Poly(3-mercapto-2-methylpropionate), a Novel α-Methylated Bio-Polythioester with Rubber-like Elasticity, and Its Copolymer with 3-hydroxybutyrate: Biosynthesis and Characterization
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strain and Plasmids
2.2. P(3HB-co-3M2MP) and P(3M2MP) Biosynthesis, Harvest, and Polymer Content
2.3. Polymer Film Preparation
2.4. Polymer Structure Characterization
2.5. Molecular Weight
2.6. Thermal Properties
2.7. Mechanical Properties
3. Results
3.1. P(3HB-co-3M2MP) and P(3M2MP) Biosynthesis and Polymer Content
3.2. Chemical Structure and Sequence Distribution Characterization
3.3. Molecular Weight and Thermal Properties
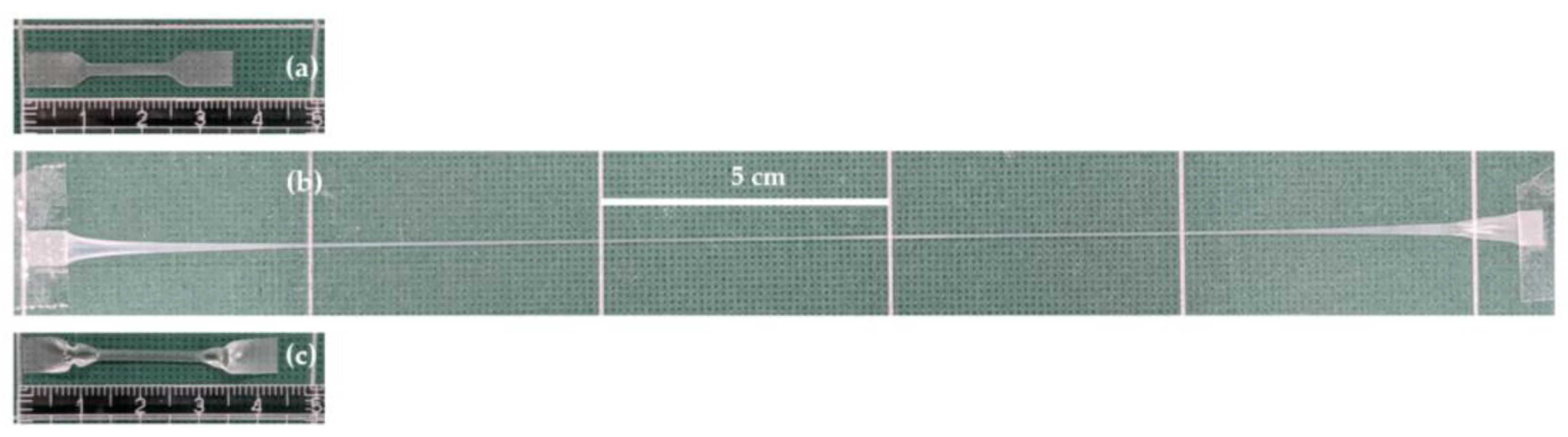
3.4. Physical and Mechanical Properties
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- PlasticsEurope. Plastics the Facts-2021. Available online: https://plasticseurope.org/wp-content/uploads/2021/12/Plastics-the-Facts-2021-web-final.pdf (accessed on 16 February 2022).
- Geyer, R.; Jambeck, J.R.; Law, K.L. Production, use, and fate of all plastics ever made. Sci. Adv. 2017, 3, e1700782. [Google Scholar] [CrossRef] [PubMed]
- Kaza, S.; Yao, L.; Bhada-Tata, P.; van Woerden, P. What a Waste 2.0: A Global Snapshot of Solid Waste Management to 2050. Urban Development Series; World Bank: Washington, DC, USA, 2018; ISBN 978-1-4648-1347-4. [Google Scholar]
- World Economic Forum; Ellen MacArthur Foundation; McKinsey & Company. The New Plastics Economy–Rethinking the Future of Plastics. Available online: https://emf.thirdlight.com/link/faarmdpz93ds-5vmvdf/@/preview/1?o (accessed on 16 February 2022).
