Impact of Various Prefermentation Treatments on the Pigment, Polyphenol, and Volatile Composition of Industrial Red Wines Made from Vitis vinifera cv Maratheftiko
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Grape Handling, Treatments, and Vinification
- Treatment C (control)—Fermentation of must was performed in contact with grape pomace for 11 days. Fermentation temperature was maintained at 13–17 °C.
- Treatment CE—Cryoextraction was applied prior to fermentation at 5 °C for 48 h, followed by saigneé. The latter technique consisted of removing from the mash a volume of must corresponding to 10% of the total volume. After racking off this volume of must, vinification was carried out as for treatment C.
- Treatment CEE—Cryoextraction was applied prior to fermentation at 5 °C for 48 h, followed by saigneé and pectolytic enzyme addition. The enzyme added was a pectolytic preparation (Laffasse HE Grand Cru, LAFFORT OENOLOGIE, Bordeaux, France) and added at a level of 2 g per 100 kg of grapes, according to the manufacturer’s specifications. After enzyme addition, vinification was carried out as for treatment C.
- Treatment CEET—Cryoextraction was applied prior to fermentation at 5 °C for 48 h, followed by saigneé, pectolytic enzyme addition, and enological tannin addition. The enological tannins used were SUBLITAN VINIF (Martin Vialatte, Thévenet, France), and added at a level of 20 g hL−1. After additions, vinification was carried out as for treatment C.

2.3. Sample Preparation and Gas Chromatography-Mass Spectrometry (GC-MS) Analysis
2.4. Liquid Chromatography Determinations
2.5. Statistical Processing
3. Results and Discussion
3.1. Impact of Treatments on Non-Pigment Polyphenols
