Oenological Performances of New White Grape Varieties
Abstract
1. Introduction
2. Materials and Methods
2.1. Must Preparation
2.2. Fermentation
2.3. Chemical Analysis of Wine
2.3.1. Metabolites of Central Carbon Metabolism
2.3.2. Analysis of Fermentative Aromas
2.3.3. Analysis of Phenolic Compounds
2.3.4. Varietal Thiol Analysis
2.4. Statistical Analysis
3. Results and Discussion
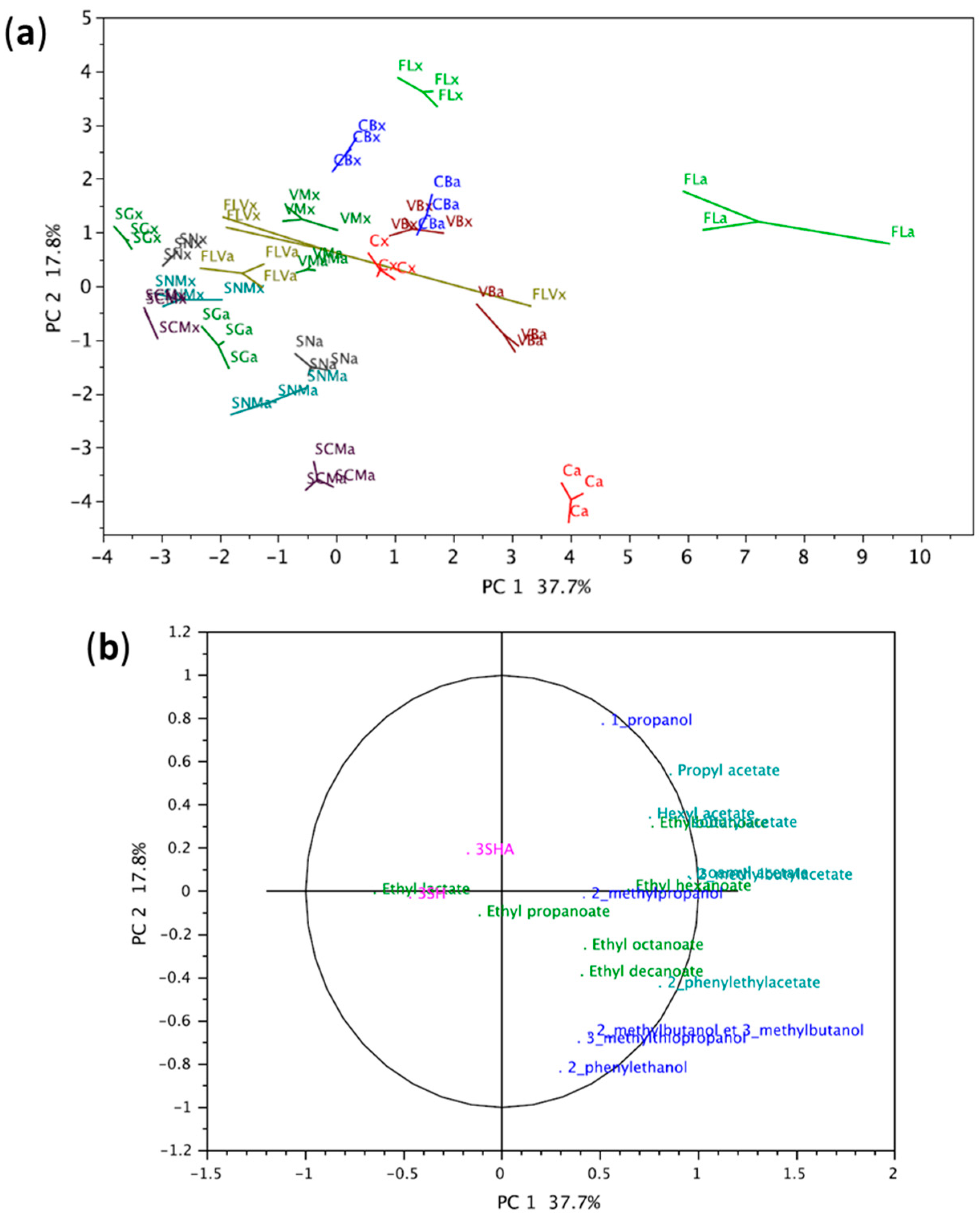
3.1. Overview of the Oenological Profiles of Wines
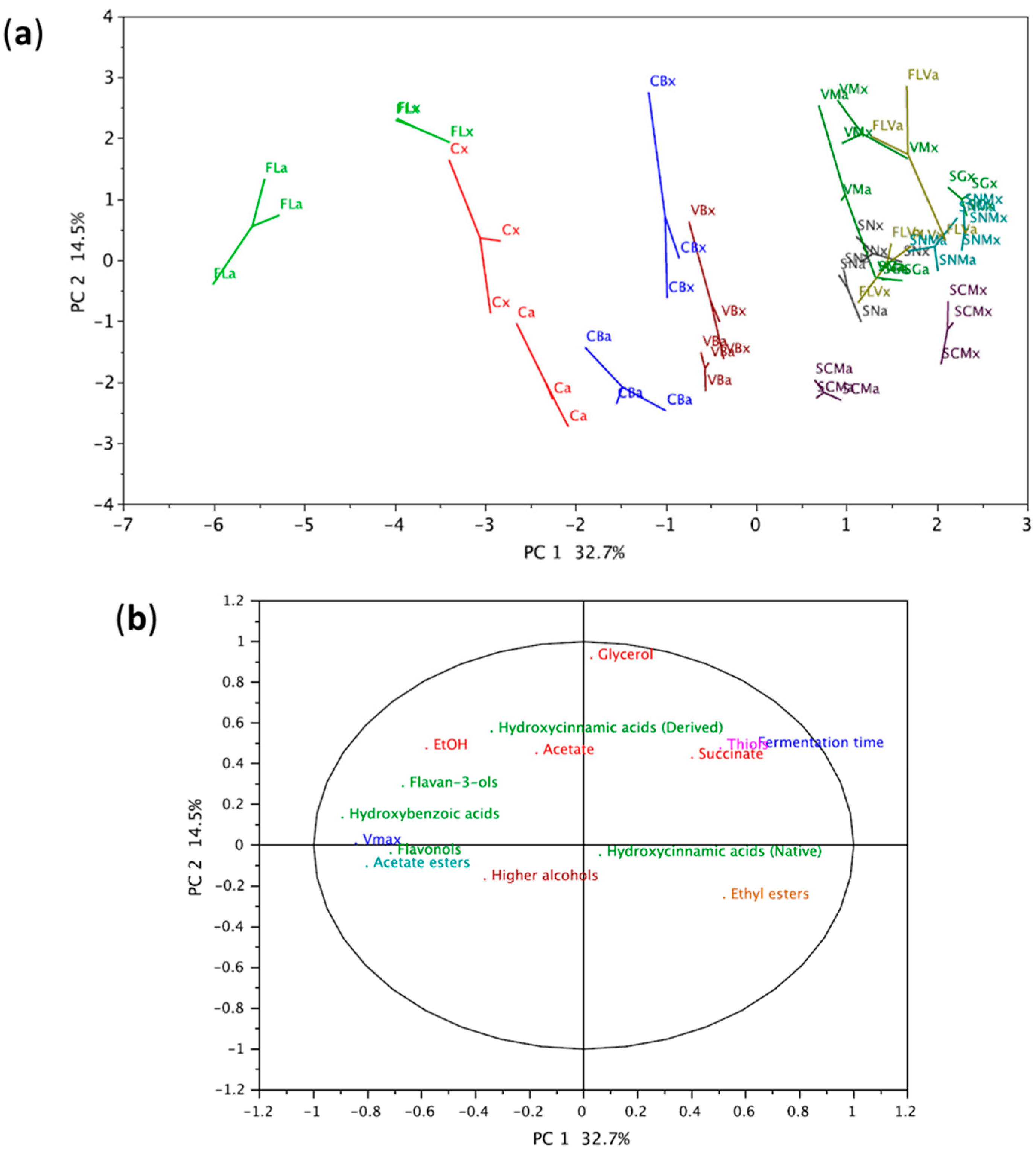
3.2. Fermentative Aromas and Varietal Thiols
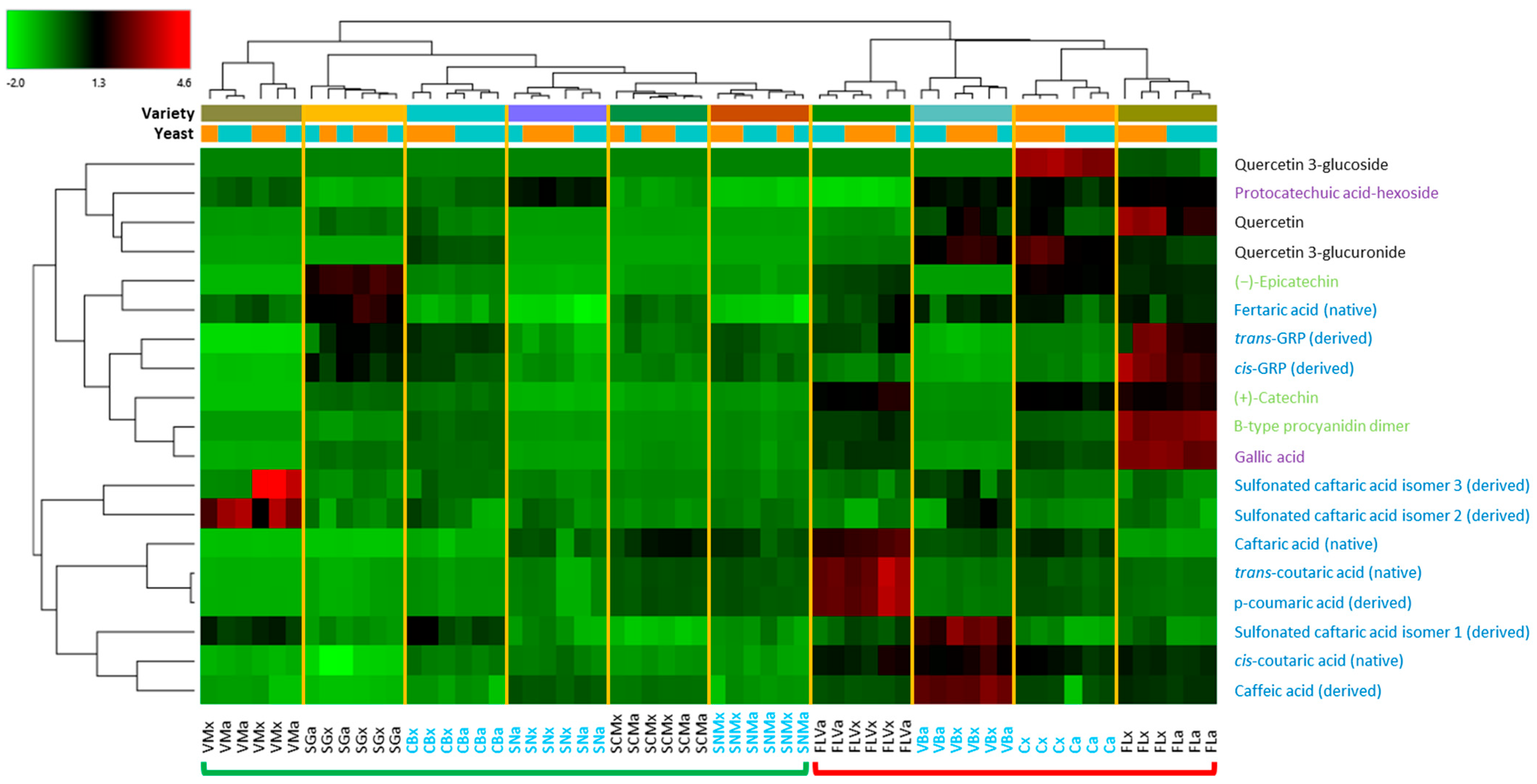
3.3. Phenolic Compounds
3.3.1. Identification
3.3.2. Comparison of Wines
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| (U)HPLC | (Ultra-)high performance liquid chromatography |
| HRMS | High resolution mass spectrometry |
| ESI | Electrospray ionization |
| RT | Retention time |
| m/z | Mass-to-charge ratio |
| PCA | Principal component analysis |
| 3SH | 3-sulfanylhexan-1-ol |
| 3SHA | 3-sulfanylhexyl acetate |
| PPO | Polyphenol oxidase |
| GSH | γ-L-glutamyl-L-cysteinyl-L-glycine |
| GRP | Grape reaction product |
| Yeast « x » | Zymaflore X5 yeast from LAFFORT® |
| Yeast « a » | Anchor Alchemy II yeast from Oenobrands SAS |
| Vmax | Maximal CO2 release rate |
References
