Neuroprotective Effects of Wine Polyphenols in Alzheimer’s and Parkinson’s Diseases: A Review of Risks and Benefits
Abstract
1. Introduction
2. Overview of Neurodegenerative Diseases
3. Chemical Composition of Wine
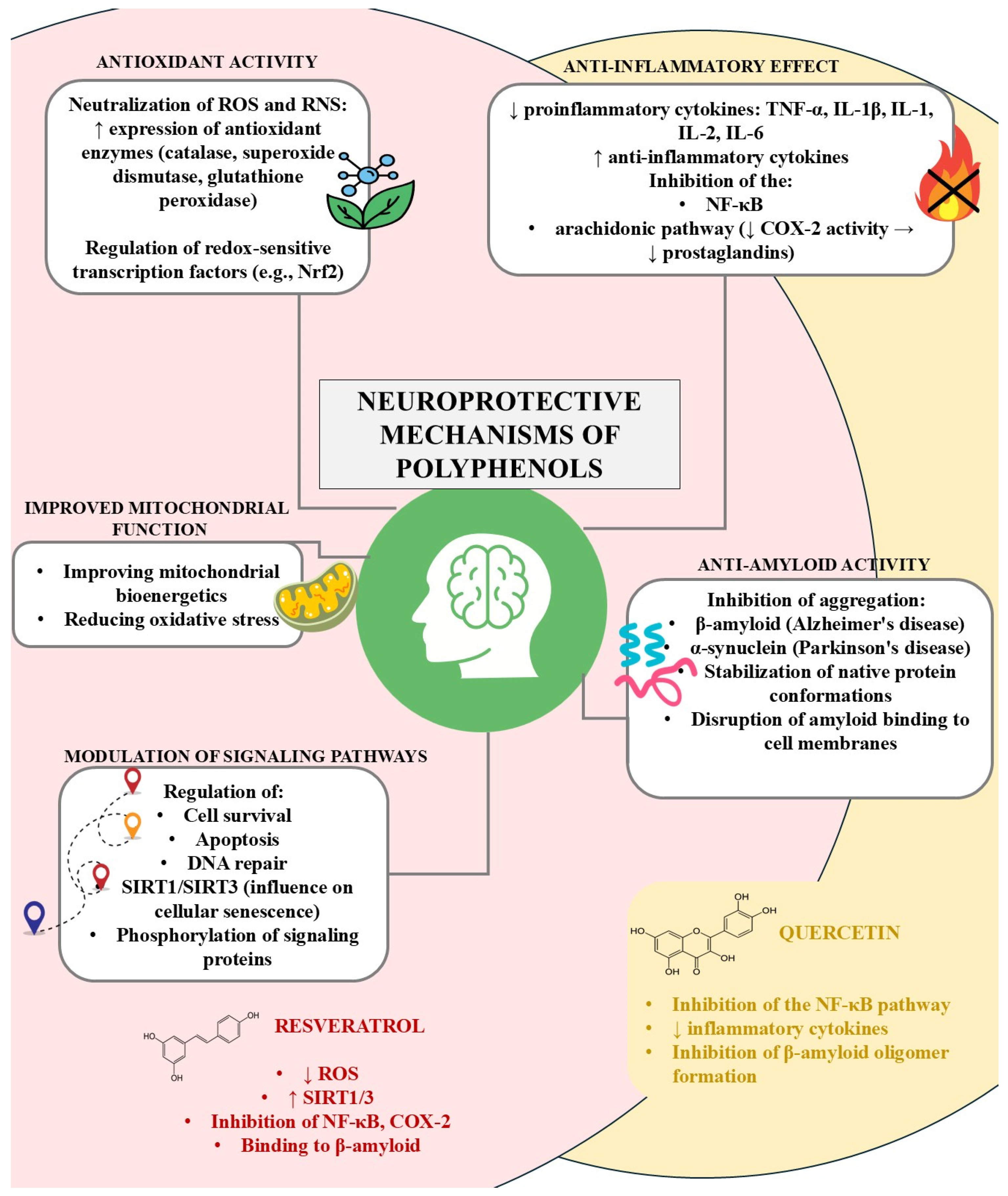
4. Potential Neuroprotective Mechanisms
5. Wine Components and Their Beneficial Effects—Review of In Vivo Studies
6. Dangers of Drinking Wine
7. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AD | Alzheimer’s Disease |
| ALS | Amyotrophic Lateral Sclerosis |
| CI | Confidence Interval |
| CNS | Central Nervous System |
| COX | Cyclooxygenase |
| HD | Huntington’s Disease |
| HR | Hazard Ratio |
| MCI | Mild Cognitive Impairment |
| OR | Odds Ratio |
| PD | Parkinson’s Disease |
| PRO-PD | Patient-Reported Outcomes in Parkinson’s Disease scale |
| RNS | Reactive Nitrogen Species |
| ROS | Reactive Oxygen Species |
References
- 2024 Alzheimer’s disease facts and figures. Alzheimer’s Dement. 2024, 20, 3708–3821. [CrossRef]
- Ben-Shlomo, Y.; Darweesh, S.; Llibre-Guerra, J.; Marras, C.; San Luciano, M.; Tanner, C. The epidemiology of Parkinson’s disease. Lancet 2024, 403, 283–292. [Google Scholar] [CrossRef]
- Lombardo, M.; Feraco, A.; Camajani, E.; Caprio, M.; Armani, A. Health effects of red wine consumption: A narrative review of an issue that still deserves debate. Nutrients 2023, 15, 1921. [Google Scholar] [CrossRef]
- Buljeta, I.; Pichler, A.; Šimunović, J.; Kopjar, M. Beneficial effects of red wine polyphenols on human health: Comprehensive review. Curr. Issues Mol. Biol. 2023, 45, 782–798. [Google Scholar] [CrossRef] [PubMed]
- Taween, F. Examining methods and effects of neurodegenerative diseases. Neuropsych. J. 2024, 14, 1–2. [Google Scholar]
- Fan, T.; Peng, J.; Liang, H.; Chen, W.; Wang, J.; Xu, R. Potential common pathogenesis of several neurodegenerative diseases. Neural Regen. Res. 2025, 21, 972–988. [Google Scholar] [CrossRef] [PubMed]
- Reale, M.; Costantini, E.; Jagarlapoodi, S.; Khan, H.; Belwal, T.; Cichelli, A. Relationship of wine consumption with alzheimer’s disease. Nutrients 2020, 12, 206. [Google Scholar] [CrossRef]
- Bianchi, V.E.; Herrera, P.F.; Laura, R. Effect of nutrition on neurodegenerative diseases. A systematic review. Nutr. Neurosci. 2019, 24, 810–834. [Google Scholar] [CrossRef]
- Zaib, S.; Javed, H.; Khan, I.; Jaber, F.; Sohail, A.; Zaib, Z.; Mehboob, T.; Tabassam, N.; Ogaly, H.A. Neurodegenerative diseases: Their onset, epidemiology, causes, and treatment. ChemistrySelect 2023, 8, e202300225. [Google Scholar] [CrossRef]
- Zhang, Y.; Liang, Y.; Gu, Y. The dopaminergic system and Alzheimer’s disease. Neural Regen. Res. 2024, 20, 2495–2512. [Google Scholar] [CrossRef]
- Zhang, J.; Kong, G.; Yang, J.; Pang, L.; Li, X. Pathological mechanisms and treatment progression of Alzheimer’s disease. Eur. J. Med. Res. 2025, 30, 625. [Google Scholar] [CrossRef]
- Castellani, R.J.; Jamshidi, P.; Plascencia-Villa, G.; Perry, G. The amyloid cascade hypothesis: A conclusion in search of support. Am. J. Pathol. 2024; in press. [Google Scholar] [CrossRef]
