A Holistic View of the Fate of Berry-Derived Adjuncts throughout Fermentation
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Gravity Measurements
2.3. pH
2.4. Titratable Acidity
2.5. Total Anthocyanins
2.6. Color
2.7. Extraction of the Volatile and Semi-Volatile Compounds
2.8. Gas Chromatography-Mass Spectrometry (GC-MS)
2.9. Statistical Methods
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, Y.; Cui, M.-Y.; Zhang, Q.; Zhu, Y.; Yang, Q.; Fu, Y.; Bi, H.-J.; Yang, X.-H.; Gao, X.-L. Enhancing blueberry wine aroma: Insights from cultivar selection and berry sorting. Curr. Res. Food Sci. 2023, 7, 100643. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Su, Q.; Jiao, J.; Kelanne, N.; Kortesniemi, M.; Xu, X.; Zhu, B.; Laaksonen, O. Exploring the Sensory Properties and Preferences of Fruit Wines Based on an Online Survey and Partial Projective Mapping. Foods 2023, 12, 1844. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Han, T.; Yang, H.; Lyu, L.; Li, W.; Wu, W. Known and potential health benefits and mechanisms of blueberry anthocyanins: A review. Food Biosci. 2023, 55, 103050. [Google Scholar] [CrossRef]
- Acosta-Estrada, B.A.; Gutiérrez-Uribe, J.A.; Serna-Saldívar, S.O. Bound phenolics in foods, a review. Food Chem. 2014, 152, 46–55. [Google Scholar] [CrossRef]
- Hornedo-Ortega, R.; Álvarez-Fernández, M.A.; Cerezo, A.B.; Garcia-Garcia, I.; Troncoso, A.M.; Garcia-Parrilla, M.C. Influence of Fermentation Process on the Anthocyanin Composition of Wine and Vinegar Elaborated from Strawberry. J. Food Sci. 2017, 82, 364–372. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Shi, Y.; Zhang, H.; Deng, X.; Wang, Y.; Ma, Y.; Zhao, X.; Zhang, C. Characterization and Comparison of Unfermented and Fermented Seed-Watermelon Juice. J. Food Qual. 2018, 2018, e4083903. [Google Scholar] [CrossRef]
- Sidor, A.; Gramza-Michałowska, A. Advanced research on the antioxidant and health benefit of elderberry (Sambucus nigra) in food—A review. J. Funct. Foods 2015, 18, 941–958. [Google Scholar] [CrossRef]
- Anton, A.M.; Pintea, A.M.; Rugină, D.O.; Sconţa, Z.M.; Hanganu, D.; Vlase, L.; Benedec, D. Preliminary Studies on the Chemical Characterization and Antioxidant Capacity of Polyphenols from Sambucus sp. Dig. J. Nanomater. Biostruct. (DJNB) 2013, 8, 973–980. [Google Scholar]
- Khoo, H.E.; Azlan, A.; Tang, S.T.; Lim, S.M. Anthocyanidins and anthocyanins: Colored pigments as food, pharmaceutical ingredients, and the potential health benefits. Food Nutr. Res. 2017, 61, 1361779. [Google Scholar] [CrossRef]
- Petruskevicius, A.; Viskelis, J.; Urbonaviciene, D.; Viskelis, P. Anthocyanin Accumulation in Berry Fruits and Their Antimicrobial and Antiviral Properties: An Overview. Horticulturae 2023, 9, 288. [Google Scholar] [CrossRef]
- Prajapati, R.; Jadeja, G. Natural Food Colorants: Extraction and Stability Study. Mater. Today Proc. 2022, 57, 2381–2395. [Google Scholar] [CrossRef]
- Osman, A.G.; Avula, B.; Katragunta, K.; Ali, Z.; Chittiboyina, A.G.; Khan, I.A. Elderberry Extracts: Characterization of the Polyphenolic Chemical Composition, Quality Consistency, Safety, Adulteration, and Attenuation of Oxidative Stress- and Inflammation-Induced Health Disorders. Molecules 2023, 28, 3148. [Google Scholar] [CrossRef] [PubMed]
- Ağalar, H.G.; Demirci, B.; Başer KH, C. The Volatile Compounds of Elderberries (Sambucus nigra L.). Nat. Vol. Essent. Oils 2014, 1, 51–54. [Google Scholar]
