Abstract
Traditional Mexican non-alcoholic, cocoa-based beverages are a relevant part of the gastronomy of the country. This study aimed to identify the volatile compound profile of three traditional beverages from Mexican gastronomy, including Pozol, Chilate, and Cacahuatole, and evaluate the acceptability of milks incorporating their traditional flavors. Chemical composition was determined, and volatile compounds were analyzed using gas chromatography–mass spectrometry (GC-MS). The results showed the Mexican beverages are rich in carbohydrates (70.3–78.2% d.b.), proteins (9.3–16% d.b.), and fat (8.8–11.7% d.b.) due to corn, rice, broad beans, and cocoa ingredients. GC revealed volatile profiles, with Pozol containing 148, Chilate 104, and Cacahuatole 109 compounds, mainly terpenes, pyrazines, aldehydes, and phenolics. Nixtamalization, cocoa roasting, and ingredient variations influenced the volatile composition and sensory attributes. Multivariate analysis distinguished the beverages based on their volatile composition, revealing ingredient-dependent variations. Artificial flavors replicating these profiles were developed and incorporated into milks, which were evaluated by a trained sensory panel using the Check-All-That-Apply (CATA) method. Sensory analysis confirmed that key aromatic compounds contributed to flavor perception. This work identified for the first time the volatile compounds of traditional Mexican cocoa-based beverages, namely, Pozol, Chilate, and Cacahuatole, and provides insight into the role of traditional preparation methods in the development of flavor profiles. On the other hand, this study highlights the potential for incorporating traditional Mexican beverage flavors into commercial milk for novel product development.
1. Introduction
Traditional Mexican cuisine is recognized as an essential part of the world’s intangible cultural heritage, designated by the United Nations Educational, Scientific, and Cultural Organization (UNESCO) in 2010 [1]. Among its culinary traditions, Mexico possesses a rich variety of cocoa-based beverages, many of which date from pre- and post-Hispanic times, are derived from indigenous traditions, and have been passed down through generations. Cocoa-based beverages represent a significant element of traditional Mexican gastronomic history. These traditional drinks, including Pozol, Cacahuatole, and Chilate, are commonly consumed in different regions of the country and are distinguished by their unique flavor profiles, which result from complex combinations of cocoa with cereals and spices, and specific preparation techniques such as nixtamalization, drying, baking, and roasting processes [2,3].
Pozol is one of the most ancient endemic Mesoamerican products still consumed in Mexico [1]. It is a corn-based beverage with cocoa, cinnamon, and other ingredients added to produce a nonstick dough that is diluted in water, and sugar is added before consumption. It is a typical beverage for the Mayan region, including Chiapas, Tabasco, Campeche, and Yucatan states. Cacahuatole, on the other hand, is a drink made from cocoa, corn, and broad beans and is mainly prepared in central Mexico, mostly in Tlaxcala. It is elaborated in the form of a paste, which must also be diluted before consumption [4]. Chilate is produced in Guerrero; the elaboration of this beverage includes cocoa, sugar, cinnamon, and rice, and it can be diluted in water or milk [5,6].
Despite their cultural and historical significance, there is a notable lack of scientific literature regarding the characterization of non-alcoholic traditional Mexican cocoa-based beverages. While previous studies have explored the sensory and nutritional properties of cocoa and corn-based foods [7,8], little research has been conducted on the volatile compound profiles that define the aroma and flavor of these traditional drinks. Aroma is a crucial factor in food acceptability, and the understanding of the volatile components of these beverages can contribute to preserving their authenticity while exploring their potential applications in food science and product development [9,10].
In addition to their cultural importance, cocoa-based beverages offer an opportunity for innovation in the dairy industry. Milk consumption in Mexico remains below the recommended intake levels set by the Food and Agriculture Organization (FAO), particularly among lower-income populations [11]. Given the affordability and nutritional potential of milk made from milk solids and vegetable fats, they present a viable means of addressing dietary gaps [12,13]. Since chocolate is one of the most widely preferred flavors in dairy beverages [14], integrating the flavor profiles of traditional Mexican cocoa-based beverages into milks could enhance their acceptability and consumption.
This work aimed to identify the volatile compound profile of three traditional cocoa-based beverages from Mexican gastronomy, namely, Pozol, Chilate, and Cacahuatole, and evaluate the acceptability of milks with their traditional flavors. Samples of the beverages were collected at local sites in different Mexican states while verbally interviewing master artisans about the fabrication process, and the volatile compounds present in the beverages were identified using gas chromatography–mass spectrometry (GC-MS) analysis. The volatile compound profiles provided valuable insights for the dairy industry, particularly allowing for the development of flavored milk-based beverages oriented to the improvement of the diet of people in precarious socioeconomic situations in Mexico; the sensory evaluation of the milks with the flavors of the traditional Mexican beverages was assessed using a semi-trained consumer panel to evaluate their acceptability. This study contributes to the preservation and valorization of Mexican cocoa-based beverages, bridging the gap between traditional culinary heritage and contemporary food science.
2. Materials and Methods
2.1. Materials and Chemicals
In total, 3–5 samples of paste/dough for traditional beverages were obtained from local markets: Pozol from Tuxtla Gutiérrez (Chiapas, Mexico), Cacahuatole from Zacatelco (Tlaxcala, Mexico), and Chilate from Acapulco (Guerrero, Mexico). Interviews were conducted with the artisans to record the beverage production processes. Milk (Fortileche Plus®, Alpura®, Cuautitlán Izcalli, Mexico) and sugar were purchased from a local store, and all HPLC-grade and reagent-grade chemicals were acquired from Sigma-Aldrich (Saint Louis, MO, USA) and JT Baker (City of Mexico, State of México, Mexico) respectively.
2.2. Chemical Composition of Pastes/Doughs
The chemical composition of Pozol, Cacahuatole, and Chilate pastes/doughs was determined according to AOAC methods [15] for moisture (930.15), fat (7.056), protein (942.05), ash (942.05), and crude fiber (973.19). Carbohydrate content was calculated on dry weight basis as follows: 100-(protein content + ashes + fat + fiber). Metabolizable energy was calculated using the Atwater conversion factors for whole cornmeal: protein 11.4 kJ/g (2.73 kcal/g), fat 35 kJ/g (8.37 kcal/g), and carbohydrate 16.9 kJ/g (4.03 kcal/g) [16].
2.3. Identification of Volatile Compounds
The volatile compound profile of the pastes/doughs was determined according to [17]. Briefly, 3 g of the samples were hermetically sealed into a 20 mL vial with an EFRP/silicone stopper and stored frozen at −22 °C until analysis. Analysis of volatile compounds was carried out using a gas chromatograph (Agilent 7890A, Santa Clara, CA, USA) coupled with an ionizing flame detector (FID), a mass spectrometer (Agilent 5975C), and an HP-5MS column (60 m × 0.25 nm ID × 0.25 μm DF, Agilent, Santa Clara, CA, USA) using Helium as a carrier gas (flow rate, of 1 mL/min; pressure, 124 kPa). Before analysis, the vials were conditioned at 50 °C for 5 min, and a 2 cm 50/30 μm divinylbenzene/carboxene/polydimethylsiloxane (DVB/CAR/PDMS) fiber (Supelco, Bellefonte, PA, USA) was exposed for 60 min at 50 °C to the headspace of the thawed aliquot in the vial for the adsorption of the analytes. The fiber was desorbed for 10 min at the injection point, and the sample was injected at 200 °C in splitless mode. The oven temperature was set at 60 °C for the first 5 min and was increased until reaching 150 °C (rate 4 °C/min), maintaining this condition for 20 min. Then, the temperature was raised to 220 °C at a rate of 3 °C/min and was held for 5 min. A final increase was made at a rate of 6 °C/min until reaching 240 °C, maintaining this condition for 5 min. The volatile compounds were detected using a temperature of 230 °C and 150 °C for the source and quadrupole, respectively. The mass spectra were acquired in a scanning mode in a range of 33–600 m/z. The equipment was operated in electron impact ionization mode at 70 eV. Data analysis was carried out using the MSD ChemStation software E.01.00.237 (Agilent Technologies Inc., Santa Clara, CA, USA). The compound identification was made by comparing the mass spectra with those found in the National Institute of Standards and Technology (NIST, Gaithersburg, MD, USA) 08 library, where the mass spectra found were compared with those available in the literature [18,19].
2.4. Flavor Development Based on Mexican Beverage Volatile Compound Profile
To replicate the characteristic sensory attributes of the traditional Mexican beverages (Pozol, Cacahuatole, and Chilate), collaborations were established with four commercial flavor manufacturers: Takasago International Co. (Tlalnepantla, State of México, Mexico), DSM Firmenich (México City, Mexico), International Flavors & Fragrances Inc. (Tlalnepantla, State of México, Mexico), and MANE Flavor & Fragrance Manufacturer (Toluca, State of México, Mexico). Each company was provided with detailed compositional data, including the ingredient lists and the volatile compound profiles previously determined for each beverage. Based on this information, the companies formulated artificial flavor prototypes designed to mimic the beverage profiles. The resulting flavor samples were supplied as liquid concentrate and applied depending on the manufacturer’s production specifications.