- Breijyeh, Z.; Karaman, R. Comprehensive review on alzheimer’s disease: Causes and treatment. Molecules 2020, 25, 5789. [Google Scholar] [CrossRef]
- Pless, A.; Ware, D.; Saggu, S.; Rehman, H.; Morgan, J.; Wang, Q. Understanding neuropsychiatric symptoms in Alzheimer’s disease: Challenges and advances in diagnosis and treatment. Front. Neurosci. 2023, 17, 1263771. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, A.L.; Rocha, N.P.; Gatchel, J. Behavioral or neuropsychiatric symptoms of Alzheimer’s disease: From psychopathology to pharmacological management. Arq Neuropsiquiatr. 2023, 81, 1152–1162. [Google Scholar] [CrossRef] [PubMed]
- Gorzkowska, A. Chapter 14: Dementia syndromes. In Neurology: A Textbook for Medical Students; Kozubski, W., Ed.; PZWL: Warszawa, Poland, 2023; pp. 325–360. (In Polish) [Google Scholar]
- Checkoway, H.; Lundin, J.; Kelada, S. Neurodegenerative diseases. IARC Sci. Publ. 2011, 163, 407–419. [Google Scholar]
- Krzymińska-Siemaszko, R.; Lewandowicz-Umyszkiewicz, M.; Wieczorowska-Tobis, K. Clinical dietetics. In Clinical Dietetics; Grzymisławski, M., Ed.; PZWL: Warszawa, Poland, 2021; pp. 97–118. (In Polish) [Google Scholar]
- Hardy, J.; Higgins, G.A. Alzheimer’s disease: The amyloid cascade hypothesis. Science 1992, 256, 184–185. [Google Scholar] [CrossRef]
- Bocwinska-Kiluk, B.; Jelski, W.; Kornhuber, J.; Piotr, L.; Barbara, M. Alzheimer’s disease: Biochemical and psychological background for diagnosis and treatment. Int. J. Mol. Sci. 2023, 24, 1059. [Google Scholar] [CrossRef] [PubMed]
- Sobów, T. Chapter 32: Psychopathological symptoms and mental disorders in diseases of the nervous system. In Neurology: A Textbook for Medical Students; Kozubski, W., Ed.; PZWL: Warszawa, Poland, 2023; pp. 325–360. (In Polish) [Google Scholar]
- Gaweł, M.; Potulska-Chromik, A. Neurodegenerative diseases: Alzheimer’s and Parkinson’s disease. Post. Nauk. Med. 2015, 28, 468–476. (In Polish) [Google Scholar]
- Giguère, N.; Nanni, S.B.; Trudeau, L.-E. On Cell Loss and Selective Vulnerability of Neuronal Populations in Parkinson’s Disease. Front. Neurol. 2018, 9, 455. [Google Scholar] [CrossRef]
- Coleman, C.; Martin, I. Unraveling Parkinson’s disease neurodegeneration: Does aging hold the clues? J. Parkinsons Dis. 2022, 12, 2321–2338. [Google Scholar] [CrossRef]
- Beheshti, I. Exploring risk and protective factors in parkinson’s disease. Cells 2025, 14, 710. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, E.; Chandrasekhar, G.; Chandrasekar, P.; Anbarasu, K.; Vickram, A.S.; Karunakaran, R.; Rajasekaran, R.; Srikumar, P.S. Alpha-synuclein aggregation in Parkinson’s disease. Front. Med. 2021, 8, 736978. [Google Scholar] [CrossRef] [PubMed]
- Peña-Zelayeta, L.; Delgado-Minjares, K.M.; Villegas-Rojas, M.M.; León-Arcia, K.; Santiago-Balmaseda, A.; Andrade-Guerrero, J.; Pérez-Segura, I.; Ortega-Robles, E.; Soto-Rojas, L.O.; Arias-Carrión, O. Redefining non-motor symptoms in parkinson’s disease. J. Pers. Med. 2025, 15, 172. [Google Scholar] [CrossRef]