3.2. Impact of Treatments on Anthocyanins
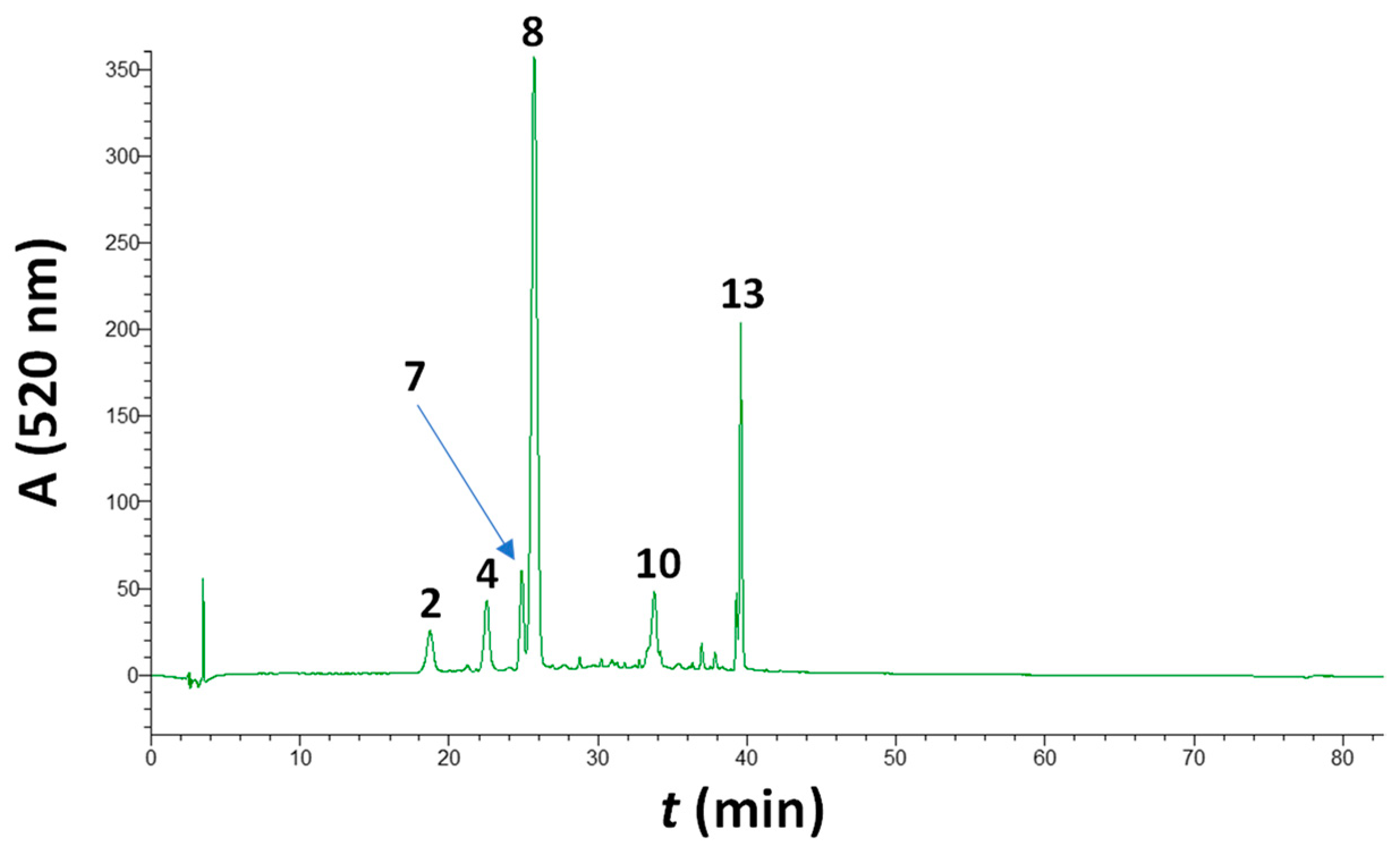
3.3. Impact of Treatments on the Volatile Profile
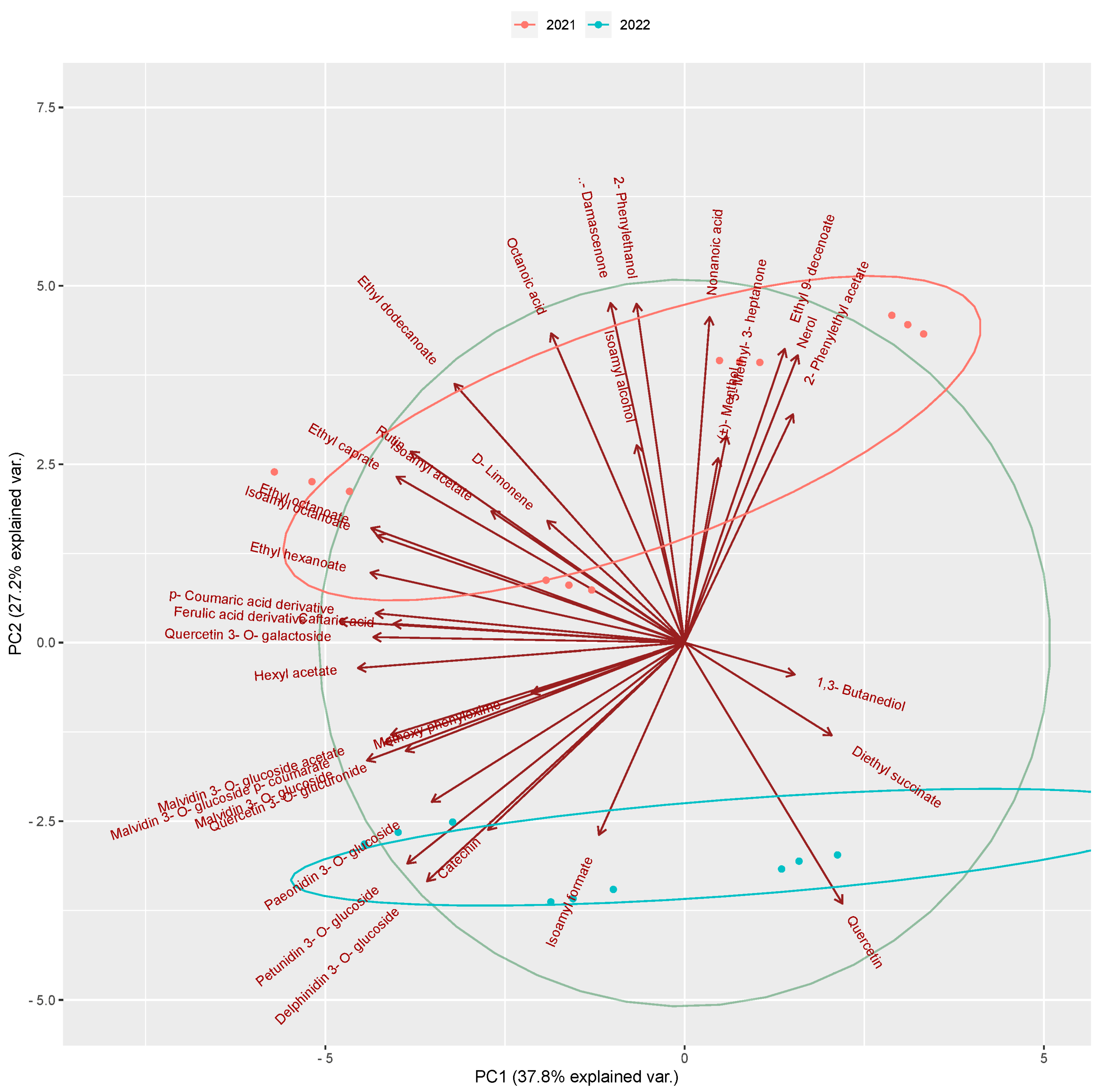
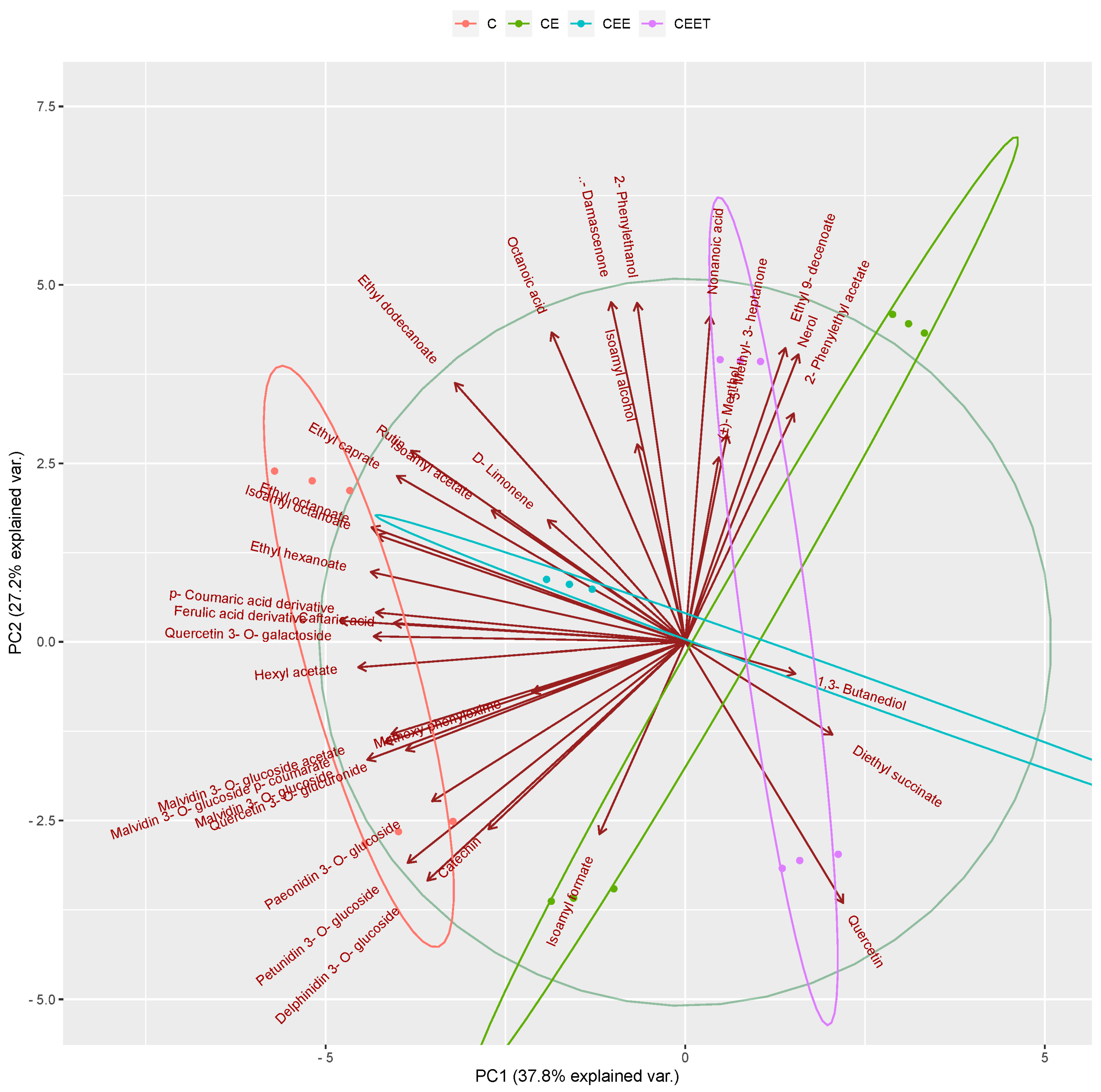
3.4. Discrimination through Principal Component Analysis (PCA) and Multivariate Correlation Analysis (MCA)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Merkytė, V.; Longo, E.; Windisch, G.; Boselli, E. Phenolic compounds as markers of wine quality and authenticity. Foods 2020, 9, 1785. [Google Scholar] [CrossRef] [PubMed]
- Pittari, E.; Moio, L.; Piombino, P. Interactions between polyphenols and volatile compounds in wine: A literature review on physicochemical and sensory insights. Appl. Sci. 2021, 11, 1157. [Google Scholar] [CrossRef]
- Basalekou, M.; Tataridis, P.; Georgakis, K.; Tsintonis, C. Measuring wine quality and typicity. Beverages 2023, 9, 41. [Google Scholar] [CrossRef]
- Cataldo, E.; Fucile, M.; Mattii, G.B. A review: Soil management, sustainable strategies and approaches to improve the quality of modern viticulture. Agronomy 2021, 11, 2359. [Google Scholar] [CrossRef]
- Gutiérrez-Escobar, R.; Aliaño-González, M.J.; Cantos-Villar, E. Wine polyphenol content and its influence on wine quality and properties: A review. Molecules 2021, 26, 718. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.K.; Jeffery, D.W.; Li, D.M.; Lan, Y.B.; Zhao, X.; Duan, C.Q. Red wine coloration: A review of pigmented molecules, reactions, and applications. Comp. Rev. Food Sci. Food Saf. 2022, 21, 3834–3866. [Google Scholar] [CrossRef] [PubMed]
- Robinson, A.L.; Boss, P.K.; Solomon, P.S.; Trengove, R.D.; Heymann, H.; Ebeler, S.E. Origins of grape and wine aroma. Part 1. Chemical components and viticultural impacts. Am. J. Enol. Vitic. 2014, 65, 1–24. [Google Scholar] [CrossRef]