- Ross, G.; Ross, S.; Tighe, B.J. Bioplastics: New routes, new products. In Brydson’s Plastics Materials, 8th ed.; Gilbert, M., Ed.; Butterworth-Heinemann: Cambridge, MA, USA, 2017; pp. 631–652. ISBN 978-0-323-35824-8. [Google Scholar]
- Chen, G.-Q.; Hajnal, I.; Wu, H.; Lv, L.; Ye, J. Engineering biosynthesis mechanisms for diversifying polyhydroxyalkanoates. Trends Biotechnol. 2015, 33, 565–574. [Google Scholar] [CrossRef] [PubMed]
- Anjum, A.; Zuber, M.; Zia, K.M.; Noreen, A.; Anjum, M.N.; Tabasum, S. Microbial production of polyhydroxyalkanoates (PHAs) and its copolymers: A review of recent advancements. Int. J. Biol. Macromol. 2016, 89, 161–174. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.Y.; Cho, I.J.; Lee, Y.; Kim, Y.-J.; Kim, K.-J.; Lee, S.Y. Microbial polyhydroxyalkanoates and nonnatural polyesters. Adv. Mater. 2020, 32, 1907138. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, M.; Tachibana, Y.; Kasuya, K.I. Biodegradability of poly (3-hydroxyalkanoate) and poly (ε-caprolactone) via biological carbon cycles in marine environments. Polym. J. 2021, 53, 47–66. [Google Scholar] [CrossRef]
- Narancic, T.; Verstichel, V.; Chaganti, S.R.; Morales-Gamez, L.; Kenny, S.T.; De Wilde, B.; Padamati, R.B.; O’Connor, K.E. Biodegradable plastic blends create new possibilities for end-of-life management of plastics but they are not a panacea for plastic pollution. Environ. Sci. Technol. 2018, 52, 10441–10452. [Google Scholar] [CrossRef]
- Choi, S.Y.; Rhie, M.N.; Kim, H.T.; Joo, J.C.; Cho, I.J.; Son, J.; Lee, Y. Metabolic engineering for the synthesis of polyesters: A 100-year journey from polyhydroxyalkanoates to non-natural microbial polyesters. Metab. Eng. 2020, 58, 47–81. [Google Scholar] [CrossRef]
- Taguchi, S.; Iwata, T.; Abe, H.; Doi, Y. Poly(hydroxyalkanoate)s. In Polymer Science: A Comprehensive Reference, 1st ed.; Matyjaszewski, K., Möller, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2012; pp. 157–182. ISBN 978-0-08-087862-1. [Google Scholar]
- McAdam, B.; Fournet, M.B.; McDonald, P.; Mojicevic, M. Production of Polyhydroxybutyrate (PHB) and Factors Impacting Its Chemical and Mechanical Characteristics. Polymers 2020, 12, 2908. [Google Scholar] [CrossRef]
- Yeo, J.C.C.; Muiruri, J.K.; Thitsartarn, W.; Li, Z.; He, C. Recent advances in the development of biodegradable PHB-based toughening materials: Approaches, advantages and applications. Mater. Sci. Eng. C Mater. Biol. Appl. 2018, 92, 1092–1116. [Google Scholar] [CrossRef]
- Sudesh, K.; Abe, H.; Doi, Y. Synthesis, structure and properties of polyhydroxyalkanoates: Biological polyesters. Prog. Polym. Sci. 2000, 25, 1503–1555. [Google Scholar] [CrossRef]
- Taguchi, S.; Matsumoto, K. Evolution of polyhydroxyalkanoate synthesizing systems toward a sustainable plastic industry. Polym. J. 2021, 53, 67–79. [Google Scholar] [CrossRef]
- Ishii-Hyakutake, M.; Mizuno, S.; Tsuge, T. Biosynthesis and characteristics of aromatic polyhydroxyalkanoates. Polymers 2018, 10, 1267. [Google Scholar] [CrossRef] [PubMed]
- Brydson, J.A. Silicone and other heat-resisting polymers. In Plastics Materials, 7th ed.; Brydson, J.A., Ed.; Butterworth-Heinemann: Oxford, UK, 1999; pp. 814–852. ISBN 0-7506-4132-0. [Google Scholar]
- Furutate, S.; Kamoi, J.; Nomura, C.T.; Taguchi, S.; Abe, H.; Tsuge, T. Superior thermal stability and fast crystallization behavior of a novel, biodegradable α-methylated bacterial polyester. NPG Asia Mater. 2021, 13, 31. [Google Scholar] [CrossRef]
- Marvel, C.S.; Kotch, A. Polythiolesters. J. Am. Chem. Soc. 1951, 73, 1100–1102. [Google Scholar] [CrossRef]
