Abstract
Synthetic plastics derived from fossil fuels—such as polyethylene, polypropylene, polyvinyl chloride, and polystyrene—are non-degradable. A large amount of plastic waste enters landfills and pollutes the environment. Hence, there is an urgent need to produce biodegradable plastics such as polyhydroxyalkanoates (PHAs). PHAs have garnered increasing interest as replaceable materials to conventional plastics due to their broad applicability in various purposes such as food packaging, agriculture, tissue-engineering scaffolds, and drug delivery. Based on the chain length of 3-hydroxyalkanoate repeat units, there are three types PHAs, i.e., short-chain-length (scl-PHAs, 4 to 5 carbon atoms), medium-chain-length (mcl-PHAs, 6 to 14 carbon atoms), and long-chain-length (lcl-PHAs, more than 14 carbon atoms). Previous reviews discussed the recent developments in scl-PHAs, but there are limited reviews specifically focused on the developments of mcl-PHAs. Hence, this review focused on the mcl-PHA production, using various carbon (organic/inorganic) sources and at different operation modes (continuous, batch, fed-batch, and high-cell density). This review also focused on recent developments on extraction methods of mcl-PHAs (solvent, non-solvent, enzymatic, ultrasound); physical/thermal properties (Mw, Mn, PDI, Tm, Tg, and crystallinity); applications in various fields; and their production at pilot and industrial scales in Asia, Europe, North America, and South America.
1. Introduction
Polyhydroxyalkanoates (PHAs) are a type of biopolymer developed as intracellular carbon/energy storage materials which have a wide range of material characteristics. PHAs are identified as granular inclusion bodies after extraction from cells, these are becoming popular as prospective replacements for traditional plastics in various applications, including food packaging industries, cultivational fields, scaffold preparation, and biomaterial implants [1,2]. PHAs are classified based on the length of the 3-hydroxyalkanoate (3HA) repeat units, the repeat units of PHAs with short chain length (scl-PHAs) are generally 4–5 carbon atoms long (e.g., 3-hydroxybutyrate, 3HB and 3-hydroxyvalerate, 3HV units), medium chain length (mcl-PHAs) are 6–14 carbon atoms long (e.g., 3-hydroxyhexanoate, 3HHx, 3-hydroxyheptanoate, 3HHo), and long chain length (lcl-PHA) are more than 14 carbon atoms (e.g., 3-hydroxyhexadecanoate) [3,4]. Rakkan et al. (2022) reported the production of mcl-co-lcl PHAs (72 to 75% of DCW) from Enterobacter sp. strains TS3 and TS1L using glucose [5]. Mcl-PHAs are soft, elastomeric, and have less crystallinity, a lower melting point, and lower glass transition temperature [6]. Nutrient limiting conditions are required for PHA generation in Pseudomonas oleovorans, Pseudomonas putida, and Ralstonia eutropha, but not in recombinant Escherichia coli and Alcaligenes latus [7]. High manufacturing costs are a key impediment to the commercialization of PHAs. Carbon conversion yield (g/g), titer or volumetric yield (g/L), and productivity (g/L/h) are crucial in this scenario [8]. Aside from production criteria, low-cost downstream processing techniques and PHA manufacturing that fulfils cost performance standards have remained difficult to achieve. This has prompted researchers to focus on improving PHA fermentation and downstream processing efficiency to lower total costs [9,10]. The PHA production and degradation cycle is explained in Figure 1.
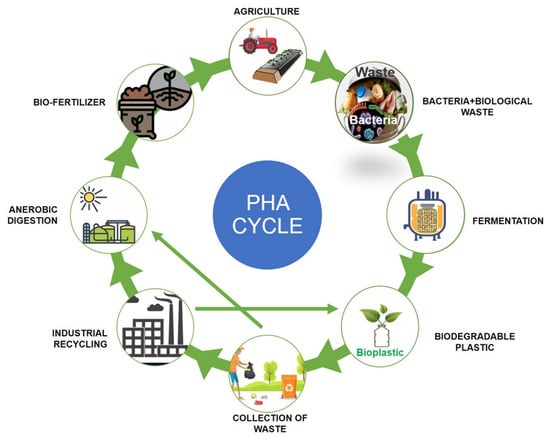
Figure 1.
Polyhydroxyalkanoates (PHA) production and degradation cycle.
A significant aim for PHA synthesis is to obtain high yields in high cell-density cultures (HCDCs). Bioethanol and yeast that produces single-cell proteins were used to construct HCDC in the beginning. Because of its benefits, such as reduced culture volume and residual liquid, cheaper production costs, and lower capital investment, HCDC technique is favored over low cell density technologies [11,12]. Dry cell weight (DCW) above 100 g/L is termed HCDC for PHA synthesis, a DCW above 50 g/L is regarded high for recombinant protein production [11,13,14]. More details on PHA production through HCDC were provided in Section 3.2. The species of Bacillus and C. necator are generally known to produce scl-PHAs, whilst Pseudomonas species known to produce mcl-PHAs. PHAs including Scl and Mcl can be developed by the species of Alcaligenes and Rhodococcus. PHAs have been used to construct a variety of drug carriers, cardiac patches, vascular grafts, nerve conduits, heart valves, artificial blood vessels, subcutaneous implants, orthopaedical pins, stents, wound dressings, and slings [15]. In addition, the FDA authorized P4HB for medical usage as absorbable sutures in the year 2007 [16]. Poly-3-hydroxyhexanoate (P3HO) has been used for soft (cardiovascular and neurologic) and hard (bone) tissue engineering [17,18]. By lowering mitochondrial damage, methyl esters of 3HB were used as drugs to treat Alzheimer’s disease [19]. Memory enhancers have also been shown to be sodium salts of 3HB monomers [20].
Unlike other polymers used before, which degrade through bulk degradation, PHAs degrade through controlled surface erosion [15]. Controlled degradation must be used to preserve implant integrity in vivo. PHAs have been thoroughly tested both inside and outside of the labs to confirm that they are biodegradable. PHAs also degrade far slower than polylactic acid (PLA), making them suited for long-term applications. This review article focused on mcl-PHA production using organic/inorganic carbon sources and various types of the fermentation strategies. We also discussed recent developments on cost-effective downstream processing methods, thermal/mechanical properties of mcl-PHAs, applications of mcl-PHAs, and their worldwide production at pilot and industrial scale.
2. Mcl-PHA production
2.1. Inorganic Carbon Sources
It is crucial to employ natural substrates for microbial PHA synthesis to reduce manufacturing costs. The ideal feedstock is one that does not compete with human food. Syngas is a carbon monoxide and hydrogen mixture that may be produced by pyrolyzing organic waste. PHAs may be synthesized by a variety of microorganisms using syngas. Heinrich et al. (2016) genetically engineered the Rhodospirillum rubrum S1 to create a heteropolymer of 3-hydroxydecanoic acid and 3-hydroxyoctanoic acid [P(3HD-co-3HO)] from artificial syngas including CO and CO2 [21]. Three genes—3-hydroxyacyl-ACP thioesterase (phaG), mcl-fatty acid CoA ligase (PP 0763), and a PHA synthase (phaC1)—from P. putida KT2440 were chosen for overexpression. To investigate the impact of syngas-mediated gene overexpression, the CO-inducible PcooF promoter was employed. A recombinant mutant produced P(3HD-co-3HO) up to 7.1% (wt/wt) of the DCW. Furthermore, enhanced mcl-PHA synthesis and increased gene expression via the PcooF promoter resulted in a greater molar fraction of 3HO in the generated copolymer than the Plac promoter, which regulated expression on the original vector. The polymer has a molecular mass of 124.3 kDa, melting point of 49.6 °C, and glass transition temperature of 41.1 °C. According to GC analysis, the polymer-isolate fractions were 55.6 mol % 3HD, 44.2 mol % 3HO, and less than 0.2 mol % 3HH. The partial disintegration of the accumulated polymer might be connected to the activity of a lipase identified in R. rubrum, as these enzymes have been shown to digest PHAs with varying chain lengths [22]. Figure 2 depicts the metabolic pathway carried in the production of mcl-PHAs from CO2 in recombinant R. rubrum. After entering into the cytoplasmic membrane, CO2 is fixed via ribulose 1,5-bisphosphate carboxylase through the Calvin cycle and generates glyceraldehyde-3-phosphate, which is subsequently converted to the pyruvate. Pyruvate is further converted into acetyl-CoA by the action of the enzyme pyruvate synthase. Acetyl-CoA is then entered into the fatty acid de novo synthesis cycle and generates 3-hydroxyacyl-ACP. The thiolysis of 3-hydroxyacyl-ACP is catalyzed by the 3-hydroxyacyl-ACP thioesterase and produces 3-hydroxy fatty acid. Activation of 3-hydroxy fatty acids occurred by the addition of CoA, which is catalyzed by the enzyme mcl-fatty acid CoA ligase. The enzyme PHA synthase polymerizes the 3-hydroxyacyl-CoA into mcl-PHA [21].
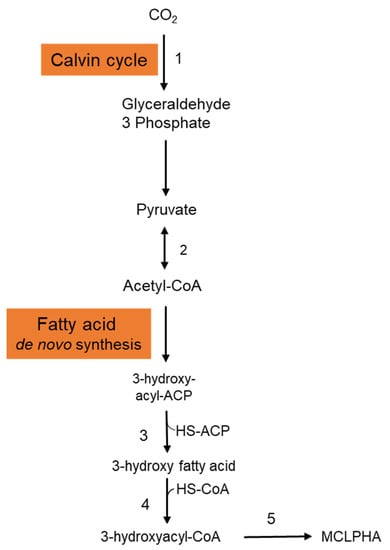
Figure 2.
Metabolic pathway involved in the synthesis of mcl-PHA from CO2 in Rhodospirillum rubrum. This figure was generated with the information from [21]. 1: Ribulose 1,5-bisphosphate carboxylase; 2: Pyruvate synthase; 3: 3-hydroxyacyl-ACP thioesterase; 4: MCL fatty acid CoA ligase; 5: PHA synthase.
PHAs were produced using C. eutrophus B-10646 with scl-mcl monomer units [23,24]. The cells were grown on a mineral medium with a main growth substrate (gas) comprising a 1:2:7 ratio of CO2: O2: H2 (by volume). The gas mixture was continuously pushed through the culture at a rate of 10–12 L/min in a 10 L bioreactor, the oxygen volume coefficient of mass transfer was 460 h−1 (kLa). By varying the butyrolactone concentration (3–5 g/L), copolymers with varying molar fractions of 3HB/4HB (10.0 to 51.3%), 3HV (0.3–0.5%), and 3HHx (0–0.4%) were obtained. Tanaka et al. (2021) used recombinant C. necator strains and a mineral salts medium [25]. A sterile filter was employed to introduce a substrate gas mixture with a ratio of H2/O2/CO2 = 8:1:1. The quantity of PHAs were determined by gas chromatography analysis. In all the cultures investigated, the PHAs generated are a copolyester of 3HB and 3HHx (47.7%). Löwe et al. (2017) used synthetic bacterial co-culture to generate mcl-PHAs (C6, C8, C10, and C12) from CO2 [26]. P. putida cscAB fixes CO2 and changes it to sucrose, and then transfers into the culture filtrate, this sugar serves as a carbon source for Synechococcus elongatus cscB, which converts it to PHAs that accumulate in the cytoplasm. Using a nitrogen-limited method, they were able to achieve a maximum PHA production rate of 23.8 mg/L/day and a maximum titer of 156 mg/L.
2.2. Organic Carbon Sources
Mcl-PHA production from various organic carbon sources were summarized in Table 1.

Table 1.
Mcl-PHA production using various carbon sources from literature reports.
2.2.1. Fatty Acids
R. eutropha grown on animal fat waste produced 45 g/L biomass which contains 60% PHA [37]. In the R. eutropha recombinant strain, the R. aetherivorans has expressed I24 PHA synthase gene, and PHA with HHx units is produced by P. aeruginosa hydratase gene (phaJ) [27]. The total biomass produced was 139 g/L with 74% of co-polymer, P (3HB-co-19 mol % 3HHx). Sato et al. observed that, in utilizing butyrate and palm kernel oil as carbon and energy sources, recombinant C. necator H16 produced good amount of P (3HB-co-19 mol % 3HHx). Furthermore, the scientists demonstrated that the 3-HHx% in KNK005 phaA-deactivated mutant strains is boosted by the butyrate. This culture environment produces higher biomass (171 g/L) and HHx copolymer in the cells (81%) with PHA titers of 139 g/L [38]. Cai et al. (2009) produced a P. putida KTMQ01 recombinant. The recombinant’s DCW was 86% mcl-PHA [39]. Genetically altered P. putida KT2440 employs inexpensive substrates, such as xylose and octanoic acid, to produce mcl-PHA at a low cost. The cells accumulated mcl-PHA to a level of 20% [40].
A PHA-negative mutant of R. eutropha was created using the phaC gene from Aeromonas caviae, produced 3HB copolymer comprising 5 mol % 3HHx using soybean oil (20 g/L) in fed-batch mode [41]. DCW and % PHA were 133 g/L, 72.5% respectively. A. hydrophila 4AK4 produced P(3HB-co-3HHx), which contains 15% HHx from dodecanoate [42]. The co-expression of phaC with phaP and phaJ resulted in a 3HHx level of 34 mol % in the copolymer. When dodecanoate was used as the only carbon source, 54 g/L DCW and 52.7% PHA were observed [43]. The wild-type strain produced 40.4 g/L of DCW, and 54.6% of P(3HB-co-3HHx) [44]. This modified strain used plant oils successfully, produced 88.3 g/L dry biomass with a polymer content of 57% (3HB-co-3HHx) [45]. Tufail et al. investigated the use of leftover braising liquid as a substrate for PHA production using P. aeruginosa (KF270353) for 72 h. PHAs (53.2%) and DCW (23.7 g/L) were detected in the highest amounts in waste braising liquid [46]. Ruiz et al. (2019) produced 159.4 g/L of DCW, and 57 g/L of mcl-PHA using fatty acids generated from waste cooking oil as the carbon source, and P. putida KT2440 as biocatalyst [28].