2.5. Flavored Milk Fabrication
The flavors were added to commercial milk (Fortileche Plus®, Alpura®, Cuautitlán Izcalli, Mexico), and the percentage of application was made according to the doses recommended by the flavorist companies. Traditional beverage controls made with Pozol, Chilate, and Cacahuatole dough combined with milk and sugar were used (Supplementary Material, Table S1).
2.6. Sensory Evaluation of Flavored Milks
The flavored milks were evaluated by a trained sensory panel composed of students from the Autonomous University of Querétaro. In previous sessions, the panel consumed the traditional drinks, one beverage per week, to identify their flavor. They were asked to describe traditional beverages about taste, smell, texture, and appearance. Then, 8–9 descriptive terms of flavor were generated (Supplementary Material, Table S2). Also, descriptive phrases specific to each beverage were established when participants were familiar with the sensorial characteristics of traditional beverages following the “free choice profiling” method [20] (Supplementary Material, Table S3). In the sensory evaluation, the panel evaluated the flavor proposals for each sample according to ISO Standard 6658:2017 [21]. Flavor proposals were given together with a control of traditional drinks prepared with milk added with sucrose. A multivariate Check All That Apply (CATA) method was applied. For this purpose, the predetermined list of phrases describing each beverage was presented, and the evaluators were asked to select the best phrases that represent the sensory attributes of milks. The samples were coded with four random numbers and presented at 10 °C in a 25 mL portion. Water and crackers were offered to cleanse the palate between each sample. The sensory evaluation protocol was approved by the Bioethics Committee of the Faculty of Chemistry (Autonomous University of Querétaro).
2.7. Statistical Analysis
At least 3 samples of traditional Mexican beverages were evaluated. The volatile compound profiles were coded binary, with 0 and 1, for the absence and presence of a specific compound, respectively. These encoded profiles were analyzed with a Venn diagram using an online tool [Bioinformatics and Systems Biology of Gent, URL: http://bioinformatics.psb.ugent.be/webtools/Venn/ (accessed on 13 August 2025)]. Then, multivariate analysis such as principal component analysis and dendrogram were carried out using the percentage of area under the chromatogram curve with R Studio version 4.4.2 and JMP 8.0 software (SAS Institute Inc., Cary, NC, USA), respectively. For CATA analysis, R Studio was used.
3. Results and Discussion
3.1. Composition and Preparation Methods
The proximate composition of pastes/doughs of the traditional beverages is presented in Table 1. Among the components examined, moisture content exhibited the greatest variation, likely due to differences in processing methods. The nixtamalization process, used in the preparation of Pozol and Cacahuatole, plays a crucial role in these variations. Nixtamalization is a traditional technique that transforms corn kernels into a cohesive dough called “masa” by cooking them in an alkaline solution with calcium hydroxide. This process induces significant physicochemical changes, facilitating starch gelatinization and its interaction with calcium, which influences pasting properties, digestibility, and hydration characteristics [22]. Consequently, nixtamalization increases moisture content, contributing to the formation of a more elastic and cohesive dough.

Table 1.
Chemical and nutritional composition of traditional beverages [a].
In contrast, Chilate exhibited a lower moisture content than Pozol and Cacahuatole, a difference attributed to its processing method. The preparation of Chilate involves soaking rice for approximately two hours, allowing for gradual water absorption. However, moisture uptake during rice soaking is influenced by factors such as temperature, initial moisture content, and rice variety. Notably, corn starch generally has a higher water absorption capacity than rice starch, resulting in lower moisture content in Chilate [23]. These findings highlight the critical role of processing techniques in shaping the hydration mechanisms of starch, which in turn modify the composition and texture of traditional Mexican beverages.
The protein and lipid content of the beverages is primarily influenced by the amount of cocoa beans used in their preparation. Cocoa beans are notable for their high lipid and protein content, with fat comprising between 40 to 57% of their composition. Their fatty acid profile consists primarily of palmitic, stearic, and oleic acids. Notably, oleic acid has recognized benefits for cardiovascular health; thus, its presence alongside saturated fatty acids may help mitigate adverse effects and present advantageous opportunities for the food industry [24]. Protein concentration in cocoa beans ranges from 11.8 to 15.7% on a dry-weight basis, higher than that of corn, which varies from approximately 9.2 to 15.8%. Cocoa proteins primarily consist of albumins (52%) and globulins (43%), which play essential roles in the structural and functional properties of the beans, influencing their nutritional profile and behavior during processing [25].
The three analyzed beverage recipes include cocoa beans as a key ingredient; cocoa beans are the most expensive component and contribute to variability among local producers. Consequently, slight differences in fat and protein content across samples can be attributed to variations in cocoa bean inclusion. Similar findings were reported by [26]. The study analyzed Pozol dough samples from Tabasco, México, and found comparable compositions. Cacahuatole dough exhibited higher protein content due to the incorporation of broad beans. Broad beans have a high protein content, typically between 27 and 34% on a dry matter basis, with globulins and albumins as the primary protein fractions [27]. Their high-quality protein profile, characterized by an adequate quantity of essential amino acids, enhances the nutritional value of Cacahuatole.
The fiber content of the beverages ranged from 2.0 to 2.4%, similar to values reported in Tejate samples by Sotelo et al. [28]. This confirms that these beverages are low in fiber. The dehulling of cocoa and broad beans after roasting contributes to this low fiber content. A notable advantage of dehulling and toasting these beans is the reduction of antinutritional factors [27].
The caloric content of the doughs ranged from 1727 to 1763 kJ/100 g DW (412–420 kcal), similar to values reported by Gonzalez-Amaro [29] for Tejate dough, a comparable Mexican beverage rich in carbohydrates and fat derived from cacao and nixtamalized corn. However, these authors found that such beverages promote better glucose metabolism compared to juices and soft drinks. Specifically, significantly lower postprandial glycemic responses were observed, attributed to the formation of starch–lipid complexes in cacao–corn mixtures. These complexes, classified as type V resistant starch, are digested slowly by gastric enzymes and may undergo fermentation in the colon, offering potential health benefits to consumers.
3.2. Volatile Compounds
The preparation methods of the studied traditional Mexican beverages are presented in Figure 1; these processes involve various artisanal processes. Crucial steps such as alkaline cooking of corn (nixtamalization), rice soaking, roasting of cocoa beans, and stone grinding have been applied since pre-Hispanic times, contributing to the release and development of volatile compounds from their ingredients. The volatile compounds in the three beverages were identified using gas chromatography. Specifically, 145, 90, and 106 different compounds were detected in samples of Pozol, Chilate, and Cacahuatole, respectively (Supplementary Material, Table S4). In Pozol, the most abundant volatile groups included terpenes (31), alcohols (14), aldehydes (13), benzenes (10), pyrazines (9), and acids (6). Less abundant groups comprised alkanes, furans, ketones, and esters. Similarly, Chilate samples contained 34 terpenes, followed by aldehydes, alcohols, and acids, each with 7 to 13 compounds. Cacahuatole also exhibited a high number of terpenes (38), along with benzenes, aldehydes, pyrazines, and alcohols, each represented by 9–18 compounds. However, not all volatiles were consistently found across samples, indicating high variability in preparation methods and ingredients. Venn diagrams (Figure 2) revealed only 12 common compounds in Pozol, 15 in Chilate, and 27 in Cacahuatole. Table 2 summarizes these common volatiles found among replicates.
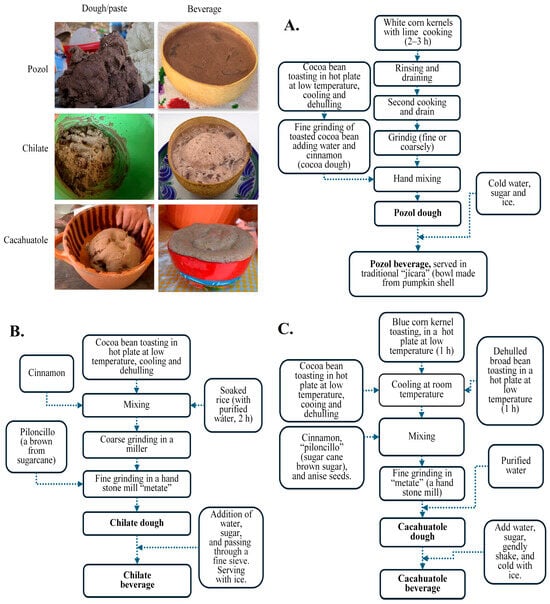
Figure 1.
Traditional fabrication process of (A) Pozol, (B) Chilate, and (C) Cacahuatole.

Figure 2.
Venn diagrams for volatile compounds of (A) Pozol, (B) Chilate, and (C) Cacahuatole.