- Schneider, C.; Spring, J.-L.; Onimus, C.; Prado, E.; Verdenal, T.; Lemarquis, G.; Lorenzini, F.; Ley, L.; Duruz, P.; Gindro, K.; et al. Programme de Collaboration Franco-Suisse Pour La Création de Nouvelles Variétés de Vigne Durablement Résistantes Au Mildiou et à l’oïdium. BIO Web Conf. 2019, 15, 01018. [Google Scholar] [CrossRef]
- Töpfer, R.; Trapp, O. A Cool Climate Perspective on Grapevine Breeding: Climate Change and Sustainability Are Driving Forces for Changing Varieties in a Traditional Market. Theor. Appl. Genet. 2022, 135, 3947–3960. [Google Scholar] [CrossRef] [PubMed]
- Duley, G.; Ceci, A.T.; Longo, E.; Boselli, E. Oenological Potential of Wines Produced from Disease-resistant Grape Cultivars. Comp. Rev. Food Sci. Food Safe 2023, 22, 2591–2610. [Google Scholar] [CrossRef] [PubMed]
- Mira De Orduña, R. Climate Change Associated Effects on Grape and Wine Quality and Production. Food Res. Int. 2010, 43, 1844–1855. [Google Scholar] [CrossRef]
- Teissedre, P.-L. Composition of Grape and Wine from Resistant Vines Varieties. OENO One 2018, 52, 211–217. [Google Scholar] [CrossRef]
- Drawert, F. Winemaking as a biotechnological sequence. In Chemistry of Winemaking; Webb, A.D., Ed.; Advances in Chemistry; American Chemical Society: Washington, DC, USA, 1974; pp. 1–10. [Google Scholar]
- Roland, A.; Schneider, R.; Razungles, A.; Cavelier, F. Varietal Thiols in Wine: Discovery, Analysis and Applications. Chem. Rev. 2011, 111, 7355–7376. [Google Scholar] [CrossRef]
