (Adansonia digitata)
Abstract
1. Introduction
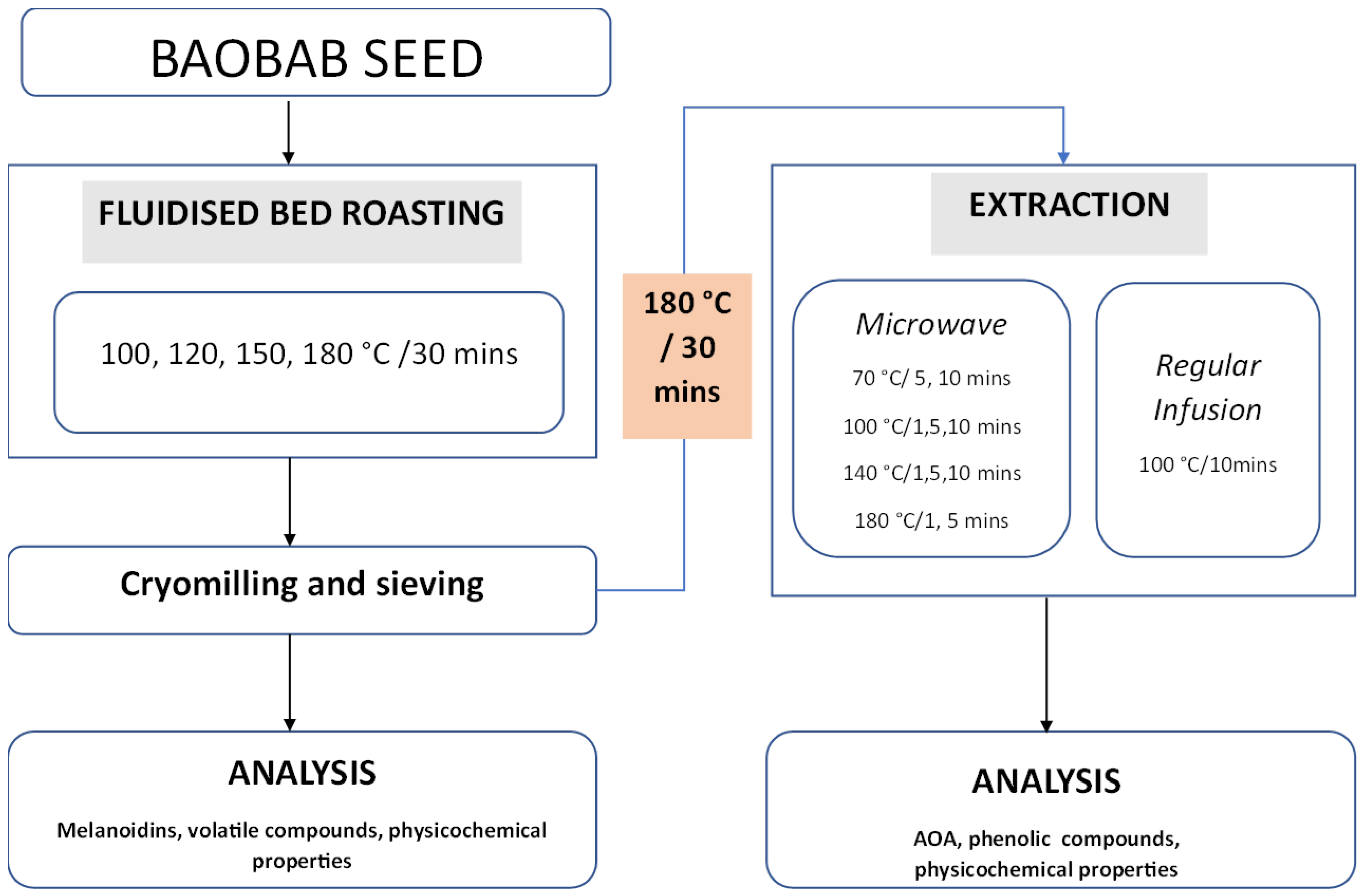
2. Materials and Methods
2.1. Experimental Design
2.1.1. Microwave-Assisted Extraction (MAE)
2.1.2. Regular Infusion
2.2. Sample Analysis of Roasted Baobab Seed Powder
2.2.1. Physico-Chemical Properties
Moisture Content and Solubility
Colour
Titratable Acidity (TA) and pH
2.2.2. Melanoidins
2.2.3. Volatile Compounds (VOC)
2.3. Sample Analysis of Baobab Seed Beverage
2.3.1. Antioxidant Activity (AOA) and Total Phenolic Compounds (TPC)
2.3.2. Quantification of Specific Phenolics Through LC-MS/MS
2.3.3. Physicochemical Properties
2.3.4. Ethanolic Precipitable Matter (EPM)
2.4. Statistical Analysis
3. Results and Discussion
3.1. Roasted Baobab Seeds
3.1.1. Physico-Chemical Properties
3.1.2. Melanoidin Content
3.1.3. Volatile Compounds
3.2. Baobab Seed Beverage
3.2.1. Physico-Chemical Properties
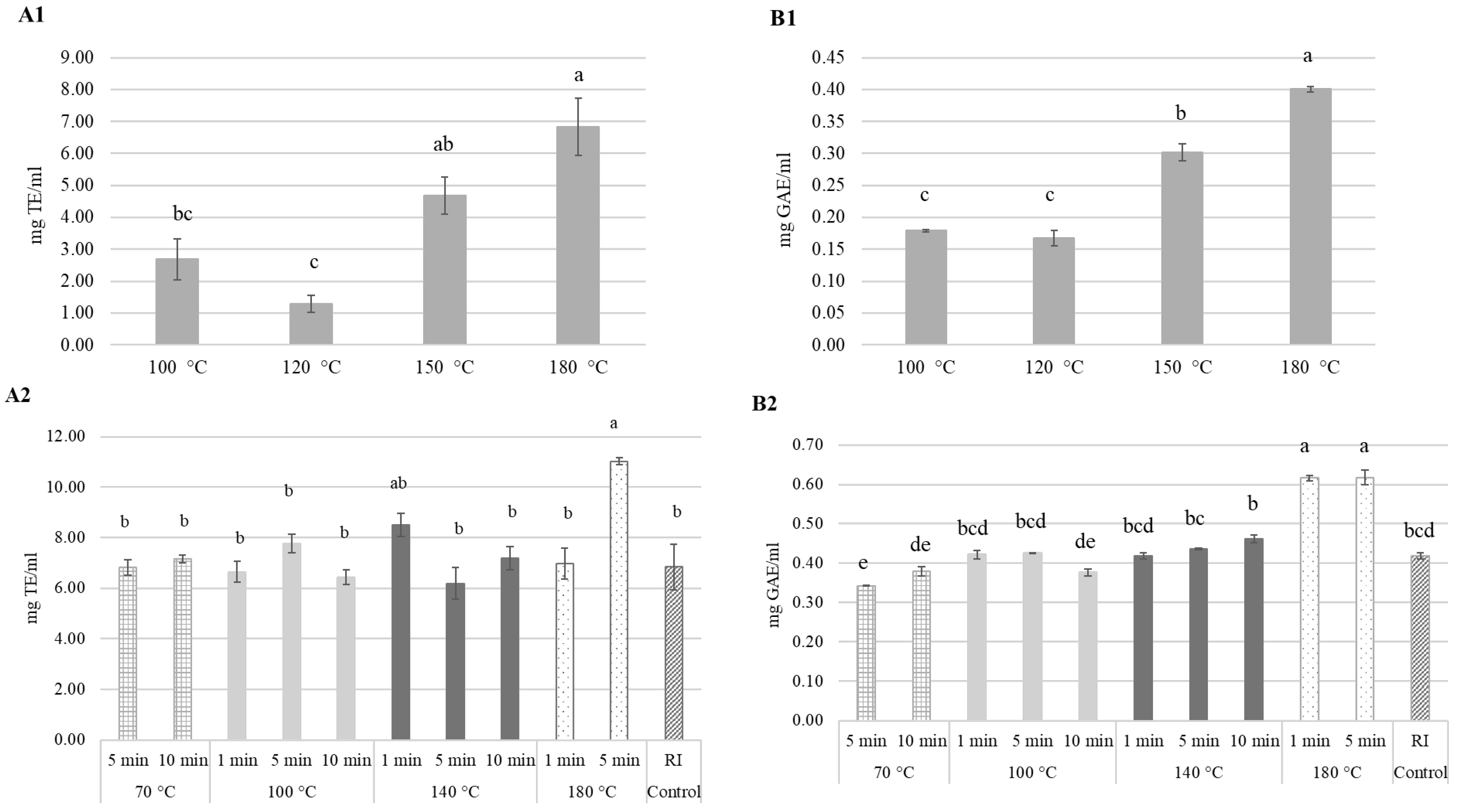
3.2.2. Antioxidant Activity, Total and Individual Phenolics
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abubakar, Y.; Sabariana, S.; Rasdiansyah, R.; Hasni, D. Sensory characteristics of espresso coffee prepared from Gayo arabica coffee roasted at various times and temperatures. IOP Conf. Ser. Earth Environ. Sci. 2021, 667, 012048. [Google Scholar] [CrossRef]
- Açar, Ö.Ç.; Gökmen, V.; Pellegrini, N.; Fogliano, V. Direct evaluation of the total antioxidant capacity of raw and roasted pulses, nuts and seeds. Eur. Food Res. Technol. 2009, 229, 961–969. [Google Scholar] [CrossRef]
- Antony, A.; Farid, M. Effect of Temperatures on Polyphenols during Extraction. Appl. Sci. 2022, 12, 4. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis of AOAC International; AOAC: Gaithersburg, MD, USA, 2005; ISBN 0935584757. [Google Scholar]
- Babalola, J.O.; Adesina, D.A.; Alabi, O.O.; Adepoju, M.R.; Bamisaiye, Y.O.; Awotunde, B.R. Effect of Processing Method on Proximate, Minerals, Phytochemicals and Anti-Nutrients Present in Baobab Seeds (Adansonia digitata). GSC Adv. Res. Rev. 2021, 6, 001–010. [Google Scholar] [CrossRef]