- Robinson, A.L.; Boss, P.K.; Solomon, P.S.; Trengove, R.D.; Heymann, H.; Ebeler, S.E. Origins of grape and wine aroma. Part 2. Chemical and sensory analysis. Am. J. Enol. Vitic. 2014, 65, 25–42. [Google Scholar] [CrossRef]
- Soares, S.; Brandão, E.; Mateus, N.; de Freitas, V. Sensorial properties of red wine polyphenols: Astringency and bitterness. Crit. Rev. Food Sci. Nutr. 2017, 57, 937–948. [Google Scholar] [CrossRef]
- Aleixandre-Tudo, J.L.; du Toit, W. Cold maceration application in red wine production and its effects on phenolic compounds: A review. LWT-Food Sci. Technol. 2018, 95, 200–208. [Google Scholar] [CrossRef]
- Casassa, L.; Vega-Osorno, A.; Hernandez, J. Chemical and chromatic effects of saignée combined with extended maceration and microwaved stem addition on three Pinot Noir clones from the Central Coast of California. Aust. J. Grape Wine Res. 2021, 27, 540–552. [Google Scholar] [CrossRef]
- Espejo, F. Role of commercial enzymes in wine production: A critical review of recent research. J. Food Sci. Technol. 2021, 58, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Sacchi, K.L.; Bisson, L.F.; Adams, D.O. A review of the effect of winemaking techniques on phenolic extraction in red wines. Am. J. Enol. Vitic. 2005, 56, 197–206. [Google Scholar] [CrossRef]
- Lukić, I.; Budić-Leto, I.; Bubola, M.; Damijanić, K.; Staver, M. Pre-fermentative cold maceration, saignée, and various thermal treatments as options for modulating volatile aroma and phenol profiles of red wine. Food Chem. 2017, 224, 251–261. [Google Scholar] [CrossRef] [PubMed]