- Tominaga, T.; Furrer, A.; Henry, R.; Dubourdieu, D. Identification of New Volatile Thiols in the Aroma of Vitis vinifera L. Var. Sauvignon Blanc Wines. Flavour Fragr. J. 1998, 13, 159–162. [Google Scholar] [CrossRef]
- Tominaga, T.; Darriet, P.; Dubordieu, D. Identification de l’acétate de 3-Mercaptohexanol, Composé à Forte Odeur de Buis, Intervenant Dans l’arôme Des Vins de Sauvignon. VITIS-J. Grapevine Res. 2015, 35, 207. [Google Scholar] [CrossRef]
- Darriet, P.; Tominaga, T.; Lavigne, V.; Boidron, J.; Dubourdieu, D. Identification of a Powerful Aromatic Component of Vitis Vinifera L. Var. Sauvignon Wines: 4-mercapto-4-methylpentan-2-one. Flavour Fragr. J. 1995, 10, 385–392. [Google Scholar] [CrossRef]
- Carlin, S.; Piergiovanni, M.; Pittari, E.; Tiziana Lisanti, M.; Moio, L.; Piombino, P.; Marangon, M.; Curioni, A.; Rolle, L.; Río Segade, S.; et al. The Contribution of Varietal Thiols in the Diverse Aroma of Italian Monovarietal White Wines. Food Res. Int. 2022, 157, 111404. [Google Scholar] [CrossRef]
- Peña-Gallego, A.; Hernández-Orte, P.; Cacho, J.; Ferreira, V. S-Cysteinylated and S-Glutathionylated Thiol Precursors in Grapes. A Review. Food Chem. 2012, 131, 1–13. [Google Scholar] [CrossRef]
- Swiegers, J.H.; Pretorius, I.S. Modulation of Volatile Sulfur Compounds by Wine Yeast. Appl. Microbiol. Biotechnol. 2007, 74, 954–960. [Google Scholar] [CrossRef] [PubMed]