- Palakurthi, B.; Burugupally, S.P. Postural instability in parkinson’s disease: A review. Brain Sci. 2019, 9, 239. [Google Scholar] [CrossRef]
- Bianchini, E.; Rinaldi, D.; Alborghetti, M.; Simonelli, M.; D’Audino, F.; Onelli, C.; Pegolo, E.; Pontieri, F.E. The story behind the mask: A narrative review on hypomimia in Parkinson’s dis-ease. Brain Sci. 2024, 14, 109. [Google Scholar] [CrossRef]
- Lemieszewska, M.; Zabłocka, A.; Rymaszewska, J. Parkinson’s disease: Etiopathogenesis, molecular basis, and potential treat-ment opportunities. Post. Hig. Med. Dosw. 2019, 73, 256–268. [Google Scholar] [CrossRef]
- Caruana, M.; Cauchi, R.; Vassallo, N. Putative role of red wine polyphenols against brain pathology in alzheimer’s and parkinson’s disease. Front. Nutr. 2016, 3, 31. [Google Scholar] [CrossRef]
- Golpich, M.; Amini, E.; Mohamed, Z.; Ali, R.A.; Ibrahim, N.M.; Ahmadiani, A. Mitochondrial dysfunction and biogenesis in neurodegenerative diseases: Pathogene-sis and treatment. CNS Neurosci. Ther. 2017, 23, 5–22. [Google Scholar] [CrossRef]
- Basli, A.; Soulet, S.; Chaher, N.; Mérillon, J.-M.; Chibane, M.; Monti, J.-P.; Richard, T. Wine Polyphenols: Potential Agents in Neuroprotection. Oxidative Med. Cell. Longev. 2012, 2012, 1–14. [Google Scholar] [CrossRef]
- Dzamko, N. Cytokine activity in Parkinson’s disease. Neuronal Signal 2023, 7, NS20220063. [Google Scholar] [CrossRef] [PubMed]
- Karampetsou, M.; Vekrellis, K.; Melachroinou, K. The promise of the TGF-β superfamily as a therapeutic target for Parkinson’s disease. Neurobiol. Dis. 2022, 171, 105805. [Google Scholar] [CrossRef] [PubMed]
- Araújo, B.; Caridade-Silva, R.; Soares-Guedes, C.; Martins-Macedo, J.; Gomes, E.D.; Monteiro, S.; Teixeira, F.G. Neuroinflammation and parkinson’s disease—From neurodegeneration to therapeutic opportunities. Cells 2022, 11, 2908. [Google Scholar] [CrossRef] [PubMed]
- Kwon, H.S.; Koh, S.-H. Neuroinflammation in neurodegenerative disorders: The roles of microglia and astrocytes. Transl. Neurodegener. 2020, 9, 42. [Google Scholar] [CrossRef]
- Yang, H.-M. Mitochondrial dysfunction in neurodegenerative diseases. Cells 2025, 14, 276. [Google Scholar] [CrossRef] [PubMed]
- Swami, S.; Thakor, N.; Divate, A. Fruit wine production: A review. J. Food Res. Technol. 2014, 2, 93–100. [Google Scholar]
- Peng, B.; Yang, Q.; Joshi, R.B.; Liu, Y.; Akbar, M.; Song, B.-J.; Zhou, S.; Wang, X. Role of alcohol drinking in alzheimer’s disease, parkinson’s disease, and amyotrophic lateral sclerosis. Int. J. Mol. Sci. 2020, 21, 2316. [Google Scholar] [CrossRef]
- Gutiérrez-Escobar, R.; Aliaño-González, M.J.; Cantos-Villar, E. Wine polyphenol content and its influence on wine quality and properties: A review. Molecules 2021, 26, 718. [Google Scholar] [CrossRef] [PubMed]
- Yahfoufi, N.; Alsadi, N.; Jambi, M.; Matar, C. The immunomodulatory and anti-inflammatory role of polyphenols. Nutrients 2018, 10, 1618. [Google Scholar] [CrossRef]