- Baggenstoss, J.; Poisson, L.; Kaegi, R.; Perren, R.; Escher, F. Coffee Roasting and Aroma Formation: Application of Different Time−Temperature Conditions. J. Agric. Food Chem. 2008, 56, 5836–5846. [Google Scholar] [CrossRef] [PubMed]
- Caudill, M.; Osborne, J.; Sandeep, K.P.; Simunovic, J.; Harris, G.K. Viability of microwave technology for accelerated cold brew coffee processing vs conventional brewing methods. J. Food Eng. 2022, 317, 110866. [Google Scholar] [CrossRef]
- Chiacchio, M.F.; Tagliamonte, S.; Visconti, A.; Ferracane, R.; Mustafa, A.; Vitaglione, P. Baobab-Fruit Shell and Fibrous Filaments Are Sources of Antioxidant Dietary Fibres. Molecules 2022, 27, 5563. [Google Scholar] [CrossRef]
- Cordoba, N.; Fernandez-Alduenda, M.; Moreno, F.L.; Ruiz, Y. Coffee extraction: A review of parameters and their influence on the physicochemical characteristics and flavour of coffee brews. Trends Food Sci. Technol. 2020, 96, 45–60. [Google Scholar] [CrossRef]
- Díaz-Rubio, M.E.; Saura-Calixto, F. Dietary Fiber in Brewed Coffee. J. Agric. Food Chem. 2007, 55, 1999–2003. [Google Scholar] [CrossRef]
- Doğan, M.; Aslan, D.; Gürmeriç, V.; Özgür, A.; Göksel Saraç, M. Powder caking and cohesion behaviours of coffee powders as affected by roasting and particle sizes: Principal component analyses (PCA) for flow and bioactive properties. Powder Technol. 2019, 344, 222–232. [Google Scholar] [CrossRef]
- Ekezie, F.-G.C.; Sun, D.-W.; Cheng, J.-H. Acceleration of microwave-assisted extraction processes of food components by integrating technologies and applying emerging solvents: A review of latest developments. Trends Food Sci. Technol. 2017, 67, 160–172. [Google Scholar] [CrossRef]
- Fikry, M.; Yusof, Y.A.; Al-Awaadh, A.M.; Rahman, R.A.; Chin, N.L.; Mousa, E.; Chang, L.S. Effect of the Roasting Conditions on the Physicochemical, Quality and Sensory Attributes of Coffee-Like Powder and Brew from Defatted Palm Date Seeds. Foods 2019, 8, 2. [Google Scholar] [CrossRef]