- Rollero, S.; Bloem, A.; Camarasa, C.; Sanchez, I.; Ortiz-Julien, A.; Sablayrolles, J.-M.; Dequin, S.; Mouret, J.-R. Combined Effects of Nutrients and Temperature on the Production of Fermentative Aromas by Saccharomyces Cerevisiae during Wine Fermentation. Appl. Microbiol. Biotechnol. 2015, 99, 2291–2304. [Google Scholar] [CrossRef]
- He, Y.; Wang, X.; Li, P.; Lv, Y.; Nan, H.; Wen, L.; Wang, Z. Research Progress of Wine Aroma Components: A Critical Review. Food Chem. 2023, 402, 134491. [Google Scholar] [CrossRef]
- Swiegers, J.H.; Bartowsky, E.J.; Henschke, P.A.; Pretorius, I.S. Yeast and Bacterial Modulation of Wine Aroma and Flavour. Aust. J. Grape Wine Res. 2005, 11, 139–173. [Google Scholar] [CrossRef]
- Ćorković, I.; Pichler, A.; Šimunović, J.; Kopjar, M. A Comprehensive Review on Polyphenols of White Wine: Impact on Wine Quality and Potential Health Benefits. Molecules 2024, 29, 5074. [Google Scholar] [CrossRef]
- Downey, M.O.; Dokoozlian, N.K.; Krstic, M.P. Cultural Practice and Environmental Impacts on the Flavonoid Composition of Grapes and Wine: A Review of Recent Research. Am. J. Enol. Vitic. 2006, 57, 257–268. [Google Scholar] [CrossRef]
- Garrido, J.; Borges, F. Wine and Grape Polyphenols—A Chemical Perspective. Food Res. Int. 2013, 54, 1844–1858. [Google Scholar] [CrossRef]
- Merkytė, V.; Longo, E.; Windisch, G.; Boselli, E. Phenolic Compounds as Markers of Wine Quality and Authenticity. Foods 2020, 9, 1785. [Google Scholar] [CrossRef]