- Xia, Y.; Wübbeler, J.H.; Qi, Q.; Steinbüchel, A. Employing a recombinant strain of Advenella mimigardefordensis for biotechnical production of homopolythioesters from 3,3′-dithiodipropionic acid. Appl. Environ. Microbiol. 2012, 78, 3286–3297. [Google Scholar] [CrossRef]
- Lütke-Eversloh, T.; Bergander, K.; Luftmann, H.; Steinbüchel, A. Identification of a new class of biopolymer: Bacterial synthesis of a sulfur-containing polymer with thioester linkages. Microbiol. Read. 2001, 147, 11–19. [Google Scholar] [CrossRef]
- Lütke-Eversloh, T.; Bergander, K.; Luftmann, H.; Steinbüchel, A. Biosynthesis of poly(3-hydroxybutyrate-co-3-mercaptobutyrate) as a sulfur analogue to poly(3-hydroxybutyrate) (PHB). Biomacromolecules 2001, 2, 1061–1065. [Google Scholar] [CrossRef]
- Lütke-Eversloh, T.; Steinbüchel, A. Novel precursor substrates for polythioesters (PTE) and limits of PTE biosynthesis in Ralstonia eutropha. FEMS Microbiol. Lett. 2003, 221, 191–196. [Google Scholar] [CrossRef]
- Lütke-Eversloh, T.; Fischer, A.; Remminghorst, U.; Kawada, J.; Marchessault, R.H.; Bögershausen, A.; Kalwei, M.; Eckert, H.; Reichelt, R.; Liu, S.J.; et al. Biosynthesis of novel thermoplastic polythioesters by engineered Escherichia coli. Nat. Mater. 2002, 1, 236–240. [Google Scholar] [CrossRef]
- Yu, F.; Dong, T.; Zhu, B.; Tajima, K.; Yazawa, K.; Inoue, Y. Mechanical properties of comonomer-compositionally fractionated poly[(3-hydroxybutyrate)-co-(3-mercaptopropionate)] with low 3-mercaptopropionate unit content. Macromol. Biosci. 2007, 7, 810–819. [Google Scholar] [CrossRef]
- Watanabe, Y.; Ishizuka, K.; Furutate, S.; Abe, H.; Tsuge, T. Biosynthesis and characterization of novel poly(3-hydroxybutyrate-co-3-hydroxy-2-methylbutyrate): Thermal behavior associated with α-carbon methylation. RSC Adv. 2015, 5, 58679. [Google Scholar] [CrossRef]
- Tappel, R.C.; Wang, Q.; Nomura, C.T. Precise control of repeating unit composition in biodegradable poly(3-hydroxyalkanoate) polymers synthesized by Escherichia coli. J. Biosci. Bioeng. 2012, 113, 480–486. [Google Scholar] [CrossRef] [PubMed]
- Ushimaru, K.; Watanabe, Y.; Hiroe, A.; Tsuge, T. A single-nucleotide substitution in phasin gene leads to enhanced accumulation of polyhydroxyalkanoate (PHA) in Escherichia coli harboring Aeromonas caviae PHA biosynthetic operon. J. Gen. Appl. Microbiol. 2015, 61, 63–66. [Google Scholar] [CrossRef] [PubMed]
- Tsuge, T.; Watanabe, S.; Shimada, D.; Abe, H.; Doi, Y.; Taguchi, S. Combination of N149S and D171G mutations in Aeromonas caviae polyhydroxyalkanoate synthase and impact on polyhydroxyalkanoate biosynthesis. FEMS Microbiol. Lett. 2007, 277, 217–222. [Google Scholar] [CrossRef]
- Arikawa, H.; Sato, S.; Fujiki, T.; Matsumoto, K. Simple and rapid method for isolation and quantitation of polyhydroxyalkanoate by SDS-sonication treatment. J. Biosci. Bioeng. 2017, 124, 250–254. [Google Scholar] [CrossRef] [PubMed]
- Kamiya, N.; Yamamoto, Y.; Inoue, Y.; Chujo, R.; Doi, Y. Microstructure of bacterially synthesized poly(3-hydroxybutyrate-co-3-hydroxyvalerate). Macromolecules 1989, 22, 1676–1682. [Google Scholar] [CrossRef]
- Socrates, G. The Carbonyl Group: C==O. In Infrared and Raman Characteristic Group Frequencies: Tables and Charts, 3rd ed.; Socrates, G., Ed.; John Wiley & Sons, Ltd.: Chichester, UK, 2001; pp. 115–156. ISBN 978-0-470-09307-8. [Google Scholar]
- Tsuge, T. Fundamental factors determining the molecular weight of polyhydroxyalkanoate during biosynthesis. Polym. J. 2016, 48, 1051–1057. [Google Scholar] [CrossRef]
- Marx, A.; Poetter, M.; Buchholz, S.; May, A.; Siegert, H.; Alber, B.; Fuchs, G.; Eggeling, L. Microbiological Production Of 3-Hydroxyisobutyric Acid. Available online: https://patentimages.storage.googleapis.com/1a/8f/54/e04f10eb8e2206/CA2654133A1.pdf (accessed on 27 February 2022).