2.2.2. Carbohydrates
P(3HB-co-3HHx) with 14 mol % HHx units was produced with gluconate using recombinant A. hydrophila 4AK4 strain [47]. Poblete-Castro et al. (2014) produced PHA using gad (gluconate dehydrogenase) deleted mutant, P. putida KT2440 [48] under fed-batch mode. The DO-stat feeding method generated the 67% of mcl-PHA with 62 g/L DCW [46]. Fed-batch cultivation of P. putida KT2440 on mixed substrates of acrylic acid, nonanoic acid, and glucose (0.05: 1.25: 1) generated 71 g/L of DCW with 75% of PHA which contains the 89 mol % 3HN [29]. However, the concentration of 3HN was decreased to 65 mol % in the absence of acrylic acid. The amount of mcl-PHA produced increased 10-fold when dodecanoic acid was utilized as the feedstock by Zhao et al. (2020) [49]. Liu et al. (2019) reported that Pseudomonas-Saccharomyces produced up to 152.3 mg/L mcl-PHA using xylose as a carbon root [50]. The inclusion of S. cerevisiae in the consortium produced cell mass sedimentation. Sugarcane biorefinery derived sucrose hydrolysate and decanoic acid used in fed-batch cell cultivations produced 33% PHA in 53.4 g/L of DCW using P. putida KT2440 [51]. A β-oxidation metabolism in the R. eutropha mutant was studied for P(3HB-co-3-HHx) development from soybean oil [52]. The removal of fadB1 (enoyl-CoA hydratase/3HACoA dehydrogenase) in R. eutropha recombinant strains with additional genes generating (R)-enoyl-CoA hydratases resulted in a 6–21% 3-HHx content in the copolymer [52]. Figure 3 depicts how the metabolic pathway operates in the production of mcl-PHAs from sugars.
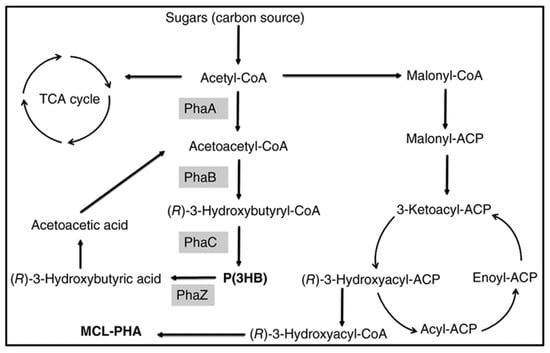
Figure 3.
Metabolic pathway involved in the synthesis of PHAs from sugars. PhaA: β-ketothiolase; PhaB: β-ketoacyl-CoA reductase; PhaC: PHA synthase; PhaZ: PHA depolymerase.
2.2.3. Organic Residues/Wastes and Others
PHA production was carried out using organic acids/or sugar-based compounds derived from renewable waste materials [53]. Davis et al. (2013) produced mcl-PHA (25–34%) from perennial ryegrass biomass using P. fluorescens 555 and P. putida W619 [54]. Awasthi et al. (2021) reported about the mcl-PHA production using watermelon waste residues [55]. Muhr et al. (2013 a,b) produced mcl-PHA (22–30% DCW) from P. chlororaphis DSM 50083 and animal-derived waste [6,56]. Grape pulp was used to produce mcl-PHA from P. resinovorans in a 3.7 L bioreactor [57]. Chicken feathers were used to produce mcl-PHA with P. putida KT2440, the polymer consisted of 3-HHx (27.2 mol %) and 3-HHo (72.8 mol %) monomer units [58]. Apple pomace, the residue which is left out after processing of apple serves as a potential carbon source to produce PHAs [59]. Blanco et al. (2021) reviewed the PHA production using various substrates [60]. Apart from the above-mentioned carbon sources, there is a scope to produce mcl-PHAs from waste materials such as food waste, molasses, lingo-cellulosic biomass, cannery waste, biodiesel industry waste, paper-mill wastewater, coffee waste, and cheese whey [61]. As an example, production from cheese whey shown in Figure 4. PHAs were produced using synthetic substrates, waste materials, and toxic compounds [62,63,64,65,66,67,68,69,70,71,72].
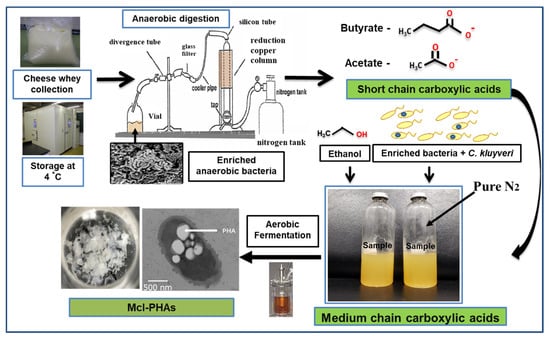
Figure 4.
Hypothetical flow chart describing steps involved in mcl-PHA production from cheese whey.
2.2.4. Vegetable Oils
Coconut oil containing both medium (C6–C14) and long (>C14) chain length fatty acids, lauric acid (C12:0) and myristic acid (C14:0) (up to 55%) are the main fatty acids [73]. Pseudomonas sp. is recognized to produce PHAs through fatty acids. Fatty acids are shortened by basically 2, 4, or 6 carbon atoms after each cycle of β-oxidation. In nature, Pseudomonas sp. is known for its adaptability, and it can make mcl-PHAs from a range of carbon feedstocks. PHAs (58% DCW) were produced in 20 L bioreactor in a batch mode by P. mendocina CH50 (NCIMB 10541) using coconut oil at a concentration of 20 g/L [74]. The optical density increased to 31 after 48 h, and the pH decreased slightly during fermentation, nitrogen content declined from 0.12 g/L to 0.015 g/L shows that the fermentation took place in a nitrogen-limiting environment. The mcl-PHAs terpolymer was composed of 3HO-3HD-3HDD. P(3HO-3HD-3HDD) has a molecular weight of 333 kDa and a PDI (polydispersity index) of 2.37, whereas mcl-PHAs with both unsaturated and saturated groups have an average molecular weight of 60–410 kDa [75]. Pseudomonas sp. Gl01 was used to produce mcl-PHA using palm oil and waste rapeseed oil. The purified polymers consisted of monomers ranging from C6 to C16 [76,77]. Song et al. (2008) used Pseudomonas sp. strain DR2 and the waste vegetable oil to produce mcl-PHA in the range of 24–38% [78]. Previous studies find out the importance of C/N balance for PHA production [79,80,81]. Carbon and nitrogen concentrations play a major role in PHA production. Mohan and Reddy (2013) applied design of experimental methodology using Taguchi orthogonal array to evaluate the influence and specific function of eight important factors and mentioned that glucose at 6 g/L and NH4Cl at 100 mg/L concentrations are best for higher PHA accumulation [80]. Reddy et al. (2015) reported that phaC gene expression was 5.37 folds higher at 100 mg/l nitrogen concentration than other concentrations [63]. The precursor molecules required for mcl-PHA synthesis in bacteria are generated through three different metabolic routes, which depend on the type of carbon source that existed in the medium. If carbohydrates are the main carbon source, the de novo fatty acid pathway is dominant, and fatty acids are the main carbon source, the β-oxidation pathway is dominant. The third pathway is chain elongation, and this pathway utilizes the precursors produced from both carbohydrates (acetyl-CoA) and fatty acids (acyl-CoA). All the three metabolic pathways generate different intermediate precursors—such as (R)-3-hydroxyacyl-acyl carrier protein, 2-trans-enoyl-CoA, (S)-3-hydroxyacyl-coA, and 3-ketoacyl-CoA—which are involved in the mcl-PHA synthesis. The hypothesis is that the (S)-3-hydroxyacyl-CoA, and 3-ketoacyl-CoA are subsequently converted to (R)-3-hydroxyacyl-CoA by the action of enzymes, 3-hydroxyacyl-coA epimerase, and 3-ketoacyl-ACP reductase respectively. The enzyme, (R)-specific enoyl-CoA hydratase (PhaJ)—which catalyzes the 2-trans-enoyl-CoA to (R)-3-hydroxyacyl-coA—plays a critical role in supplying monomer units from β-oxidation to PHA synthesis. (R)-3-hydroxyacyl-ACP-CoA transferase (PhaG), which has been identified in P. putida and P. aeruginosa plays, an important role in the metabolic connection of de novo fatty acid biosynthesis with mcl-PHA synthesis. PhaG catalyzes the conversion of (R)-3-hydroxyacyl-ACP to (R)-3-hydroxyacyl-CoA and contribute to mcl-PHA synthesis from gluconate or other carbohydrate sources. In the final step of mcl-PHA synthesis, the enzyme PHA synthase (PhaC) catalyzes the conversion of (R)-3-hydroxyacyl-CoA molecules into mcl-PHA [6,28,40,48,52,54,56].
3. Mcl-PHA Production at Various Modes of Operations
Mcl-PHA yields are optimized in batch, fed-batch, and continuous operation methods (Table 2). Sugar has been the most common carbon source used by most industries to manufacture PHAs during the last 20 years. Sugar can be produced from several biomass sources such as sugarcane, beet, molasses, and bagasse. They are plentiful and simple to obtain, and bacteria can swiftly digest and convert them to PHAs. Large PHA enterprises have selected this strategy due to the ample availability of raw materials and the ease of operation.

Table 2.
Mcl-PHA production at various modes of operations from literature reports.
3.1. Batch Mode
In batch mode, carbon/nitrogen sources will be added into the reactor at initial hours of incubation, and no extra nutrients will be introduced afterwards [90]. Batch fermentation procedures, in general, lead to a lower PHA yields. This is due to the breakdown of PHAs that produced in the cultivation process [91]. Under batch conditions, Rai et al. (2011) achieved a DCW of 0.8 g/L which contains 31% homopolymer, P3HO using P. mendocina [82].
3.2. Continuous Mode
This mode maintains a constant growth rate of microbes under ideal conditions. Consequently, by continuing to cultivate at high specific growth rates, production may be enhanced. Continuous culture is also beneficial since it reduces the need for bioreactor shutdowns and cleaning. Furthermore, the continuous culture prevents washout even at high dilution rates; therefore, product concentration and production may rise. The continuous culture is maintained by new media to the reactor, which feeds the cells with new nutrients. Products and effluent are continuously removed to maintain a consistent bioreactor workload. Jung et al. used P. oleovorans to derive mcl-PHAs from n-octane in a two-step continuous process. With two-stage fermentation, one can focus on accumulating PHAs in one reactor while accumulating biomass in another. Under these conditions, 18 g/L DCW, 63% of PHAs were achieved [83]. Egli et al. (1991) employed chemostat cultivation atmosphere to produce PHAs from P. putida GPo1 [92]. According to this study, PHAs may form if nitrogen limitation is applied [93].
3.3. Fed-Batch Mode
In the fed-batch method, cells proliferate until the desired cell density is attained with a steady supply of carbon sources and necessary nutrients. The feeding of nutrients and carbon sources maintains a consistent rate of growth, limiting the formation of by-products. There are two different types of feed-batch operations, one is the development of growth-related products, and the other one is product formation that occurs only under the non-growth associated conditions. PHA development usually occurs in two stages. First, the log stage is performed so that the cells gained the sufficient biomass. The second stage of polymer synthesis entails feeding all the essential materials into the bioreactor [94]. The second stage usually occurs when an essential nutrient, such as nitrogen, phosphorous, and oxygen limited conditions.
Most of the mcl-PHA production studies were conducted in a batch-fed mode. The pH and DO stats are utilized during the fermentation process to keep the pH and DO at specific levels. Lee et al. (2000) got 51% of PHAs, 141 g/L of DCW, using P. putida and oleic acid [84]. Davis et al. (2015) employed P. putida KT2440 in a two-stage fed-batch mode with glucose and nonanoic acid [13]. Cells were fed glucose during the biomass developing phase, while nonanoic acid was provided during the PHAs developmental phase and obtained 32% of PHAs, 102 g/L of DCW [13]. An oxygen-limited fed-batch growth approach with P. putida LS46 and octanoic acid as a substrate in a 7 L bioreactor achieved 29 g/L of DCW, 61% of PHA [30]. Octanoic acid toxicity in P. putida LS46 cells might explain the low biomass accumulation. Gao et al. (2016) created mcl-PHA using a mixture of decanoic and acetic acids in a fed-batch culture of P. putida KT2440 [85]. Acetic acid used to inhibit the crystallization of decanoic acid. Different glucose/acetic acid/decanoic acid ratios were used to find co-feed ratios that resulted in higher mcl-PHA yields. At the ideal ratio (4:1:5), 75 g/L of total DCW and 74% of PHA content were attained. Sun et al. (2009) demonstrated that P. putida KT2440 developed mcl-PHA by co-feeding glucose and nonanoic acid [31]. After exponential, as well as subsequent constant feed, with 1:1 (w/w) nonanoic acid: glucose, 71 g/L of total DCW and 56% of PHA were observed. Cerrone et al. (2014) demonstrated that the P. putida CA-3 HCDC was formed in a two-stage fermentation using decanoic and butyric acid [86]. To boost the maximal yield of mcl-PHA, the isolates were cultivated first on butyric acid (biomass growth phase) and then on a combination of butyric and decanoic acid (20:80 v/v ratio) during the PHA synthesis stage. This approach resulted in 71.3 g/L of DCW with 65% of PHA [86].