- Song, Y.; Zhang, Y.; Liu, N.; Ye, D.; Gong, X.; Qin, Y.; Liu, Y. Volatile compounds in wild strawberry and their odorants of wild strawberry wines: Effects of different stages of fermentation. Int. J. Food Prop. 2017, 20 (Suppl. S1), S399–S415. [Google Scholar] [CrossRef]
- Romano, P.; Braschi, G.; Siesto, G.; Patrignani, F.; Lanciotti, R. Role of Yeasts on the Sensory Component of Wines. Foods 2022, 11, 1921. [Google Scholar] [CrossRef] [PubMed]
- American Society of Brewing Chemists (ASBC). ASBC Methods of Analysis; Instrumental Method for Alcohol and Original Gravity; Method Beer-4; Alcohol; American Society of Brewing Chemists (ASBC): St. Paul, MN, USA, 2018. [Google Scholar]
- Sims, C.A.; Morris, J.R. Effects of pH, Sulfur Dioxide, Storage Time, and Temperature on the Color and Stability of Red Muscadine Grape Wine. Am. J. Enol. Vitic. 1984, 35, 35–39. [Google Scholar] [CrossRef]
- Lee, J. Determination of total monomeric anthocyanin pigment content of fruit juices, beverages, natural colorants, and wines. J. AOAC Int. 2005, 88, 1269–1278. [Google Scholar] [CrossRef]
- Glories, Y. La couleur des vins rouges: Mesure, origine et interpretation: Partie I. Connaiss. Connaiss. Vigne Vin. 1984, 18, 253–271. [Google Scholar]
- Thompson-Witrick, K.A.; Rouseff, R.L.; Cadawallader, K.R.; Duncan, S.E.; Eigel, W.N.; Tanko, J.M.; O’Keefe, S.F. Comparison of Two Extraction Techniques, Solid-Phase Microextraction Versus Continuous Liquid–Liquid Extraction/Solvent-Assisted Flavor Evaporation, for the Analysis of Flavor Compounds in Gueuze Lambic Beer. J. Food Sci. 2015, 80, C571–C576. [Google Scholar] [CrossRef]
- Wnuk, S.; Kinastowski, S.; Kaminski, E. Synthesis and analysis of 1-octen-3-ol, the main flavour component of mushrooms. Nahrung 1983, 27, 479–486. [Google Scholar] [CrossRef]
- ASBC. ASBC Beer Flavor Database. 2012. Available online: https://www.asbcnet.org/Methods/SensoryAnalysis/Pages/default.aspx (accessed on 1 May 2024).
- Available online: https://cameochemicals.noaa.gov/chris/BAL.pdf (accessed on 1 May 2024).
- Burdock, G.A. Fenaroli’s Handbook of Flavor Ingredients, 6th ed.; Taylor & Francis Group: Oxfordshire, UK, 2010. [Google Scholar]
- Available online: https://www.nj.gov/health/eoh/rtkweb/documents/fs/1039.pdf (accessed on 1 May 2024).
- Available online: https://www.env.go.jp/en/air/odor/measure/02_3_2.pdf (accessed on 1 May 2024).
- The Good Scents Company. TGSC Flavor, Fragrance, Food and Cosmetics Ingredients Information. Available online: http://www.thegoodscentscompany.com/ (accessed on 1 May 2024).
- Cai, J.; Zhu, B.-Q.; Wang, Y.-H.; Lu, L.; Lan, Y.-B.; Reeves, M.J.; Duan, C.-Q. Influence of pre-fermentation cold maceration treatment on aroma compounds of Cabernet Sauvignon wines fermented in different industrial scale fermenters. Food Chem. 2014, 154, 217–229. [Google Scholar] [CrossRef]
- Available online: https://wwwn.cdc.gov/TSP/substances/ToxSubstance.aspx?toxid=74#:~:text=It%20is%20also%20known%20as,it%20a%20sharp%2C%20unpleasant%20smell (accessed on 1 May 2024).
- Lytra, G.; Tempere, S.; Revel, G.; Barbe, J.-C. Document Distribution and organoleptic impact of ethyl 2-hydroxy-4-methylpentanoate enantiomers in wine. J. Agric. Food Chem. 2012, 60, 1503–1509. [Google Scholar] [CrossRef] [PubMed]
- Elsharif, S.; Banerjee, A.; Buettner, A. Structure-odor relationships of linalool, linalyl acetate and their corresponding oxygenated derivatives. Front. Chem. 2015, 3, 57. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.osha.gov/sites/default/files/methods/osha-pv2142.pdf (accessed on 1 May 2024).