- Orbanić, F.; Rossi, S.; Bestulić, E.; Budić-Leto, I.; Kovačević Ganić, K.; Horvat, I.; Plavša, T.; Bubola, M.; Lukić, I.; Jeromel, A. Applying different vinification techniques in Teran red wine production: Impact on bioactive compounds and sensory attributes. Foods 2023, 12, 3838. [Google Scholar] [CrossRef] [PubMed]
- Roufas, K.; Chatzimitakos, T.; Athanasiadis, V.; Lalas, S.I.; Makris, D.P. Changes in Polyphenols and Anthocyanin Pigments during Ripening of Vitis vinifera cv Maratheftiko: A Two-Year Study. Beverages 2023, 9, 39. [Google Scholar] [CrossRef]
- Galanakis, C.M.; Kotanidis, A.; Dianellou, M.; Gekas, V. Phenolic content and antioxidant capacity of Cypriot wines. Czech J. Food Sci. 2015, 33, 126–136. [Google Scholar] [CrossRef]
- Copper, A.; Johnson, T.; Danner, L.; Bastian, S.; Collins, C. Preliminary sensory and chemical profiling of Cypriot wines made from indigenous grape varieties Xynisteri, Maratheftiko and Giannoudhi and acceptability to Australian consumers. OENO One 2019, 53, 229–248. [Google Scholar] [CrossRef]
- Tsiakkas, O.; Escott, C.; Loira, I.; Morata, A.; Rauhut, D.; Suárez-Lepe, J.A. Determination of anthocyanin and volatile profile of wines from varieties Yiannoudi and Maratheftiko from the island of Cyprus. Beverages 2020, 6, 4. [Google Scholar] [CrossRef]
- Copper, A.W.; Collins, C.; Bastian, S.E.; Johnson, T.E.; Capone, D.L. Preliminary investigation of potent thiols in Cypriot wines made from indigenous grape varieties Xynisteri, Maratheftiko and Giannoudhi. OENO One 2021, 55, 223–234. [Google Scholar] [CrossRef]
- International Code of Œnological Practices; OIV-35; International Organisation of Vine and Wine: Paris, France, 2022; ISBN 978-2-85038-059-4.