- Gawel, R.; Smith, P.A.; Cicerale, S.; Keast, R. The Mouthfeel of White Wine. Crit. Rev. Food Sci. Nutr. 2018, 58, 2939–2956. [Google Scholar] [CrossRef]
- Li, H.; Guo, A.; Wang, H. Mechanisms of Oxidative Browning of Wine. Food Chem. 2008, 108, 1–13. [Google Scholar] [CrossRef]
- Hayasaka, Y.; Black, C.A.; Hack, J.; Smith, P. Structural Characterization of Reaction Products of Caftaric Acid and Bisulfite Present in a Commercial Wine Using High Resolution Mass Spectrometric and Nuclear Magnetic Resonance Techniques. Food Chem. 2017, 230, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Cheynier, V.F.; Trousdale, E.K.; Singleton, V.L.; Salgues, M.J.; Wylde, R. Characterization of 2-S-Glutathionyl Caftaric Acid and Its Hydrolysis in Relation to Grape Wines. J. Agric. Food Chem. 1986, 34, 217–221. [Google Scholar] [CrossRef]
- Singleton, V.L.; Salgues, M.; Zaya, J.; Trousdale, E. Caftaric Acid Disappearance and Conversion to Products of Enzymic Oxidation in Grape Must and Wine. Am. J. Enol. Vitic. 1985, 36, 50–56. [Google Scholar] [CrossRef]
- Dournes, G.; Casalta, E.; Samson, A.; Aguera, E.; Mouret, J.-R.; Roland, A. Characterization of the Thiol Aromatic Potential of a New Resistant Grape Variety: Floreal. IVES. Available online: https://ives-openscience.eu/6950/ (accessed on 4 March 2025).