Sun et al. (2007) used the P. putida KT2440 to manufacture mcl-PHA from nonanoic acid, obtained 70 g/L of DCW with 75% PHAs [87]. Diniz et al. (2004) employed P. putida IPT 046 to explore various feeding patterns such as exponential preceded by constant feed for production of mcl-PHA [32]. The exponential feeding method yields a maximum 40 g/L DCW and a 21% of PHAs. However, when phosphate was limited, 50 g/L of DCW with 63% PHAs was obtained [32]. P. oleovorans ATCC 29347 was cultivated under pH-stat fed-batch conditions with octanoic acid as the carbon source and reported 63 g/L of DCW with 62% PHAs [88]. Kim et al. (1997) investigated a two-stage fed-batch approach using P. putida BM01 [89]. Glucose and octanoate were supplied in growth and PHAs development stages. This approach yielded 55 g/L of DCW with 66% of PHA. Higher DCW (125.6 g/L) with 55% PHAs observed using P. putida KT2440 [95].
4. Industries Producing PHAs
Scl-PHA are produced by various industries; however, very few industries produce mcl-PHAs. In this section, we provide information regarding industries that produce PHAs in various regions.
4.1. Europe
In 2007, Biomer Biotechnology Co. (Starnberg, Germany) produced 10 tonnes of Biomer bio polyester. Biomer’s objective has been to integrate with agricultural food industries, which they believe will offer the technology needed to create PHAs from its waste [96]. Bio-on founded LUX-ON with the purpose of generating PHAs using CO2 as organic load. The system also gathers sustainable solar energy to power the bioproduction process. Sugar cane molasses, sugar cane, food wastes, waste cooking oil, glycerol, and carbohydrates are among the feedstocks utilized [97]. Paques, a Dutch company, employs natural bacteria and methods to generate biodegradable PHBV biopolymers from waste streams [98]. Bioextrax is a Sweden-based company producing PHAs using bacteria from sucrose [99]. BASF is a German-based company producing biopolymer, Ecovio® with special material properties such as flexibility and toughness. Guzik (2021) summarized the recent developments occurred in Jerzy Haber Institute of Catalysis and Surface Chemistry of the Polish Academy of Sciences in collaboration with other groups [100].
4.2. Asia
Tianan Biologic [101] is a biopolymer specialist in the production and use of PHBV and PHB. To make the copolymer, C. necator is fermented with D-glucose and propionic acid. The fermentation facilities are in the city of Ningbo, China. Since its founding in 2000, the company has generally been recognized as the largest maker of PHBV, with an annual capacity of 2000 metric tons. In 2004, they were the first company in the world to commercially synthesize PHBV using water-based extraction technology. The extraction method has been patent-protected. Tianjin GreenBio Material Co. is China’s first company to produce 10,000 tonnes of PHAs per year. They have developed completely biodegradable granules for the manufacturing of blown film (SoGreen 2013). They also developed PHAs foam pellets, which can be converted into completely biodegradable foams for usage in food service and appliance packaging. The PHAs are created using a P(3HB-co-4HB) copolymer.
Mitsubishi (Tokyo, Japan) manufactures P(3HB) at both research and pilot scales under the brand name Biogreen® [102]. Kaneka Corporation (Tokyo, Japan) manufactures P(3HB-co-3HHx) under the brand names Kaneka PHBH® and AONILEX®, with an annual capacity of 100 tonnes. To make it, plant oils and fatty acids are used as the primary raw material in a microbial fermentation process [103]. Metabolix, located in Massachusetts, has completed the sale of its PHA biopolymer intellectual property to an associate company, CJ Cheil Jedang Co (Seoul, Korea) for 10 million USD. The acquisition includes patents on production and application, as well as microbes used in Metabolix’s manufacturing procedures [104].
4.3. North and South America
PHAs are being commercialized for high-value biological applications by several PHA manufacturers. These firms include Terra Verdae Bioworks (Edmonton, AB, Canada), PolyFerm (Kingston, ON, Canada), and Tepha Inc. (Lexington, MA, USA). Among the products provided are heart valves, scaffolds, ecological sutures, and materials for regulated distribution. These product categories have lower volume but higher profit margins. Polyferm Canada [105] produces mcl-PHAs under the brand name. Versa MerTM using naturally selected microorganisms and basic materials such as vegetable oils and sugars. The applications they are now working on include medical devices, sealants, adhesives, and polymer modifiers. Danimer Scientific manufactures produces PHAs under the trade name NodaxTM in the United States (Bainbridge, GA, USA). They entered the PHAs industry in 2007 after receiving knowledge from Procter & Gamble (Cincinnati, OH, USA). Danimer created a copolymer with a 3HB unit and a mcl-PHA repeat unit [106]. NodaxTM PHAs are manufactured from easily accessible feedstock and are completely renewable. Mango Materials, a company based in the United States, is producing PHAs from methane.
PHB Industrial S/A is a Brazil based company that manufactures and sells Biocycle® disposable polymers, PHB, and P (3HB-co-3HV) since September 2000 [107]. Until 2015, the firm functioned on a small scale and sold its commodities to Japan. Metabolix, Inc. released Yield10 in 2015, and it was listed on NASDAQ. PHB and its copolymers are manufactured by Yield10 Bioscience under the MillerTM brand [108]. MillerTM was marketed by Telles, a joint venture between Archer Daniels Midland Firm and Metabolix. TephaFLEX® produces P4HB. After detoxification, P4HB is converted into healthcare products such as films, sutures, and fabrics [109]. P4HB is digested to yield 4HB, a naturally occurring element of the vertebrate body. Tepha’s TephELAST is more elastic than TephaFLEX and has been employed in the production of medical devices. These polymers are created using pilot and research platforms. Newlight Technologies developed a unique biocatalyst to produce PHAs from CO2 and biogas using microorganisms obtained from the Pacific Ocean. Newlight created the AirCarbon polymer by combining methane from a California cattle farm with air [110,111].
5. News on PHAs
The PHAs market is predicted to reach 121 million USD by 2025, according to Markets and Markets [112]. The rising demand for PHAs in various industries—such as packaging, biomedical, and agricultural—is driving market expansion. Several factors will propel the PHAs sector, including public awareness of the depletion of hydrocarbons and the development of sustainable, eco-friendly bioplastics. Europe is the world’s largest PHAs market in terms of volume and value, followed by North America and Asia. PHAs are more expensive than standard polymers, which is one of the key impediments to the market’s growth. Biodegradable polymers, such as PHAs, have higher production costs than normal plastics, ranging from 20% to 80% more. This is mostly due to the high polymerization cost of biodegradable polymers, which is because most of the technologies are still in the research stage. These bio-based manufacturing methods and materials are still in the early stages of development and have not yet achieved the level of commercialization that their petroleum-based equivalents have.
The company Mars plans to reduce virgin plastic use by 25% by the year 2025 and make all plastic packaging reusable, recyclable, or biodegradable [113]. Mars is aiming for 100% biodegradable, recyclable, or biological plastic packaging by 2025, as well as a 25% reduction in virgin plastic consumption, a 30% average recycled content in plastic packaging, and recycling requirements for customers in all important markets. Maltesers replaced the plastic interior of the candy box with a water-based coating, reducing 82 metric tons of plastic waste. The first-ever Mars Wrigley Gum bottle with 30% recycled content is now available on the German market. This is an industry-leading move, reducing unused plastic use by approximately 350 tons annually. Orbit Megapack has released an on-pack recycling guide on How2Recycle, which provides a step-by-step guide on whether and how to recycle each part of the gum pack. Balisto has partnered with German retailer EDEKA Minden-Hannover to offer the first chocolate bar with paper-based packaging, reducing packaging plastic use by approximately 440 kg. M&M’s launched recyclable packaging in the M&M’s Choco 300 g pouch in France. Mars Wrigley partnered with Danimer Scientific, a leading developer and manufacturer of biodegradable materials, to develop an innovative home compostable packaging for its products. Colgate designed recyclable toothpaste tubes so they can go into curbside recycling bins. This could eventually keep a billion tubes out of landfills each year [114].
6. Extraction of PHAs
In terms of product quality, price, and environmental effect, downstream processing is a significant stage in PHAs manufacture. Following fermentation, there are two fundamental ways for recovering PHAs: (i) dissolve the biomass in acid, alkaline solutions, detergents, protease and extracting the pellets; or (ii) direct solvent separation of PHAs from the bacteria [115]. Enzymatic degradation of non-PHA biomass is one solvent-free approach for releasing PHA granules from cells. Certain proteins have been found on the membrane of PHA granules, such proteins include synthases, PHA depolymerize enzymes, polymer membrane expressed protein (e.g., phasins), and other proteins that influence pellet organelle distribution [116]. The chemical composition of PHAs determines their soluble nature in various solvents, mcl-PHAs are mostly soluble in a lot of solvents which include acetone and ethyl acetate. Another critical aspect of PHA pellet separation appears to be that the processes used must allow the polymers’ molecular size to change as little as possible.
6.1. Solvent Extraction
This was a popular technique because of its easy of usage with accessibility. Solvents induce the cellular surface to rupture, and increase diffusivity. Fluid interaction with PHA particles causes polymer solubilization. To recover the item, it should be precipitated with a non-solvent. Acetone and ethyl acetate are the solvents preferred for the separation of mcl-PHAs, whereas cold methanol or ethanol are acceptable non-solvents used for precipitation. The advantage of solvent separation is that it yields high-purity PHAs with nearly no change in particle size [117]. Many extraction methods use chloroform, although it is dangerous and increases the cost of the process. Endotoxin content is low in chloroform extracted PHAs, which is crucial for PHAs used in pharmaceutical applications. In several countries, chlorinated agents are no longer permitted in consumer goods. At room temperature, mcl-PHAs can be isolated using diethyl ether, tetrahydrofuran, or acetone [118]. Endotoxins (e.g., lipopolysaccharides) must be removed during PHA separation, especially if the product is to be used as a pharmaceutical. Thermally controlled separation procedures, as reported by Ferrur et al. (2007) for isolation of P(3HO-co-3HHx) from Pseudomonas putida GPo1, are one possible approach [119]. Organic solvents such as 2-propanol and n-hexane were used to achieve PHAs quality of more than 97% (w/w) and very less endotoxin limits (i.e., 10–15 endotoxin units per gram of PHO). PHO was also redissolved in 2-propanol at a temperature of 45 °C and precipitated at a temperature of 10 °C, providing a high purity product with low endotoxicity (2 per gram PHO). Green PHA isolation processes will necessitate less energy requirement, no harmful chemical usage, and excellent purity and yields [120]. Yabueng et al. (2018) examined green solvents such as 1,3-propanediol, 2-methyltetrahydrofuran, ethyl lactate, and 1,3-dioxolane for PHA separation from Cupriavidus necator [121].
6.2. Ultrasound-Assisted and Aqueous Two-Phase Extraction
Ishak et al. (2016) used ultra-high-frequency sound to irradiate mcl-PHAs with cells mixed with an excellent and minimal non-solute-solvent combination [122]. When that solvent is mixed in a sufficient proportion with a suitable solvent, the PHAs stay in suspension [123]. If the marginal solvent concentration is raised, the PHAs will be dissolved. These researchers evaluated impacts of ultrasonic irradiation maximal dissipation on energy, solvent/marginal non-solvent ratio, and time on mcl-PHA removal efficiencies. Furthermore, employing ultrasound during PHA extraction decreases solvent quantities, allowing safer solvents to be used, and it also shortens process times [124].
Aqueous two-phase extraction (ATPE) has the advantage of having a larger water concentration (up to 90% w/w), which makes biopolymer separation more ecologically friendly. Furthermore, the phase-separation components of ATPE can be harmless and relatively favorable. ATPE is said to be a scalable, cost-effective PHAs separation technique [9,125,126]. Leong et al. reexamined the parameters for purifying PHA from C. necator [127]. Additional centrifugation stages were avoided under these circumstances, and the recovery yield and purity factor increased by 72.2% and 1.61-fold, respectively [127]. As previously stated, non-PHA organic matter was removed to isolate PHAs from C. necator H16. The procedure was optimized using EOPO 3900 (5%), pH 6, and a fermenting temperature of 30 °C, purity factor and recovery yield were 1.36 and 97.6%, respectively [128].
6.3. Chemical and Enzymatic Digestion Method
By dissolving non-PHA cell material (NPCM), enzymatic and chemical digestion techniques aim to preserve unbroken PHA granules. The idea behind these methods is to break down microbial cell walls and release PHAs from the microorganism [129,130]. Early research looked towards removing NPCM from cells using powerful oxidizing agents such as sodium hydroxide and sodium hypochlorite. Extreme circumstances favorable for oxidation of both NPCM and PHAs—and therefore keen command of oxidizing agent content, heat generated, and reaction rate—is critical to this technique (i.e., reduced molecular weight). Dong et al. investigated surfactant and sodium hypochlorite mixtures for recovering PHAs from Azotobacter cerococcid G-3 [131]. Proteolytic enzymes catalyze hydrolytic processes involving proteins. According to preliminary research, lysozyme, bromelain, and trypsin are the most promising enzymes for this procedure. Yasotha et al. recovered and purified mcl-PHAs using alcalase, SDS, EDTA, and lysozyme [132]. Studies demonstrated that alcalase was the most important element in NPCM degradation and mcl-PHA purification. The counter flow filtration process successfully eliminated NPCM and allowed for high-purity mcl-PHA recovery [132]. PHA was purified by an enzyme method, according to Kachrimanidou et al. (2016) they developed a blend of unrefined enzyme to catalysis of C. necator lysis and release the PHAs compound using solid-state cultivation of Aspergillus oryzae. The enzymatic reaction was carried out at 48 °C with no control of pH. The product yield and quality of PHA achieved is 98% and 96.7%, respectively [133]. For the extraction of PHAs, Israni et al. took use of Streptomyces albus strong lytic activity [134]. S. albus and PHAs producing cultures are injected together into a reactor. The lytic enzymes from S. albus are added for PHA extraction in the second stage.