- Available online: https://www.degruyter.com/document/doi/10.1515/znc-1992-3-403/pdf (accessed on 1 May 2024).
| Parameter | Variety 1 Juice | Variety 2 Juice | Variety 1 Wine | Variety 2 Wine |
|---|---|---|---|---|
| pH | 4.61 ± 0.01 | 4.86 ± 0.02 | 3.97 ± 0.01 | 3.96 ± 0.01 |
| Total Acidity as Citric Acid (g/L) | 6.87 ± 0.30 | 4.74 ± 0.26 | 8.53 ± 0.74 | 8.96 ± 0.10 |
| Solids (°Brix) | 11.87 ± 0.10 | 10.30 ± 0.10 | 1.87 ± 0.06 | 2.33 ± 0.10 |
| Total Anthocyanins (mg/L) | 3931.1 ± 60.5 | 6765.8 ± 334.6 | 2288.8 ± 531.3 | 4005.4 ± 38.8 |
| Color (Red%) | 50.72 ± 1.93 | 49.61 ± 0.37 | 56.69 ± 1.42 | 58.53 ± 0.40 |
| Color Hex Code | 331C0E | 321C0F | 391909 | 3B190A |
| Color |  | 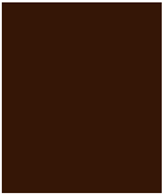 |  | 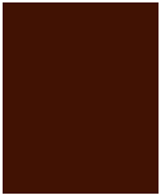 |
| Parameter | Commercial Cider Base | Commercial Elderberry Cider |
|---|---|---|
| pH | 4.12 ± 0.01 | 4.07 ± 0.01 |
| Total Acidity as Malic Acid (g/L) | 3.80 ± 0.39 | 3.80 ± 0.39 |
| Solids (°Brix) | 0.3 ± 0.1 | 0.57 ± 0.1 |
| Total Anthocyanins (mg/L) | 0 ± 0 | 355.7 ± 114.4 |
| Color (Red%) | 32.65 ± 0.48 | 37.11 ± 0.84 |
| Compound | Odor Descriptors | Odor Threshold (μg/L) | Concentration (μg/L) | |||
|---|---|---|---|---|---|---|
| Variety 1 Juice ** | Variety 2 Juice * | Variety 1 Wine * | Variety 2 Wine * | |||
| Alcohols | ||||||
| 1-Octen-3-ol | Mushroom [21] | 0.1 [21] | 314 ± 544 | 679 ± 204 | 29.1 ± 0.05 | - |
| Benzyl alcohol | Almonds, bitter [22] | 5500 [23] | 835 ± 1099 | 524 ± 126 | 58.2 ± 28.0 | 54.2 ± 67.5 |
| Isoamyl alcohol | Whiskey, fusel oil [24] | 42 [25] | - | 2210 ± 1448 | 22,492 ± 3128 | 4956 ± 1491 |
| 2-Decanol | Coconut, aniseed [22] | 0.77 [26] | 9.09 ± 7.89 | - | 5.21 ± 9.03 | 3.70 ± 6.41 |
| 1-Hexanol | Green, fruity, sweet alcohol [27] | 6.0 [26] | - | - | 38.3 ± 33.4 | 40.7 ± 70.5 |
| p-Methoxybenzyl alcohol | - | - | 428 ± 742 | - | - | - |
| Phenylethyl alcohol | Floral, rose, dried rose, bready, sweet [24] | 140,000 [28] | 764 ± 958 | 15,062 ± 3579 | 10,188 ± 1895 | 12,789 ± 2082 |
| SUBTOTAL | 3026 ± 289 | 21,392 ± 2842 | 35,685 ± 5396 | 19,410 ± 3155 | ||
| Benzene | ||||||
| Styrene | Sweet [29] | 35 [26] | 32.98 ± 57.12 | - | - | 315 ± 55.2 |
| SUBTOTAL | 32.98 ± 57.12 | - | - | 315 ± 55.2 | ||
| Esters | ||||||
| Isoamyl acetate | Sweet, winy [24] | 3.4 [27] | 12.3 ± 21.4 | 95 ± 134 | 2209 ± 1464 | 647 ± 126 |
| Ethyl 2-hydroxy-4-methylpentanoate | Fresh blackberry [30] | 51 [30] | 40.0 ± 46.0 | 410 ± 285 | 740 ± 165 | 192 ± 8.14 |
| Ethyl hexanoate | Fruity, pineapple–banana, winy [24] | 154,600 [28] | 51.8 ± 89.8 | 54.4 ± 77.0 | 1300 ± 516 | 798 ± 250 |
| Ethyl octanoate | Fruity, floral, winy, apricot [24] | 580 [28] | 286 ± 323 | 426 ± 169 | 8817 ± 1098 | 4323 ± 587 |
| Ethyl decanoate | Oily, cognac-/brandy-like [24] | 200 [30] | 476 ± 615 | 360 ± 128 | 5690 ± 373 | 2985 ± 296 |
| Ethyl dodecanoate | Oily, fatty, fruity [28] | 1500 [28] | 194 ± 261 | 105 ± 46.7 | 875 ± 231 | 517 ± 47.6 |
| SUBTOTAL | 2497 ± 181 | 4081 ± 179 | 23,394 ± 1621 | 12,336 ± 846 | ||
| Terpenes | ||||||
| Linalool | Floral, fresh [24] | 0.0032 [30,31] | 1035 ± 451 | 11,920 ± 5134 | 691 ± 110 | 4757 ± 643 |