- Toulaki, A.K.; Athanasiadis, V.; Chatzimitakos, T.; Kalompatsios, D.; Bozinou, E.; Roufas, K.; Mantanis, G.I.; Dourtoglou, V.G.; Lalas, S.I. Investigation of Xinomavro red wine aging with various wood chips using pulsed electric field. Beverages 2024, 10, 13. [Google Scholar] [CrossRef]
- Makris, D.P.; Psarra, E.; Kallithraka, S.; Kefalas, P. The effect of polyphenolic composition as related to antioxidant capacity in white wines. Food Res. Inter. 2003, 36, 805–814. [Google Scholar] [CrossRef]
- Makris, D.; Kefalas, P. Characterization of polyphenolic phytochemicals in red grape pomace. Int. J. Waste Resour. 2013, 3, 126. [Google Scholar] [CrossRef]
- Aleixandre-Tudo, J.L.; Alvarez, I.; Lizama, V.; Nieuwoudt, H.; Garcia, M.J.; Aleixandre, J.L.; Du Toit, W.J. Modelling phenolic and volatile composition to characterize the effects of pre-fermentative cold soaking in Tempranillo wines. LWT-Food Sci. Technol. 2016, 66, 193–200. [Google Scholar] [CrossRef]
- Wu, Y.; Xing, K.; Zhang, X.; Wang, H.; Wang, F.; Wang, Y.; Li, J. Effect of pre-fermentation saignée treatment on phenolic compound profile in wine made of Cabernet Sauvignon. J. Food Biochem. 2017, 41, e12380. [Google Scholar] [CrossRef]
- Cheng, Y.; Watrelot, A.A. Effects of saignée and bentonite treatment on phenolic compounds of Marquette red wines. Molecules 2022, 27, 3482. [Google Scholar] [CrossRef] [PubMed]
- Wojdyło, A.; Samoticha, J.; Chmielewska, J. Effect of different pre-treatment maceration techniques on the content of phenolic compounds and color of Dornfelder wines elaborated in cold climate. Food Chem. 2021, 339, 127888. [Google Scholar] [CrossRef] [PubMed]
- Álvarez, I.; Aleixandre, J.; García, M.; Lizama, V. Impact of prefermentative maceration on the phenolic and volatile compounds in Monastrell red wines. Anal. Chim. Acta 2006, 563, 109–115. [Google Scholar] [CrossRef]
- Paladines-Quezada, D.F.; Moreno-Olivares, J.D.; Fernández-Fernández, J.I.; Bleda-Sánchez, J.A.; Martínez-Moreno, A.; Gil-Muñoz, R. Elicitors and pre-fermentative cold maceration: Effects on polyphenol concentration in Monastrell grapes and wines. Biomolecules 2019, 9, 671. [Google Scholar] [CrossRef]
- Ghanem, C.; Bouajila, J.; Rizk, Z.; El Beyrouthy, M.; Sadaka, C.; Gürer, E.S.; Souchard, J.P.; Taillandier, P.; Sharifi-Rad, J.; Nehme, N. Comparative analysis of pre-fermentation treatments on phenolic compounds and bioactivity in Vitis Vinifera var. Syrah and var. Cabernet Sauvignon grapes. Nutrire 2023, 48, 25. [Google Scholar] [CrossRef]
- Lasanta, C.; Cejudo, C.; Gómez, J.; Caro, I. Influence of prefermentative cold maceration on the chemical and sensory properties of red wines produced in warm climates. Processes 2023, 11, 374. [Google Scholar] [CrossRef]
- Ortega-Heras, M.; Pérez-Magariño, S.; González-Sanjosé, M. Comparative study of the use of maceration enzymes and cold pre-fermentative maceration on phenolic and anthocyanic composition and colour of a Mencía red wine. LWT-Food Sci. Technol. 2012, 48, 1–8. [Google Scholar] [CrossRef]