- Peña-Correa, R.F.; Ataç Mogol, B.; van Boekel, M.A.J.S.; Fogliano, V. Fluidized bed roasting of cocoa nibs speeds up processing and favors the formation of pyrazines. Innov. Food Sci. Emerg. Technol. 2022, 79, 103062. [Google Scholar] [CrossRef]
- Vilas-Franquesa, A.; Fryganas, C.; Casertano, M.; Montemurro, M.; Fogliano, V. Upcycling mango peels into a functional ingredient by combining fermentation and enzymatic-assisted extraction. Food Chem. 2024, 434, 137515. [Google Scholar] [CrossRef]
- Innocentia, O.N.; Ojotu, E.M.; Emmanuel, A. Quality evaluation of coffee-like beverage from baobab (Adansonia digitata) seed. Adv. J. Food Sci. Technol. 2014, 6, 1050–1055. [Google Scholar] [CrossRef]
- Ismail, B.B.; Huang, R.; Liu, D.; Ye, X.; Guo, M. Potential valorisation of baobab (Adansonia digitata) seeds as a coffee substitute: Insights and comparisons on the effect of roasting on quality, sensory profiles, and characterisation of volatile aroma compounds by HS-SPME/GC–MS. Food Chem. 2022, 394, 133475. [Google Scholar] [CrossRef]
- Hatamian, M.; Noshad, M.; Abdanan-Mehdizadeh, S.; Barzegar, H. Effect of roasting treatment on functional and antioxidant properties of chia seed flours. NFS J. 2020, 21, 1–8. [Google Scholar] [CrossRef]
- Kim, W.-M.; Lee, G.-H. Comparison of Imported Wheat Flour Bread Making Properties and Korean Wheat Flour Bread Making Properties Made by Various Bread Making Methods. J. Korean Soc. Food Sci. Nutr. 2015, 44, 434–441. [Google Scholar] [CrossRef]
- Kim, S.J.; Yoon, H.N.; Rhee, J.S. The Effects of Roasting Temperatures on the Formation of Headspace Volatile Compounds in Perilla Seed Oil. J. Am. Oil Chem. Soc. 2000, 77, 451. [Google Scholar] [CrossRef]
- Kipkorir, R.K.; Patrick, M.; Simon, M. Effects of coffee processing technologies on physico chemical properties and sensory qualities of coffee. Afr. J. Food Sci. 2015, 9, 230–236. Available online: https://repository.dkut.ac.ke:8080/xmlui/handle/123456789/258 (accessed on 10 September 2025). [CrossRef]