- Vermeer, C.M.; Bons, L.J.; Kleerebezem, R. Production of a newly discovered PHA family member with an isobutyrate-fed enrichment culture. Appl. Microbiol. Biotechnol. 2022, 106, 605–618. [Google Scholar] [CrossRef]
- Kajita, T.; Noro, A.; Matsushita, Y. Design and properties of supramolecular elastomers. Polymer 2017, 128, 297–310. [Google Scholar] [CrossRef]
- Furutate, S.; Abe, H.; Tsuge, T. Thermal properties of poly(3-hydroxy-2-methylbutyrate-co-3-hydroxybutyrate) copolymers with narrow comonomer-unit compositional distributions. Polym. J. 2021, 53, 1451–1457. [Google Scholar] [CrossRef]
- Kawada, J.; Lütke-Eversloh, T.; Steinbüchel, A.; Marchessault, R.H. Physical properties of microbial polythioesters: Characterization of poly(3-mercaptoalkanoates) synthesized by engineered Escherichia coli. Biomacromolecules 2003, 4, 1698–1702. [Google Scholar] [CrossRef]
- Thakor, N.; Lütke-Eversloh, T.; Steinbüchel, A. Application of the BPEC pathway for large-scale biotechnological production of poly(3-mercaptopropionate) by recombinant Escherichia coli, including a novel in situ isolation method. Appl. Environ. Microbiol. 2005, 71, 835–841. [Google Scholar] [CrossRef]
- Hu, D.; Chung, A.L.; Wu, L.P.; Zhang, X.; Wu, Q.; Chen, J.C.; Chen, G.Q. Biosynthesis and characterization of polyhydroxyalkanoate block copolymer P3HB-b-P4HB. Biomacromolecules 2011, 12, 3166–3173. [Google Scholar] [CrossRef]