7. Properties of PHAs
One of the most significant characteristics for processing and producing polymers’ end-use is their thermal and physical qualities. The molecular structure of polymers is largely responsible for their physical and thermal characteristics. The physicochemical features of scl/mcl PHAs are distinct. Because PHAs are susceptible to increasing in temperature and breaking down during the melt process, their heat stability is essential. Plasticizers can help to prevent PHAs from degrading too much during the melting process [135]. Both P3HO and P3HO-P3HD have higher thermal degradation kinetic parameters than PHB and PHBV due to complexation on the development of six-membered transitional configuration. The thermal breakdown temperatures of P3HO with epoxy and hydroxy groups are varied. The P3HO with epoxy pendant groups might have a higher heat breakdown temperature after raising the temperature due to the bridging potential of epoxy groups.
7.1. Melting Point, Crystallization, Gas Barrier, and Solubility
Polymer performance is influenced by their crystallization kinetics. In PHAs, the length of the side chains can have a big impact on how they crystallize; for example, mcl-PHAs can form a layered crystal structure, increasing the side chain length of mcl-PHAs (e.g., C7 or more) and resulting in the formation of a new smectic liquid crystalline phase [136]. Both the main and side chains of mcl-PHAs with smectic crystals can cause cold crystallization during heating [32]; therefore, two melting points in mcl-PHAs have been found, which can be ascribed to the development of two distinct crystal phases (phase I and phase II) [137]. Crystallization rates have been shown to be improved by reactive chemical modification of PHAs. When compared to unmodified PHAs, modified PHAs with lengthy chain branching and low cross-linking densities demonstrated enhanced crystallization rates [138,139,140]. These chemical alterations also enhanced the rheological characteristics and processability of the material. Scl-PHAs require halogenated solvents to dissolve due to their high crystallinity [141]. The low crystallinity of mcl-PHAs permits them to be dissolved in non-halogenated solvents. The extraction, purification, and recycling of mcl-PHA copolymers are made easier and more cost-effective because of their solubility profile. Sobieski et al. (2017) reported the physical gel formation of P(3HB-co-3HHx) copolymer, and the gel’s thermal reversibility was established by heating it to 85 °C [142]. The composition of 3HHx in P(3HB-co-3HHx) might affect physical and mechanical strength, and rheological qualities in addition to polymer concentration. P(3HB-co-3HHx) gels with a low concentration of 3HHx (3.9 mol %) exhibit a greater opacity and better structure preservation when tilted than gels with a higher 3HHx content (13 mol %). After removing the solvent, the PHBHx gels revealed a sub-micron scale interconnected porous topology, indicating a viable material for biological applications such as tissue engineering [142].
When varying monomers with three carbon units, the melting point and crystallinity were reduced [143]. The quantity of side-chain carbons affects their melting and glass transition temperatures [144]. By copolymerizing P3HB with mcl-PHAs, the glass transition temperature of P3HB may be greatly decreased. The glass transition temperature of the mcl-PHAs generated from the C1–C7 side chain was between 0 °C and 50 °C, with a melting point of 175–69 °C [136]. Mcl-PHAs with slow crystallization rate and low melting point have limited melt processability; reactive modifications—including crosslinking, mixing with other polymers, and grafting techniques—can be used to solve these problems in the PHAs [145].
The oxygen and water vapor barrier qualities of polymers are important as their physical and thermal properties when it comes to using them for effective packaging applications such as food packing. When compared to traditional polymers, such as polypropylene and polyethylene, PHAs have better gas barrier qualities [146]. In particular, PHA copolymers have significantly better oxygen, carbon dioxide, and odor barrier characteristics than polyethylene and polypropylene [106]. PHBV has the best resistance to oxygen and water vapor penetration among biodegradable polymers [147]. When compared to PLA and PBS, the PHBV offers good potential for usage in fruit, and vegetable packaging as it has an appropriate oxygen and water vapor transfer rate [147]. However, the PHBV’s gas barrier qualities are insufficient for the packaging of meat, cheese, and coffee. Graphene oxide and clay supplementation to PHAs has been found to increase barrier characteristics by creating a convoluted route for gas molecules [148].
7.2. Mechanical Properties of PHAs
Because of their molecular weight and chemical makeup, the mechanical characteristics of PHAs can vary greatly. Tensile strength and Young’s modulus are 43 MPa and 3.5 GPa, respectively, for a typical P3HB with a molecular weight of less than 1000 Kda [149]. Because of secondary crystallization at room temperature, the brittleness of the P3HB was demonstrated to rise even more with age [150]. Copolymerization with other monomers can compensate for P3HB’s lack of toughness [149]. It was also shown that employing a modified E. coli strain to increase the molecular weight of the P3HB (2200 KDa) during synthesis might improve the mechanical qualities even further [151,152]. Molecular weight of the mcl-PHAs, varies from 45 to 462 Kda. Mw, Mn, and PDI of mcl-PHAs produced using various carbon sources and bacteria were summarized in Table 3. Mcl-PHAs are soft, flexible, sticky depending on the monomer composition. Copolymerization using as little as 5 mol % mcl monomers can improve the attributes of scl-PHAs (P3HB), such as toughness and thermal properties [143,153]. When P3HB was copolymerized with 17 mol % 3HHx, the elongation at break rose from 6% to 850%, while the P3HB’s tensile strength reduced from 43 to 20 MPa [153]. PHA copolymers with differing side group chain lengths or co-monomer compositions have variable modulus and elongation properties, as predicted.

Table 3.
Gel permeation chromatography (GPC) results of mcl-PHAs from literature reports.
8. Applications of PHAs
PHAs have shown considerable promise in various applications over the last two decades in the packaging, agricultural, and medical sectors in applications such as drug carriers, medical implants, and biocontrol agents [160]. The key criteria that govern the use of PHAs are their molecular weights and co-monomer concentration [143]. When compared to P3HB, P3HBV copolymer offers superior physical qualities such as impact resistance, hardness, flexibility, lower processing temperature, and a wider processing window [161]. Mcl-PHAs can be used as adhesives [162], coatings [163], plasticizers, medical devices, fibers [164], non-woven composites [165], and as bio-carriers in the slow release of fertilizers [166].
Crosslinking improves the mechanical qualities of PHAs, they can be employed in cartilage and ligament substitutes [167]. TEPHA Medical Devices Inc., based in the United States, has been manufacturing FDA-approved PHA sutures for medical absorbable sutures under the TephaFLEX® brand since 2007 [168]. Unilever developed and launched PHA micro powder-based sun creams in 2019 to improve sun creams’ water resistance while lowering their environmental effect. This sun lotion is a natural alternative to microbead-based sunscreens, which can damage the seas and oceans. Danimer Scientific and Cove have produced entirely biodegradable straw and bottles made of PHAs. Nestle has also teamed up with Danimer Scientific to develop biodegradable water bottles, with the goal of making all plastic packaging recyclable or reusable by 2025 [169]. Overall, PHAs have applications in plasticizers, fishing lines [170], wastewater treatment [171], food packaging, and fertilizers [172].
Research on mcl-PHAs and their derivatives, which are more elastomeric, has intensified in recent years. Due to the limitations of large-scale production of mcl-PHAs, most recent studies have focused on the copolymers P(3HB-co-3HHx) and P(3HO), which have better mechanical characteristics and many more elastomers than scl-PHAs in addition to having been manufactured in considerable amounts. Because many organs in the body have elastomeric qualities, mcl-PHA polymer scaffolds that can withstand and retake from numerous structures without harming other nearby tissue can be used. Tim et al. employed a polymeric scaffold made up of two components fashioned into a tubular conduit, the inner layer was made using a polyglycollic acid mesh and the outer layer was made with three layers of non-porous P(3HO-co-3HHx) with 10% 3HHx for tissue engineering studies [172].
P(3HB-co-3HHx) scaffolds can thus be employed for vascular grafting, according to these findings [173]. Sodian et al. conducted one of the first investigations employing an elastomeric P(3HO) for the creation of a tri-leaflet heart valve scaffold [174]. Based on the findings of these experiments, it was shown that P(3HO) manufactured TEHVs may be implanted in the pulmonary location and operate properly for 120 days in lambs [174]. Stock et al. (2000) investigated the possibility of developing three-leaflet, valved pulmonary conduits in lambs using autologous ovine vascular cells and thermoplastic P(3HO) [175]. Sodian et al. (2002) employed a new stereolithography manufacturing approach to create P(4HB) and P(3HO) scaffolds of cuspid and semilunar valves based on X-ray computed tomography and appropriate software [176]. Wu et al. (2007) created unique hybrid valves made of decellularized swine aortic valves covered with PVC (3HB-co-3HHx) [177]. Yang et al. (2002) conducted one of the first experiments on P(3HB-co-3HHx) as a nerve regeneration conduit material [178]. Ying et al. (2008) used electro spun copolymers of P(3HB-co-5 mol % 3HHx), P(3HB-co-7 mol % 4HB) and P(3HB-co-97 mol % 4HB) to make nanostructured fibrous scaffolds [179]. The generated scaffolds have tensile strength and Young’s modulus in the ranges of 5–30 and 15–150 MPa, which are equivalent to human skin. P3HO homopolymer fabricated 2D films were also investigated as a matrix material for skin tissue engineering. Wang et al. (2004) tested the in vitro biocompatibility of rabbit bone marrow cells injected on PLA, P3HB, and P(3HB-co-3HHx) 3D scaffolds [180]. On the P(3HB-co-3HHx) scaffolds, the cells proliferated the most. Several studies on three-dimensional polymer scaffold systems using a blend of P(3HB) and P(3HB-co-3HHx) for use as a matrix for cartilage tissue engineering have been conducted [181,182,183]. Wang et al. conducted one of the things is to first investigations of the use of PHA with dendrimer matrix for effective skin drug delivery systems (SDS) [184]. Vermeer et al. (2021) demonstrated a new application of PHAs in self-healing concrete [185]. Harazna et al. (2020) used ceramic-polymer bounded diclofenac with the biocompatible P(3HO) [186].
9. Conclusions and Future Perspectives
This review summarized the recent developments in mcl-PHA production using organic/inorganic carbon sources and various types of the fermentation strategies such as batch, continuous, and fed-batch. We also discussed downstream processing methods, properties and applications of mcl-PHAs, and their worldwide production at an industrial scale. Current mcl-PHA production through fermentation is facing several problems, such as high levels of foaming in the reactor due to toxicity of fatty acids which are used as a carbon source, inconsistency in the PHA titers (varying from 3 to 102 g/L), polymer property alterations due to fluctuations in molecular weights (from 45 to 462 Kda of Mw). Fatty acid (high) concentration in the fermentation broth inhibiting the growth of microorganisms and creating problems in down-stream processing, such as centrifugation and lyophilization. High-cell-density fermentation requires a continuous oxygen supply (7 L/min) which is expensive and creates safety issues in large-scale fermentation. Before scaling-up the mcl-PHA production process, researchers should focus on solving the above-mentioned problems by using recombinant strains and wild type bacteria other than Pseudomonas.