Due to the great diversity of identified volatiles, their analysis can only be interpreted from a general point of view and is mainly related to the ingredients and processing methods used. Across all beverages, terpenes dominated the volatile profiles (Table S4 and Table 2). These compounds impart fruity, floral, spicy, and citrus-like aromas. Major contributors included (-)-β-pinene, (-)-terpinen-4-ol, 1(e)-β-ocimene, α-caryophyllene, α-phellandrene, α-thujene, β-phellandrene, camphene, caryophyllene, γ-terpinene, and sabinene, which originate mainly from cocoa beans, cereals such as corn, rice [30,31], and legumes like broad beans [32].
Focusing on used ingredients, cocoa features a range of volatile compounds, derived from its inherent components as well as those formed during roasting. In this way, cocoa-derived volatiles include aldehydes such as 2-methyl propanal, 2-methyl butanal, and 3-methyl butanal, and ketones such as 2-heptanone, 2-pentanone, 2-nonanone, acetophenone, and acetoin, imparting chocolate and malty notes and enhancing cocoa flavor [33,34,35]. In this work, 3-methylbutanal (in PZ2 and CA2), 2-methylbutanal (in PZ2, CH2, CH5, and CA2), and 2-nonanone (in CH5) were detected (Table S4). In addition, cocoa roasting was a key source of pyrazines responsible for nutty, roasted aromas, with tetramethylpyrazine accounting for up to 90% of the total [34]. This pyrazine appeared in Chilate samples, while 2,3-dimethylpyrazine, 2,3-diethyl-5-methylpyrazine, 2,5-dimethylpyrazine, 3,5-diethyl-2-methylpyrazine, ethylpyrazine, and methylpyrazine were detected in Cacahuatole (Table 2). Concerning Pozol, several pyrazines were identified (e.g., methylpyrazine, ethylpyrazine, tetramethylpyrazine, etc.) (Table S4); however, they were not common to all the samples evaluated. Overall, results suggest differences in roasting intensity or cocoa bean quality among beverages [34].
Roasting processes involved in beverage production also promoted the formation of floral, fruity, and sweet esters and alcohols, including 1-butanol-3-methyl-acetate, 2-propen-1-ol-3-phenyl-acetate, 3-phenyl-2-propen-1-ol, and linalool (Table 2). Linalool is a key floral note in South American cocoa [35]; thus, its natural presence in cocoa beans may also be possible and would explain their detection in all the analyzed samples. Other detected volatiles, such as 2-phenylethyl acetate, 2-heptanol, hexanal, nonanal, benzeneacetaldehyde, and furfural, enriched the aroma [34,35,36].

Table 2.
Volatile compounds a in Pozol, Chilate, and Cacahuatole.
Table 2.
Volatile compounds a in Pozol, Chilate, and Cacahuatole.
| Compound | CAS | Aroma Descriptor b | PZ | CH | CA | Compound | CAS | Aroma Descriptor b | PZ | CH | CA |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Terpenes: | Pyrazines: | ||||||||||
| (-)-Beta-Pinene | 000127-91-3 | Pine, wood | X | X | - | Pyrazine, 2,3-dimethyl | 005910-89-4 | Caramel, cocoa, hazelnut, peanut butter, toasted | - | - | X |
| (-)-Terpinen-4-ol | 020126-76-5 | - | - | - | X | Pyrazine, 2,3-diethyl-5-methyl | 018138-04-0 | Earthy, meat, potato, roasted | - | - | X |
| 1(E)-Beta-Ocimene | 003779-61-1 | Floral | - | X | - | Pyrazine, 2,5-dimethyl | 000123-32-0 | Cocoa, roast beef, toasted walnut | - | - | X |
| Alpha-Caryophyllene | 006753-98-6 | Fried, spices, wood | - | X | - | Pyrazine, 3,5-diethyl-2-methyl | 018138-05-1 | Baked, cocoa, toasted, rum | - | - | X |
| Alpha-Phellandrene | 000099-83-2 | Citrus, fresh, mint, pepper, spices, and wood | - | X | X | Pyrazine, ethyl | 013925-00-3 | Burnt, must, peanut butter, toasted, rum, wood | - | - | X |
| Alpha-Thujene | 002867-05-2 | - | - | X | X | Pyrazine, methyl | 000109-08-0 | Cocoa, green, hazelnut, popcorn, roasted | - | - | X |
| Beta-Phellandrene | 000555-10-2 | - | - | - | X | Pyrazine, tetramethyl | 001124-11-4 | Cocoa, coffee, green, mocha, roasted | - | X | - |
| Camphene | 000079-92-5 | Menthol, citrus | - | X | X | ||||||
| Caryophyllene | 000087-44-5 | Fried, spices, wood | X | - | X | Esters: | |||||
| Gamma-Terpinene | 000099-85-4 | Bitter and citrusy | - | X | X | 1-Butanol, 3-methyl-, acetate | 000123-92-2 | Apple, banana, glue, pear | X | - | - |
| Sabinene | 003387-41-5 | - | - | - | X | 2-Propen-1-ol, 3-phenyl-, acetate | 000103-54-8 | Floral, fruit, honey | - | - | X |
| Acids: | Aldehydes: | ||||||||||
| Dodecanoic Acid/Lauric Acid | 000143-07-7 | - | X | - | - | Benzaldehyde | 000100-52-7 | Bitter almond | - | X | X |
| Nonanoic Acid/Pelargonic Acid | 000112-05-0 | Fat and bitter | X | - | - | Benzene acetaldehyde | 000122-78-1 | - | - | X | |
| Cinnamaldehyde, (E) | 014371-10-9 | - | - | X | |||||||
| Alcohols: | Benzene derivatives: | ||||||||||
| 2-Propen-1-ol, 3-Phenyl | 000104-54-1 | - | X | - | X | Benzene, 1-methoxy-4-(1- propenyl) | 000104-46-1 | - | - | - | X |
| Ethanol | 000064-17-5 | - | X | X | X | Benzene, 1-methyl-4-(1- methylethyl) | 000099-87-6 | Fresh, citrusy | - | X | - |
| Linalool | 000078-70-6 | Coriander, floral, lavender, lemon, rose | X | X | X | Estragole | 000140-67-0 | - | - | - | X |
| Phenylethyl alcohol | 000060-12-8 | Fruit, honey, lilac, rose, wine | - | X | - | ||||||
| Phenols: | Others: | ||||||||||
| 2-Methoxy-4-Vinylphenol | 007786-61-0 | Clove, curry, spices | X | - | X | 2-Furanmethanol | 000098-00-0 | Burnt, caramel, cooked | - | - | X |
| Eugenol | 000097-53-0 | Burnt, cloves, spices | X | - | X | Hexane | 000110-54-3 | - | X | X | - |
| Styrene | 000100-42-5 | Sweet and floral | X | X | X | ||||||
a Compounds found in all samples of each beverage. b Most frequently associated aroma descriptors [37]. PZ, Pozol. CH, Chilate. CA, Cacahuatole.
Beyond flavor, cocoa roasting reduces astringency and polyphenol content, producing melanoidins that contribute to bean darkening. A balanced polyphenol retention is desirable to preserve antioxidant capacity while preventing flavor loss [34]. Detected phenols included 2-methoxy-4-vinylphenol (clove, curry, spicy notes) and eugenol (burnt, clove, spicy notes) in Cacahuatole and Pozol beverages (Table 2).
All the drinks analyzed contained cocoa, but their distinct ingredients led to unique volatile profiles. Pozol, which is made from cocoa and nixtamalized corn, differed noticeably from Chilate (featuring cocoa and rice) and Cacahuatole (which includes cocoa, corn, and broad beans), creating clear differences among them. For instance, the use of nixtamalized maize in the preparation of Pozol and Cacahuatole was anticipated to confer relevant volatile compounds. Nixtamalization is known to hydrolyze bound phytochemicals, enhancing their extractability [38]. Lipid-oxidation aldehydes such as hexanal, heptanal, octanal, and nonanal are common in nixtamalized corn [39]. These compounds are also related to broad beans [31,36]. Nevertheless, hexanal and nonanal were only detected in CA1; however, their absence in the other Cacahuatole samples and Pozol may be related to subsequent thermal decomposition. The use of broad beans in Cacahuatole, on the other hand, could be responsible for the detection of a greater amount of pyrazines, compared to Pozol and Chilate, since pyrazines were predominant in roasted broad beans [31].
Spices played a role in enhancing the beverage’s aroma. Analytical results indicated the presence of cinnamaldehyde, eugenol, and benzaldehyde from cinnamon in multiple samples. Cinnamaldehyde was found in Pozol (PZ2, PZ3, PZ5), Chilate (CH1, CH3), and Cacahuatole (CA1-3). In addition, eugenol was found in all samples of Pozol and Cacahuatole as well as CH1,2,5, and benzaldehyde was found in all samples of Chilate and Cacahuatole as well as PZ2-4. Furthermore, the detection of styrene in all three beverages (Table 2) can be attributed to the presence of cinnamon, which is known to naturally contain elevated levels of styrene [40,41] and may also generate it through the degradation of cinnamaldehyde [42]. Estragole, on the other hand, was found only in Cacahuatole samples (Table 2) and is chiefly associated with the anise seeds used in its preparation [43].