- Leis, D.; Renner, W.; Leitner, E. Characterisation of Wines Produced from Fungus Resistant Grape Varieties. In Flavour Science: Proceedings of the XV Weurman Flavour Research Symposium, Graz, Austria, 18–22 September 2017; Graz University of Technology: Graz, Austria, 2018. [Google Scholar]
- Forino, M.; Cassiano, C.; Gambuti, A.; Picariello, L.; Aversano, R.; Villano, C.; Basile, B.; Moio, L.; Frusciante, L. Aging Behavior of Two Red Wines from the PIWI Pathogen-Resistant Grapevines “Cabernet Eidos” and “Merlot Khorus”. ACS Food Sci. Technol. 2022, 2, 638–646. [Google Scholar] [CrossRef]
- Nicolini, G.; Roman, T.; Flamini, R.; Tonidandel, L.; Gardiman, M.; Larcher, R. Thiol Precursors in Vitis Mould-tolerant Hybrid Varieties. J. Sci. Food Agric. 2020, 100, 3262–3268. [Google Scholar] [CrossRef]
- Versari, A.; Laurie, V.F.; Ricci, A.; Laghi, L.; Parpinello, G.P. Progress in Authentication, Typification and Traceability of Grapes and Wines by Chemometric Approaches. Food Res. Int. 2014, 60, 2–18. [Google Scholar] [CrossRef]
- Claudel, P.; Dumas, V.; Thibon, C.; Lemarquis, G.; Jaegli, N.; Sivsivadzé, A.; Baltenweck, R.; Hugueney, P.; Duchêne, É. A Test-Tube Vinification Method for High-Throughput Characterisation of the Oenological and Aromatic Potential of White Wines. OENO One 2024, 58, 1–14. [Google Scholar] [CrossRef]
- Gratl, V.; Sturm, S.; Zini, E.; Letschka, T.; Stefanini, M.; Vezzulli, S.; Stuppner, H. Comprehensive Polyphenolic Profiling in Promising Resistant Grapevine Hybrids Including 17 Novel Breeds in Northern Italy. J. Sci. Food Agric. 2021, 101, 2380–2388. [Google Scholar] [CrossRef]
- Guittin, C.; Maçna, F.; Barreau, A.; Poitou, X.; Sablayrolles, J.-M.; Mouret, J.-R.; Farines, V. The Aromatic Profile of Wine Distillates from Ugni Blanc Grape Musts Is Influenced by the Nitrogen Nutrition (Organic vs. Inorganic) of Saccharomyces Cerevisiae. Food Microbiol. 2023, 111, 104193. [Google Scholar] [CrossRef]
- Dias, A.L.D.S.; Fenger, J.-A.; Meudec, E.; Verbaere, A.; Costet, P.; Hue, C.; Coste, F.; Lair, S.; Cheynier, V.; Boulet, J.-C.; et al. Shades of Fine Dark Chocolate Colors: Polyphenol Metabolomics and Molecular Networking to Enlighten the Brown from the Black. Metabolites 2023, 13, 667. [Google Scholar] [CrossRef] [PubMed]
- Dournes, G.; Dufourcq, T.; Suc, L.; Roland, A.; Mouret, J.-R. Unravelling Copper Effect on the Production of Varietal Thiols during Colombard and Gros Manseng Grape Juices Fermentation by Saccharomyces Cerevisiae. Front. Microbiol. 2023, 14, 1101110. [Google Scholar] [CrossRef] [PubMed]
- Flores, D.; Meudec, E.; Dias, A.L.D.S.; Sommerer, N. A Versatile Ultra-High-Performance Liquid Chromatography-Full-Scan High-Resolution Mass Spectrometry Method to Quantify Wine Polyphenols. MPs 2024, 7, 82. [Google Scholar] [CrossRef]
- Perestrelo, R.; Lu, Y.; Santos, S.A.O.; Silvestre, A.J.D.; Neto, C.P.; Câmara, J.S.; Rocha, S.M. Phenolic Profile of Sercial and Tinta Negra Vitis vinifera L. Grape Skins by HPLC–DAD–ESI-MSn. Food Chem. 2012, 135, 94–104. [Google Scholar] [CrossRef]