- Li, D.-C.; Jiang, J.-G. Optimization of the microwave-assisted extraction conditions of tea polyphenols from green tea. Int. J. Food Sci. Nutr. 2010, 61, 837–845. [Google Scholar] [CrossRef] [PubMed]
- Lopes, G.R.; Passos, C.P.; Rodrigues, C.; Teixeira, J.A.; Coimbra, M.A. Impact of microwave-assisted extraction on roasted coffee carbohydrates, caffeine, chlorogenic acids and coloured compounds. Food Res. Int. 2020, 129, 108864. [Google Scholar] [CrossRef]
- Marcolino, E.; Salavarria, D.; da Silva, L.G.M.; Almeida, A.; da Silva, F.M.O.; Dias, J. Valorization of baobab seeds (Adansonia digitata) as a coffee-like beverage: Evaluation of roasting time on bioactive compounds. J. Food Sci. Technol. 2024, 61, 727–733. [Google Scholar] [CrossRef] [PubMed]
- Mokrzycki, W.; Tatol, M. Color difference Delta E—A survey. Mach. Graph. Vis. 2011, 20, 383–411. [Google Scholar]
- Montemurro, M.; Casertano, M.; Vilas-Franquesa, A.; Rizzello, C.G.; Fogliano, V. Exploitation of spent coffee ground (SCG) as a source of functional compounds and growth substrate for probiotic lactic acid bacteria. LWT 2024, 198, 115974. [Google Scholar] [CrossRef]
- Morales, F.J.; Somoza, V.; Fogliano, V. Physiological relevance of dietary melanoidins. Amino Acids 2012, 42, 1097–1109. [Google Scholar] [CrossRef]
- Munthali, C.R.Y.; Chirwa, P.W.; Akinnifesi, F.K. Phenotypic variation in fruit and seed morphology of Adansonia digitata L. (baobab) in five selected wild populations in Malawi. Agrofor. Syst. 2012, 85, 279–290. [Google Scholar] [CrossRef]
- Nielsen Pm Petersen, D.; Dambmann, C. Improved Method for Determining Food Protein Degree of Hydrolysis. J. Food Sci. 2001, 66, 642–646. [Google Scholar] [CrossRef]
- Nouruddeen, Z.B.; Mohammed, A.; Ghazali, H.M.; Karim, R. Baobab Tree (Adansonia digitata L.) Parts: Nutrition, Applications in Food and Uses in Ethno-medicine—A Review. Ann. Nutr. Disord. Ther. 2014, 1, 11011. [Google Scholar] [CrossRef]