Author Contributions
Conceptualization, M.V.R. and Y.-C.C.; Writing—original draft preparation, V.U.N.R. and M.V.R.; Writing—review and editing, S.V.R. and Y.-C.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is contained within the article or corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Lemoigne, M. Dehydration and polymerization product of β-oxybutyric acid. Bull. Soc. Chim. Biol. 1926, 8, 770–782. [Google Scholar]
- Muhammadi, S.; Muhammad, A.; Shafqat, H. Bacterial polyhydroxyalkanoates-eco-friendly next generation plastic: Production, biocompatibility, biodegradation, physical properties and applications. Green Chem. Lett. Rev. 2015, 8, 56–77. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.Y.; Chang, H.N. Production of poly (hydroxy alkanoic acid). Adv. Biochem. Eng. Biotechnol. 1995, 52, 27–58. [Google Scholar]
- Choonut, A.; Prasertsan, P.; Klomklao, S.; Sangkharak, K. A Novel Green Process for Synthesis of 3-Hydroxyalkanoate Methyl Ester Using Lipase and Novel mcl-co-lcl PHA as Catalyst and Substrate. J. Polym. Environ. 2021, 30, 1423–1434. [Google Scholar] [CrossRef]
- Rakkan, T.; Chana, N.; Chirapongsatonkul, N.; Sangkharak, K. Screening and identification of newly isolated basic red 9-degrading bacteria from textile wastewater and their ability to produce medium-co-long-chain-length polyhydroxyalkanoates. J. Polym. Environ. 2022, 30, 415–423. [Google Scholar] [CrossRef]
- Muhr, A.; Rechberger, E.M.; Salerno, A.; Reiterer, A.; Schiller, M.; Kwicien, M.; Adamus, G.; Kowalczuk, M.; Strohmeier, K.; Schober, S.; et al. Biodegradable latexes from animal-derived waste: Biosynthesis and characterization of mcl-PHA accumulated by P. citronellolis. React. Funct. Polym. 2013, 73, 1391–1398. [Google Scholar] [CrossRef]
- Nitschke, M.; Costa, S.G.V.A.O.; Contiero, J. Rhamnolipids and PHAs: Recent reports on Pseudomonas-derived molecules of increasing industrial interest. Process Biochem. 2011, 46, 621–630. [Google Scholar] [CrossRef]
- Chen, G.-Q.; Jiang, X.-R. Engineering bacteria for enhanced polyhydroxyalkanoates (PHA) biosynthesis. Synth. Syst. Biotechnol. 2017, 2, 192–197. [Google Scholar] [CrossRef]
- Kourmentza, C.; Plácido, J.; Venetsaneas, N.; Burniol-Figols, A.; Varrone, C.; Gavala, H.N.; Reis, M.A.M. Recent advances and challenges towards sustainable polyhydroxyalkanoate (PHA) production. Bioengineering 2017, 4, 55. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.Y.; Wong, H.H.; Choi, J.; Lee, S.H.; Lee, S.C.; Han, C.S. Production of Medium-Chain-Length Polyhydroxyalkanoates by High-Cell-Density Cultivation of Pseudomonas putida Under Phosphorus Limitation. Biotechnol. Bioeng. 2000, 68, 466–470. [Google Scholar] [CrossRef]
- Riesenberg, D.; Guthke, R. High-cell-density cultivation of microorganisms. Appl. Microbiol. Biotechnol. 1999, 51, 422–430. [Google Scholar] [CrossRef]
- Cavalheiro, J.M.B.T.; Raposo, S.R.; Almeida, M.C.M.D.; Sevrin, M.T.C.C.; Grandfils, C.; Fonseca, M.M.R. Effect of cultivation parameters on the production of poly (3-hydroxybutyrate-co-4-hydroxybutyrate) and poly (3-hydroxybutyrate-4-hydroxybutyrate-3-hydroxyvalerate) by Cupriavidus necator using waste glycerol. Bioresour. Technol. 2012, 111, 391–397. [Google Scholar] [CrossRef] [PubMed]
- Davis, R.; Duane, G.; Kenny, S.T.; Cerrone, F.; Guzik, M.W.; Babu, R.P.; Casey, E.; O’Connor, K.E. High cell density cultivation of Pseudomonas putida KT2440 using glucose without the need for oxygen enriched air supply. Biotechnol. Bioeng. 2015, 112, 725–733. [Google Scholar] [CrossRef] [PubMed]
- Ryu, H.W.; Hahn, S.K.; Chang, Y.K.; Chang, H.N. Production of poly(3-hydroxybutyrate) by high cell density fed-batch culture of Alcaligenes eutrophus with phosphate limitation. Biotechnol. Bioeng. 1997, 55, 25–32. [Google Scholar] [CrossRef]
- Chen, G.-O.; Zhang, J. Microbial polyhydroxyalkanoates as medical implant biomaterials. Artif. Cells Nanomed. Biotechnol. 2018, 46, 1–18. [Google Scholar] [CrossRef]
- Williams, S.F.; Rizk, S.; Martin, D.P. Poly-4-hydroxybutyrate (P4HB): A new generation of resorbable medical devices for tissue repair and regeneration. Biomed. Eng. 2013, 58, 439–452. [Google Scholar] [CrossRef]
- Skibiński, S.; Cichoń, E.; Haraźna, K.; Marcello, E.; Roy, I.; Witko, M.; Ślósarczyk, A.; Czechowska, J.; Guzik, M.; Zima, A. Functionalized tricalcium phosphate and poly (3-hydroxyoctanoate) derived composite scaffolds as platforms for the controlled release of diclofenac. Ceram. Int. 2021, 47, 3876–3883. [Google Scholar] [CrossRef]
- Cichoń, E.; Haraźna, K.; Skibiński, S.; Witko, T.; Zima, A.; Ślósarczyk, A.; Zimowska, M.; Witko, M.; Leszczyński, B.; Wróbel, A.; et al. Novel bioresorbable tricalcium phosphate/polyhydroxyoctanoate (TCP/PHO) composites as scaffolds for bone tissue engineering applications. J. Mech. Behav. Biomed. Mater. 2019, 98, 235–245. [Google Scholar] [CrossRef]
- Zhang, J.; Cao, Q.; Li, S.; Lu, X.; Zhao, Y.; Guan, J.-S.; Chen, J.-C.; Wu, Q.; Chen, G.-Q. 3-Hydroxybutyrate methyl ester as a potential drug against Alzheimer’s disease via mitochondria protection mechanism. Biomaterials 2013, 34, 7552–7562. [Google Scholar] [CrossRef]
- Xiao, X.-Q.; Zhao, Y.; Chen, G.-Q. The effect of 3-hydroxybutyrate and its derivatives on the growth of glial cells. Biomaterials 2007, 28, 3608–3616. [Google Scholar] [CrossRef]
- Heinrich, D.; Raberg, M.; Fricke, P.; Kenny, S.T.; Gamez, L.M.; Babu, R.P.; O’Connor, K.; Steinbüchel, A. Synthesis gas (syngas)-derived medium-chain-length polyhydroxyalkanoate synthesis in engineered Rhodospirillum Rubrum. Appl. Environ. Microbiol. 2016, 82, 6132–6140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jaeger, K.E.; Steinbüchel, A.; Jendrossek, D. Substrate specificities of bacterial polyhydroxyalkanoate depolymerases and lipases: Bacterial lipases hydrolyze poly(ω-hydroxyalkanoates). Appl. Environ. Microbiol. 1995, 61, 3113–3118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Volova, T.G.; Kiselev, E.G.; Shishatskaya, E.I.; Zhila, N.O.; Boyandin, A.N.; Syrvacheva, D.A.; Vinogradova, O.N.; Kalacheva, G.S.; Vasiliev, A.D.; Peterson, I.V. Cell growth and accumulation of polyhydroxyalkanoates from CO2 and H2 of a hydrogen-oxidizing bacterium, Cupriavidus eutrophus B-10646. Bioresour. Technol. 2013, 146, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Volova, T.G.; Shishatskaya, E.I.; Zhila, N.O.; Shishatskii, O.N.; Kiselev, Y.G.; Mironov, P.V.; Vasiliev, A.D.; Peterson, I.V.; Sinskey, A.J. Fundamental basis of production and application of biodegradable polyhydroxyalkanoates. J. Sib. Fed. Univ. Biol. 2012, 3, 280–299. [Google Scholar]
- Tanaka, K.; Yoshida, K.; Orita, I.; Fukui, T. Biosynthesis of Poly (3-hydroxybutyrate-co-3-hydroxyhexanoate) from CO2 by a recombinant Cupriavidusnecator. Bioengineering 2021, 8, 179. [Google Scholar] [CrossRef]
- Löwe, H.; Hobmeier, K.; Moos, M.; Kremling, A.; Pflüger-Grau, K. Photoautotrophic production of polyhydroxyalkanoates in a synthetic mixed culture of Synechococcus elongatus cscB and Pseudomonas putida cscAB. Biotechnol. Biofuels 2017, 10, 1–14. [Google Scholar] [CrossRef]
- Riedel, S.L.; Bader, J.; Brigham, C.J.; Budde, C.F.; Yusof, Z.A.; Rha, C.; Sinskey, A.J. Production of poly (3-hydroxybutyrate- co-3-hydroxyhexanoate) by Ralstonia eutropha in high cell density palm oil fermentations. Biotechnol. Bioeng. 2012, 109, 74–83. [Google Scholar] [CrossRef] [Green Version]
- Ruiz, C.; Kenny, S.T.; Babu, P.R.; Walsh, M.; Narancic, T.; O’Connor, K.E. High cell density conversion of hydrolyzed waste cooking oil fatty acids into medium chain length polyhydroxyalkanoate using Pseudomonas putida KT2440. Catalysts 2019, 9, 468. [Google Scholar] [CrossRef] [Green Version]
- Jiang, X.J.; Sun, Z.; Ramsay, J.A.; Ramsay, B.A. Fed-batch production of MCL-PHA with elevated 3-hydroxynonanoate content. AMB Express 2013, 50, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Blunt, W.; Dartiailh, C.; Sparling, R.; Gapes, D.J.; Levin, D.B.; Cicek, N. Development of high cell density cultivation strategies for improved medium chain length polyhydroxyalkanoate productivity using Pseudomonas putida LS46. Bioengineering 2019, 6, 89. [Google Scholar] [CrossRef] [Green Version]
- Sun, Z.; Ramsay, J.A.; Guay, M.; Ramsay, B.A. Enhanced yield of medium-chain-length polyhydroxyalkanoates from nonanoic acid by co-feeding glucose in carbon-limited, fed-batch culture. J. Biotechnol. 2009, 143, 262–267. [Google Scholar] [CrossRef] [PubMed]
- Diniz, S.C.; Taciro, M.K.; Gomez, J.G.C.; Pradella, J.G. High-cell-density cultivation of Pseudomonas putida IPT 046 and medium-chain-length polyhydroxyalkanoate production from sugarcane carbohydrates. Appl. Biochem. Biotech. 2004, 119, 1–69. [Google Scholar] [CrossRef]
- Kellerhals, M.B.; Hazenberg, W.; Witholt, B. High cell density fermentations of Pseudomonas oleovorans for the production of mcl-PHAs in two-liquid phase media. Enz. Microbiol. Technol. 1999, 24, 111–116. [Google Scholar] [CrossRef]
- Kellerhals, M.B.; Kessler, B.; Witholt, B.; Tchouboukov, A.; Brand, H. Renewable long-chain fatty acids for production of biodegradable medium-chain-length polyhydroxyalkanoates (mcl-PHAs) at laboratory and pilot plant scales. Macromolecules 2000, 33, 4690–4698. [Google Scholar] [CrossRef]
- Preusting, H.; Hazenberg, W.; Witholt, B. Continuous production of poly(3-hydroxyalkanoates) by Pseudomonas oleovorans in a high-ceil-density, two-liquid-phase chemostat. Enzym. Microb. Technol. 1993, 15, 311–316. [Google Scholar] [CrossRef]
- Preusting, H.; Houten, R.; Hoefs, A.; Langenberghe, E.K.; Favre-Bulle, O.; Witholt, B. High cell density cultivation of Pseudomonas oleovorans: Growth and production of poly (3-hydroxyalkanoates) in two-liquid phase batch and fed-batch systems. Biotechnol. Bioeng. 1993, 41, 550–556. [Google Scholar] [CrossRef] [PubMed]
- Riedel, S.L.; Jahns, S.; Koenig, S.; Bock, M.C.; Brigham, C.J.; Bader, J.; Stahl, U. Polyhydroxyalkanoates production with Ralstonia eutropha from low quality waste animal fats. J. Biotechnol. 2015, 214, 119–127. [Google Scholar] [CrossRef]
- Sato, S.; Maruyama, H.; Fujiki, T.; Matsumoto, K. Regulation of 3-hydroxyhexanoate composition in PHBH synthesized by recombinant Cupriavidus necator H16 from plant oil by using butyrate as a co-substrate. J. Biosci. Bioeng. 2015, 120, 246–251. [Google Scholar] [CrossRef]
- Cai, L.; Yuan, M.Q.; Liu, F.; Jian, J.; Chen, G.Q. Enhanced production of medium-chain-length polyhydroxyalkanoates (PHA) by PHA depolymerase knockout mutant of Pseudomonas putida KT2442. Bioresour. Technol. 2009, 100, 2265–2270. [Google Scholar] [CrossRef]
- LeMeur, S.; Zinn, M.; Egli, T.; Thöny-Meyer, L.; Ren, Q. Production of medium-chain-length polyhydroxyalkanoates by sequential feeding of xylose and octanoic acid in engineered Pseudomonas putida KT2440. BMC Biotechnol. 2012, 12, 53. [Google Scholar]
- Kahar, P.; Tsuge, T.; Taguchi, K.; Doi, Y. High yield production of polyhydroxyalkanoates from soybean oil by Ralstonia eutropha and its recombinant strain. Polym. Degrad. Stab. 2004, 83, 79–86. [Google Scholar] [CrossRef]
- Jing, H.; Yuan-Zheng, Q.; Dai-Cheng, L.; Guo-Qiang, C. Engineered Aeromonas hydrophila for enhanced production of poly (3-hydroxybutyrate-co-3-hydroxyhexanoate) with alterable monomers composition. FEMS Microbiol. Lett. 2004, 239, 195–201. [Google Scholar]
- Ouyang, S.; Han, J.; Qiu, Y.; Qin, L.; Chen, S.; Wu, Q.; Leski, M.L.; Chen, G. Poly (3-hydroxybutyrate-co-3-hydroxyhexanoate) production in recombinant Aeromonas hydrophila 4ak4 harboring phba, phbb and vgb genes. Macromol. Symp. 2005, 224, 21–34. [Google Scholar] [CrossRef]
- Budde, C.F.; Riedel, S.L.; Willis, L.B.; Rha, C.; Sinskey, A.J. Production of poly (3-hydroxybutyrate-co-3-hydroxyhexanoate) from plant oil by engineered Ralstonia eutropha strains. Appl. Environ. Microbiol. 2011, 77, 2847–2854. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thinagaran, L.; Sudesh, K. Evaluation of sludge palm oil as feedstock and development to efficient method for its utilization to produce polyhydroxyalkanoate. Waste Biomass Valoriz. 2017, 10, 709–720. [Google Scholar] [CrossRef]
- Tufail, S.; Munir, S.; Jamil, N. Variation analysis of bacterial polyhydroxyalkanoates production using saturated and unsaturated hydrocarbons. Braz. J. Microbiol. 2017, 48, 629–636. [Google Scholar] [CrossRef]
- Qiu, Y.Z.; Han, J.; Guo, J.J.; Chen, G.Q. Production of poly (3-hydroxybutyrate-co-3-hydroxyhexanoate) from gluconate and glucose by recombinant Aeromonas hydrophila and Pseudomonas putida. Biotechnol. Lett. 2005, 27, 1381–1386. [Google Scholar] [CrossRef]
