Accurate Determination of 24 Water-Soluble Synthetic Colorants in Premade Cocktail Using Ultra-Performance Liquid Chromatography with Diode Array Detection
Abstract
1. Introduction
2. Experimental Section
2.1. Chemicals and Reagents
2.2. Standard Solution Preparation
2.3. Instrumentation and Conditions
2.4. Sample Preparation
2.5. Method Validation
3. Results and Discussion
3.1. HPLC-DAD Method Development
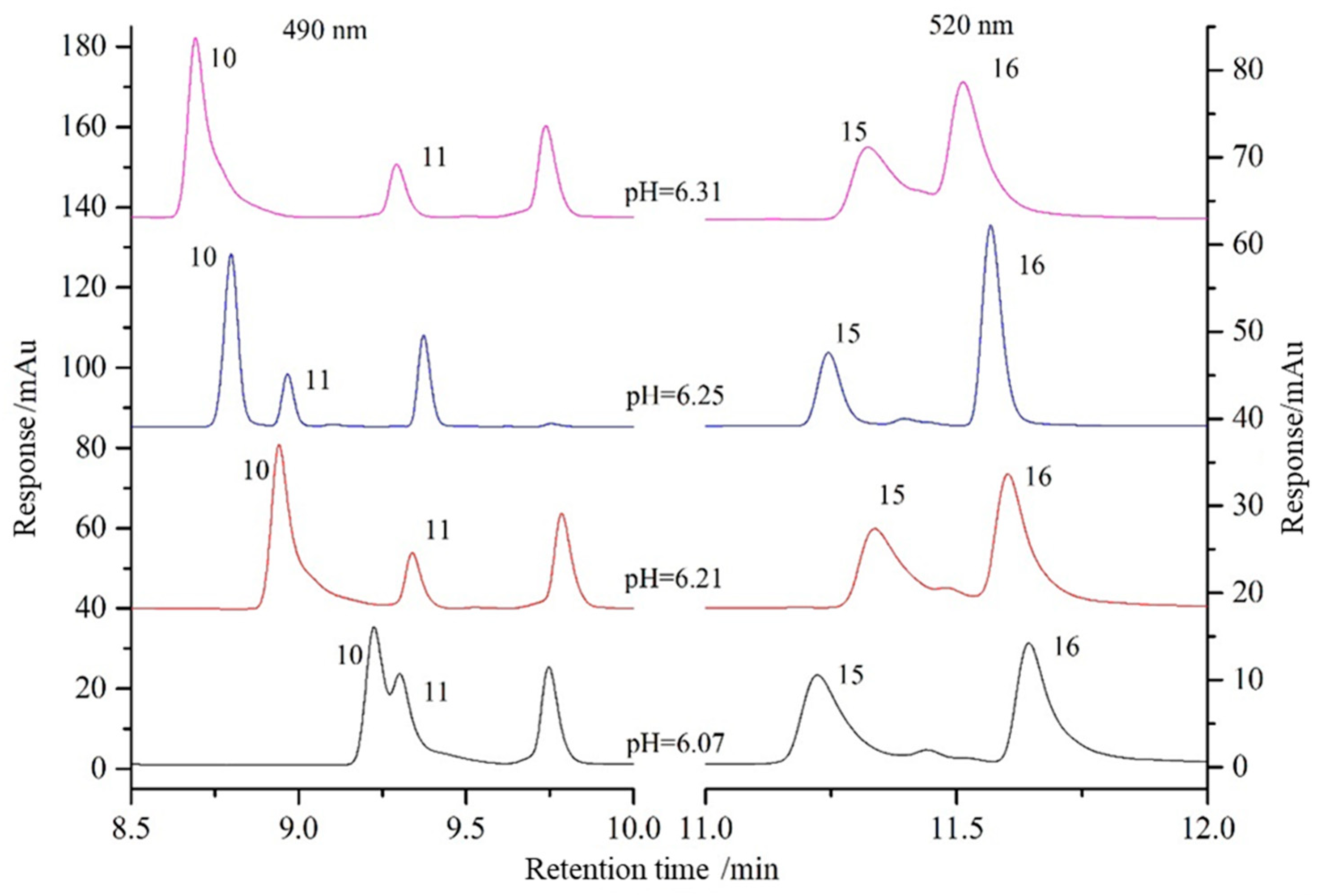
3.1.1. Mobile Phase
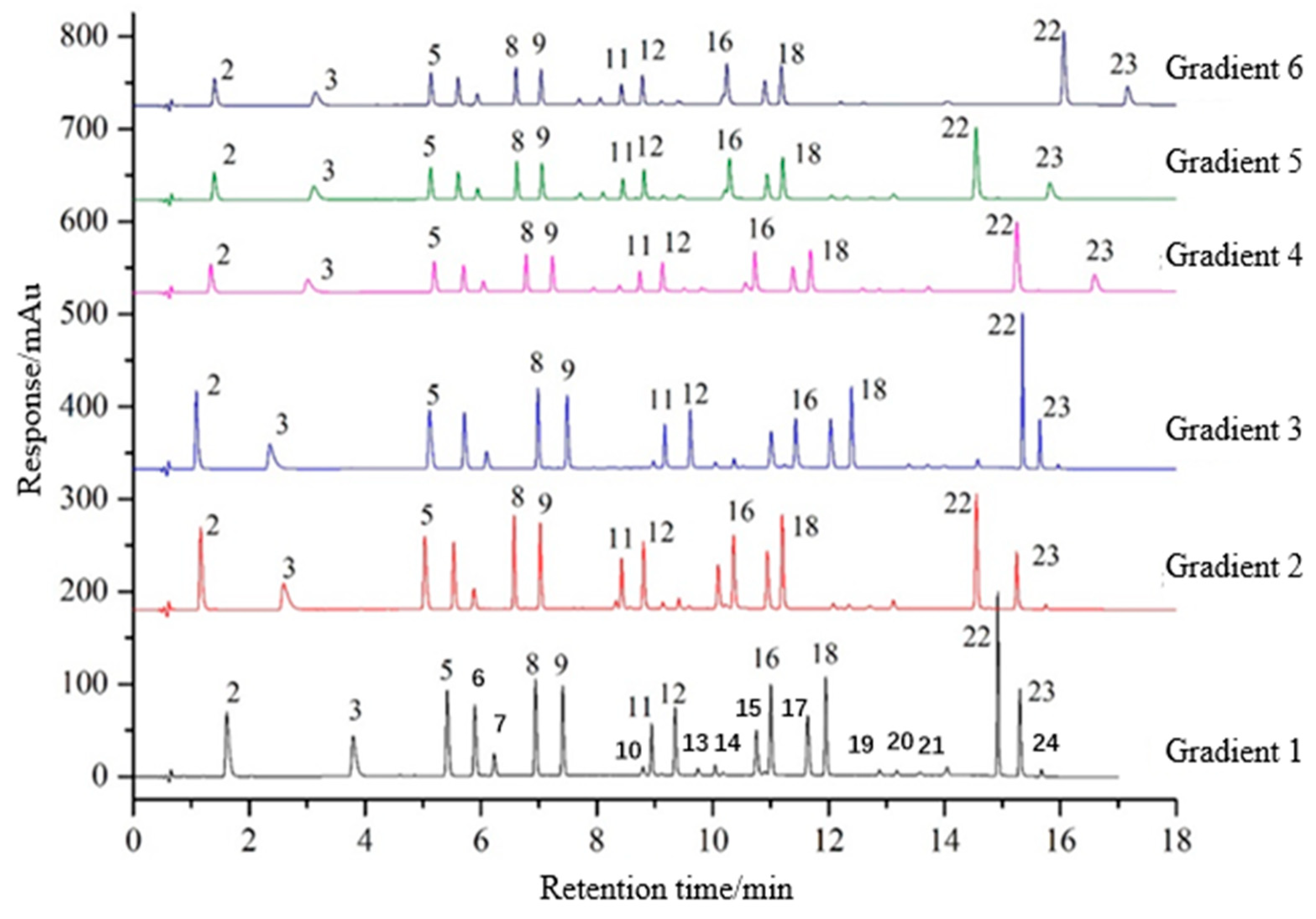
3.1.2. Gradient Program
3.2. Method Validation Result
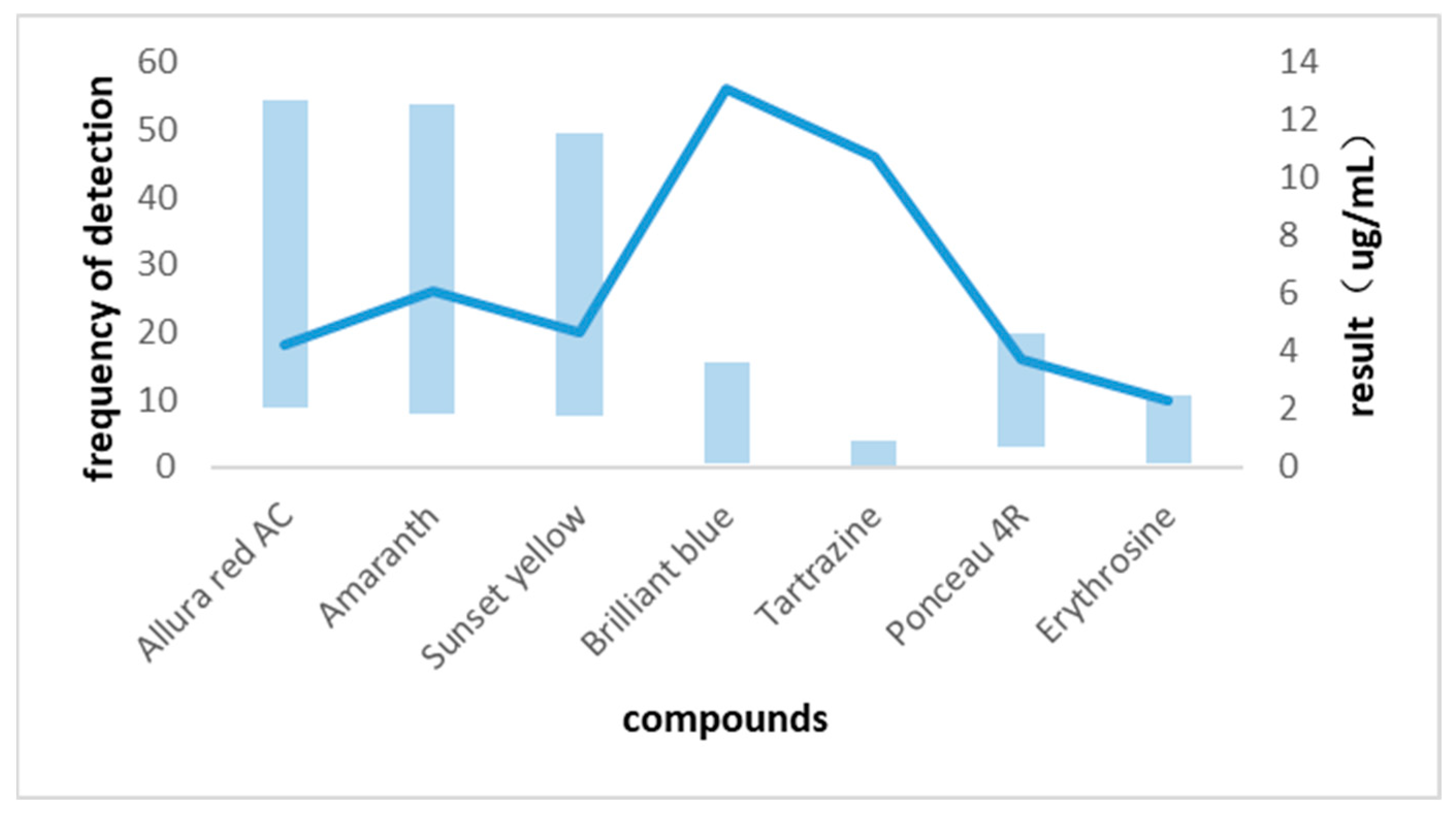
3.3. Comparison with Published Results
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Carocho, M.; Barreiro, M.F.; Morales, P.; Ferreira, I.C.F.R. Adding Molecules to Food, Pros and Cons: A Review on Synthetic and Natural Food Additives. Compr. Rev. Food Sci. Food Saf. 2014, 13, 377–399. [Google Scholar] [CrossRef]