- Parley, A.; Vanhanen, L.; Heatherbell, D. Effects of pre-fermentation enzyme maceration on extraction and colour stability in Pinot Noir wine. Aust. J. Grape Wine Res. 2001, 7, 146–152. [Google Scholar] [CrossRef]
- Wang, J.; Huo, S.; Zhang, Y.; Liu, Y.; Fan, W. Effect of different pre-fermentation treatments on polyphenols, color, and volatile compounds of three wine varieties. Food Sci. Biotech. 2016, 25, 735–743. [Google Scholar] [CrossRef]
- Tang, D.; Chen, M.; Huang, X.; Zhang, G.; Zeng, L.; Zhang, G.; Wu, S.; Wang, Y. SRplot: A free online platform for data visualization and graphing. PLoS ONE 2023, 18, e0294236. [Google Scholar] [CrossRef] [PubMed]

| Parameter | Harvest | |
|---|---|---|
| 2021 | 2022 | |
| Total acidity (g TAE L−1) * | 5.89 ± 0.2 | 6.53 ± 0.3 |
| pH | 3.27 ± 0.01 | 3.30 ± 0.01 |
| Density (g mL−1) | 1.095 ± 0.001 | 1.105 ± 0.001 |
| Potential alcoholic title (% v/v) | 13.0 ± 0.1 | 14.7 ± 0.2 |
| Reducing sugar concentration (g L−1) | 222 ± 3 | 251 ± 4 |
| Wine | d (g mL−1) | Alcohol Content (% v/v) | Titratable Acidity (g L−1) | Volatile Acidity (g L−1) | pH |
|---|---|---|---|---|---|
| Harvest 2021 | |||||
| C | 0.989 ± 0.001 | 13.1 ± 0.1 | 6.9 ± 0.2 | 0.47 ± 0.01 | 2.96 ± 0.01 |
| CE | 0.989 ± 0.001 | 12.6 ± 0.1 | 7.6 ± 0.2 | 0.52 ± 0.02 | 2.83 ± 0.02 |
| CEE | 0.989 ± 0.001 | 13.1 ± 0.1 | 7.7 ± 0.1 | 0.42 ± 0.01 | 2.70 ± 0.01 |
| CEET | 0.988 ± 0.002 | 13.3 ± 0.1 | 6.8 ± 0.1 | 0.52 ± 0.02 | 3.07 ± 0.02 |
| Harvest 2022 | |||||
| C | 0.998 ± 0.001 | 15.5 ± 0.2 | 9.0 ± 0.1 | 0.49 ± 0.02 | 2.53 ± 0.01 |
| CE | 0.985 ± 0.002 | 15.1 ± 0.1 | 7.6 ± 0.1 | 0.49 ± 0.01 | 2.87 ± 0.01 |
| CEE | 0.999 ± 0.001 | 13.9 ± 0.1 | 7.2 ± 0.1 | 0.57 ± 0.01 | 3.09 ± 0.02 |
| CEET | 0.989 ± 0.001 | 14.6 ± 0.1 | 7.7 ± 0.2 | 0.49 ± 0.01 | 2.86 ± 0.02 |
| Compound | Year 2021 | Year 2022 | ||||||
|---|---|---|---|---|---|---|---|---|
| C | CE | CEE | CEET | C | CE | CEE | CEET | |
| Non-pigment polyphenols | ||||||||
| Caftaric acid | 87.75 ± 2.24 a | 60.61 ± 2.81 b | 74.81 ± 2.61 c | 76.90 ± 3.46 c | 90.12 ± 5.66 a | 62.14 ± 3.19 b | 54.93 ± 2.58 d | 76.63 ± 5.69 c |
| Catechin | 53.48 ± 1.22 a | 35.11 ± 0.56 b | 45.63 ± 1.82 c | 29.98 ± 0.86 d | 47.23 ± 1.06 c | 81.86 ± 3.42 e | 25.29 ± 1.03 f | 56.50 ± 4.12 a |
| p-coumaric acid derivative | 8.51 ± 0.18 a | 6.00 ± 0.11 b | 7.34 ± 0.32 c | 8.00 ± 0.21 a | 8.62 ± 0.45 a | 6.72 ± 0.11 d | 5.54 ± 0.44 e | 7.26 ± 0.52 c |
| Ferulic acid derivative | 48.71 ± 2.21 a | 29.21 ± 0.87 b | 41.70 ± 0.96 c | 39.69 ± 0.82 c | 49.13 ± 1.06 a | 35.39 ± 1.43 d | 21.87 ± 0.85 e | 36.98 ± 0.88 d |
| Rutin | 29.09 ± 1.04 a | 13.16 ± 0.35 b | 24.90 ± 0.24 c | 18.69 ± 0.44 d | 12.32 ± 0.67 b | 14.12 ± 0.98 b | 2.52 ± 0.10 e | 8.15 ± 0.32 f |
| Quercetin 3-O-galactoside | 13.92 ± 0.65 a | 6.71 ± 0.23 b | 11.81 ± 0.64 c | 9.22 ± 0.13 d | 9.81 ± 0.84 d, e | 10.79 ± 0.32 e | 7.32 ± 0.47 b | 8.43 ± 0.44 f |
| Quercetin 3-O-glucuronide | 156.64 ± 7.40 a | 75.69 ± 1.97 b | 133.20 ± 2.47 c | 97.07 ± 3.56 d | 121.17 ± 9.43 c | 130.31 ± 6.44 c | 103.00 ± 5.66 d | 111.93 ± 6.56 d |
| Quercetin | 4.00 ± 0.24 a | 2.11 ± 0.05 b | 3.34 ± 0.09 c | 2.39 ± 0.06 d | 4.95 ± 0.19 e | 4.21 ± 0.10 a | 9.12 ± 0.52 f | 8.56 ± 0.49 f |