| SUBTOTAL | 1212 ± 321 | 13,084 ± 3166 | 756 ± 278 | 5436 ± 1484 | ||
| Other | ||||||
| 2,3-Butanediol | Rubber, sweet, warming, diacetyl, butterscotch [22] | 1500 [28] | 197 ± 180 | 1010 ± 419 | 2687 ± 1194 | 1432 ± 399 |
| m-Di-tert-butylbenzene | - | - | 1.45 ± 2.51 | - | 301 ± 467 | 11.1 ± 10.0 |
| Compound | LRI | Odor Descriptors | Odor Threshold (μg/L) | Concentration (μg/L) | |
|---|---|---|---|---|---|
| Commercial Cider Base * | Commercial Elderberry Cider * | ||||
| Alcohols | |||||
| 1-Octen-3-ol | 970 | Mushroom [16] | 0.1 [16] | - | - |
| Benzyl alcohol | 1036 | Almonds, bitter [17] | 5500 [18] | - | - |
| Isoamyl alcohol | - | Whiskey, fusel oil [19] | 42 [20] | 8313 ± 3656 | 23,690 ± 726 |
| 2-Decanol | 1207 | Coconut, aniseed [17] | 0.77 [21] | 368 ± 303 | 26.8 ± 16.7 |
| Phenylethyl alcohol | 1115 | Floral, rose, dried rose, bready, sweet [19] | 140,000 [24] | 6086 ± 3102 | 7112 ± 690 |
| SUBTOTAL | 15,703 ± 2225 | 31,472 ± 5723 | |||
| Esters | |||||
| Isoamyl acetate | 882 | Sweet, winy [19] | 3.4 [27] | 5113 ± 2195 | 7870 ± 739 |
| Ethyl 2-hydroxy-4-methylpentanoate | 1057 | Fresh blackberry [33] | 51 [33] | - | 17.0 ± 29.5 |
| Ethyl hexanoate | 1003 | Fruity, pineapple–banana, winy [19] | 154,600 [24] | 1728 ± 723 | 1739 ± 626 |
| Ethyl octanoate | 1177 | Fruity, floral, winy, apricot [19] | 580 [24] | 11,697 ± 3100 | 1191 ± 693 |
| Ethyl decanoate | 1385 | Oily, cognac-/brandy-like [19] | 200 [24] | 5208 ± 996 | 595 ± 207 |
| Ethyl dodecanoate | 1514 | Oily, fatty, fruity [24] | 1500 [24] | 163 ± 27.2 | 26.9 ± 46.5 |
| SUBTOTAL | 26,729 ± 2738 | 13,924 ± 1596 | |||
| Ketones | |||||
| β-Damascenone | 1368 | Stewed apple, honey, kettle hop, tobacco, coconut [17] | 0.001 [17] | 391 ± 645 | 49.9 ± 45.8 |
| SUBTOTAL | 428 ± 189 | ||||
| Terpenes | |||||
| Linalool | 1097 | Floral, fresh [19] | 0.0032 [28] | - | 48.8 ± 84.5 |
| SUBTOTAL | 812 ± 172 | 125 ± 19.8 | |||
| Other | |||||
| 2,3-Butanediol | 804 | Rubber, sweet, warming, diacetyl, butterscotch [17] | 1500 [24] | 79.2 ± 117 | 9.37 ± 16.2 |
| m-Di-tert-butylbenzene | 1244 | - | - | 427 ± 372 | 118 ± 38.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Serviss, M.T.; Wendrick, N.A.; MacIntosh, A.J.; Thompson-Witrick, K.A. A Holistic View of the Fate of Berry-Derived Adjuncts throughout Fermentation. Beverages 2024, 10, 38. https://doi.org/10.3390/beverages10020038
Serviss MT, Wendrick NA, MacIntosh AJ, Thompson-Witrick KA. A Holistic View of the Fate of Berry-Derived Adjuncts throughout Fermentation. Beverages. 2024; 10(2):38. https://doi.org/10.3390/beverages10020038
Chicago/Turabian StyleServiss, Mary T., Nicholas A. Wendrick, Andrew J. MacIntosh, and Katherine A. Thompson-Witrick. 2024. "A Holistic View of the Fate of Berry-Derived Adjuncts throughout Fermentation" Beverages 10, no. 2: 38. https://doi.org/10.3390/beverages10020038
APA StyleServiss, M. T., Wendrick, N. A., MacIntosh, A. J., & Thompson-Witrick, K. A. (2024). A Holistic View of the Fate of Berry-Derived Adjuncts throughout Fermentation. Beverages, 10(2), 38. https://doi.org/10.3390/beverages10020038