Although the pastes/doughs of the traditional Mexican beverages are not deliberately fermented, ethanol was consistently found in all beverage samples (Table 2), likely due to the activity of lactic acid bacteria (LAB) during storage at ambient temperature between preparation and consumption [2,44]. Additional cocoa fermentation-associated compounds, including guaiacol and terpineol [45], were also detected in specific samples. Guaiacol was present in PZ2, while terpineol was identified in PZ2, CH1-4, and CA3.
The results of this study provided an initial assessment of the volatiles identified in traditional Mexican beverages. Quantifying these concentrations was not part of the current analysis. Future research should include quantification, as the sensory relevance of volatiles is determined by both their presence and concentration, as well as interactions with other compounds within the beverage matrix. The relationship between volatile compound concentrations and their odor thresholds plays a key role in understanding aroma perception and overall acceptability. The olfactory threshold refers to the lowest concentration at which a particular compound can be detected by the human nose. Volatile compounds with concentrations above this threshold contribute to aroma, while those below the threshold may still affect perception through interactions with other volatiles. Some key volatile compounds in cocoa have well-established olfactory thresholds, which are used by the chocolate industry to monitor and control flavor quality. Compounds such as pyrazines, esters, acids, and alcohols have thresholds in the low microgram per kilogram range. A few threshold values for some of the identified compounds in this work are noted: eugenol, threshold ≈0.71–470 µg/Kg; linalool, threshold ≈140–600 µg/Kg; β-pinene, ≈6–1500 µg/Kg; caryophyllene, ≈64–1540 µg/Kg; 3-methylbutanal, threshold ≈0.35–7 µg/Kg; and benzaldehyde, threshold ≈300–2200 µg/Kg [46].
Importantly, it is expected that the sensory perception of volatiles is also strongly modulated by the beverage matrix. For example, the high starch content from nixtamalized corn or soaked rice, along with proteins from cocoa and broad beans, can bind hydrophobic volatiles through hydrogen bonding and hydrophobic interactions [17,22]. Additionally, the lipid fraction of cocoa butter can act as a solvent for non-polar compounds, prolonging their retention and modifying their release rate during consumption [36]. Furthermore, Maillard-derived compounds formed during cocoa roasting may interact with aroma-active molecules, influencing not only flavor perception but also aroma stability during storage [47]. This matrix effect highlights the importance of integrating both chemical and sensory data when evaluating the flavor potential of complex, traditional beverages.
On the other hand, given the complexity of the volatiles profiles of Pozol, Chilate, and Cacahuatole, a principal component analysis (PCA) was performed. It showed high variability, with PC1 + PC2/PC1 + PC3 explaining less than 30% and PC1 + PC2 + PC3 explaining less than 50% of total variance (Figure 3A–C). Nonetheless, hierarchical clustering (Figure 3D) separated the samples into two groups: one containing all Cacahuatole samples and another comprising Pozol and Chilate. It seems Cacahuatole’s distinct aldehyde profile, linked to broad bean lipid oxidation [48], contributed to this separation. The clustering also showed some samples (PZ4, CA2, CH5) diverged from their groups due to unique volatile signatures, consistent with their Venn diagram patterns, and attributed to differences in raw materials and methods used.

Figure 3.
Principal component analysis (A–C) and color map dendrogram (D) for volatile compound characterization of traditional Mexican beverages. PZ, Pozol. CH, Chilate. CA, Cacahuatole. The number after the two capital letters indicates the sample number for each beverage.
3.3. Sensory Evaluation of Milks with Flavors of Traditional Mexican Beverages
The processing methods, composition, volatile compound profiles, and ingredient lists were used to develop artificial flavors for each beverage in collaboration with commercial flavor houses. These flavors were then added to plain milk at the recommended doses. A trained sensory panel, familiarized with the traditional beverages, evaluated the similarity between the developed flavors and their authentic counterparts. Control samples for sensory analysis were prepared by mixing plain milk with dough samples of the traditional beverages and adding sugar. The Check-All-That-Apply (CATA) method was used to assess sensory attributes.
Figure 4 presents the CATA analysis map for the flavor proposals from commercial flavor houses (Supplementary Material, Table S1), aiming to replicate the sensory perception of traditional Mexican beverages. The panel described Pozol as having flavor notes of vanilla, sweetness, cinnamon, coffee, toasted cocoa, cooked corn, burnt corn, and an earthy taste (Supplementary Material, Table S3). Figure 4A shows that the Pozol control (FPZ0), located in the upper left quadrant, was close to descriptors associated with “optimal” flavors. As expected, the milks containing Pozol dough were perceived as having a flavor profile similar to the original beverage. FPZ0 was distant from all the flavor proposals. To the right of the y-axis, descriptors indicating the absence of key Pozol flavor notes, such as vanilla and cinnamon, as well as “unpleasant to the palate,” were found. In this area, the proposals FPZ1 and FPZ2 were located. FPZ3 and FPZ5 appeared in the lower right quadrant, near descriptors such as “light” flavors, “no cooked corn taste,” and “no toasted cocoa flavor,” highlighting their lack of essential Pozol notes. The proposal closest to the control, FPZ4, positioned in the lower left quadrant, was evaluated as “pleasant to the palate” and featured “optimal” notes of coffee, sweetness, and cinnamon.

Figure 4.
CATA analysis map of milk formulas with flavor of traditional Mexican beverages. (A) Pozol. (B) Chilate. (C) Cacahuatole. FPZ, flavor proposal for Pozol. FCH, flavor proposal for Chilate. FCA, flavor proposal for Cacahuatole.
Figure 4B displays the CATA map for Chilate-flavored samples. The panel characterized Chilate with notes of sweetness, cinnamon, vanilla, sourness, bitterness, rice, cocoa, and toasted cocoa (Supplementary Material, Table S3). Only three proposals were evaluated. The control (FCH0) was positioned near descriptors of “optimal” notes, including sweet, cocoa, and vanilla, along with slight acidity, bitterness, and rice taste. Thus, the control sample was perceived as the most similar to traditional Chilate. Proposals FCH2 and FCH3 clustered together, associated with the absence of key flavors like bitterness, roasted cocoa, and sweetness. Some descriptors indicating a slight presence of cinnamon were also nearby. In contrast, proposal FCH1 was located near descriptors such as “no vanilla, no cocoa, and no cinnamon taste” and “unpleasant to the palate.” This suggests that the developed flavors or their dosages failed to replicate the complexity of Chilate.
For Cacahuatole-flavored samples, Figure 4C illustrates the CATA analysis map, which shows similarly unsatisfactory results. The panel described Cacahuatole as a sweet drink with notes of cinnamon, vanilla, anise, roasted beans, cocoa, toasted cocoa, bitterness, and corn (Supplementary Material, Table S3). As in previous cases, the control (FCA0) was positioned near “optimal” descriptors, indicating it closely resembled the original beverage. FCA3 and FCA4, grouped in the lower right quadrant, lacked key notes such as anise, cinnamon, toasted cocoa, and corn. In the upper right quadrant, FCA1 was associated with “excessive” notes and was also perceived as “unpleasant.” Finally, FCA2 was near the center of the map, surrounded by descriptors indicating a “slight” presence of important flavors such as cinnamon, broad bean, and anise, along with “slightly similar to Cacahuatole”.
Overall, the results indicate that replicating the complex flavors of traditional beverages using artificial formulations remains challenging, owing mainly to the intricate flavor profiles of these drinks. While flavors from original Mexican beverages are mixtures with hundreds of volatiles that synergistically influence their aroma, artificial formulations generally concentrate just a few of them. Moreover, the reduced sensory fidelity of the flavored milks may also result from milk matrix effects. Milk proteins and lipids can bind hydrophobic aroma compounds, decreasing their volatility and sensory perception. Similarly, polar compounds may bind to casein micelles, slowing their release. Differences between authentic and formulated flavors appear to be due to the absence or sub-threshold levels of key volatiles and flavor suppression due to milk–volatile interactions. Further research is necessary to refine flavor development and optimize dosages to improve consumer perception since the complexity of traditional Mexican beverage aromas exceeds the capabilities of the current formulations. While this study represents an initial step in this area, deeper analysis and advanced technological development are required to achieve a more accurate replication of these flavors. This will allow the diversification of the options to taste highly culturally relevant Mexican flavors and enable their integration into milk and other dairy-based matrices, enhancing product diversity while preserving traditional sensory heritage.
4. Conclusions
Traditional Mexican cocoa-based beverages, including Pozol, Chilate, and Cacahuatole, exhibit compositions and preparation methods that impact their nutritional profiles and sensory characteristics. The volatile compound analysis revealed a diverse range of molecules influenced by ingredient inclusion and processing techniques. Terpenes, aldehydes, ketones, pyrazines, and phenolics played a crucial role in defining the sensory profiles of these beverages. Additionally, roasting cocoa beans enhanced their characteristic flavors, leading to the formation of complex aroma compounds. Future research should include quantitative analyses and compare them with odor thresholds to better understand the contribution of these volatile compounds to beverage aroma.