- Berlina, A.N.; Zherdev, A.V.; Dzantiev, B.B. ELISA and Lateral Flow Immunoassay for the Detection of Food Colorants: State of the Art. Crit. Rev. Anal. Chem. 2019, 49, 209–223. [Google Scholar] [CrossRef] [PubMed]
- Gosetti, F.; Chiuminatto, U.; Mazzucco, E.; Calabrese, G.; Gennaroa, M.C.; Marengo, E. Identification of photodegradation products of Allura Red AC (E129) in a beverage by ultra high performance liquid chromatography–quadrupole-time-of-flight mass spectrometry. Anal. Chim. Acta 2012, 746, 84–89. [Google Scholar] [CrossRef]
- Gosetti, F.; Frascarolo, P.; Mazzucco, E.; Gianotti, V.; Bottaro, M.; Gennaro, M.C. Photo degradation of E110 and E122 dyes in a commercial aperitif A high performance liquid chromatography–diode array–tandem mass spectrometry study. J. Chromatogr. A 2008, 1202, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Perez-Ibarbia, L.; Majdanski, T.; Schubert, S.; Windhab, N.; Schubert, U.S. Safety and Regulatory Review of Dyes Commonly Used as Excipients in Pharmaceutical and Nutraceutical Applications. Eur. J. Pharm. Sci. 2016, 93, 264–273. [Google Scholar] [CrossRef]
- Oplatowska-Stachowiak, M.; Elliott, C.T. Food Colors: Existing and Emerging Food Safety Concerns. Crit. Rev. Food Sci. Nutr. 2017, 57, 524–548. [Google Scholar] [CrossRef]
- Lehto, S.; Buchweitz, M.; Klimm, A.; Straßburger, R.; Bechtold, C.; Ulberth, F. Comparison of Food Colour Regulations in the EU and the US: A Review of Current Provisions. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2017, 34, 335–355. [Google Scholar] [CrossRef] [PubMed]
- GB 2760-2024; Hygienic Standards for Uses of Food Additives. National Standards Administration: Beijing, China, 2024.
- FDA. Toxicological Principles for the Safety Assessment of Direct Food Additives and Color Additives Used in Food; National Technical Information Service: Springfield, VA, USA, 2019.
- Hwang, S.L.; Kim, M.; Lee, G.; Shin, J.-W. Dietary Exposure Assessment of Synthetic Food Colours Using Analytical Concentrations in Korea. Food Addit. Contam. Part A 2019, 36, 1453–1466. [Google Scholar]
- Pressman, P.; Clemens, R.; Hayes, W.C. Food Additive Safety: A Review of Toxicologic and Regulatory Issues. Toxicol. Res. Appl. 2017, 1, 2397847317723572. [Google Scholar] [CrossRef]
- GB 7718-2011; National Food Safety Standard General Rules for Prepackaged Food Labeling. National Standards Administration: Beijing, China, 2011.