- Obizoba, I.C.; Amaechi, N.A. The Effect of Processing Methods on the Chemical Composition of Baobab (Adansonia digitata L.) Pulp and Seed. Ecol. Food Nutr. 1993, 29, 199–205. [Google Scholar] [CrossRef]
- Pérez-Hernández, L.M.; Chávez-Quiroz, K.; Medina-Juárez, L.Á.; Gámez Meza, N. Phenolic characterization, melanoidins, and antioxidant activity of some commercial coffees from Coffea arabica and Coffea canephora. J. Mex. Chem. Soc. 2012, 56, 430–435. [Google Scholar]
- Petisca, C.; Pérez-Palacios, T.; Farah, A.; Pinho, O.; Ferreira, I.M.P.L.V.O. Furans and Other Volatile Compounds in Ground Roasted and Espresso Coffee Using Headspace Solid-Phase Microextraction: Effect of Roasting Speed. Food Bioprod. Process. 2013, 91, 233–241. [Google Scholar] [CrossRef]
- Pettinato, M.; Casazza, A.A.; Perego, P. The role of heating step in microwave-assisted extraction of polyphenols from spent coffee grounds. Food Bioprod. Process. 2019, 114, 227–234. [Google Scholar] [CrossRef]
- Pușcaș, A.; Tanislav, A.E.; Marc, R.A.; Mureșan, V.; Mureșan, A.E.; Pall, E.; Cerbu, C. Cytotoxicity Evaluation and Antioxidant Activity of a Novel Drink Based on Roasted Avocado Seed Powder. Plants 2022, 11, 1083. [Google Scholar] [CrossRef] [PubMed]
- Robards, K.; Prenzler, P.D.; Tucker, G.; Swatsitang, P.; Glover, W. Phenolic compounds and their role in oxidative processes in fruits. Food Chem. 1999, 66, 401–436. [Google Scholar] [CrossRef]
- Sacchetti, G.; Ioannone, F.; De Gregorio, M.; Di Mattia, C.; Serafini, M.; Mastrocola, D. Non-enzymatic browning during cocoa roasting as affected by processing time and temperature. J. Food Eng. 2016, 169, 44–52. [Google Scholar] [CrossRef]
- Salih, N.K.-E.M.; Yahia, E.M. Phenolics and fatty acids compositions of vitex and baobab seeds used as coffee substitutes in Nuba Mountains, Sudan. Agric. Biol. J. N. Am. 2015, 6, 90–93. Available online: http://www.scihub.org/ABJNA/PDF/2015/3/ABJNA-6-3-90-93.pdf (accessed on 10 September 2025).