Despite attempts to replicate the traditional beverage flavors and their addition to milk-based formulations, commercial flavor proposals struggled to capture the sensory attributes of traditional beverages. Sensory analysis highlighted the challenge of artificially reproducing the balance of roasted notes inherent in these drinks. This underscores the need for further research into refining flavor replication to preserve the authenticity of these traditional beverages. Ultimately, the study emphasizes the cultural and nutritional significance of these drinks, warranting continued exploration into flavor development and their use in food products that improve the nutritional support for the Mexican population.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/beverages11050130/s1, Table S1. Flavor proposals for milks. Table S2. Descriptors for traditional Mexican beverages. Table S3. Phrases to describe traditional Mexican beverages. Table S4A. Volatile compound profile of Pozol. Table S4B. Volatile compound profile of Chilate. Table S4C. Volatile compound profile of Cacahuatole [49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112,113,114].
Author Contributions
L.A.-G.: Conceptualization, Investigation, Acquisition of data, Formal analysis, Writing-Original draft; E.A.-P.: Investigation, Visualization, Acquisition of data, Formal analysis; P.A.V.-L.: Supervision, Data curation, Writing-review and editing; M.d.C.C.-T.: Visualization, Formal Analysis, Writing-review and editing; S.L.A.-L.: Project administration, Resources, Supervision, Writing-review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Ganaderos Productores de Leche Pura S.A. de C.V. (ALPURA, México), SUV/DVS-EXT-2021-003.
Institutional Review Board Statement
This work was approved by the Ethics Committee of the Facultad de Química, Universidad Autónoma de Querétaro (México) CBQ20/086.
Informed Consent Statement
Participants in the sensory analysis signed informed consent forms.
Data Availability Statement
Data are available from the corresponding author on reasonable request.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| PZ | Pozol |
| CA | Cacahuatole |
| CH | Chilate |
| FPZ | Flavor proposal for Pozol |
| FCA | Flavor proposal for Cacahuatole |
| FCH | Flavor proposal for Chilate |
| PCA | Principal component analysis |
| CATA | Check All That Apply |
References
- Pérez-Armendáriz, B.; Cardoso-Ugarte, G.A. Traditional fermented beverages in Mexico: Biotechnological, nutritional, and functional approaches. Food Res. Int. 2020, 136, 109307. [Google Scholar] [CrossRef]
- Barros, C.; Buenrostro, M. Pozol, Popo, Champurrado. Rev. Digit. Univ. de la Univ. Autónoma de México 2011, 12, 1–9. Available online: http://www.revista.unam.mx/vol.12/num4/art41/art41.pdf (accessed on 30 November 2021).
- Robledo-Márquez, K.; Ramírez, V.; González-Córdova, A.F.; Ramírez-Rodríguez, Y.; García-Ortega, L.; Trujillo, J. Research opportunities: Traditional fermented beverages in Mexico. Cultural, microbiological, chemical, and functional aspects. Food Res. Int. 2021, 147, 110482. [Google Scholar] [CrossRef] [PubMed]
- Garcia Aguilar, M.; Martinez Dorado, G.; Pulido Meneses, I.; Flores Ambrosio, E.; Avila Rojas, J.; Flores Morales, A.; Sánchez Contreras, A. Desarrollo de dos formulaciones de una bebida de cacao tradicional del municipio de Zacatelco, Tlaxcala. In Investigación y Desarrollo en Ciencia y Tecnología de Alimentos; Universidad Autónoma de Nuevo León: San Nicolas de los Garza, Nuevo León, Mexico, 2019; Volume 4. [Google Scholar]
- Gobierno de México, G. El Chilate Bebida Tradicional del Estado de Guerrero. 2018. Available online: https://www.gob.mx/agricultura/guerrero/articulos/el-chilate-bebida-tradicional-del-estado-de-guerrero?idiom=es (accessed on 18 March 2025).
- Ramirez, R. Propuesta de organización de productores de cacao para la sustentabilidad y desarrollo local en Tepango, municipio de Ayulta de los libres, Guerrero. In Desarrollo Sustentable y Participación Social; Rivera, R., Ed.; Universidad Autónoma Chapingo: Texcoco, Mexico; Universidad Autónoma de Guerrero: Chilpancingo, Guerrero, Mexico, 2018; pp. 435–452. [Google Scholar]
- Soleri, D.; Cleveland, D.A.; Cuevas, F.A. Food globalization and local diversity: The case of tejate. Curr. Anthropol. 2008, 49, 281–290. [Google Scholar] [CrossRef]
- Staller, J.; Carrasco, M. (Eds.) Pre-Columbian Foodways: Interdisciplinary Approaches to Food, Culture, and Markets in Ancient Mesoamerica; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2009. [Google Scholar] [CrossRef]
- Schieberle, P. New developments in methods for analysis of volatile flavor compounds and their precursors. In Characterization of Food; Elsevier Science BV: Amsterdam, The Netherlands, 1995; pp. 403–431. [Google Scholar] [CrossRef]
- Song, H.; Liu, J. GC-O-MS technique and its applications in food flavor analysis. Food Res. Int. 2018, 114, 187–198. [Google Scholar] [CrossRef] [PubMed]
- González, L. Producción mexicana de leche se incrementó en 2.1% en 2020, dice la Canilec. El Economista. 25 October 2021. Available online: https://www.eleconomista.com.mx/empresas/Produccion-mexicana-de-leche-se-incremento-en-2.1-en-2020-dice-la-Canilec-20211025-0075.html (accessed on 21 October 2022).
- Hernandez, G.; Parrish, M.R. Mexico Dairy and Products Semi-annual Mexico Seeks to Improve Dairy Quality. In Global Agricultural Information Network; Report Number MX7020; USDA: Washington, DC, USA, 2017. Available online: https://apps.fas.usda.gov/newgainapi/api/report/downloadreportbyfilename?filename=Dairy%20and%20Products%20Semi-annual_Mexico%20City_Mexico_5-24-2017.pdf (accessed on 16 March 2025).
- Sallyards, M.; Kuypers, K.; Lara, G. Mexico Dairy and Products Semi-annual High Demand Drives Greater Cheese Production and Imports. In Global Agricultural Information Network; Report number MX9019; USDA: Washington, DC, USA, 2019. Available online: https://apps.fas.usda.gov/newgainapi/api/report/downloadreportbyfilename?filename=Dairy%20and%20Products%20Semi-annual_Mexico%20City_Mexico_5-24-2019.pdf (accessed on 15 March 2025).
- Barišić, V.; Icyer, N.C.; Akyil, S.; Toker, O.S.; Flanjak, I.; Ačkar, Đ. Cocoa based beverages–Composition, nutritional value, processing, quality problems and new perspectives. Trends Food Sci. Technol. 2023, 132, 65–75. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis of the Association of Official Analytical Chemists, 17th ed.; AOAC: Washington, DC, USA, 2000. [Google Scholar]
- FAO. Food Energy—Methods of analysis and conversion factors. In Report of a Technical Workshop. FAO Food and Nutrition Paper 77; FAO: Rome, Italy, 2003. [Google Scholar]
- Pérez-Ramírez, I.F.; Cariño-Sarabia, A.; Castaño-Tostado, E.; Vázquez-Landaverde, P.A.; Ramos-Gómez, M.; Reynoso-Camacho, R.; Amaya-Llano, S.L. Chemical and sensorial characterization of Tejate, a Mexican traditional maize-cocoa beverage, and improvement of its nutritional value by protein addition. J. Food Sci. Technol. 2021, 58, 3548–3560. [Google Scholar] [CrossRef] [PubMed]
- Cecilia, K.; Glaston, K.; Simon, M.; Renaud, B.; Fredrick, N. Volatile organic compounds in brewed Kenyan Arabica coffee genotypes by solid phase extraction gas chromatography mass spectrometry. Food Sci. Qual. Manag. 2012, 8, 18–26. [Google Scholar]
- Duarte, W.F.; Dias, D.R.; Oliveira, J.M.; Teixeira, J.A.; de Almeida e Silva, J.B.; Schwan, R.F. Characterization of different fruit wines made from cacao, cupuassu, gabiroba, jaboticaba and umbu. LWT 2010, 43, 1564–1572. [Google Scholar] [CrossRef]
- Varela, P.; Ares, G. Sensory profiling, the blurred line between sensory and consumer science. A review of novel methods for product characterization. Food Res. Int. 2012, 48, 893–908. [Google Scholar] [CrossRef]
- ISO 6658; Sensory Analysis: Methodology: General Guidance. International Organization for Standardization: Geneva, Switzerland, 2017.