- Tripathi, L.; Wu, L.P.; Chen, J.; Chen, G.Q. Synthesis of Diblock copolymer poly-3-hydroxybutyrate -block-poly-3-hydroxyhexanoate [PHB-b-PHHx] by a β-oxidation weakened Pseudomonas putida KT2442. Microb. Cell Fact. 2012, 11, 11. [Google Scholar] [CrossRef]
- Impallomeni, G.; Steinbüchel, A.; Lütke-Eversloh, T.; Barbuzzi, T.; Ballistreri, A. Sequencing microbial copolymers of 3-hydroxybutyric and 3-mercaptoalkanoic acids by NMR, electrospray ionization mass spectrometry, and size exclusion chromatography NMR. Biomacromolecules 2007, 8, 985–991. [Google Scholar] [CrossRef]
- Huang, P.; Furutate, S.; Mizuno, S.; Tsuge, T. Thermal degradation behavior of bacterial poly (3-hydroxybutyrate-co-3-mercaptopropionate). Polym. Degrad. Stab. 2019, 165, 35–42. [Google Scholar] [CrossRef]
- Goff, J.; Sulaiman, S.; Arkles, B.; Lewicki, J.P. Soft Materials with Recoverable Shape Factors from Extreme Distortion States. Adv. Mater. 2016, 28, 2393–2398. [Google Scholar] [CrossRef]
- Arkles, B.; Goff, J.; Sulaiman, S.; Sikorsky, A. Ultra-High Elongation Silicone Elastomers. Available online: https://www.gelest.com/wp-content/uploads/Ultra-high-Elongation-Silicone-Elastomers.pdf (accessed on 27 February 2022).
- Hiroe, A.; Ishii, N.; Ishii, D.; Kabe, T.; Abe, H.; Iwata, T.; Tsuge, T. Uniformity of monomer composition and material properties of medium-chain-length polyhydroxyalkanoates biosynthesized from pure and crude fatty acids. ACS Sustain. Chem. Eng. 2016, 4, 6905–6911. [Google Scholar] [CrossRef]
- Saito, Y.; Doi, Y. Microbial synthesis and properties of poly(3-hydroxybutyrate-co-4-hydroxybutyrate) in Comamonas acidovorans. Int. J. Biol. Macromol. 1994, 16, 99–104. [Google Scholar] [CrossRef]