- Haseeb, S.; Alexander, B.; Baranchuk, A. Wine and Cardiovascular Health. Circulation 2017, 136, 1434–1448. [Google Scholar] [CrossRef]
- Hrelia, S.; Di Renzo, L.; Bavaresco, L.; Bernardi, E.; Malaguti, M.; Giacosa, A. Moderate wine consumption and health: A narrative review. Nutrients 2022, 15, 175. [Google Scholar] [CrossRef]
- Meng, T.; Xiao, D.; Muhammed, A.; Deng, J.; Chen, L.; He, J. Anti-inflammatory action and mechanisms of resveratrol. Molecules 2021, 26, 229. [Google Scholar] [CrossRef]
- Sejbuk, M.; Mirończuk-Chodakowska, I.; Karav, S.; Witkowska, A.M. Dietary polyphenols, food processing and gut microbiome: Recent find-ings on bioavailability, bioactivity, and gut microbiome interplay. Antioxidants 2024, 13, 1220. [Google Scholar] [CrossRef]
- Bié, J.; Sepodes, B.; Fernandes, P.C.B.; Ribeiro, M.H.L. Polyphenols in health and disease: Gut microbiota, bioaccessibility, and bioavailability. Compounds 2023, 3, 40–72. [Google Scholar] [CrossRef]
- Scott, M.B.; Styring, A.K.; McCullagh, J.S.O. Polyphenols: Bioavailability, Microbiome Interactions and Cellular Effects on Health in Humans and Animals. Pathogens 2022, 11, 770. [Google Scholar] [CrossRef] [PubMed]
- Rudrapal, M.; Rakshit, G.; Singh, R.P.; Garse, S.; Khan, J.; Chakraborty, S. Dietary polyphenols: Review on chemistry/sources, bioavailability/metabolism, antioxidant effects, and their role in disease management. Antioxidants 2024, 13, 429. [Google Scholar] [CrossRef] [PubMed]
- Manach, C.; Scalbert, A.; Morand, C.; Rémésy, C.; Jiménez, L. Polyphenols: Food sources and bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar] [CrossRef] [PubMed]
- Gambini, J.; Inglés, M.; Olaso, G.; Lopez-Grueso, R.; Bonet-Costa, V.; Gimeno-Mallench, L.; Mas-Bargues, C.; Abdelaziz, K.M.; Gomez-Cabrera, M.C.; Vina, J.; et al. Properties of resveratrol: In vitro and in vivo studies about metabolism, bioavailability, and biological effects in animal models and humans. Oxid. Med. Cell Longev. 2015, 2015, 837042. [Google Scholar] [CrossRef]
- Tsouh Fokou, P.V.; Kamdem Pone, B.; Appiah-Oppong, R.; Ngouana, V.; Bakarnga-Via, I.; Ntieche Woutouoba, D.; Donfack Donkeng, V.F.; Tchokouaha Yamthe, L.R.; Fekam Boyom, F.; Arslan Ateşşahin, D.; et al. An update on antitumor efficacy of catechins: From molecular mechanisms to clinical applications. Food Sci. Nutr. 2025, 13, e70169. [Google Scholar] [CrossRef]
- Tsang, C.; Higgins, S.; Duthie, G.G.; Duthie, S.J.; Howie, M.; Mullen, W.; Lean, M.E.J.; Crozier, A. The influence of moderate red wine consumption on antioxidant status and indices of oxidative stress associated with CHD in healthy volunteers. Br. J. Nutr. 2005, 93, 233–240. [Google Scholar] [CrossRef]
- Donovan, J.L.; Bell, J.R.; Kasim-Karakas, S.; German, J.B.; Walzem, R.L.; Hansen, R.J.; Waterhouse, A.L. Catechin is present as metabolites in human plasma after consumption of red wine. J. Nutr. 1999, 129, 1662–1668. [Google Scholar] [CrossRef]