- Schulte, H.; Vhangani, L.N.; Toepfl, S. Physicochemical and antioxidant properties of baobab seed. Int. J. Food Sci. Technol. 2025, 60, vvae025. [Google Scholar] [CrossRef]
- Tarapatskyy, M.; Zaguła, G.; Bajcar, M.; Puchalski, C.; Saletnik, B. Magnetic Field Extraction Techniques in Preparing High-Quality Tea Infusions. Appl. Sci. 2018, 8, 1876. [Google Scholar] [CrossRef]
| Parameter | Moisture Content (%) | ΔE | L* | Solubility (%) | Clarity (% Transmittance at 660 nm) | pH | TA (g/L) | Melanoidin Concentration (g/100 g) |
|---|---|---|---|---|---|---|---|---|
| FB1 | 2.54 ± 0.07 a | 24.94 | 47.52 ± 0.02 a | 24.28 ± 0.39 a | 29.70 ± 0.01 a | 6.15 ± 0.02 a | 0.011 ± 0.005 a | 9.91 ± 0.01 a |
| FB2 | 1.56 ± 0.07 b | 20.79 | 43.23 ± 0.01 b | 32.77 ± 0.40 b | 25.58 ± 0.02 b | 6.13 ± 0.02 a | 0.026 ± 0.005 b | 9.99 ± 0.01 b,c |
| FB3 | 0.78 ± 0.06 c | 11.36 | 33.61 ± 0.02 c | 33.41 ± 0.46 c | 24.58 ± 0.01 b,d | 5.97 ± 0.01 b | 0.041 ± 0.005 c | 10.04 ± 0.01 b,d,e |
| FB4 | 0.44 ± 0.07 d | 3.56 | 25.61 ± 0.02 d | 34.93 ± 0.17 d | 15.51 ± 0.01 c | 5.22 ± 0.07 c | 0.109 ± 0.005 d | 10.09 ± 0.05 e |
| Traditional pan-roasted | 0.68 ± 0.07 e | 16.73 | 38.44 ± 0.02 e | 29.22 ± 0.28 e | 22.81 ± 0.01 d | 5.77 ± 0.03 d | 0.079 ± 0.005 e | 9.99 ± 0.04 c,d |
| Control | 1.65 ± 0.06 b | - | 22.86 ± 0.01 f | 25.28 ± 0.37 f | 18.12 ± 0.01 e | 5.16 ± 0.05 e | 0.150 ± 0.02 f | 10.20 ± 0.06 f |
| FB4 | Traditionally Roasted | Control |
|---|---|---|
| (2-Aziridinylethyl)amine | (2-Aziridinylethyl)amine | 3-Trifluoroacetoxypentadecane |
| Acetone | Acetone | Pyrazine |
| Acetic acid | 1-Decyne | Pyrazine, methyl- |
| 1-Decyne | Pyrazine, methyl- | Pyrazine, 2,5-dimethyl |
| Pyrazine, methyl- | 2-Propanone, 1-hydroxy- | Pyrazine, 2-ethyl-3-methyl |
| Pyrazine, 2, 6-dimethyl | Pyrimidine, 4,6-dimethyl- | 3(2H)-Furanone, dihydro-2-methyl |
| Pyrazine, 2-ethyl-6-methyl | Ammonium acetate | 2-Propanone, 1-hydroxy- |
| Pyrazine, 2-ethyl-3-methyl | Furfural | Disulfide |
| Pyrimidine, 4,6-dimethyl- | 2(1H)-Pyridinone | Furfural |
| 2(1H)-Pyridinone | Propanoic acid | Furan, 3-methyl |
| Propanoic acid | 2-Furanmethanol | 2(1H)-Pyridinone |
| Furfural | 2-Furancarboxaldehyde | Propanoic acid |
| 2-Furanmethanol | Paromomycin | |
| 2-Furancarboxaldehyde | 2-Furanmethanol |
| Roasting Temperature (°C) | Fat (g/100 g) | EPM (%) | Protein (g/100 g) | DH (%) |
|---|---|---|---|---|
| 100 °C | 13.906 ± 0.106 | 1.443 ± 0.140 b | 0.329 ± 0.044 | 0.287 ± 0.001 b |
| 120 °C | 13.541 ± 0.330 | 1.866 ± 0.133 a | 0.282 ± 0.049 | 0.294 ± 0.000 a |
| 150 °C | 14.073 ± 0.052 | 2.156 ± 0.182 a | 0.207 ± 0.028 | 0.293 ± 0.000 a |
| 180 °C | 13.385 ± 0.240 | 2.875 ± 0.194 a | 0.230 ± 0.0366 | 0.292 ± 0.001 a |