- Poblete-Castro, I.; Rodriguez, A.L.; Lam, C.M.; Kessler, W. Improved production of medium-chain-length polyhydroxyalkanoates in glucose-based fed-batch cultivations of metabolically engineered Pseudomonas putida strains. J. Microbiol. Biotechnol. 2014, 24, 59–69. [Google Scholar] [CrossRef] [Green Version]
- Zhao, F.; He, F.; Liu, X.; Shi, J.; Liang, J.; Wang, S.; Yang, C.; Liu, R. Metabolic engineering of Pseudomonas mendocina NK-01 for enhanced production of medium-chain-length polyhydroxyalkanoates with enriched content of the dominant monomer. Int. J. Biol. Macromol. 2020, 154, 1596–1605. [Google Scholar] [CrossRef]
- Liu, C.; Qi, L.; Yang, S.; He, Y.; Jia, X. Increased sedimentation of a Pseudomonas–Saccharomyces microbial consortium producing medium chain length polyhydroxyalkanoates. Chin. J. Chem. Eng. 2019, 27, 1659–1665. [Google Scholar] [CrossRef]
- Oliveira, G.H.D.; Zaiat, M.; Rodrigues, J.A.D.; Ramsay, J.A.; Ramsay, B.A. Towards the production of mcl-pha with enriched dominant monomer content: Process development for the sugarcane biorefinery context. J. Polym. Environ. 2020, 28, 844–853. [Google Scholar] [CrossRef]
- Insomphun, C.; Mifune, J.; Orita, I.; Numata, K.; Nakamura, S.; Fukui, T. Modification of β-oxidation pathway in Ralstonia eutropha for production of poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) from soybean oil. J. Biosci. Bioeng. 2014, 117, 184–190. [Google Scholar] [CrossRef]
- Serafim, L.S.; Lemos, P.C.; Albuquerque, M.G.; Reis, M.A. Strategies for PHA production by mixed cultures and renewable waste materials. Appl. Microbiol. Biotechnol. 2008, 81, 615–628. [Google Scholar] [CrossRef] [PubMed]
- Davis, R.; Kataria, R.; Cerrone, F.; Woods, T.; Kenny, S.; O’Donovan, A.; Guzik, M.; Shaikh, H.; Duane, G.; Gupta, V.K.; et al. Conversion of grass biomass into fermentable sugars and its utilization for medium chain length polyhydroxyalkanoate (mcl-PHA) production by Pseudomonas strains. Bioresour. Technol. 2013, 150, 202–209. [Google Scholar] [CrossRef] [PubMed]
- Awasthi, M.K.; Kumar, V.; Yadav, V.; Sarsaiya, S.; Awasthi, S.K.; Sindhu, R.; Zhang, Z. Current state of the art biotechnological strategies for conversion of watermelon wastes residues to biopolymers production: A review. Chemosphere 2022, 290, 133310. [Google Scholar] [CrossRef]
- Muhr, A.; Rechberger, E.M.; Salerno, A.; Reiterer, A.; Malli, K.; Strohmeier, K.; Koller, M. Novel description of mcl-PHA biosynthesis by Pseudomonas chlororaphis from animal-derived waste. J. Biotechnol. 2013, 165, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Andler, R.; Valdés, C.; Urtuvia, V.; Andreeßen, C.; Díaz-Barrera, A. Fruit residues as a sustainable feedstock for the production of bacterial polyhydroxyalkanoates. J. Clean. Prod. 2021, 307, 127236. [Google Scholar] [CrossRef]
- Pernicova, I.; Enev, V.; Marova, I.; Obruca, S. Interconnection of waste chicken feather biodegradation and keratinase and mcl-PHA production employing Pseudomonas putida KT2440. Appl. Food Biotechnol. 2019, 6, 83–90. [Google Scholar]
- Liu, H.; Kumar, V.; Jia, L.; Sarsaiya, S.; Kumar, D.; Juneja, A.; Awasthi, M.K. Biopolymer poly-hydroxyalkanoates (PHA) production from apple industrial waste residues: A review. Chemosphere 2021, 284, 131427. [Google Scholar] [CrossRef]
- Blanco, F.G.; Hernández, N.; Rivero-Buceta, V.; Maestro, B.; Sanz, J.M.; Mato, A.; Prieto, M.A. From residues to added-value bacterial biopolymers as nanomaterials for biomedical applications. Nanomaterials 2021, 11, 1492. [Google Scholar] [CrossRef]
- Yadav, B.; Pandey, A.; Kumar, L.R.; Tyagi, R.D. Bioconversion of waste (water)/residues to bioplastics-A circular bioeconomy approach. Bioresour. Technol. 2020, 298, 122584. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.C.; Venkateswar Reddy, M.; Imura, K.; Onodera, R.; Kamada, N.; Sano, Y. Two-Stage polyhydroxyalkanoates (PHA) production from cheese whey using Acetobacter pasteurianus C1 and Bacillus sp. CYR1. Bioengineering 2021, 8, 157. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.C.; Venkateswar Reddy, M.; Choi, D.B. Cometabolic degradation of toxic trichloroethene or cis-1,2-dichloroethene with phenol and production of poly-β-hydroxybutyrate (PHB). Green Chem. 2021, 23, 2729–2737. [Google Scholar] [CrossRef]
- Reddy, M.V.; Watanabe, A.; Onodera, R.; Mawatari, Y.; Tsukiori, Y.; Watanabe, A.; Kudou, M.; Chang, Y.C. Polyhydroxyalkanoates (PHA) production using single or mixture of fatty acids with Bacillus sp. CYR1: Identification of PHA synthesis genes. Bioresour. Technol. Rep. 2020, 11, 100483–100491. [Google Scholar] [CrossRef]
- Reddy, M.V.; Mawatari, S.; Onodera, R.; Nakamura, Y.; Yajima, Y.; Chang, Y.C. Bacterial conversion of waste into polyhydroxybutyrate (PHB): A new approach of bio-circular economy for treating waste and energy generation. Bioresour. Technol. Rep. 2019, 7, 100246–100254. [Google Scholar] [CrossRef]
- Reddy, M.V.; Mawatari, Y.; Onodera, R.; Nakamura, Y.; Yajima, Y.; Chang, Y.C. Polyhydroxyalkanoates (PHA) production from synthetic waste using Pseudomonas pseudoflava: PHA synthase enzyme activity analysis from P. pseudoflava and P. palleronii. Bioresour. Technol. 2017, 234, 99–105. [Google Scholar] [CrossRef] [Green Version]
- Reddy, M.V.; Mawatari, Y.; Yajima, Y.; Satoh, K.; Venkata Mohan, S.; Chang, Y.C. Production of poly-3-hydroxybutyrate (P3HB) and poly-3-(hydroxybutyrate-co-hydroxyvalerate) P(3HB-co-3HV) from synthetic wastewater using Hydrogenophaga palleronii. Bioresour. Technol. 2016, 215, 155–162. [Google Scholar] [CrossRef] [Green Version]
- Amulya, K.; Venkateswar Reddy, M.; Rohit, M.V.; Mohan, S.V. Wastewater as renewable feedstock for polyhydroxyalkanoates (PHA) production: Understanding the role of reactor microenvironment and system pH. J. Clean. Prod. 2016, 112, 4618–4627. [Google Scholar] [CrossRef]
- Amulya, K.; Venkateswar Reddy, M.; Mohan, S.V. Acidogenic spent wash valorization through polyhydroxyalkanoate (PHA) synthesis coupled with fermentative biohydrogen production. Bioresour. Technol. 2014, 158, 336–342. [Google Scholar] [CrossRef]
- Reddy, M.V.; Mohan, S.V. Influence of aerobic and anoxic microenvironments on polyhydroxyalkanoates (PHA) production from food waste and acidogenic effluents using aerobic consortia. Bioresour. Technol. 2012, 103, 313–321. [Google Scholar] [CrossRef]
- Srikanth, S.; Venkateswar Reddy, M.; Mohan, S.V. Microaerophilic microenvironment at biocathode enhances electrogenesis with simultaneous synthesis of polyhydroxyalkanoates (PHA) in bioelectrochemical system (BES). Bioresour. Technol. 2012, 125, 291–299. [Google Scholar] [CrossRef] [PubMed]
- Mohan, S.V.; Venkateswar Reddy, M.; Subhash, G.V.; Sarma, P.N. Fermentative effluents from hydrogen producing bioreactor as substrate for poly (β-OH) butyrate production with simultaneous treatment: An integrated approach. Bioresour. Technol. 2010, 101, 9382–9386. [Google Scholar] [CrossRef] [PubMed]
- Appaiah, P.; Sunil, L.; Prasanth Kumar, P.K.; Gopala Krishna, A.G. Composition of Coconut Testa, Coconut Kernel and its Oil. J. Am. Oil Chem. Soc. 2014, 91, 917–924. [Google Scholar] [CrossRef]
- Basnett, P.; Marcello, E.; Lukasiewicz, B.; Panchal, B.; Nigmatullin, B.; Knowles, J.C.; Roy, I. Biosynthesis and characterization of a novel, biocompatible medium chain length polyhydroxyalkanoate by Pseudomonas mendocina CH50 using coconut oil as the carbon source. J. Mater. Sci. Mater. Med. 2018, 29, 1–11. [Google Scholar] [CrossRef]
- Valappil, S.P.; Misra, S.K.; Boccaccini, A.R.; Roy, I. Biomedical applications of polyhydroxyalkanoates (PHAs), an overview of animal testing and in vivo responses. Expert Rev. Med. Devices 2006, 3, 853–868. [Google Scholar] [CrossRef]
- Możejko, J.; Ciesielski, S. Saponified waste palm oil as an attractive renewable resource for mcl-polyhydroxyalkanoate synthesis. J. Biosci. Bioeng. 2013, 116, 485–492. [Google Scholar] [CrossRef]
- Mozejko, J.; Wilke, A.; Przybylek, G.; Ciesielski, S. Mcl-PHAs produced by Pseudomonas sp. Gl01 using fed-batch cultivation with waste rapeseed oil as carbon source. J. Microbiol. Biotechnol. 2012, 22, 371–377. [Google Scholar] [CrossRef] [Green Version]
- Song, J.H.; Jeon, C.O.; Choi, M.H.; Yoon, S.C.; Park, W.J. Polyhydroxyalkanoate (PHA) production using waste vegetable oil by Pseudomonas sp. strain DR2. J. Microbiol. Biotechnol. 2008, 18, 1408–1415. [Google Scholar]
- Reddy, M.V.; Yajima, Y.; Mawatari, Y.; Hoshino, T.; Chang, Y.C. Degradation and conversion of toxic compounds into useful bioplastics by Cupriavidus sp. CY-1: Relative expression of PhaC gene under phenol and nitrogen stress. Green Chem. 2015, 17, 4560–4569. [Google Scholar] [CrossRef] [Green Version]
- Mohan, S.V.; Venkateswar Reddy, M. Optimization of critical factors to enhance polyhydroxyalkanoates (PHA) synthesis by mixed culture using Taguchi design of experimental methodology. Bioresour. Technol. 2013, 128, 409–416. [Google Scholar] [CrossRef]
- Reddy, M.V.; Mohan, S.V. Effect of substrate load and nutrients concentration on the Polyhydroxyalkanoates (PHA) production using mixed consortia through wastewater treatment. Bioresour. Technol. 2012, 114, 573–582. [Google Scholar] [CrossRef] [PubMed]
- Rai, R.; Keshavarz, T.; Roether, J.A.; Boccaccini, A.R.; Roy, L. Medium chain length polyhydroxyalkanoates, promising new biomedical materials for the future. Mater. Sci. Eng. R Rep. 2011, 72, 29–47. [Google Scholar] [CrossRef]
- Jung, K.; Hazenberg, W.; Prieto, M.; Witholt, B. Two-stage continuous process development for the production of medium-chain-length poly(3-hydroxyalkanoates). Biotech. Bioeng. 2001, 71, 19–24. [Google Scholar] [CrossRef]
- Lee, M.Y.; Park, W.H.; Lenz, R.W. Hydrophilic bacterial polyesters modified with pendant hydroxyl groups. Polymer 2000, 41, 1703–1709. [Google Scholar] [CrossRef]
- Gao, J.; Ramsay, J.A.; Ramsay, B.A. Fed-batch production of poly-3-hydroxydecanoate from decanoic acid. J. Biotechnol. 2016, 218, 102–107. [Google Scholar] [CrossRef]
- Cerrone, F.; Duane, G.; Casey, E.; Davis, R.; Belton, I.; Kenny, S.T.; Guzik, M.W.; Woods, T.; Babu, R.P.; O’Connor, K. Fed-batch strategies using butyrate for high cell density cultivation of Pseudomonas putida and its use as a biocatalyst. Appl. Microbiol. Biotechnol. 2014, 98, 9217–9228. [Google Scholar] [CrossRef]
- Sun, Z.; Ramsay, J.A.; Guay, M.; Ramsay, B.A. Carbon-limited fed-batch production of medium-chain-length polyhydroxyalkanoates from nonanoic acid by Pseudomonas putida KT2440. Appl. Microbiol. Biotechnol. 2007, 74, 69–77. [Google Scholar] [CrossRef]
- Kim, B.S. Production of medium chain length polyhydroxyalkanoates by fed-batch culture of Pseudomonas oleovorans. Biotechnol. Lett. 2002, 24, 125–130. [Google Scholar] [CrossRef]
- Kim, G.J.; Lee, I.Y.; Yoon, S.C.; Shin, Y.C.; Park, Y.H. Enhanced yield and a high production of medium-chain-length poly(3-hydroxyalkanoates) in a two-step fed-batch cultivation of Pseudomonas putida by combined use of glucose and octanoate. Enzym. Microb. Technol. 1997, 20, 500–505. [Google Scholar] [CrossRef]
- Kaur, G.; Roy, I. Strategies for large-scale production of polyhydroxyalkanoates. Chem. Biochem. Eng. Q. 2015, 29, 157–172. [Google Scholar] [CrossRef]
- Zinn, M.; Witholt, B.; Egli, T. Occurrence, synthesis and medical application of bacterial polyhydroxyalkanoate. Adv. Drug. Del. Rev. 2001, 53, 5–21. [Google Scholar] [CrossRef]
- Egli, T. On multiple-nutrient-limited growth of microorganisms, with special reference to dual limitation by carbon and nitrogen substrates. Antonie Van Leeuwenhoek 1991, 60, 225–234. [Google Scholar] [CrossRef] [PubMed]
- Huijberts, G.N.M.; Eggink, G. Production of poly(3-hydroxyalkanoates) by Pseudomonas putida KT2442 in continuous cultures. Appl. Microbiol. Biotechnol. 1996, 46, 233–239. [Google Scholar] [CrossRef]
- McNeil, B.; Harvey, L.M. Fermentation, a Practical Approach; IRL: Tokyo, Japan, 1990. [Google Scholar]
- Andin, N.; Longieras, A.; Veronese, T.; Marcato, F.; Molina-Jouve, C.; Uribelarrea, J.-L. Improving carbon and energy distribution by coupling growth and medium chain length polyhydroxyalkanoate production from fatty acids by Pseudomonas putida KT2440. Biotechnol. Bioprocess Eng. 2017, 22, 308–318. [Google Scholar] [CrossRef]
- Bio-On Declares Bankruptcy. Available online: http://www.plasticsnews.com/news/bio-declares-bankruptcy (accessed on 3 January 2020).