- Ferruzzi, M.G.; Lobo, J.K.; Janle, E.M.; Cooper, B.; Simon, J.E.; Wu, Q.-L.; Welch, C.; Ho, L.; Weaver, C.; Pasinetti, G.M. Bioavailability of gallic acid and catechins from grape seed polyphenol extract is im-proved by repeated dosing in rats: Implications for treatment in Alzheimer’s disease. J. Alzheimer’s Dis. 2009, 18, 113–124. [Google Scholar] [CrossRef]
- Bell, J.R.; Donovan, J.L.; Wong, R.; Waterhouse, A.L.; German, J.B.; Walzem, R.L.; Kasim-Karakas, S.E. (+)-Catechin in human plasma after ingestion of a single serving of reconstituted red wine. Am. J. Clin. Nutr. 2000, 71, 103–108. [Google Scholar] [CrossRef]
- Donovan, J.L.; Kasim-Karakas, S.; German, J.B.; Waterhouse, A.L. Urinary excretion of catechin metabolites by human subjects after red wine consumption. Br. J. Nutr. 2002, 87, 31–37. [Google Scholar] [CrossRef]
- Morris, M.C.; Tangney, C.C.; Wang, Y.; Sacks, F.M.; Barnes, L.L.; Bennett, D.A.; Aggarwal, N.T. MIND diet slows cognitive decline with aging. Alzheimer’s Dement. 2015, 11, 1015–1022. [Google Scholar] [CrossRef]
- Morris, M.C.; Tangney, C.C.; Wang, Y.; Sacks, F.M.; Bennett, D.A.; Aggarwal, N.T. MIND diet associated with reduced incidence of Alzheimer’s disease. Alzheimer’s Dement. 2015, 11, 1007–1014. [Google Scholar] [CrossRef]
- Davis, C.; Bryan, J.; Hodgson, J.; Murphy, K. Definition of the Mediterranean Diet; A Literature Review. Nutrients 2015, 7, 9139–9153. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.-D.; Luo, M.; Huang, S.-Y.; Saimaiti, A.; Shang, A.; Gan, R.-Y.; Li, H.-B.; Teodoro, A.J. Effects and mechanisms of resveratrol on aging and age-related diseases. Oxidative Med. Cell. Longev. 2021, 2021, 9932218. [Google Scholar] [CrossRef]
- Cosín-Tomàs, M.; Senserrich, J.; Arumí-Planas, M.; Alquézar, C.; Pallàs, M.; Martín-Requero, Á.; Suñol, C.; Kaliman, P.; Sanfeliu, C. Role of resveratrol and selenium on oxidative stress and expression of antioxidant and anti-aging genes in immortalized lymphocytes from alzheimer’s disease patients. Nutrients 2019, 11, 1764. [Google Scholar] [CrossRef]
- Marchal, J.; Dal-Pan, A.; Epelbaum, J.; Blanc, S.; Mueller, S.; Kieffer, M.W.; Metzger, F.; Aujard, F. Calorie restriction and resveratrol supplementation prevent age-related DNA and RNA oxidative damage in a non-human primate. Exp. Gerontol. 2013, 48, 992–1000. [Google Scholar] [CrossRef]
- Fernández-Pachón, M.S.; Berná, G.; Otaolaurruchi, E.; Troncoso, A.M.; Martín, F.; García-Parrilla, M.C. Changes in antioxidant endogenous enzymes (activity and gene expression levels) after repeated red wine intake. J. Agric. Food Chem. 2009, 57, 6578–6583. [Google Scholar] [CrossRef]
- Bellaver, B.; Souza, D.; Souza, D.; Quincozes-Santos, A. Resveratrol increases antioxidant defenses and decreases proinflammatory cytokines in hippocampal astrocyte cultures from newborn, adult, and aged Wistar rats. Toxicol. In Vitro 2014, 28, 479–484. [Google Scholar] [CrossRef]
- Abraham, J.; Johnson, R.W. Consuming a diet supplemented with resveratrol reduced infection-related neuroinflammation and deficits in working memory in aged mice. Rejuvenation Res. 2009, 12, 445–453. [Google Scholar] [CrossRef]