- Santiago-Ramos, D.; Figueroa-Cárdenas, J.; Mariscal-Moreno, R.; Escalante-Aburto, A.; Ponce-García, N.; Véles-Medina, J. Physical and chemical changes undergone by pericarp and endosperm during corn nixtamalization-A review. J. Cereal Sci. 2018, 81, 108–117. [Google Scholar] [CrossRef]
- Bello, M.O.; Tolaba, M.P.; Suarez, C. Water absorption and starch gelatinization in whole rice grain during soaking. LWT 2007, 40, 313–318. [Google Scholar] [CrossRef]
- Mustiga, G.; Morrissey, J.; Stack, J.; Duval, A.; Royaert, S.; Jansen, J.; Bizzotto, C.; Villela-Dias, C.; Mei, L.; Cahoon, E.; et al. Identification of climate and genetic factors that control fat content and fatty acid composition of Theobroma cacao L. Beans. Front. Plant Sci. 2019, 10, 1159. [Google Scholar] [CrossRef]
- Bertazzo, A.; Comai, S.; Brunato, I.; Zancato, M.; Costa, C. The content of protein and non-protein (free and protein-bound) tryptophan in Theobroma cacao beans. Food Chem. 2011, 124, 93–96. [Google Scholar] [CrossRef]
- Rizo, J.; Rogel, M.A.; Guillén, D.; Wacher, C.; Martinez-Romero, E.; Encarnación, S.; Rodríguez-Sanoja, R. Nitrogen fixation in pozol, a traditional fermented beverage. Appl. Environ. Microbiol. 2020, 86, e00588-20. [Google Scholar] [CrossRef] [PubMed]
- Shi, D.; Stone, A.K.; Marinangeli, C.P.; Carlin, J.; Nickerson, M.T. Faba bean nutrition: Macronutrients, antinutrients, and the effect of processing. Cereal Chem. 2024, 101, 1181–1197. [Google Scholar] [CrossRef]
- Sotelo, A.; Soleri, D.; Wacher, C.; Sánchez-Chinchillas, A.; Argote, R.M. Chemical and nutritional composition of tejate, a traditional maize and cacao beverage from the Central Valleys of Oaxaca, Mexico. Plant Foods Hum. Nutr. 2012, 67, 148–155. [Google Scholar] [CrossRef]
- González-Amaro, R.M.; de Dios Figueroa-Cárdenas, J.; Perales, H.; Santiago-Ramos, D. Maize races on functional and nutritional quality of tejate: A maize-cacao beverage. LWT 2015, 63, 1008–1015. [Google Scholar] [CrossRef]
- Kaul, P.N.; Bhattacharya, A.K.; Rajeswara Rao, B.R.; Syamasundar, K.V.; Ramesh, S. Volatile constituents of essential oils isolated from different parts of cinnamon (Cinnamomum zeylanicum Blume). J. Sci. Food Agric. 2003, 83, 53–55. [Google Scholar] [CrossRef]
- Karolkowski, A.; Guichard, E.; Briand, L.; Salles, C. Volatile compounds in pulses: A review. Foods 2021, 10, 3140. [Google Scholar] [CrossRef]
- Pico, J.; Tapia, J.; Bernal, J.; Gómez, M. Comparison of different extraction methodologies for the analysis of volatile compounds in gluten-free flours and corn starch by GC/QTOF. Food Chem. 2018, 267, 303–312. [Google Scholar] [CrossRef] [PubMed]
- Quelal, O.M.; Hurtado, D.P.; Benavides, A.A.; Alanes, P.V.; Alanes, N.V. Key aromatic volatile compounds from roasted cocoa beans, cocoa liquor, and chocolate. Fermentation 2023, 9, 166. [Google Scholar] [CrossRef]
- Rojas, M.; Hommes, A.; Heeres, H.J.; Chejne, F. Physicochemical phenomena in the roasting of cocoa (Theobroma cacao L.). Food Eng. Rev. 2022, 14, 509–533. [Google Scholar] [CrossRef]
- Aprotosoaie, A.C.; Hăncianu, M.; Costache, I.I.; Miron, A. Linalool: A review on a key odorant molecule with valuable biological properties. Flavour Fragr. J. 2014, 29, 193–219. [Google Scholar] [CrossRef]
- Utrilla-Vázquez, M.; Rodríguez-Campos, J.; Avendaño-Arazate, C.H.; Gschaedler, A.; Lugo-Cervantes, E. Analysis of volatile compounds of five varieties of Maya cocoa during fermentation and drying processes by Venn diagram and PCA. Food Res. Int. 2020, 129, 108834. [Google Scholar] [CrossRef]
- National Center for Biotechnology Information. PubChem Compound Summaries. Available online: https://pubchem.ncbi.nlm.nih.gov (accessed on 13 June 2025).
- Kamau, E.H.; Nkhata, S.G.; Ayua, E.O. Extrusion and nixtamalization conditions influence the magnitude of change in the nutrients and bioactive components of cereals and legumes. Food Sci. Nutr. 2020, 8, 1753–1765. [Google Scholar] [CrossRef] [PubMed]
- Buzgau, G.; Marc, R.A.; Muresan, C.C.; Farcas, A.; Socaci, S.A.; Muresan, A.; Muste, S. The study of the quality parameters of the tortilla chips products formulated from mixtures of corn flour and legumes. Turk J. Agric. For. 2023, 47, 772–786. [Google Scholar] [CrossRef]
- Steele, D.H.; Thornburg, M.J.; Stanley, J.S.; Miller, R.R.; Brooke, R.; Cushman, J.R.; Cruzan, G. Determination of styrene in selected foods. J. Agric. Food Chem. 1994, 42, 1661–1665. [Google Scholar] [CrossRef]
- Cao, X.L.; Sparling, M.; Pelletier, L.; Dabeka, R. Styrene in foods and dietary exposure estimates. Food Addit. Contam. Part A 2018, 35, 2045–2051. [Google Scholar] [CrossRef]
- Fragnière, C.; Aebischer, J.N.; Dudler, V.; Sager, F. A short study on the formation of styrene in cinnamon. Mitteilungen Leb. Hygie. 2003, 94, 609–620. [Google Scholar]
- Shojaii, A.; Fard, M.A. Review of pharmacological properties and chemical constituents of Pimpinella anisum. Int. Sch. Res. Not. 2012, 2012, 510795. [Google Scholar] [CrossRef] [PubMed]
- Fuentes-Manzo, M.I. Cuantificación de Antioxidantes en Bebidas de Maíz (Zea mays). Master’s Thesis, Universidad de ciencias y artes de Chiapas, Tuxtla Gutierrez, Chiapas, Mexico, 2018. Available online: https://repositorio.unicach.mx/handle/20.500.12753/552?locale-attribute=en (accessed on 8 October 2024).
- da Veiga Moreira, I.M.; de Figueiredo Vilela, L.; Santos, C.; Lima, N.; Schwan, R.F. Volatile compounds and protein profiles analyses of fermented cocoa beans and chocolates from different hybrids cultivated in Brazil. Food Res. Int. 2018, 109, 196–203. [Google Scholar] [CrossRef]
- Rychlik, M.; Schieberle, P.; Grosch, W. Compilation of Odor Thresholds, Odor Qualities and Retention Indices of Key Food Odorants; Dt. Forschungsanst. für Lebensmittelchemie: Freising, Germany, 1998. [Google Scholar]
- Engel, E.; Ratel, J. Correction of the data generated by mass spectrometry analyses of biological tissues: Application to food authentication. J. Chromatogr. A 2007, 1154, 331–341. [Google Scholar] [CrossRef] [PubMed]
- Badjona, A.; Bradshaw, R.; Millman, C.; Howarth, M.; Dubey, B. Faba bean flavor effects from processing to consumer acceptability. Foods 2023, 12, 2237. [Google Scholar] [CrossRef]
- Bonaïti, C.; Irlinger, F.; Spinnler, H.E.; Engel, E. An iterative sensory procedure to select odor-active associations in complex consortia of microorganisms: Application to the construction of a cheese model. J. Dairy Sci. 2005, 88, 1671–1684. [Google Scholar] [CrossRef]
- Wu, S.; Zorn, H.; Krings, U.; Berger, R.G. Volatiles from submerged and surface-cultured beefsteak fungus. Fistulina hepatica. Flavour Fragr. J. 2007, 22, 53–60. [Google Scholar] [CrossRef]
- Insausti, K.; Goñi, V.; Petri, E.; Gorraiz, C.; Beriain, M.J. Effect of weight at slaughter on the volatile compounds of cooked beef from Spanish cattle breeds. Meat Sci. 2005, 70, 83–90. [Google Scholar] [CrossRef]
- Avsar, Y.K.; Karagul-Yuceer, Y.; Drake, M.A.; Singh, T.K.; Yoon, Y.; Cadwallader, K.R. Characterization of nutty flavor in Cheddar cheese. J. Dairy Sci. 2004, 87, 1999–2010. [Google Scholar] [CrossRef] [PubMed]
- Jarunrattanasri, A.; Theerakulkait, C.; Cadwallader, K.R. Aroma components of acid- hydrolyzed vegetable protein made by partial hydrolysis of rice bran protein. J. Agric. Food Chem. 2007, 55, 3044–3050. [Google Scholar] [CrossRef]
- Tret’yakov, K.V. Retention Data NIST Mass Spectrometry Data Center; NIST Mass Spectrom. Data Cent. Gaithersburg, MD, USA. 2007. Available online: https://chemdata.nist.gov (accessed on 13 November 2023).