- Arroz, E.; Jordan, M.; Dumancas, G.G. Development of a Direct Spectrophotometric and Chemometric Method for Determining Food Dye Concentrations. Appl. Spectrosc. 2017, 71, 1633–1639. [Google Scholar] [CrossRef]
- Ou, Y.; Wang, X.; Lai, K.; Huang, Y.; Rasco, B.A.; Fan, Y. Gold Nanorods as Surface-Enhanced Raman Spectroscopy (SERS) Substrates for Rapid and Sensitive Analysis of Allura Red and Sunset Yellow in Beverages. J. Agric. Food Chem. 2018, 66, 2954–2961. [Google Scholar] [CrossRef] [PubMed]
- Mejia, E.; Ding, Y.; Mora, M.F.; Garcia, C.D. Determination of Banned Sudan Dyes in Chili Powder by Capillary Electrophoresis. Food Chem. 2007, 102, 1027–1033. [Google Scholar] [CrossRef]
- Lipskikh, O.I.; Nikolaeva, A.A.; Korotkova, E.I. Voltammetric Determination of Tartrazine in Food. J. Anal. Chem. 2017, 72, 396–401. [Google Scholar] [CrossRef]
- Jampasa, S.; Siangproh, W.; Duangmal, K.; Chailapakul, O. Electrochemically Reduced Graphene Oxide-Modified Screen-Printed Carbon Electrodes for a Simple and Highly Sensitive Electrochemical Detection of Synthetic Colorants in Beverages. Talanta 2016, 160, 113–124. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Na, L.; Jiang, Y.; Han, D.; Lou, D.; Jin, L. A Fluorescence-Quenching Method for Quantitative Analysis of Ponceau 4R in Beverage. Food Chem. 2017, 221, 803–808. [Google Scholar] [CrossRef]
- Jiao, J.; Wang, J.; Li, M.; Li, J.; Li, Q.; Quan, Q.; Chen, J. Simultaneous Determination of Three Azo Dyes in Food Product by Ion Mobility Spectrometry. J. Chromatogr. B 2016, 1025, 105–109. [Google Scholar] [CrossRef]
- Kucharska, M.; Grabka, J. A Review of Chromatographic Methods for Determination of Synthetic Food Dyes. Talanta 2010, 80, 1045–1051. [Google Scholar] [CrossRef]
- De Andrade Silva, B.W.; Lima, B.P.; Paim, A.P. Simultaneous Determination of Synthetic Colorants in Yogurt by HPLC. Food Chem. 2015, 183, 154–160. [Google Scholar]
- Zhu, S.; Zhou, J.; Jia, H.; Zhang, H. Liquid-Liquid Microextraction of Synthetic Pigments in Beverages Using a Hydrophobic Deep Eutectic Solvent. Food Chem. 2018, 243, 351–356. [Google Scholar] [CrossRef]
- Tang, B.; Xi, C.; Yun, Z.; Wang, G.; Li, X.; Zhang, L.; Chen, D.; Zhang, J. Simultaneous Determination of 16 Synthetic Colorants in Hotpot Condiment by High Performance Liquid Chromatography. J. Chromatogr. B 2014, 960, 87–91. [Google Scholar] [CrossRef]
- Rejczak, T.; Tuzimski, T. Application of High-Performance Liquid Chromatography with Diode Array Detector for Simultaneous Determination of 11 Synthetic Dyes in Selected Beverages and Foodstuffs. Food Anal. Methods 2017, 10, 3572–3588. [Google Scholar] [CrossRef]
- Floriano, L.; Ribeiro, L.C.; Saibt, N.; Bandeira, N.; Prestes, O.; Zanella, R. Determination of Six Synthetic Dyes in Sports Drinks by Dispersive Solid-Phase Extraction and HPLC-UV-Vis. J. Braz. Chem. Soc. 2018, 29, 602–608. [Google Scholar] [CrossRef]
- Karanikolopoulos, G.; Gerakis, A.; Papadopoulou, K.; Mastrantoni, I. Determination of Synthetic Food Colorants in Fish Products by an HPLC-DAD Method. Food Chem. 2015, 177, 197–203. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, H.; Wang, Y.; Wu, X.; Zhao, Y. Simultaneous Determination of Seven Synthetic Colorants in Wine by Dispersive Micro-Solid-Phase Extraction Coupled with Reversed-Phase High-Performance Liquid Chromatography. J. Chromatogr. Sci. 2015, 53, 210–218. [Google Scholar] [CrossRef]