- Xu, Y.; Liang, P.-L.; Chen, X.-L.; Gong, M.-J.; Zhang, L.; Qiu, X.-H.; Zhang, J.; Huang, Z.-H.; Xu, W. The Impact of Citrus-Tea Cofermentation Process on Chemical Composition and Contents of Pu-Erh Tea: An Integrated Metabolomics Study. Front. Nutr. 2021, 8, 737539. [Google Scholar] [CrossRef]
- Bravo, M.N.; Silva, S.; Coelho, A.V.; Boas, L.V.; Bronze, M.R. Analysis of Phenolic Compounds in Muscatel Wines Produced in Portugal. Anal. Chim. Acta 2006, 563, 84–92. [Google Scholar] [CrossRef]
- Tominaga, T.; Peyrot Des Gachons, C.; Dubourdieu, D. A New Type of Flavor Precursors in Vitis v Inifera L. Cv. Sauvignon Blanc: S -Cysteine Conjugates. J. Agric. Food Chem. 1998, 46, 5215–5219. [Google Scholar] [CrossRef]
- Serni, E.; Tomada, S.; Haas, F.; Robatscher, P. Characterization of Phenolic Profile in Dried Grape Skin of Vitis vinifera L. Cv. Pinot Blanc with UHPLC-MS/MS and Its Development during Ripening. J. Food Compos. Anal. 2022, 114, 104731. [Google Scholar] [CrossRef]
- Cejudo-Bastante, M.J.; Pérez-Coello, M.S.; Hermosín-Gutiérrez, I. Identification of New Derivatives of 2- S -Glutathionylcaftaric Acid in Aged White Wines by HPLC-DAD-ESI-MSn. J. Agric. Food Chem. 2010, 58, 11483–11492. [Google Scholar] [CrossRef]
- Šikuten, I.; Štambuk, P.; Andabaka, Ž.; Tomaz, I.; Marković, Z.; Stupić, D.; Maletić, E.; Kontić, J.K.; Preiner, D. Grapevine as a Rich Source of Polyphenolic Compounds. Molecules 2020, 25, 5604. [Google Scholar] [CrossRef]
- Ginjom, I.; D’Arcy, B.; Caffin, N.; Gidley, M. Phenolic Compound Profiles in Selected Queensland Red Wines at All Stages of the Wine-Making Process. Food Chem. 2011, 125, 823–834. [Google Scholar] [CrossRef]
- Singleton, V.L.; Zaya, J.; Trousdale, E.K. Caftaric and Coutaric Acids in Fruit of Vitis. Phytochemistry 1986, 25, 2127–2133. [Google Scholar] [CrossRef]
- Singleton, V.L.; Timberlake, C.F.; Whiting, G.C. Chromatography of Natural Phenolic Cinnamate Derivatives on Sephadex LH-20 and G-25. J. Chromatogr. A 1977, 140, 120–124. [Google Scholar] [CrossRef]
- Cheynier, V.; Owe, C.; Rigaud, J. Oxidation of Grape Juice Phenolic Compounds in Model Solutions. J. Food Sci. 1988, 53, 1729–1732. [Google Scholar] [CrossRef]
- Tarara, J.M.; Lee, J.; Spayd, S.E.; Scagel, C.F. Berry Temperature and Solar Radiation Alter Acylation, Proportion, and Concentration of Anthocyanin in Merlot Grapes. Am. J. Enol. Vitic. 2008, 59, 235–247. [Google Scholar] [CrossRef]
- Price, S.F.; Breen, P.J.; Valladao, M.; Watson, B.T. Cluster Sun Exposure and Quercetin in Pinot Noir Grapes and Wine. Am. J. Enol. Vitic. 1995, 46, 187–194. [Google Scholar] [CrossRef]
- Pérez-Magariño, S.; González-San José, M.L. Evolution of Flavanols, Anthocyanins, and Their Derivatives during the Aging of Red Wines Elaborated from Grapes Harvested at Different Stages of Ripening. J. Agric. Food Chem. 2004, 52, 1181–1189. [Google Scholar] [CrossRef]