- Ames, J.; Guy, R.C.E.; Kipping, G.J. Effect of pH and temperature on the formation of volatile compounds in cysteine/reducing sugar/starch mixtures during extrusion cooking. J. Agric. Food Chem. 2001, 49, 1885–1894. [Google Scholar] [CrossRef]
- Pino, J.A.; Mesa, J.; Muñoz, Y.; Martí, M.P.; Marbot, R. Volatile components from mango (Mangifera indica L.) cultivars. J. Agric. Food Chem. 2005, 53, 2213–2223. [Google Scholar] [CrossRef] [PubMed]
- Nakahara, K.; Yoshida, S.; Komatsu, K.; Ishiwata, K.; Sakamoto, O. Polymer analysis by improved pyrolysis-Gas Chromatography hyphenated with four specific detectors. J. Soc. Cosmet. Chem. Jpn. 2000, 34, 142–151. [Google Scholar] [CrossRef]
- Weissbecker, B.; Holighaus, G. Gas chromatography with mass spectrometric and electroantennographic detection: Analysis of wood odorants by direct coupling of insect olfaction and mass spectrometry. J. Chromatogr. A 2004, 1056, 209–216. Available online: https://ediss.uni-goettingen.de/bitstream/handle/11858/00-1735-0000-000D-F05D-C/holighaus.pdf?sequence=1 (accessed on 13 November 2023).
- Siegmund, B.; Murkovic, M. Changes in chemical composition of pumpkin seeds during the roasting process for production of pumpkin seed oil (Part 2: Volatile compounds). Food Chem. 2004, 84, 367–374. [Google Scholar] [CrossRef]
- Kotowska, U.; Żalikowski, M.; Isidorov, V.A. HS-SPME/GC-MS analysis of volatile and semi-volatile organic compounds emitted from municipal sewage sludge. Environ. Monit. Assess. 2012, 184, 2893–2907. [Google Scholar] [CrossRef]
- Isidorov, V.A.; Krajewska, U.; Dubis, E.N.; Jdanova, M.A. Partition coefficients of alkyl aromatic hydrocarbons and esters in a hexane-acetonitrile system. J. Chromatogr. A 2001, 923, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, T.; Plotto, A.; Goodner, K.; Gmitter, F.G. Distribution of aroma volatile compounds in tangerine hybrids and proposed inheritance. J. Sci. Food Agric. 2011, 91, 449–460. [Google Scholar] [CrossRef]
- Benkaci-Ali, F.; Baaliouamer, A.; Meklati, B.Y.; Chemat, F. Chemical composition of seed essential oils from Algerian Nigella sativa extracted by microwave and hydrodistillation. Flavour Fragr. J. 2007, 22, 148–153. [Google Scholar] [CrossRef]
- Zeller, A.; Rychlik, M. Character impact odorants of fennel fruits and fennel tea. J. Agric. Food Chem. 2006, 54, 3686–3692. [Google Scholar] [CrossRef]
- Rezazadeh, S.; Hamedani, M.P.; Dowlatabadi, R.; Yazdani, D.; Shafiee, A. Chemical composition of the essential oils of Stachys schtschegleevii Sosn. and Stachys balansae Boiss & Kotschy from Iran. Flavour Fragr. J. 2006, 21, 290–293. [Google Scholar] [CrossRef]
- Couladis, M.; Chinou, I.B.; Tzakou, O.; Petrakis, P.V. Composition and antimicrobial activity of the essential oil of Hypericum rumeliacum subsp. Apollonis (Boiss. & Heldr.). Phytother. Res. 2003, 17, 152–154. [Google Scholar] [CrossRef]
- Zeng, Y.X.; Zhao, C.X.; Liang, Y.Z.; Yang, H.; Fang, H.Z.; Yi, L.Z.; Zeng, Z.D. Comparative analysis of volatile components from Clematis species growing in China. Anal. Chim. Acta. 2007, 595, 328–339. [Google Scholar] [CrossRef]
- Rembold, H.; Wallner, P.; Nitz, S.; Kollmannsberger, H.; Drawert, F. Volatile Components of Chickpea (Cicer arietinum L.) Seed. J. Agric. Food Chem. 1989, 37, 659–662. [Google Scholar] [CrossRef]
- Kundakovic, T.; Fokialakis, N.; Chinou, I. Essential oil composition of Achillea lingulata and A. umbellata. Flavour Fragr. J. 2007, 22, 184–187. [Google Scholar] [CrossRef]
- Lalel, H.J.D.; Singh, Z.; Tan, S.C. Glycosidically-bound aroma volatile compounds in the skin and pulp of “Kensington Pride” mango fruit at different stages of maturity. Postharvest Biol. Technol. 2003, 29, 205–218. [Google Scholar] [CrossRef]
- Beaulieu, J.C.; Grim, C.C. Identification of volatile compounds in cantaloupe at various developmental stages using solid phase microextraction. J. Agric. Food Chem. 2001, 49, 1345–1352. [Google Scholar] [CrossRef]
- Kallio, M.; Jussila, M.; Rissanen, T.; Anttila, P.; Hartonen, K.; Reissell, A.; Vreuls, R.; Adahchour, M.; Hyötyläinen, T. Comprehensive two-dimensional gas chromatography coupled to time-of-flight mass spectrometry in the identification of organic compounds in atmospheric aerosols from coniferous forest. J. Chromatogr. A 2006, 1125, 234–243. [Google Scholar] [CrossRef]
- Xu, X.; Van Stee, L.L.P.; Williams, J.; Beens, J.; Adahchour, M.; Vreuls, R.J.J.; Brinkman, U.A.T.; Lelieveld, J. Comprehensive two-dimensional gas chromatography (GC×GC) measurements of volatile organic compounds in the atmosphere. Atmos. Chem. Phys. 2003, 3, 665–682. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, Z.P.; Gao, S.H.; Ren, H.Q.; Zhong, R.Q.; Chen, W.S. The effects of salvia przewalskii total phenolic acid extract on immune complex glomerulonephritis. Pharm. Biol. 2017, 55, 2153–2160. [Google Scholar] [CrossRef] [PubMed]
- Radulović, N.; Lazarević, J.; Ristić, N.; Palić, R. Chemotaxonomic significance of the volatiles in the genus Stachys (Lamiaceae): Essential oil composition of four Balkan Stachys species. Biochem. Syst. Ecol. 2007, 35, 196–208. [Google Scholar] [CrossRef]
- Estévez, M.; Ventanas, S.; Ramírez, R.; Cava, R. Influence of the addition of rosemary essential oil on the volatiles pattern of porcine frankfurters. J. Agric. Food Chem. 2005, 53, 8317–8324. [Google Scholar] [CrossRef]
- Belsito, E.L.; Carbone, C.; Di Gioia, M.L.; Leggio, A.; Liguori, A.; Perri, F.; Siciliano, C.; Viscomi, M.C. Comparison of the volatile constituents in cold-pressed bergamot oil and a volatile oil isolated by vacuum distillation. J. Agric. Food Chem. 2007, 55, 7847–7851. [Google Scholar] [CrossRef]
- Kartal, N.; Sokmen, M.; Tepe, B.; Daferera, D.; Polissiou, M.; Sokmen, A. Investigation of the antioxidant properties of Ferula orientalis L. using a suitable extraction procedure. Food Chem. 2007, 100, 584–589. [Google Scholar] [CrossRef]
- De Pooter, H.L.; Montena, J.P.; Willaert, G.A.; Dirinck, P.J.; Schamp, N.M. Treatment of golden delicious apples with aldehydes and carboxylic acids: Effect on the Headspace Composition. J. Agric. Food Chem. 1983, 31, 813–818. [Google Scholar] [CrossRef]
- Agnihotri, V.K.; Agarwal, S.G.; Dhar, P.L.; Thappa, R.K.; Baleshwar Kapahi, B.K.; Saxena, R.K.; Qazi, G.N. Essential oil composition of Mentha pulegium L. growing wild in the north-western Himalayas India. Flavour Fragr. J. 2005, 20, 607–610. [Google Scholar] [CrossRef]
- Vagionas, K.; Ngassapa, O.; Runyoro, D.; Graikou, K.; Gortzi, O.; Chinou, I. Chemical analysis of edible aromatic plants growing in Tanzania. Food Chem. 2007, 105, 1711–1717. [Google Scholar] [CrossRef]
- Cerny, C.; Guntz-Dubini, R. Role of the solvent glycerol in the Maillard reaction of D- fructose and L-alanine. J. Agric. Food Chem. 2006, 54, 574–577. [Google Scholar] [CrossRef] [PubMed]
- Hazzit, M.; Baaliouamer, A.; Faleiro, M.L.; Miguel, M.G. Composition of the essential oils of Thymus and Origanum species from Algeria and their antioxidant and antimicrobial activities. J. Agric. Food Chem. 2006, 54, 6314–6321. [Google Scholar] [CrossRef] [PubMed]