- Mazdeh, F.Z.; Khorrami, A.R.; Moradi-Khatoonabadi, Z.; EsmaeiliAftabdari, F.; Ardekani, M.R.S.; Moghaddam, G.; Hajimahmoodi, M. Determination of Eight Synthetic Food Dyes by Solid Phase Extraction and Reversed-Phase High Performance Liquid Chromatography. Trop. J. Pharm. Res. 2016, 15, 173–181. [Google Scholar] [CrossRef]
- Sun, H.W.; Sun, N.; Li, H.; Zhang, J.X.; Yang, Y.L. Development of Multiresidue Analysis for 21 Synthetic Colorants in Meat by Microwave-Assisted Extraction-Solid Phase Extraction-Reversed-Phase Ultrahigh Performance Liquid Chromatography. Food Anal. Methods 2013, 6, 1291–1299. [Google Scholar] [CrossRef]
- Amorello, D.; Barreca, S.; Pensato, F.; Orecchio, S. Potentiometric analysis of fluoride in commonly consumed beverages: Method development, evaluation, and risk assessment. J. Food Compos. Anal. 2025, 137, 106836. [Google Scholar] [CrossRef]
- Tsai, C.F.; Kuo, C.H.; Shih, Y.C. Determination of 20 Synthetic Dyes in Chili Powders and Syrup-Preserved Fruits by Liquid Chromatography/Tandem Mass Spectrometry. J. Food Drug Anal. 2015, 23, 453–462. [Google Scholar] [CrossRef] [PubMed]
- Qi, P.; Zhou, Q.; Chen, G.; Lin, Z.; Zhao, J.; Xu, H.; Gao, H.; Liu, D.; Mao, X. Simultaneous Qualitative and Quantitative Determination of 104 Fat-Soluble Synthetic Dyes in Foods Using Disperse Solid-Phase Extraction and UHPLC-Q-Orbitrap HRMS Analysis. Food Chem. 2023, 427, 136665. [Google Scholar] [CrossRef]
- Ma, K.; Li, X.J.; Wang, H.F.; Zhao, M. Rapid and Sensitive Method for the Determination of Eight Food Additives in Red Wine by Ultra-Performance Liquid Chromatography Tandem Mass Spectrometry. Food Anal. Methods 2015, 8, 203–212. [Google Scholar] [CrossRef]
- Chen, X.H.; Zhao, Y.G.; Shen, H.Y.; Zhou, L.-X.; Pan, S.-D.; Jin, M.-C. Fast Determination of Seven Synthetic Pigments from Wine and Soft Drinks Using Magnetic Dispersive Solid-Phase Extraction Followed by Liquid Chromatography-Tandem Mass Spectrometry. J. Chromatogr. A 2014, 1346, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Martin, F.; Oberson, J.M.; Meschiari, M.; Munari, C. Determination of 18 Water-Soluble Artificial Dyes by LC-MS in Selected Matrices. Food Chem. 2016, 197, 1249–1255. [Google Scholar] [CrossRef] [PubMed]
- Al Tamim, A.; AlRabeh, M.; Al Tamimi, A.; AlAjlan, A.; Alowaifeer, A. Fast and Simple Method for the Detection and Quantification of 15 Synthetic Dyes in Sauce, Cotton Candy, and Pickle by LC-MS/MS. Arab. J. Chem. 2019, 13, 3882–3888. [Google Scholar]
- Sun, X.D.; Wu, H.L.; Liu, Z.; Chen, Y.; Liu, Q.; Ding, Y.J.; Yu, R.Q. Rapid and Sensitive Detection of Multi-Class Food Additives in Beverages for Quality Control by Using HPLC-DAD and Chemometrics Methods. Food Anal. Methods 2019, 12, 381–393. [Google Scholar] [CrossRef]
- Gosetti, F.; Chiuminatto, U.; Mastroianni, R.; Mazzucco, E.; Manfredi, M. Retrospective analysis for the identification of 4-aminocarminic acid photo-degradation products in beverages. Food Add. Contam. A 2015, 32, 285–292. [Google Scholar] [CrossRef] [PubMed]
- GB 5009.35-2023; National Food Safety Standard—Determination of Synthetic Colorants in Food. National Standards Administration: Beijing, China, 2023.