| Extraction Temperature (°C) | Extraction Time (min) | Fat (g/100 g) | EPM (%) | Protein (g/100 g) | DH (%) |
|---|---|---|---|---|---|
| 70 °C | 5 min | 16.87 ± 0.025 a | 0.961 ± 0.050 b | 0.169 ± 0.005 b | 0.294 ± 0.001 a |
| 10 min | 16.579 ± 0.015 a | 0.936 ± 0.127 b | 0.169 ± 0.000 b | ||
| 100 °C | 1 min | 16.607 ± 0.445 a | 2.131 ± 0.289 a | 0.191 ± 0.013 b | |
| 5 min | 14.448 ±0.298 bc | 1.930 ± 0.289 ab | 0.173 ± 0.001 b | 0.296 ± 0.001 a | |
| 10 min | 15.549 ± 0.657 ab | 1.987 ± 0.326 ab | 0.181 ± 0.029 b | ||
| 140 °C | 1 min | 13.498 ± 0.091 cde | 2.041 ± 0.136 ab | 0.169 ± 0.009 b | |
| 5 min | 14.123 ± 0.318 bcd | 1.98 ± 0.234 ab | 0.184 ± 0.004 b | 0.294 ± 0.001 a | |
| 10 min | 13.665 ± 0.281 cde | 2.610 ± 0.076 a | 0.197 ± 0.004 b | ||
| 180 °C | 1 min | 12.234 ± 0.314 e | 2.716 ± 0.052 a | 0.330 ± 0.002 a | |
| 5 min | 12.525 ± 0.250 de | 2.943 ± 0.188 a | 0.393 ± 0.055 a | 0.284 ± 0.001 b | |
| Control | RI | 13.385 ± 0.240 cde | 2.875 ± 0.194 a | 0.230 ± 0.0366 b | 0.292 ± 0.001 a |
| Regular infusion | Infusion time (min) | Roasting temperature (°C) | Gallic acid (ppm) | Vanillin (ppm) |
| 10 | 100 | 9.260 a ± 1.231 | <LOD | |
| 10 | 120 | 3.489 b ± 2.471 | <LOD | |
| 10 | 150 | 8.963 a ± 0.742 | <LOD | |
| 10 | 180 | 3.579 b ± 0.130 | <LOD | |
| Microwave | Extraction time (min) | Extraction temperature (°C) | ||
| 5 | 70 | 3.096 b ± 0.180 | 1.104 c ± 0.010 | |
| 10 | 70 | 3.028 b ± 0.201 | 1.115 c ± 0.112 | |
| 1 | 100 | 3.425 b ± 0.071 | 1.232 bc ± 0.055 | |
| 5 | 100 | 3.510 b ± 0.113 | 1.191 bc ± 0.064 | |
| 10 | 100 | 3.405 b ± 0.027 | 1.148 bc ± 0.028 | |
| 1 | 140 | 4.007 b ± 0.275 | 1.258 bc ± 0.006 | |
| 5 | 140 | 4.289 b ± 0.370 | 1.326 b ± 0.038 | |
| 10 | 140 | 4.597 b ± 0.119 | 1.262 bc ± 0.043 | |
| 1 | 180 | 5.095 b ± 0.360 | 1.317 b ± 0.075 | |
| 5 | 180 | 5.486 b ± 0.043 | 1.557 a ± 0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ngadze, R.T.; Casertano, M.; Vilas-Franquesa, A. Design of a Coffee Alternative by Brewing Roasted Seeds from Baobab (Adansonia digitata). Beverages 2025, 11, 155. https://doi.org/10.3390/beverages11060155
Ngadze RT, Casertano M, Vilas-Franquesa A. Design of a Coffee Alternative by Brewing Roasted Seeds from Baobab (Adansonia digitata). Beverages. 2025; 11(6):155. https://doi.org/10.3390/beverages11060155
Chicago/Turabian StyleNgadze, Ruth T., Melania Casertano, and Arnau Vilas-Franquesa. 2025. "Design of a Coffee Alternative by Brewing Roasted Seeds from Baobab (Adansonia digitata)" Beverages 11, no. 6: 155. https://doi.org/10.3390/beverages11060155
APA StyleNgadze, R. T., Casertano, M., & Vilas-Franquesa, A. (2025). Design of a Coffee Alternative by Brewing Roasted Seeds from Baobab (Adansonia digitata). Beverages, 11(6), 155. https://doi.org/10.3390/beverages11060155