- Mallard, W.G.; Andriamaharavo, N.R.; Mirokhin, Y.A.; Halket, J.M.; Stein, S.E. Creation of libraries of recurring mass spectra from large data sets assisted by a dual-column workflow. Anal. Chem. 2014, 86, 10231–10238. [Google Scholar] [CrossRef]
- Steinhaus, P.; Schieberle, P. Characterization of the key aroma compounds in soy sauce using approaches of molecular sensory science. J. Agric. Food Chem. 2007, 55, 6262–6269. [Google Scholar] [CrossRef]
- Buchin, S.; Salmon, J.C.; Carnat, A.P.; Berger, T.; Bugaud, C.; Bosset, J.O. Identification de composés monoterpéniques, sesquiterpéniques et benzéniques dans un lait d’alpage tres riche en ces substances. Mitt. Geb. Leb. 2002, 93, 199–216. [Google Scholar] [CrossRef]
- Alves, R.J.V.; Pinto, C.A.; Da Costa, A.V.M.; Rezende, C.M. Zizyphus mauritiana Lam. (Rhamnaceae) and the chemical composition of its floral fecal odor. J. Braz. Chem. Soc. 2005, 16, 654–656. [Google Scholar] [CrossRef]
- Zhao, C.; Li, X.; Liang, Y.; Fang, H.; Huang, L.F.; Guo, F. Comparative analysis of chemical components of essential oils from different samples of Rhododendron with the help of chemometrics methods. Chemom. Intell. Lab. Syst. 2006, 82, 218–228. [Google Scholar] [CrossRef]
- Skaltsa, H.D.; Mavrommati, A.; Constantinidis, T. A chemotaxonomic investigation of volatile constituents in Stachys subsect. Swainsonianeae (Labiatae). Phytochemistry 2001, 57, 235–244. [Google Scholar] [CrossRef]
- Oruna-Concha, M.J.; Craig Duckham, S.; Ames, J.M. Comparison of volatile compounds isolated from the skin and flesh of four potato cultivars after baking. J. Agric. Food Chem. 2001, 49, 2414–2421. [Google Scholar] [CrossRef]
- Pino, J.A.; Marbot, R.; Vazquez, C. Volatile Components of tamarind (Tamarindus indica L.) grown in cuba australian species of palmeria (monimiaceae). J. Essent. Oil Res. 2004, 16, 318–320. [Google Scholar] [CrossRef]
- Shalit, M.; Katzir, N.; Tadmor, Y.; Larkov, O.; Burger, Y.; Shalekhet, F.; Lastochkin, E.; Ravid, U.; Amar, O.; Edelstein, M.; et al. Acetyl-CoA: Alcohol acetyltransferase activity and aroma formation in ripening melon fruits. J. Agric. Food Chem. 2001, 49, 794–799. [Google Scholar] [CrossRef] [PubMed]
- Solina, M.; Baumgartner, P.; Johnson, R.L.; Whitfield, F.B. Volatile aroma components of soy protein isolate and acid-hydrolysed vegetable protein. Food Chem. 2005, 90, 861–873. [Google Scholar] [CrossRef]
- Boulanger, R.; Crouzet, J. Identification of the aroma components of acerola (Malphigia glabra L.): Free and bound flavour compounds. Food Chem. 2001, 74, 209–216. [Google Scholar] [CrossRef]
- Mondello, L.; Sciarrone, D.; Casilli, A.; Tranchida, P.Q.; Dugo, P.; Dugo, G. Fast gas chromatography-full scan quadrupole mass spectrometry for the determination of allergens in fragrances. J. Sep. Sci. 2007, 30, 1905–1911. [Google Scholar] [CrossRef]
- Pino, J.; Marbot, R.; Rosado, A. Volatile constituents of star apple (Chrysophyllum cainito L.) from Cuba. Flavour Fragr. J. 2002, 17, 401–403. [Google Scholar] [CrossRef]
- Masoudi, S.; Esamaeili, A.; Khalilzadeh, M.A.; Rustaiyan, A.; Moazami, N.; Akhgar, M.R.; Varavipoor, M. Volatile constituents of Dorema aucheri Boiss., Seseli libanotis (L.) W. D. Koch var. armeniacum Bordz. and Conium maculatum L. three Umbelliferae herbs growing wild in Iran. Flavour Fragr. J. 2006, 21, 801–804. [Google Scholar] [CrossRef]
- Saroglou, V.; Marin, P.D.; Rancic, A.; Veljic, M.; Skaltsa, H. Composition and antimicrobial activity of the essential oil of six Hypericum species from Serbia. Biochem. Syst. Ecol. 2007, 35, 146–152. [Google Scholar] [CrossRef]
- Paulo, P.C.; Bittrich, V.; Shepherd, G.J.; Lopes, A.V.; Marsaioli, A.J. The ecological and taxonomic importance of flower volatiles of Clusia species (Guttiferae). Phytochemistry 2001, 56, 443–452. [Google Scholar] [CrossRef] [PubMed]
- Salido, S.; Valenzuela, L.R.; Altarejos, J.; Nogueras, M.; Sánchez, A.; Cano, E. Composition and infraspecific variability of Artemisia herba-alba from southern Spain. Biochem. Syst. Ecol. 2004, 32, 265–277. [Google Scholar] [CrossRef]
- Isidorov, V.A.; Vinogorova, V.T.; Rafałowski, K. HS-SPME analysis of volatile organic compounds of coniferous needle litter. Atmos. Environ. 2003, 37, 4645–4650. [Google Scholar] [CrossRef]
- Liu, J.; Nan, P.; Tsering, Q.; Tsering, T.; Bai, Z.; Wang, L.; Liu, Z.; Zhong, Y. Volatile constituents of the leaves and flowers of Salvia przewalskii Maxim. from Tibet. Flavour Fragr. J. 2006, 21, 435–438. [Google Scholar] [CrossRef]
- Majcher, M.A.; Jeleń, H.H. Effect of cysteine and cystine addition on sensory profile and potent odorants of extruded potato snacks. J. Agric. Food Chem. 2007, 55, 5754–5760. [Google Scholar] [CrossRef]
- Tuberoso, C.I.G.; Kowalczyk, A.; Coroneo, V.; Russo, M.T.; Dessì, S.; Cabras, P. Chemical composition and antioxidant, antimicrobial, and antifungal activities of the essential oil of Achillea ligustica All. J. Agric. Food Chem. 2005, 53, 10148–10153. [Google Scholar] [CrossRef]
- Saroglou, V.; Dorizas, N.; Kypriotakis, Z.; Skaltsa, H.D. Analysis of the essential oil composition of eight Anthemis species from Greece. J. Chromatogr. A 2006, 1104, 313–322. [Google Scholar] [CrossRef]
- Tsiri, D.; Kretsi, O.; Chinou, I.B.; Spyropoulos, C.G. Composition of fruit volatiles and annual changes in the volatiles of leaves of Eucalyptus camaldulensis Dehn. growing in Greece. Flavour Fragr. J. 2003, 18, 244–247. [Google Scholar] [CrossRef]
- Shang, C.; Hu, Y.; Deng, C.; Hu, K. Rapid determination of volatile constituents of Michelia alba flowers by gas chromatography-mass spectrometry with solid-phase microextraction. J. Chromatogr. A 2002, 942, 283–288. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.X.; Liang, Y.Z.; Fang, H.Z.; Li, X.N. Temperature-programmed retention indices for gas chromatography-mass spectroscopy analysis of plant essential oils. J. Chromatogr. A 2005, 1096, 76–85. [Google Scholar] [CrossRef] [PubMed]
- Mevy, J.P.; Bousquet-Mélou, A.; Greff, S.; Millongo, J.; Fernandez, C. Chemical composition of the volatile oil of Laggera aurita Schulz from Burkina-Faso. Biochem. Syst. Ecol. 2006, 34, 815–818. [Google Scholar] [CrossRef]
- Molo, L.; Rillo, L.; Ledda, A.; Addeo, F. Odorous constituents of ovine milk in relationship to diet. J. Dairy Sci. 1996, 79, 1322–1331. [Google Scholar] [CrossRef]
- Harangi, J. Retention index calculation without n-alkanes—The virtual carbon number. J. Chromatogr. A 2003, 993, 187–195. [Google Scholar]
- Bruni, R.; Bianchi, A.; Bellardi, M.G. Essential oil composition of Agastache anethiodora Britton (Lamiaceae) infected by cucumber mosaic virus (CMV). Flavour Fragr. J. 2007, 22, 66–70. [Google Scholar] [CrossRef]
- Gómez, E.; Ledbetter, C.A.; Hartsell, P.L. Volatile Compounds in Apricot, Plum, and Their Interspecific Hybrids. J. Agric. Food Chem. 1993, 41, 1669–1676. [Google Scholar] [CrossRef]
- Vahirua-Lechat, I.; Menut, C.; Roig, B.; Bessiere, J.M.; Lamaty, G. Isoprene related esters, significant components of Pandanus tectorius. Phytochemistry 1996, 43, 1277–1279. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).