- A Bio-on e a Hera Criam a Lux-on, o Novo Desafio Para Produzir Bioplástico a Partir de CO2. Available online: http://www.bio-on.it/project.php?lin=portoghese (accessed on 10 December 2018).
- Upcycling Waste to Natural Biopolymers. Available online: http://www.paquesbiomaterials.nl (accessed on 10 December 2018).
- Bioextrax develops world-leading bio-based technologies – accelerating the transition to a sustainable global economy. Available online: http://bioextrax.com (accessed on 25 March 2022).
- Guzik, M.W. Polyhydroxyalkanoates, bacterially synthesized polymers, as a source of chemical compounds for the synthesis of advanced materials and bioactive molecules. Appl. Microbiol. Biotechnol. 2021, 105, 7555–7566. [Google Scholar] [CrossRef]
- Titan An Biopolymers/Enmat. Available online: http://www.tianan-enmat.com/ (accessed on 25 March 2022).
- Mitsubishi Gas Chemical (MGC). Available online: https://www.mgc.co.jp/ (accessed on 25 March 2022).
- Completion of Kaneka Biodegradable Polymer PHBH™ Plant with Annual Production of 5,000 Tons. Available online: http://www.kaneka.co.jp/en/service/news/nr20191219/ (accessed on 19 December 2019).
- CJ Acquires Intellectual Property and Lab Equipment from US Venture Firm. Available online: http://www.ajudaily.com/view/20160823101739592 (accessed on 23 August 2016).
- Polymeron. Available online: http://www.polymerofcanada.com/versamerphas.html (accessed on 25 March 2022).
- Noda, I.; Lindsey, S.B.; Caraway, D. NodaxTM class PHA copolymers: Their properties and applications. In Plastics from Bactria: Natural Functions and Applications; Chen, G.-Q., Ed.; Springer: Berlin/Heidelberg, Germany, 2010; pp. 237–255. [Google Scholar]
- Biocycle. Available online: http://ww.biocycle.com.br (accessed on 25 March 2022).
- Yield10 BIOSCIENCE. Available online: http://www.yield10bio.com (accessed on 25 March 2022).
- Tepha Medical Devices. Tepha Medical Devices. Available online: http://www.tepha.com/technology/polymer-processing-material-attributes (accessed on 25 March 2022).
- Jennifer, B.; Emily, G. Plastic from thin air. Popul.Sci. 2014, 285, 24. [Google Scholar]
- Can Plastic Be Made Environmentally Friendly? Available online: http://www.scientificamerican.com/article/can-plastic-be-made-environmentally-friendly (accessed on 29 January 2014).
- Polyhydroxyalkanoate (PHA) Market by Type (Short Chain Length, Medium Chain Length), Production Method (Sugar Fermentation, Vegetable Oil Fermentation, Methane Fermentation), Application, and Region - Global Forecast to 2025. Available online: http://www.marketsandmarkets.com/Market-Reports/pha-market-395.html (accessed on 1 February 2021).
- MARS. Available online: http://www.mars.com/sustainability-plan/healthy-planet/sustainable-packaging (accessed on 25 March 2022).
- After 149 Years, Colgate’s Toothpaste Tubes are Finally Recyclable. Available online: http://www.fastcompany.com/90713412/after-149-years-colgates-toothpaste-tubes-are-finally-recyclable (accessed on 19 January 2022).
- Kosseva, M.R.; Rusbandi, E. Trends in the biomanufacturer of polyhydroxyalkanoates with focus on downstream processing. Int. J. Biol. Macromol. 2018, 107, 762–778. [Google Scholar] [CrossRef]
- Bresan, S.; Sznajder, A.; Hauf, W.; Forchhammer, K.; Pfeiffer, D.; Jendrossek, D. Polyhydroxyalkanoate (PHA) granules have no phospholipids. Sci. Rep. 2016, 6, 26612. [Google Scholar] [CrossRef] [Green Version]
- Jacquel, N.; Lo, C.W.; Wei, Y.H.; Wu, H.S.; Wang, S.S. Isolation and purification of bacterial poly (3-hydroxyalkanoates). Biochem. Eng. J. 2008, 39, 15–27. [Google Scholar] [CrossRef]
- Koller, M.; Niebelschütz, H.; Braunegg, G. Strategies for recovery and purification of poly[(R)-3-hydroxyalkanoates] (PHA) bio polyesters from surrounding biomass. Eng. Life Sci. 2013, 13, 549–562. [Google Scholar] [CrossRef]
- Furrer, P.; Panke, S.; Zinn, M. Efficient recovery of low endotoxin medium-chain length poly([R]-3-hydroxyalkanoate) from bacterial biomass. J. Microbiol. Methods 2007, 69, 206–213. [Google Scholar] [CrossRef] [PubMed]
- Chemat, F.; Vian, M.A.; Cravotto, G. Green extraction of natural products: Concept and principles. Int. J. Mol. Sci. 2012, 13, 8615–8627. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yabueng, N.; Napathorn, S.C. Toward non-toxic and simple recovery process of poly (3-hydroxybutyrate) using the green solvent 1,3-dioxolane. Process Biochem. 2018, 69, 197–207. [Google Scholar] [CrossRef]
- Ishak, K.A.; Annuar, M.S.M.; Heidelberg, T.; Gumel, A.M. Ultrasound-assisted rapid extraction of bacterial intra cellular medium- chain-length poly(3-hydroxyalkanoates) (mcl-phas) in medium mixture of solvent/marginal non-solvent. Arab. J. Sci. Eng. 2016, 41, 33–44. [Google Scholar] [CrossRef]
- Noda, I. Process for Recovering Polyhydroxyalkanotes Using Air Classification. U.S. Patent US5849854A, 15 December 1998. [Google Scholar]
- Vilkhu, K.; Mawson, R.; Simons, L.; Bates, D. Applications and opportunities for ultrasound assisted extraction in the food industry-A review. Innov. Food Sci. Emerg. Technol. 2008, 9, 161–169. [Google Scholar] [CrossRef]
- Leong, Y.K.; Koroh, F.E.; Show, P.L.; Lan, J.C.W.; Loh, H.S. Optimization of extractive bioconversion for green polymer via aqueous two-phase system. Chem. Eng. Trans. 2015, 45, 1495–1500. [Google Scholar]
- Leong, Y.K.; Lan, J.C.W.; Loh, H.S.; Ling, T.C.; Ooi, C.W.; Show, P.L. Cloud-point extraction of green-polymers from Cupriavidus necator lysate using thermo separating-based aqueous two-phase extraction. J. Biosci. Bioeng. 2016, 123, 3270–3375. [Google Scholar]
- Leong, Y.K.; Show, P.L.; Lan, J.C.-W.; Loh, H.-S.; Yap, Y.-J.; Ling, T.C. Extraction and purification of polyhydroxyalkanoates (PHAs): Application of thermo separating aqueous two-phase extraction. J. Polym. Res. 2017, 24, 158. [Google Scholar] [CrossRef]
- Leong, K.; Show, P.L.; Lan, J.C.W.; Krishna Moorthy, R.; Chu, D.T.; Nagarajan, D.; Yen, H.W.; Chang, J.S. Application of thermo-separating aqueous two-phase system in extractive bioconversion of polyhydroxyalkanoates by Cupriavidus necator H16. Bioresour. Technol. 2019, 287, 121474. [Google Scholar] [CrossRef]
- López-Abelairas, M.; García-Torreiro, M.; Lú-Chau, T.; Lema, J.M.; Steinbüchel, A. Comparison of several methods for the separation of poly(3-hydroxybutyrate) from Cupriavidus necator H16 cultures. Biochem. Eng. J. 2015, 93, 250–259. [Google Scholar] [CrossRef]
- Gumel, A.M.; Annuar, M.S.M.; Chisti, Y. Recent advances in the production, recovery, and applications of polyhydroxyalkanoates. J. Polym. Environ. 2013, 21, 580–605. [Google Scholar] [CrossRef]
- Dong, Z.; Sun, X.; Zhaolin, D.; Xuenan, S.U.N. A new method of recovering polyhydroxyalkanoate from Azotobacter chroococcum. Chin. Sci. Bull. 2000, 45, 252–256. [Google Scholar] [CrossRef]
- Yasotha, K.; Aroua, M.K.; Ramachandran, K.B.; Tan, I.K.P. Recovery of medium-chain-length polyhydroxyalkanoates (PHAs) through enzymatic digestion treatments and ultrafiltration. Biochem. Eng. J. 2006, 30, 260–268. [Google Scholar] [CrossRef]
- Kachrimanidou, V.; Kopsahelis, N.; Vlysidis, A.; Papanikolaou, S.; Kookos, I.K.; Martínez, B.M.; Rondan, M.C.E.; Kautinas, A.A. Downstream separation of poly(hydroxyalkanoates) using crude enzyme consortia produced via solid state fermentation integrated in a biorefinery concept. Food Bioprod. Process 2016, 100, 323–334. [Google Scholar] [CrossRef]
- Israni, N.; Thapa, S.; Shivakumar, S. Biolytic extraction of poly (3-hydroxybutyrate) from Bacillus megaterium Ti3 using the lytic enzyme of Streptomyces albus Tia1. J. Genet. Eng. Biotechnol. 2018, 16, 265–271. [Google Scholar] [CrossRef]
- Panaitescu, D.M.; Lupescu, I.; Frone, A.N.; Chiulan, I.; Nicolae, C.A.; Tofan, V.; Stefaniu, A.; Somoghi, R.; Trusca, R. Medium chain-length polyhydroxyalkanoate copolymer modified by bacterial cellulose for medical devices. Biomacromolecules 2017, 18, 3222–3232. [Google Scholar] [CrossRef]
- Abe, H.; Ishii, N.; Sato, S.; Tsuge, T. Thermal properties and crystallization behaviors of medium-chain-length poly (3-hydroxyalkanoate) s. Polymer 2012, 53, 3026–3034. [Google Scholar] [CrossRef]
- Chen, G.Q. A microbial polyhydroxyalkanoates (PHA) based bio and material industry. Chem. Soc. Rev. 2009, 38, 2434–2446. [Google Scholar] [CrossRef]
- Gopi, S.; Kontopoulou, M.; Ramsay, B.A.; Ramsay, J.A. Manipulating the structure of medium-chain-length polyhydroxyalkanoate (MCL-PHA) to enhance thermal properties and crystallization kinetics. Int. J. Biol. Macromol. 2018, 119, 1248–1255. [Google Scholar] [CrossRef]
- Nerkar, M.; Ramsay, J.A.; Ramsay, B.A.; Kontopoulou, M. Melt compounded blends of short and medium chain-length poly-3-hydroxyalkanoates. J. Polym. Environ. 2014, 22, 236–243. [Google Scholar] [CrossRef]
- Xiang, H.; Chen, W.; Chen, Z.; Sun, B.; Zhu, M. Significant accelerated crystallization of long chain branched poly (3-hydroxybutyrate-co-3-hydroxyvalerate) with high nucleation temperature under fast cooling rate. Compos. Sci. Technol. 2017, 142, 207–213. [Google Scholar] [CrossRef]
- Noda, I.; Schechtman, L.A. Solvent extraction of polyhydroxyalkanoates from biomass. U.S. Patent 5,942,597, 24 August 1999. [Google Scholar]
- Sobieski, B.J.; Gong, L.; Aubuchon, S.R.; Noda, I.; Chase, D.B.; Rabolt, J.F. Thermally reversible physical gels of poly[(R)-3-hydroxybutyrate-co-(R)-3-hydroxyhexanoate]: Part 1 gelation in dimethylformamide. J. Polym. 2017, 131, 217–223. [Google Scholar] [CrossRef]
- Noda, I.; Green, P.R.; Satkowski, M.M.; Schechtman, L.A. Preparation and properties of a novel class of polyhydroxyalkanoate copolymers. Biomacromolecules 2005, 6, 580–586. [Google Scholar] [CrossRef]
- Tanadchangsaeng, N.; Tsuge, T.; Abe, H. Comonomer compositional distribution, physical properties, and enzymatic degradability of bacterial poly (3-hydroxybutyrate-co-3-hydroxy-4-methylvalerate) polyesters. Biomacromolecules 2010, 11, 1615–1622. [Google Scholar] [CrossRef] [PubMed]