- Nikolantonaki, M.; Jourdes, M.; Shinoda, K.; Teissedre, P.-L.; Quideau, S.; Darriet, P. Identification of Adducts between an Odoriferous Volatile Thiol and Oxidized Grape Phenolic Compounds: Kinetic Study of Adduct Formation under Chemical and Enzymatic Oxidation Conditions. J. Agric. Food Chem. 2012, 60, 2647–2656. [Google Scholar] [CrossRef]
- Magdas, D.A.; Pirnau, A.; Feher, I.; Guyon, F.; Cozar, B.I. Alternative Approach of Applying 1H NMR in Conjunction with Chemometrics for Wine Classification. LWT 2019, 109, 422–428. [Google Scholar] [CrossRef]
- Jordão, A.M.; Ricardo-da-Silva, J.M.; Laureano, O. Evolution of Catechins and Oligomeric Procyanidins during Grape Maturation of Castelão Francês and Touriga Francesa. Am. J. Enol. Vitic. 2001, 52, 230–234. [Google Scholar] [CrossRef]
| Sample Code | Grape Variety | Origin (City—Postal Code | Resistance to Downy Mildew and Powdery Mildew a | Variety Number VIVC b | Pedigree b |
|---|---|---|---|---|---|
| SN | Sauvignon Blanc | Nantes—44000 | NO | 10790 | Traminer X unknown |
| SNM | Sauvignon Blanc | Vix—85770 | NO | 10790 | Traminer X unknown |
| SCM | Sauvignac | Vix—85770 | YES | 22322 | (Sauvignon X Riesling) X unknown |
| SG | Souvignier Gris | Vix—85770 | YES | 22629 | Cabernet Sauvignon X Bronner |
| FL | Floreal | Vix—85770 | YES | 25805 | Villaris X a descendant of Muscadinia rotundifolia (MTP 3159-2-12) |
| FLV | Floreal | Nantes—44000 | YES | 25805 | Villaris X a descendant of Muscadinia rotundifolia (MTP 3159-2-12) |
| VM | Voltis | Rodilhan—30230 | YES | 25807 | Villaris X a descendant of Muscadinia rotundifolia (MTP 3159-2-12) |
| C | Chardonnay | Gruissan—11170 | NO | 2455 | Heunisch Weiss X Pinot noir |
| CB | Chardonnay | Bourdic—30190 | NO | 2455 | Heunisch Weiss X Pinot noir |
| VB | Viognier | Bourdic—30190 | NO | 13106 | Unknown |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Sousa Dias, A.L.; Guittin-Leignadier, C.; Roy, A.; Sachot, S.; Maçna, F.; Flores, D.; Meudec, E.; Boulet, J.-C.; Sommerer, N.; Roland, A.; et al. Oenological Performances of New White Grape Varieties. Beverages 2025, 11, 90. https://doi.org/10.3390/beverages11030090
de Sousa Dias AL, Guittin-Leignadier C, Roy A, Sachot S, Maçna F, Flores D, Meudec E, Boulet J-C, Sommerer N, Roland A, et al. Oenological Performances of New White Grape Varieties. Beverages. 2025; 11(3):90. https://doi.org/10.3390/beverages11030090
Chicago/Turabian Stylede Sousa Dias, Aécio Luís, Charlie Guittin-Leignadier, Amélie Roy, Somaya Sachot, Faïza Maçna, Damien Flores, Emmanuelle Meudec, Jean-Claude Boulet, Nicolas Sommerer, Aurélie Roland, and et al. 2025. "Oenological Performances of New White Grape Varieties" Beverages 11, no. 3: 90. https://doi.org/10.3390/beverages11030090
APA Stylede Sousa Dias, A. L., Guittin-Leignadier, C., Roy, A., Sachot, S., Maçna, F., Flores, D., Meudec, E., Boulet, J.-C., Sommerer, N., Roland, A., Ducasse, M.-A., & Mouret, J.-R. (2025). Oenological Performances of New White Grape Varieties. Beverages, 11(3), 90. https://doi.org/10.3390/beverages11030090