- Kim, D.Y.; Lütke-Eversloh, T.; Elbanna, K.; Thakor, N.; Steinbüchel, A. Poly(3-mercaptopropionate): A nonbiodegradable biopolymer? Biomacromolecules 2005, 6, 897–901. [Google Scholar] [CrossRef]
- Steinbüchel, A. Non-biodegradable biopolymers from renewable resources: Perspectives and impacts. Curr. Opin. Biotechnol. 2005, 16, 607–613. [Google Scholar] [CrossRef]
- Kato, M.; Toshima, K.; Matsumura, S. Preparation of aliphatic poly(thioester) by the lipase-catalyzed direct polycondensation of 11-mercaptoundecanoic acid. Biomacromolecules 2005, 6, 2275–2280. [Google Scholar] [CrossRef]


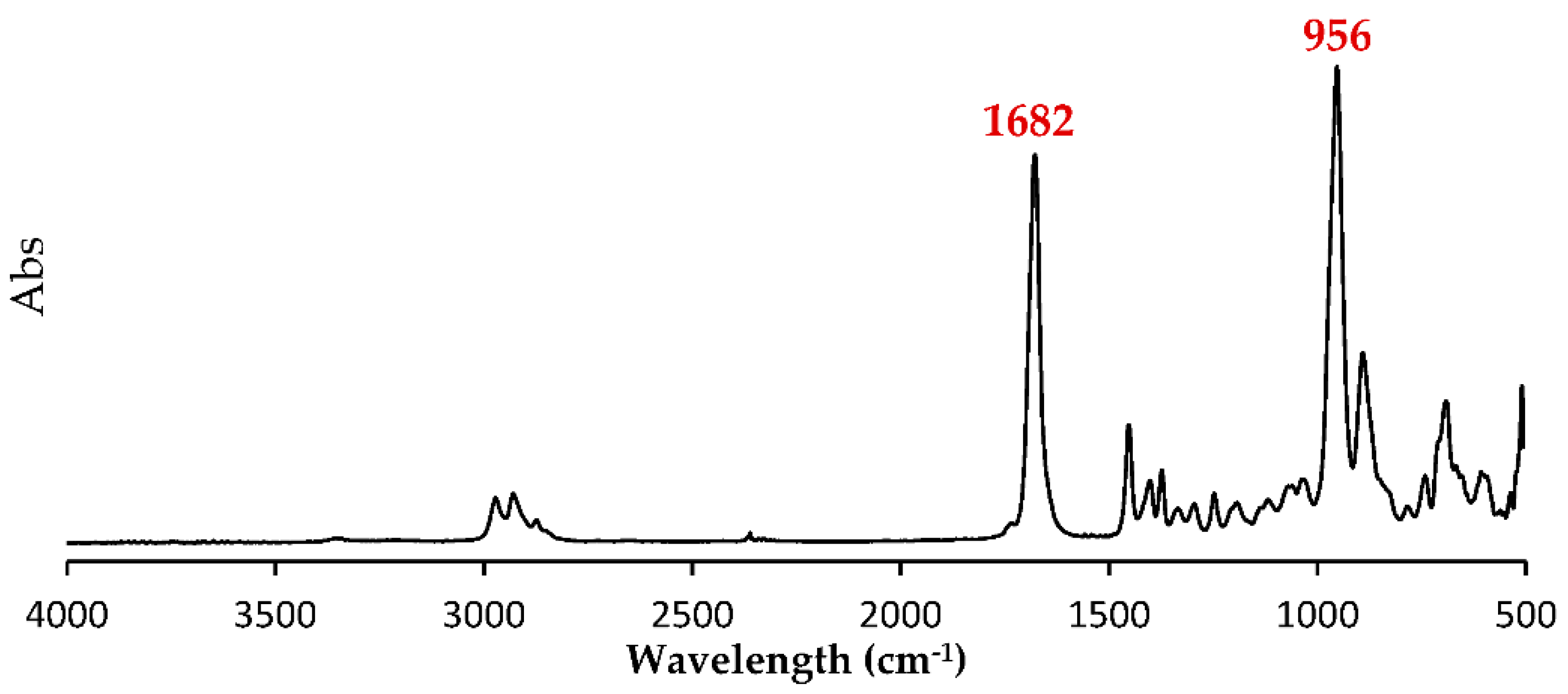
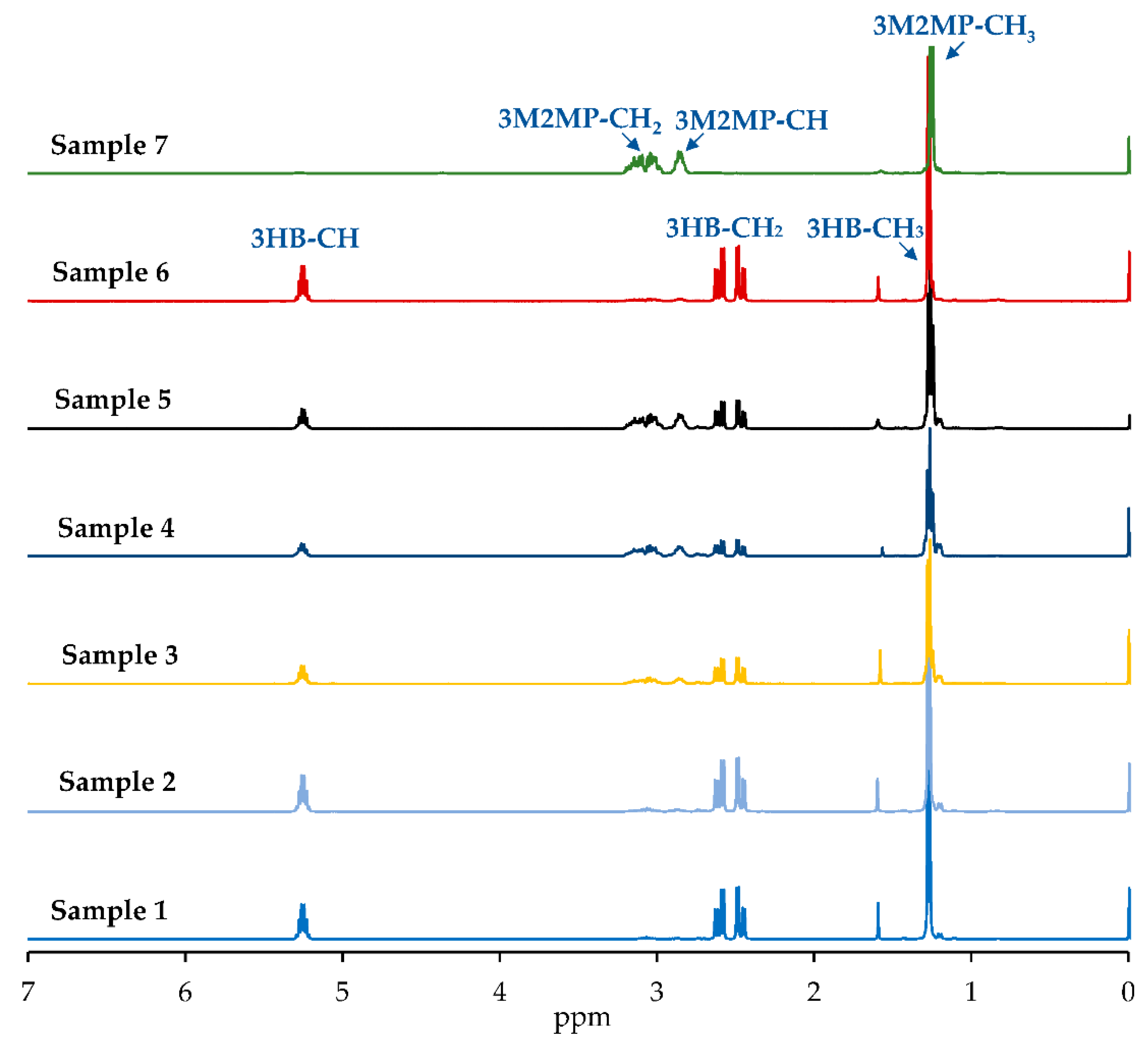
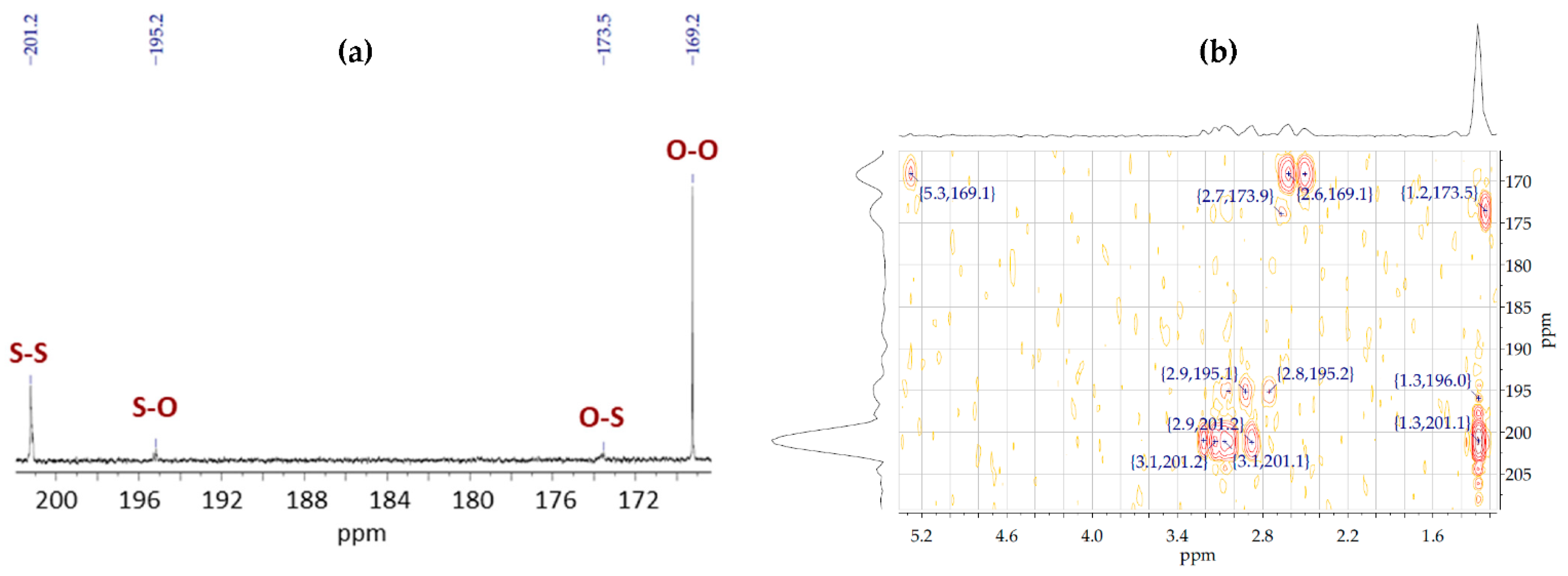
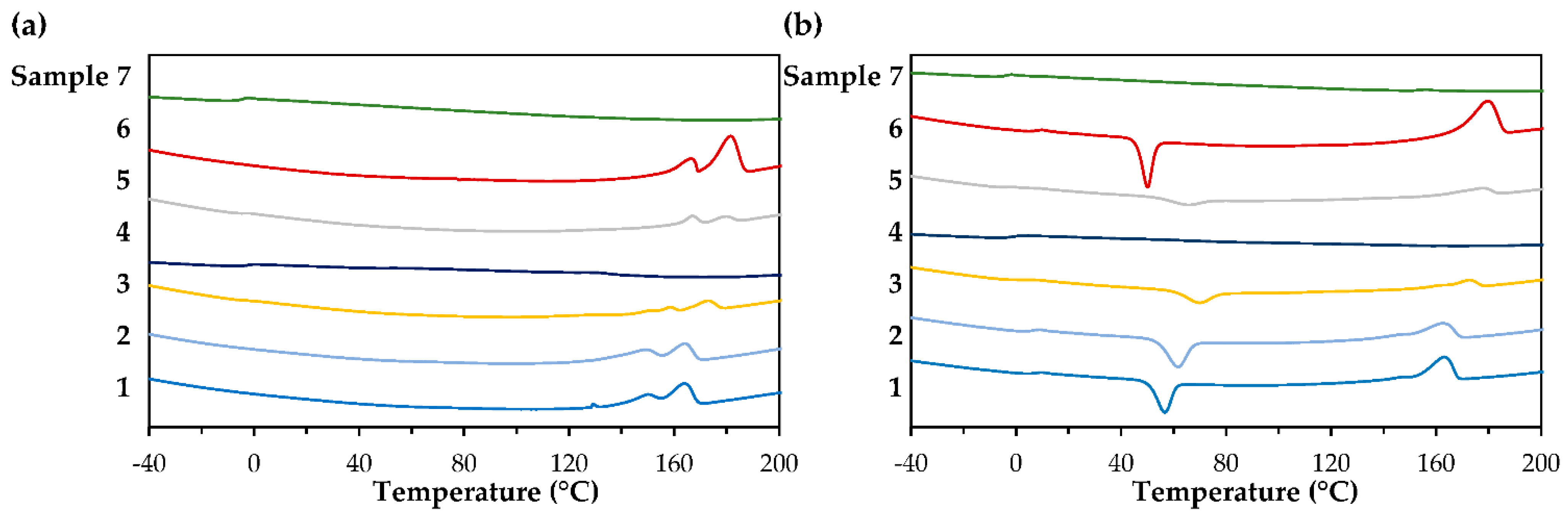


| PhaAB and PhaJ Expression | Precursor (g/L) 1 | Dry Cell wt. (g/L) | Polymer Content (wt%) | Monomer Composition (mol%) 2 | Sample ID | |
|---|---|---|---|---|---|---|
| 3HB | 3M2MP | |||||
| + 3 | 0.25 | 3.50 ± 0.03 | 69.1 ± 1.9 | 94.5 | 5.5 | 1 |
| + 3 | 0.50 | 3.69 ± 0.25 | 68.7 ± 1.0 | 89.9 | 10.1 | 2 |
| + 3 | 1.00 | 3.48 ± 0.02 | 67.3 ± 0.1 | 65.8 | 34.2 | 3 |
| + 3 | 1.50 | 3.63 ± 0.02 | 69.1 ± 0.2 | 46.1 | 53.9 | 4 |
| + 3 | 2.00 | 4.29 ± 0.07 | 72.1 ± 0.8 | 45.2 | 54.8 | 5 |
| + 3 | 2.50 | 5.06 ± 0.04 | 77.2 ± 0.3 | 89.3 | 10.7 | 6 |
| − 4 | 1.20 | 1.28 ± 0.01 | 8.4 ± 1.3 | 0 | 100 | 7 |
| Sample ID | 3M2MP (mol%) | Diad Sequence Distribution | D | Degree of Block Sequence 1 | |||
|---|---|---|---|---|---|---|---|