- Hu, Z.; Qi, P.; Wang, N.; Zhou, Q.-Q.; Lin, Z.-H.; Chen, Y.-Z.; Mao, X.-W.; Jiang, J.-J.; Li, C. Simultaneous Determination of Multiclass Illegal Dyes with Different Acidic-Basic Properties in Foodstuffs by LC-MS/MS via Polarity Switching Mode. Food Chem. 2020, 309, 125745. [Google Scholar] [CrossRef]
- Arabkhani, P.; Sadegh, N.; Asfaram, A. Nanostructured Magnetic Graphene Oxide/UIO-66 Sorbent for Ultrasound-Assisted Dispersive Solid-Phase Microextraction of Food Colorants Prior to HPLC Analysis. Microchem. J. 2023, 184, 108149. [Google Scholar] [CrossRef]
- Li, F.; Wang, Y.; Wang, H.; Tang, A. Study on the Adsorption of Crystal Violet from Water Using Citric Acid-Modified Sorghum Straw. J. Wuhan Inst. Technol. 2010, 32, 47–51. [Google Scholar]
- Park, J.; Lee, M.H.; Cho, Y.S.; Seo, D.W.; Choi, J.Y. Rapid Identification and Quantitation of 34 Synthetic Colorants in Beverages and Candies by UPLC-PDA. J. Food Compos. Anal. 2024, 133, 106479. [Google Scholar] [CrossRef]



| Name | Molecular Formula | No. CI | CAS Number | MW | λ (max) a | Detection Wavelength | Purity (g/g) b | Provider/CRM Number c | Solubility d |
|---|---|---|---|---|---|---|---|---|---|
| Tartrazine | C16H9N4Na3O9S2 | 19140 | 1934-21-0 | 534.36 | 427 nm | 420 nm | / | NIM, GBW(E)100001a 0.5 mg/mL ± 1% | 260 g/L |
| Amaranth | C20H11N2Na3O10S3 | 16185 | 915-67-3 | 604.47 | 521 nm | 520 nm | / | NIM, GBW(E)100002a 0.5 mg/mL ± 2% | 50 g/L |
| Ponceau 4R | C20H11N2Na3O10S3 | 16255 | 2611-82-7 | 604.47 | 508 nm | 520 nm | / | NIM, GBW(E)100004a 0.5 mg/mL ± 2% | 25 g/L |
| Sunset yellow | C16H10N2Na2O7S2 | 15985 | 2783-94-0 | 452.37 | 483 nm | 490 nm | / | NIM, GBW(E)100003a 0.5 mg/mL ± 1% | 50–100 g/L |
| Allura red AC | C18H14N2Na2O8S2 | 16035 | 25956-17-6 | 496.42 | 509 nm | 520 nm | / | NIM, GBW(E)100192 0.5 mg/mL ± 2% | 53.878 g/L |
| Brilliant blue | C37H34N2Na2O9S3 | 42090 | 3844-45-9 | 792.85 | 629 nm | 620 nm | / | NIM, GBW(E)100005a 0.5 mg/mL ± 2% | 25 g/L |
| Crystal violet | C25H30ClN3 | 42555 | 548-62-9 | 407.99 | 590 nm | 520 nm | 98.1% ± 1% | NIM, GBW06420 98.1% ± 1% | 16 g/L |
| Erythrosine | C20H6I4Na2O5 | 45430 | 568-63-8 | 879.86 | 526 nm | 520 nm | / | NIM, GBW(E)100191 0.2 mg/mL ± 2% | 100 g/L |
| Rhodamine B | C28H31ClN2O3 | 45170 | 81-88-9 | 479.01 | 550 nm | 520 nm | 99.0% ± 0.4% | NIM, GBW06146 99.0% ± 0.4% | 10 g/L |
| Orange G | C16H10N2Na2O7S2 | 16230 | 1936-15-8 | 452.37 | 480 nm | 490 nm | 98.5% ± 0.5% | Sigma–Aldrich | 50 g/L |
| Ponceau S | C22H12N4Na4O13S4 | 27195 | 6226-79-5 | 760.57 | 520 nm | 520 nm | 98.5% ± 0.6% | Sigma–Aldrich | 10 g/L |
| Red 2G | C18H13N3Na2O8S2 | 18050 | 3734-67-6 | 509.42 | 532 nm | 520 nm | 98.3% ± 0.8% | Sigma–Aldrich | |
| Azorubine | C20H12N2Na2O7S2 | 14720 | 3567-69-9 | 502.43 | 518 nm | 520 nm | 98.8% ± 0.8% | Sigma–Aldrich | |
| Ponceau SX | C18H14N2Na2O7S2 | 14700 | 4548-53-2 | 480.42 | 504 nm | 520 nm | 96.6% ± 1.6% | Sigma–Aldrich | |