- Wang, J.; Ho, L.; Zhao, Z.; Seror, I.; Humala, N.; Dickstein, D.L.; Thiyagarajan, M.; Percival, S.S.; Talcott, S.T.; Pasinetti, G.M. Moderate consumption of Cabernet Sauvignon attenuates A neuropathology in a mouse model of Alzheimer’s disease. FASEB J. 2006, 20, 2313–2320. [Google Scholar] [CrossRef]
- Orgogozo, J.M.; Dartigues, J.F.; Lafont, S.; Letenneur, L.; Commenges, D.; Salamon, R.; Renaud, S.; Breteler, M.M. Wine consumption and dementia in the elderly: A prospective community study in the Bordeaux area. Rev. Neurol. 1997, 153, 185–192. [Google Scholar]
- Pinder, R. Does wine prevent dementia? Int. J. Wine Res. 2009, 1, 41–52. [Google Scholar] [CrossRef]
- Letenneur, L. Risk of Dementia and Alcohol and Wine Consumption: A Review of Recent Results. Biol. Res. 2004, 37, 189–193. [Google Scholar] [CrossRef]
- Lindsay, J.; Laurin, D.; Verreault, R.; Hébert, R.; Helliwell, B.; Hill, G.B.; McDowell, I. Risk Factors for Alzheimer’s Disease: A Prospective Analysis from the Canadian Study of Health and Aging. Am. J. Epidemiol. 2002, 156, 445–453. [Google Scholar] [CrossRef] [PubMed]
- Fischer, K.; van Lent, D.M.; Wolfsgruber, S.; Weinhold, L.; Kleineidam, L.; Bickel, H.; Scherer, M.; Eisele, M.; Bussche, H.V.D.; Wiese, B.; et al. Prospective associations between single foods, alzheimer’s dementia and memory decline in the elderly. Nutrients 2018, 10, 852. [Google Scholar] [CrossRef] [PubMed]
- Weyerer, S.; SchäUfele, M.; Wiese, B.; Maier, W.; Tebarth, F.; Bussche, H.v.D.; Pentzek, M.; Bickel, H.; Luppa, M.; Riedel-Heller, S.G. Current alcohol consumption and its relationship to incident dementia: Results from a 3-year follow-up study among primary care attenders aged 75 years and older. Age Ageing 2011, 40, 456–463. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; Zhou, D.H.; Li, J.; Wang, Y.J.; Gao, C.; Chen, M. A 2-year follow-up study of alcohol consumption and risk of dementia. Clin. Neurol. Neurosurg. 2006, 108, 378–383. [Google Scholar] [CrossRef]
- Heymann, D.; Stern, Y.; Cosentino, S.; Tatarina-Nulman, O.; Dorrejo, J.N.; Gu, Y. The association between alcohol use and the progression of Alzheimer’s disease. Curr. Alzheimer Res. 2016, 13, 1356–1362. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Grossman, H.; Neugroschl, J.; Parker, S.; Burden, A.; Luo, X.; Sano, M. A randomized, double-blind, placebo-controlled trial of resveratrol with glucose and malate (RGM) to slow the progression of Alzheimer’s disease: A pilot study. Alzheimer’s Dement. Transl. Res. Clin. Interv. 2018, 4, 609–616. [Google Scholar] [CrossRef] [PubMed]
- Turner, R.S.; Thomas, R.G.; Craft, S.; van Dyck, C.H.; Mintzer, J.; Reynolds, B.A.; Brewer, J.B.; Rissman, R.A.; Raman, R.; Aisen, P.S. A randomized, double-blind, placebo-controlled trial of resveratrol for Alzheimer disease. Neurology 2015, 85, 1383–1391. [Google Scholar] [CrossRef] [PubMed]