- Kolahchi, A.R.; Kontopoulou, M. Chain extended poly (3-hydroxybutyrate) with improved rheological properties and thermal stability, through reactive modification in the melt state. Polym. Degrad. Stab. 2015, 121, 222–229. [Google Scholar] [CrossRef] [Green Version]
- Muthuraj, R.; Misra, M.; Mohanty, A.K. Biodegradable compatibilized polymer blends for packaging applications: A literature review. J. Appl. Polym. Sci. 2018, 135, 45726. [Google Scholar] [CrossRef] [Green Version]
- Wu, F.; Misra, M.; Mohanty, A.K. Challenges and new opportunities on barrier performance of biodegradable polymers for sustainable packaging. Prog. Polym. Sci. 2021, 117, 101395. [Google Scholar] [CrossRef]
- Xu, P.; Yang, W.; Niu, D.; Yu, M.; Du, M.; Dong, W.; Chen, M.; Lemstra, P.J.; Ma, P. Multifunctional and robust polyhydroxyalkanoate nanocomposites with superior gas barrier, heat resistant and inherent antibacterial performances. Chem. Eng. J. 2020, 382, 122864. [Google Scholar] [CrossRef]
- Choi, S.Y.; Cho, I.J.; Lee, Y.; Kim, Y.; Kim, K.; Lee, S.Y. Microbial polyhydroxyalkanoates and nonnatural polyesters. Adv. Mater. 2020, 32, 1907138. [Google Scholar] [CrossRef]
- Bugnicourt, E.; Cinelli, P.; Lazzeri, A.; Alvarez, V.A. Polyhydroxyalkanoate (PHA): Review of synthesis, characteristics, processing and potential applications in packaging. Express Polym. Lett. 2014, 8, 791–808. [Google Scholar] [CrossRef] [Green Version]
- Kusaka, S.; Abe, H.; Lee, S.; Doi, Y. Molecular mass of poly [(R)-3-hydroxybutyric acid] produced in a recombinant Escherichia coli. Appl. Microbiol. Biotechnol. 1997, 47, 140–143. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Lee, S.Y. High level production of supra molecular weight poly (3-hydroxybutyrate) by metabolically engineered Escherichia coli. Biotechnol. Bioprocess Eng. 2004, 9, 196–200. [Google Scholar] [CrossRef]
- Doi, Y.; Kitamura, S.; Abe, H. Microbial synthesis and characterization of poly (3-hydroxybutyrate-co-3-hydroxyhexanoate). Macromolecules 1995, 28, 4822–4828. [Google Scholar] [CrossRef]
- Acuña, J.M.; Rohde, M.; Saldias, C.; Castro, I.P. Fed-Batch mcl-Polyhydroxyalkanoates production in Pseudomonas putida KT2440 and phaZ mutant on biodiesel-derived crude glycerol. Front. Bioeng. Biotechnol. 2021, 9, 1–10. [Google Scholar]
- Dartiailh, C.; Blunt, W.; Sharma, P.K.; Liu, S.; Cicek, N.; Levin, D.B. The thermal and mechanical properties of medium chain-length polyhydroxyalkanoates produced by Pseudomonas putida LS46 on various substrates. Front. Bioeng. Biotechnol. 2021, 8, 1–9. [Google Scholar] [CrossRef]
- Liu, W.; Chen, G.Q. Production and characterization of mediumchain-length polyhydroxyalkanoate with high 3-hydroxytetradecanoate monomer content by fadB and fadA knockout mutant of Pseudomonas putida KT2442. Appl. Microbiol. Biotechnol. 2007, 76, 1153–1159. [Google Scholar] [CrossRef]
- Solaiman, D.K.Y.; Ashby, R.D.; Foglia, T.A. Physiological characterization and genetic engineering of Pseudomonas corrugata for medium-chain-length polyhydroxyalkanoates synthesis from triacylglycerols. Curr. Microbiol. 2002, 44, 189–195. [Google Scholar] [CrossRef]
- Ashby, R.D.; Foglia, T.A. Poly(hydroxyalkanoate) biosynthesis from triglyceride substrates. Appl. Microbiol. Biotechnol. 1998, 49, 431–437. [Google Scholar] [CrossRef]
- Hazer, D.B.; Hazer, B.; Kaymaz, F. Synthesis of microbial elastomers based on soybean oily acids. Biocompatibility studies. Biomed. Mater. 2009, 4, 1–9. [Google Scholar] [CrossRef]
- Kalia, V.C.; Patel, S.K.S.; Shanmugam, R.; Lee, J.K. Polyhydroxyalkanoates: Trends and advances toward biotechnological applications. Bioresour. Technol. 2021, 326, 124737. [Google Scholar] [CrossRef]
- Dill, S.; Demicheva, M.; Fleschutz, B.; Weinlein, R.; Demicheva, M.; Fleschutz, B.; Weinlein, R. Influence of polyhydroxybutyrate content on the crystallization behavior of polyamide 6-polyhydroxy-butyrate blends. Macromol. Symp. 2019, 384, 1800170. [Google Scholar] [CrossRef]
- Wang, S.; Chen, W.; Xiang, H.; Yang, J.; Zhou, Z.; Zhu, M. Modification and potential application of short-chain-length polyhydroxyalkanoate (SCL-PHA). Polymers 2016, 8, 273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rastogi, V.; Samyn, P. Bio-based coatings for paper applications. Coatings 2015, 5, 887–930. [Google Scholar] [CrossRef] [Green Version]
- Xiang, H.; Chen, Z.; Zheng, N.; Zhang, X.; Zhu, L.; Zhou, Z.; Zhu, M. Melt-spun microbial poly (3-hydroxybutyrate-co-3-hydroxyvalerate) fibers with enhanced toughness: Synergistic effect of heterogeneous nucleation, long-chain branching and drawing process. Int. J. Biol. Macromol. 2019, 122, 1136–1143. [Google Scholar] [CrossRef] [PubMed]
- Wagner, A.; Poursorkhabi, V.; Mohanty, A.K.; Misra, M. Analysis of porous electrospun fibers from poly (l-lactic acid)/Poly (3-hydroxybutyrate-co-3-hydroxyvalerate) blends. ACS Sustain. Chem. Eng. 2014, 2, 1976–1982. [Google Scholar] [CrossRef]
- Ivanov, V.; Stabnikov, V.; Ahmed, Z.; Dobrenko, S.; Saliuk, A. Production, and applications of crude polyhydroxyalkanoate-containing bioplastic from the organic fraction of municipal solid waste. Int. J. Environ. Sci. Technol. 2015, 12, 725–738. [Google Scholar] [CrossRef] [Green Version]
- Ang, S.L.; Sivashankari, R.; Shaharuddin, B.; Chuah, J.-A.; Tsuge, T.; Abe, H.; Sudesh, K. Potential applications of polyhydroxyalkanoates as a biomaterial for the aging population. Polym. Degrad. Stab. 2020, 181, 109371. [Google Scholar] [CrossRef]
- Tepha Medical Devices Overview. Available online: http://www.tepha.com/technology/overview/ (accessed on 25 March 2022).
- Development of Biodegradable Water Bottle. Available online: http://www.foodpackagingforum.org/news/development-of-biodegradable-water-bottle (accessed on 23 January 2019).
- Wang, Y.; Yin, J.; Chen, G.-Q. Polyhydroxyalkanoates, challenges and opportunities. Curr. Opin. Biotechnol. 2014, 30, 59–65. [Google Scholar] [CrossRef]
- Choonut, A.; Prasertsan, P.; Klomklao, S.; Sangkharak, K. An environmentally friendly process for textile wastewater treatment with a medium-chain-length polyhydroxyalkanoate film. J. Polym. Environ. 2021, 29, 3335–3346. [Google Scholar] [CrossRef]
- Tim, D.S.; Stock, U.; Hrkach, J.; Shinoka, T.; Lien, B.; Moses, M.A.; Stamp, A.; Taylor, G.; Moran, A.M.; Landis, W.; et al. Tissue engineering of autologous aorta using a new biodegradable polymer. Ann. Thorac. Surg. 1999, 68, 2298–2305. [Google Scholar]
- Zhang, L.; Zheng, Z.; Xi, J.; Gao, Y.; Ao, Q.; Gong, Y.; Zhao, N.; Zhang, X. Improved mechanical property and biocompatibility of poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) for blood vessel tissue engineering by blending with poly(propylene carbonate). Eur. Polym. J. 2007, 43, 2975–2986. [Google Scholar] [CrossRef]
- Sodian, R.; Hoerstrup, S.P.; Sperling, J.S.; Daebritz, S.; Martin, D.P.; Moran, A.M.; Kim, B.S.; Schoen, F.J.; Vacanti, J.P.; Mayer Jr, J.E. Early in vivo experience with tissue-engineered trileaflet heart valves. Circulation 2007, 102, 22–29. [Google Scholar]
- Stock, U.A.; Nagashima, M.; Kahalil, P.N.; Nollert, G.D.; Herden, T.; Sperling, J.S.; Moran, A.M.; Lien, B.; Martin, D.P.; Schoen, F.J.; et al. Tissue-engineered valved conduits in the pulmonary circulation. J. Thorac. Cardiovasc. Surg. 2000, 119, 732–740. [Google Scholar] [CrossRef] [Green Version]
- Sodian, R.; Loebe, M.; Hein, A.; Martin, D.P.; Hoerstrup, S.P.; Potapov, E.V.; Hausmann, H.; Leuth, T.; Hetzer, R. Application of stereolithography for scaffold fabrication for tissue engineered heart valves. ASAIO J. 2002, 48, 12–16. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Liu, Y.L.; Cui, B.; Qu, X.H.; Chen, G.Q. Study on decellularized porcine aortic valve/poly (3-hydroxybutyrate-co-3-hydroxyhexanoate) hybrid heart valve in sheep model. Artif. Organs 2007, 31, 689–697. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Li, X.; Li, G.; Zhao, N.; Zhang, X. Study on chitosan and PHBHHx used as nerve regeneration conduit material. J. Biomed. Eng. 2002, 19, 25–29. [Google Scholar]
- Ying, H.T.; Ishii, D.; Mahara, A.; Murakami, S.; Yamaoka, T.; Sudesh, K.; Samian, R.; Fujita, M.; Maeda, M.; Iwata, T. Scaffolds from electrospun polyhydroxyalkanoate copolymers: Fabrication, characterization, bioabsorption and tissue response. Biomaterials 2008, 29, 1307–1317. [Google Scholar] [CrossRef]
- Wang, Y.W.; Wu, Q.O.; Chen, G.Q. Attachment, proliferation and differentiation of osteoblasts on random biopolyester poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) scaffolds. Biomaterials 2004, 25, 669–675. [Google Scholar] [CrossRef]
- Zhao, K.; Deng, Y.; Chen, J.C.; Chen, G.Q. Polyhydroxyalkanoate (PHA) scaffolds with good mechanical properties and biocompatibility. Biomaterials 2003, 24, 1041–1054. [Google Scholar] [CrossRef]
- Deng, Y.; Zhao, K.; Zhang, X.F.; Hu, P.; Chen, G.Q. Study on the three-dimensional proliferation of rabbit articular cartilage-derived chondrocytes on polyhydroxyalkanoate scaffolds. Biomaterials 2002, 23, 4049–4056. [Google Scholar] [CrossRef]
- Deng, Y.; Lin, X.J.; Zheng, Z.; Deng, J.G.; Chen, J.C.; Ma, H.; Chen, G.Q. Poly(hydroxybutyrate-co-hydroxyhexanoate) promoted production of extracellular matrix of articular cartilage chondrocytes in vitro. Biomaterials 2003, 24, 4273–4281. [Google Scholar] [CrossRef]
- Wang, Z.; Itoh, Y.; Hosaka, Y.; Kobayashi, I.; Nakano, Y.; Maeda, I.; Umeda, F.; Yamakawa, J.; Kawase, M.; Yagi, K. Novel transdermal drug delivery system with polyhydroxyalkanoate and starburst polyamidoamine dendrimer. J. Biosci. Bioeng. 2003, 95, 541–543. [Google Scholar] [CrossRef]
- Vermeer, C.M.; Rossi, E.; Tamis, J.; Jonkers, H.M.; Kleerebezem, R. From waste to self-healing concrete: A proof-of-concept of a new application for polyhydroxyalkanoate. Resour. Conserv. Recycl. 2021, 164, 105206. [Google Scholar] [CrossRef]
- Haraźna, K.; Cichoń, E.; Skibiński, S.; Witko, T.; Solarz, D.; Kwiecień, I.; Guzik, M. Physicochemical and biological characterisation of diclofenac oligomeric poly (3-hydroxyoctanoate) hybrids as β-TCP ceramics modifiers for bone tissue regeneration. Int. J. Mol. Sci. 2020, 21, 9452. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).