| FOO | FOS | FSO | FSS | ||||
| 1 | 5.5 | 0.960 | 0.016 | 0.009 | 0.015 | 100 | High |
| 2 | 10.1 | 0.934 | 0.012 | 0.006 | 0.048 | 623 | High |
| 3 | 34.2 | 0.741 | 0.006 | 0.037 | 0.216 | 720 | High |
| 4 | 53.9 | 0.329 | 0.182 | 0.152 | 0.337 | 4 | Low |
| 5 | 54.8 | 0.449 | 0.067 | 0.045 | 0.439 | 65 | Medium |
| 6 | 10.7 | 0.868 | 0.057 | 0.021 | 0.054 | 39 | Medium |
| Sample ID | 3M2MP (mol%) | Mw (×105) | Mw/Mn | Tg (°C) | Tcc (°C) | Tm (°C) | ΔHm (J/g) |
|---|---|---|---|---|---|---|---|
| P(3HB) 1 | 0 | 5.2 | 2.3 | 4.0 | NA | 176 | 79 |
| 1 | 5.5 | 14.6 | 3.3 | 8.1 | 57 | 150, 164 | 28 |
| 2 | 10.1 | 12.0 | 3.2 | 6.6 | 62 | 149, 164 | 26 |
| 3 | 34.2 | 10.0 | 3.6 | 5.4 | 70 | 159, 173 | 13 |
| 4 | 53.9 | 13.7 | 4.4 | −1.2 | - | - | - |
| 5 | 54.8 | 7.7 | 4.2 | −3.1, 7.0 | 65 | 167, 179 | 9 |
| 6 | 10.7 | 17.5 | 5.6 | 8.4 | 50 | 166, 181 | 51 |
| 7 | 100 | 15.0 | 2.6 | −3.1 | - | - | - |
| Sample ID | 3M2MP (mol%) | Yield Strength (MPa) | Tensile Strength (MPa) | Elongation at Break (%) | Young’s Modulus (MPa) |
|---|---|---|---|---|---|
| P(3HB) 1 | 0 | NA 2 | 58 ± 7 | 12 ± 0 | 1420 ± 80 |
| 1 | 5.5 | 13.6 ± 2.0 | 23.0 ± 2.3 | 24 ± 13 | 880 ± 75 |
| 2 | 10.1 | 14.6 ± 2.2 | 19.3 ± 3.0 | 470 ± 131 | 644 ± 41 |
| 3 | 34.2 | 6.5 ± 0.3 | 17.1 ± 0.8 | 825 ± 21 | 87 ± 8 |
| 4 | 53.9 | 2.2 ± 0.1 | 12.7 ± 0.2 | 1549 ± 103 | 13 ± 1 |
| 5 | 54.8 | 1.3 ± 0.2 | 5.8 ± 0.4 | 436 ± 49 | 18 ± 1 |
| 6 | 10.7 | 8.2 ± 3.5 | 15.5 ± 1.4 | 158 ± 58 | 709 ± 303 |