- Fall, P.; Fredrikson, M.; Axelson, O.; Granérus, A. Nutritional and occupational factors influencing the risk of Parkinson’s disease: A case-control study in southeastern Sweden. Mov. Disord. 1999, 14, 28–37. [Google Scholar] [CrossRef]
- Nicoletti, A.; Pugliese, P.; Nicoletti, G.; Arabia, G.; Annesi, G.; De Mari, M.; Lamberti, P.; Grasso, L.; Marconi, R.; Epifanio, A.; et al. Voluptuary habits and clinical subtypes of Parkinson’s disease: The FRAGAMP case–control study. Mov. Disord. 2010, 25, 2387–2394. [Google Scholar] [CrossRef]
- Liu, R.; Guo, X.; Park, Y.; Wang, J.; Huang, X.; Hollenbeck, A.; Blair, A.; Chen, H. Alcohol consumption, types of alcohol, and Parkinson’s disease. PLoS ONE 2013, 8, e66452. [Google Scholar] [CrossRef]
- Shao, C.; Wang, X.; Wang, P.; Tang, H.; He, J.; Wu, N. Parkinson’s Disease Risk and Alcohol Intake: A Systematic Review and Dose-Response Meta-Analysis of Prospective Studies. Front. Nutr. 2021, 8, 709846. [Google Scholar] [CrossRef]
- Jiménez-Jiménez, F.J.; Alonso-Navarro, H.; García-Martín, E.; Agúndez, J.A.G. Alcohol consumption and risk for Parkinson’s disease: A systematic review and meta-analysis. J. Neurol. 2018, 266, 1821–1834. [Google Scholar] [CrossRef]
- Zhang, D.; Jiang, H.; Xie, J. Alcohol intake and risk of Parkinson’s disease: A meta-analysis of observational studies. Mov. Disord. 2014, 29, 819–822. [Google Scholar] [CrossRef]
- Gao, X.; Cassidy, A.; Schwarzschild, M.; Rimm, E.; Ascherio, A. Habitual intake of dietary flavonoids and risk of Parkinson disease. Neurology 2012, 78, 1138–1145. [Google Scholar] [CrossRef]
- Zhang, X.; Molsberry, S.A.; Yeh, T.-S.; Cassidy, A.; Schwarzschild, M.A.; Ascherio, A.; Gao, X. Intake of flavonoids and flavonoid-rich foods and mortality risk among individuals with parkinson disease. Neurology 2022, 98, e1064–e1076. [Google Scholar] [CrossRef]
- Mischley, L.K.; Lau, R.C.; Bennett, R.D.; Giudetti, A.M. Role of diet and nutritional supplements in Parkinson’s disease progression. Oxidative Med. Cell. Longev. 2017, 2017, 6405278. [Google Scholar] [CrossRef] [PubMed]
- Boffetta, P.; Hashibe, M.; La Vecchia, C.; Zatonski, W.; Rehm, J. The burden of cancer attributable to alcohol drinking. Int. J. Cancer 2006, 119, 884–887. [Google Scholar] [CrossRef] [PubMed]
- Rehm, J.; Mathers, C.; Popova, S.; Thavorncharoensap, M.; Teerawattananon, Y.; Patra, J. Global burden of disease and injury and economic cost attributable to alcohol use and alcohol-use disorders. Lancet 2009, 373, 2223–2233. [Google Scholar] [CrossRef]
- Stickel, F.; Hampe, J. Genetic determinants of alcoholic liver disease. Gut 2011, 61, 150–159. [Google Scholar] [CrossRef] [PubMed]
- Volkow, N.D.; Koob, G.F.; McLellan, A.T. Neurobiologic advances from the brain disease model of addiction. N. Engl. J. Med. 2016, 374, 363–371. [Google Scholar] [CrossRef]
- World Health Organization. Global Status Report on Alcohol and Health 2018; WHO Press: Geneva, Switzerland, 2018; Available online: https://www.who.int/publications/i/item/9789241565639 (accessed on 2 July 2025).