| Total | 402.11 | 228.63 | 342.73 | 281.94 | 343.35 | 345.54 | 229.59 | 314.44 |
| Compound | Year 2021 | Year 2022 | ||||||
|---|---|---|---|---|---|---|---|---|
| C | CE | CEE | CEET | C | CE | CEE | CEET | |
| Anthocyanin pigments | ||||||||
| Delphinidin 3-O-glucoside | 49.34 ± 1.25 a | 18.94 ± 0.58 b | 41.97 ± 1.09 c | 29.13 ± 1.10 d | 63.37 ± 2.83 e | 57.94 ± 1.56 f | 23.43 ± 1.06 g | 57.37 ± 2.22 f |
| Petunidin 3-O-glucoside | 68.93 ± 2.33 a | 32.59 ± 0.94 b | 56.41 ± 1.98 c | 39.11 ± 1.23 d | 90.08 ± 4.00 e | 83.55 ± 2.83 e | 31.08 ± 1.88 b | 70.40 ± 2.69 a |
| Paeonidin 3-O-glucoside | 106.83 ± 3.21 a | 32.45 ± 1.03 b | 89.73 ± 3.33 c | 90.17 ± 2.34 c | 95.87 ± 6.01 c | 143.74 ± 7.77 d | 43.17 ± 2.44 e | 101.06 ± 6.31 a |
| Malvidin 3-O-glucoside | 750.74 ± 14.32 a | 438.29 ± 12.33 b | 631.71 ± 22.11 c | 520.02 ± 12.89 d | 863.52 ± 43.02 e | 928.07 ± 56.04 e | 222.05 ± 12.09 f | 600.00 ± 17.44 c |
| Malvidin 3-O-glucoside acetate | 44.88 ± 1.06 a | 35.08 ± 0.86 b | 36.96 ± 1.55 b | 33.16 ± 0.98 c | 58.40 ± 1.22 d | 61.05 ± 1.99 e | 12.78 ± 0.74 f | 34.85 ± 1.58 b |
| Malvidin 3-O-glucoside p-coumarate | 177.01 ± 9.58 a | 114.43 ± 2.69 b | 144.46 ± 3.62 c | 98.13 ± 2.93 d | 194.99 ± 11.53 e | 239.15 ± 11.05 f | 27.68 ± 1.11 g | 128.69 ± 7.56 h |
| Total | 1197.73 | 671.78 | 1001.24 | 809.72 | 1366.23 | 1513.5 | 360.19 | 992.37 |
| Compound | CAS Number | Retention Time (min) | Year 2021 | Year 2022 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| C | CE | CEE | CEET | C | CE | CEE | CEET | |||
| Isoamyl alcohol | 123-51-3 | 3.11 | 5.83 ± 0.98 a | 4.46 ± 0.21 b | 2.66 ± 0.09 c | 2.82 ± 0.15 c | Nd d | 2.83 ± 0.11 c | 1.68 ± 0.09 e | 3.45 ± 0.12 f |
| Isoamyl formate | 110-45-2 | 3.60 | Nd a | Nd a | 5.46 ± 0.12 b | Nd a | 8.43 ± 0.23 c | 3.39 ± 0.13 d | 4.01 ± 0.08 e | Nd a |
| 1,3-butanediol | 107-88-0 | 4.78 | Nd a | Nd a | 0.04 ± 0.00 b | Nd a | Nd a | Nd a | 0.03 ± 0.00 c | Nd a |
| Isoamyl acetate | 123-92-2 | 7.08 | 0.78 ± 0.01 a | 0.58 ± 0.01 b | 0.46 ± 0.01 c | 0.52 ± 0.01 d | 0.98 ± 0.03 e | Nd f | 0.21 ± 0.00 g | 0.37 ± 0.00 h |
| 5-methyl-3-heptanone | 541-85-5 | 10.01 | 0.16 ± 0.00 a | 0.12 ± 0.00 b | 0.08 ± 0.01 c | 0.17 ± 0.00 d | 0.06 ± 0.00 e | 0.12 ± 0.01 b | 0.13 ± 0.00 f | 0.08 ± 0.00 c |
| Methoxy phenyloxime | 1000222-86-6 | 10.48 | Nd a | Nd a | 0.08 ± 0.00 b | 0.03 ± 0.00 c | 0.09 ± 0.00 d | Nd a | Nd a | 0.02 ± 0.00 e |
| Ethyl hexanoate | 123-66-0 | 13.81 | 0.38 ± 0.01 a | 0.11 ± 0.00 b | 0.20 ± 0.00 c | 0.11 ± 0.00 b | 0.22 ± 0.00 d | 0.14 ± 0.00 e | 0.06 ± 0.00 f | 0.09 ± 0.00 g |
| Hexyl acetate | 142-92-7 | 14.69 | 0.04 ± 0.00 a | 0.01 ± 0.00 b | 0.02 ± 0.00 c | 0.02 ± 0.00 c | 0.03 ± 0.00 d | 0.04 ± 0.00 a | Nd e | 0.01 ± 0.00 b |
| D-limonene | 5989-27-5 | 15.60 | 0.03 ± 0.00 a | 0.01 ± 0.00 b | Nd c | Nd c | Nd c | Nd c | Nd c | 0.01 ± 0.00 b |
| 2-phenylethanol | 60-12-8 | 20.49 | 1.36 ± 0.05 a, b | 1.29 ± 0.07 a | 1.33 ± 0.08 a, b | 1.45 ± 0.05 b | 0.49 ± 0.00 c | 0.55 ± 0.01 d | 0.67 ± 0.02 e | 0.53 ± 0.00 f |