| 7 | 100 | 1.0 ± 0.1 | 4.2 ± 0.5 | 2605 ± 4 | 0.8 ± 0.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ceneviva, L.V.S.; Mierzati, M.; Miyahara, Y.; Nomura, C.T.; Taguchi, S.; Abe, H.; Tsuge, T. Poly(3-mercapto-2-methylpropionate), a Novel α-Methylated Bio-Polythioester with Rubber-like Elasticity, and Its Copolymer with 3-hydroxybutyrate: Biosynthesis and Characterization. Bioengineering 2022, 9, 228. https://doi.org/10.3390/bioengineering9050228
Ceneviva LVS, Mierzati M, Miyahara Y, Nomura CT, Taguchi S, Abe H, Tsuge T. Poly(3-mercapto-2-methylpropionate), a Novel α-Methylated Bio-Polythioester with Rubber-like Elasticity, and Its Copolymer with 3-hydroxybutyrate: Biosynthesis and Characterization. Bioengineering. 2022; 9(5):228. https://doi.org/10.3390/bioengineering9050228
Chicago/Turabian StyleCeneviva, Lucas Vinicius Santini, Maierwufu Mierzati, Yuki Miyahara, Christopher T. Nomura, Seiichi Taguchi, Hideki Abe, and Takeharu Tsuge. 2022. "Poly(3-mercapto-2-methylpropionate), a Novel α-Methylated Bio-Polythioester with Rubber-like Elasticity, and Its Copolymer with 3-hydroxybutyrate: Biosynthesis and Characterization" Bioengineering 9, no. 5: 228. https://doi.org/10.3390/bioengineering9050228
APA StyleCeneviva, L. V. S., Mierzati, M., Miyahara, Y., Nomura, C. T., Taguchi, S., Abe, H., & Tsuge, T. (2022). Poly(3-mercapto-2-methylpropionate), a Novel α-Methylated Bio-Polythioester with Rubber-like Elasticity, and Its Copolymer with 3-hydroxybutyrate: Biosynthesis and Characterization. Bioengineering, 9(5), 228. https://doi.org/10.3390/bioengineering9050228