- American Cancer Society. Alcohol Use and Cancer; American Cancer Society: Atlanta, Georgia, 2022; Available online: https://www.cancer.org/cancer/cancer-causes/diet-physical-activity/alcohol-use-and-cancer.html (accessed on 2 July 2025).

| (A) | ||||
| Type of Alcohol | Amount/Frequency | Effect | Observations | Reference |
| red wine | 3–4 servings/day | ↓ risk of AD and dementia | protective effect lost above 4 servings/day | [68] |
| wine (general) | ≥once per week | ↓ risk of AD | general alcohol: OR = 0.68 (0.47–1.00); no effect for beer or spirits | [71] |
| red and white wine | higher intake | ♂: ↓ AD risk ♀: ↑ AD risk | white wine increased risk in APOEε4 carriers | [72] |
| alcohol (general) | 20–29 g alcohol/day | lowest risk of AD and dementia | U-shaped dose–response | [7,39,73] |
| wine/alcohol | low/moderate intake | ↓ risk of AD and dementia | no protective effect at high intake | [74] |
| alcohol (general) | 1–7 drinks/week | ↓ cognitive decline rate | spirits associated with faster decline; wine and beer neutral | [75] |
| resveratrol (supplement) | ≥500 mg/day for 52 weeks | ↓ decline in Aβ40 and daily functioning | suggesting potential neuroprotective effects | [77] |
| resveratrol (low dose) | – | trend toward ↓ cognitive decline | difference not statistically significant | [76] |
| (B) | ||||
| Type of Alcohol | Amount/Frequency | Effect | Observations | Reference |
| red wine | 1–4 bottles/month | ↓ risk of PD | dose-dependent effect | [78] |
| wine | 1–2 glasses/day | ↓ risk of PD | – | [79] |
| alcohol (general)/wine | 1–2 servings wine/day | trend to ↓ PD risk (not significant) | large cohort; self-reported intake | [80] |
| alcohol (general) | 26–35 g alcohol/day | greatest ↓ risk of PD | U-shaped relationship; no effect for wine | [81] |
| flavonoids (diet) | quintile 5 vs. 1 | ↓ PD risk in men | no direct association with wine | [84] |
| wine (pre/post diagnosis) | ≥3 servings/week | ↓ mortality risk | no effect at <1 serving/week | [85] |
| wine | increased frequency | ↓ PD progression (PRO-PD score) | observational; adjusted for confounders | [86] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zięba, A.; Wiśniowska, A.; Bronowicka-Adamska, P.; Kuśnierz-Cabala, B.; Zagrodzki, P.; Tyszka-Czochara, M. Neuroprotective Effects of Wine Polyphenols in Alzheimer’s and Parkinson’s Diseases: A Review of Risks and Benefits. Beverages 2025, 11, 131. https://doi.org/10.3390/beverages11050131
Zięba A, Wiśniowska A, Bronowicka-Adamska P, Kuśnierz-Cabala B, Zagrodzki P, Tyszka-Czochara M. Neuroprotective Effects of Wine Polyphenols in Alzheimer’s and Parkinson’s Diseases: A Review of Risks and Benefits. Beverages. 2025; 11(5):131. https://doi.org/10.3390/beverages11050131
Chicago/Turabian StyleZięba, Aleksandra, Aleksandra Wiśniowska, Patrycja Bronowicka-Adamska, Beata Kuśnierz-Cabala, Paweł Zagrodzki, and Malgorzata Tyszka-Czochara. 2025. "Neuroprotective Effects of Wine Polyphenols in Alzheimer’s and Parkinson’s Diseases: A Review of Risks and Benefits" Beverages 11, no. 5: 131. https://doi.org/10.3390/beverages11050131
APA StyleZięba, A., Wiśniowska, A., Bronowicka-Adamska, P., Kuśnierz-Cabala, B., Zagrodzki, P., & Tyszka-Czochara, M. (2025). Neuroprotective Effects of Wine Polyphenols in Alzheimer’s and Parkinson’s Diseases: A Review of Risks and Benefits. Beverages, 11(5), 131. https://doi.org/10.3390/beverages11050131