| Diethyl succinate | 123-25-1 | 25.36 | 0.01 ± 0.00 a | Nd b | 0.01 ± 0.00 a | Nd b | Nd b | Nd b | 0.02 ± 0.00 c | 0.01 ± 0.00 a |
| (±)-menthol | 89-78-1 | 25.41 | Nd a | 0.06 ± 0.00 b | Nd a | 1.70 ± 0.03 c | Nd a | Nd a | Nd a | Nd a |
| Ethyl octanoate | 106-32-1 | 27.43 | 1.44 ± 0.01 a | 0.72 ± 0.02 b | 0.83 ± 0.03 c | 0.74 ± 0.01 b | 1.39 ± 0.03 d | 0.51 ± 0.01 e | 0.10 ± 0.00 f | 0.28 ± 0.00 g |
| Octanoic acid | 124-07-2 | 28.74 | 0.33 ± 0.02 a | 0.22 ± 0.00 b | 0.24 ± 0.01 c | 0.23 ± 0.00 c | 0.03 ± 0.00 d | 0.01 ± 0.00 e | Nd f | 0.07 ± 0.00 g |
| 2-phenylethyl acetate | 103-45-7 | 29.85 | 0.30 ± 0.00 a | 4.24 ± 0.11 b | 0.33 ± 0.01 c | 0.58 ± 0.01 d | 0.17 ± 0.00 e | 0.19 ± 0.00 f | 0.09 ± 0.00 g | 0.12 ± 0.00 h |
| Nonanoic acid | 112-05-0 | 34.35 | 0.05 ± 0.00 a | 0.16 ± 0.01 b | 0.14 ± 0.00 c | 0.18 ± 0.00 d | Nd e | Nd e | Nd e | Nd e |
| β-damascenone | 23726-93-4 | 38.37 | 0.01 ± 0.00 a | 0.01 ± 0.00 a | 0.01 ± 0.00 a | 0.01 ± 0.00 a | Nd b | Nd b | Nd b | Nd b |
| Nerol | 106-25-2 | 39.06 | Nd a | 0.01 ± 0.00 b | Nd a | 0.01 ± 0.00 b | Nd a | Nd a | Nd a | Nd a |
| Ethyl 9-decenoate | 67233-91-4 | 39.58 | 0.04 ± 0.00 a | 0.29 ± 0.01 b | 0.03 ± 0.00 c | 0.16 ± 0.00 d | 0.03 ± 0.00 c | Nd e | 0.01 ± 0.00 f | 0.02 ± 0.00 g |
| Ethyl caprate | 110-38-3 | 40.53 | 1.46 ± 0.01 a | 0.90 ± 0.02 b | 1.09 ± 0.06 c | 0.60 ± 0.01 d | 1.16 ± 0.04 c | 0.46 ± 0.01 e | 0.05 ± 0.00 f | 0.14 ± 0.00 g |
| Isoamyl octanoate | 2035-99-6 | 43.41 | 0.02 ± 0.00 a | 0.01 ± 0.00 b | 0.02 ± 0.00 a | 0.01 ± 0.00 b | 0.02 ± 0.00 a | 0.01 ± 0.00 b | Nd c | Nd c |
| Ethyl dodecanoate | 106-33-2 | 52.80 | 0.13 ± 0.01 a | 0.09 ± 0.00 b | 0.11 ± 0.00 c | 0.13 ± 0.00 a | 0.10 ± 0.00 d | 0.04 ± 0.00 e | Nd f | Nd f |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roufas, K.; Athanasiadis, V.; Chatzimitakos, T.; Lalas, S.I.; Toulaki, A.; Makris, D.P. Impact of Various Prefermentation Treatments on the Pigment, Polyphenol, and Volatile Composition of Industrial Red Wines Made from Vitis vinifera cv Maratheftiko. Beverages 2024, 10, 39. https://doi.org/10.3390/beverages10020039
Roufas K, Athanasiadis V, Chatzimitakos T, Lalas SI, Toulaki A, Makris DP. Impact of Various Prefermentation Treatments on the Pigment, Polyphenol, and Volatile Composition of Industrial Red Wines Made from Vitis vinifera cv Maratheftiko. Beverages. 2024; 10(2):39. https://doi.org/10.3390/beverages10020039
Chicago/Turabian StyleRoufas, Kosmas, Vassilis Athanasiadis, Theodoros Chatzimitakos, Stavros I. Lalas, Artemis Toulaki, and Dimitris P. Makris. 2024. "Impact of Various Prefermentation Treatments on the Pigment, Polyphenol, and Volatile Composition of Industrial Red Wines Made from Vitis vinifera cv Maratheftiko" Beverages 10, no. 2: 39. https://doi.org/10.3390/beverages10020039
APA StyleRoufas, K., Athanasiadis, V., Chatzimitakos, T., Lalas, S. I., Toulaki, A., & Makris, D. P. (2024). Impact of Various Prefermentation Treatments on the Pigment, Polyphenol, and Volatile Composition of Industrial Red Wines Made from Vitis vinifera cv Maratheftiko. Beverages, 10(2), 39. https://doi.org/10.3390/beverages10020039