| Orange II | C16H11N2NaO4S | 15510 | 633-96-5 | 350.32 | 485 nm | 490 nm | 98.6% ± 0.7% | Toronto Research Chemicals | 116 g/L |
| Orange IV | C18H14N3NaO3S | 13080 | 554-73-4 | 375.38 | 428 nm | 420 nm | 98.5% ± 0.6% | Toronto Research Chemicals | |
| Metanil yellow orange MNO | C18H14N3NaO3S | 13065 | 587-98-4 | 375.38 | 420 nm | 420 nm | 98.4% ± 1.6% | Toronto Research Chemicals | 25 g/L |
| Chrysoidine Y | C12H13ClN4 | 11270 | 532-82-1 | 248.71 | 408 nm | 420 nm | 99.0% ± 0.8% | Sigma–Aldrich | |
| Fast green FCF | C37H34N2O10S3Na2 | 42053 | 2353-45-9 | 808.86 | 623 nm | 620 nm | 96.2% ± 2.0% | Toronto Research Chemicals | |
| Brilliant green | C27H34N2O4S | 42040 | 633-03-4 | 482.63 | 626 nm | 620 nm | 97.2% ± 1.4% | Toronto Research Chemicals | 100 g/L |
| Acid red 52 | C27H29N2NaO7S2 | 45100 | 3520-42-1 | 580.65 | 562 nm | 520 nm | 96.0% ± 2.0% | Sigma–Aldrich | |
| Naphthol yellow | C10H4N2Na2O8S | 10316 | 846-70-8 | 358.19 | 431 nm | 420 nm | 99.3% ± 0.7% | Dr. Ehrenstorfer | |
| Neutral Red | C15H17ClN4 | 50040 | 553-24-2 | 288.78 | 461 nm | 490 nm | 95.8% ± 2.4% | Dr. Ehrenstorfer | 50 g/L |
| Uranine | C20H10Na2O5 | 45350 | 518-47-8 | 376.27 | 488 nm | 490 nm | 99.6% ± 0.4% | Dr. Ehrenstorfer | 500 g/L |
| Flavor in Label of Premade Cocktails | Color of Premade Cocktails | Commonly Detected Colorants |
|---|---|---|
| Strawberry | Red | Amaranth, Ponceau 4R, Allura red AC |
| Peach | Pink | Amaranth, Ponceau 4R, |
| Orange | Orange yellow | Tartrazine, Sunset yellow, Allura red AC |
| Pineapple | Yellow | Tartrazine, Sunset yellow |
| Mango | Golden yellow | Amaranth, Tartrazine |
| Grape | Purple | Amaranth, Brilliant blue |
| Breezer | Green | Tartrazine, Brilliant blue |
| Apple | Cyan | Tartrazine, Sunset yellow Brilliant blue |
| Blueberry | Blue | Brilliant blue |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, K.; Zhang, Y.; Wu, T. Accurate Determination of 24 Water-Soluble Synthetic Colorants in Premade Cocktail Using Ultra-Performance Liquid Chromatography with Diode Array Detection. Beverages 2025, 11, 91. https://doi.org/10.3390/beverages11030091
Ma K, Zhang Y, Wu T. Accurate Determination of 24 Water-Soluble Synthetic Colorants in Premade Cocktail Using Ultra-Performance Liquid Chromatography with Diode Array Detection. Beverages. 2025; 11(3):91. https://doi.org/10.3390/beverages11030091
Chicago/Turabian StyleMa, Kang, Yiwen Zhang, and Taipeng Wu. 2025. "Accurate Determination of 24 Water-Soluble Synthetic Colorants in Premade Cocktail Using Ultra-Performance Liquid Chromatography with Diode Array Detection" Beverages 11, no. 3: 91. https://doi.org/10.3390/beverages11030091
APA StyleMa, K., Zhang, Y., & Wu, T. (2025). Accurate Determination of 24 Water-Soluble Synthetic Colorants in Premade Cocktail Using Ultra-Performance Liquid Chromatography with Diode Array Detection. Beverages, 11(3), 91. https://doi.org/10.3390/beverages11030091






