Abstract
Current work investigates the fabrication and performance of nanocomposite membranes, modified with varying concentrations of hybrid nanostructures comprising titanium nanowires coated with iron nanoparticles (TiO2 NWs-Fe2O3), for the removal of Naphthol Blue Black (NBB) dye from industrial wastewater. A series of analytical tools were employed to confirm the successful modification including scanning electron microscopy and EDX analysis, porosity and hydrophilicity measurements, Fourier-transform infrared spectroscopy, and X-Ray Diffraction. The incorporation of TiO2 NWs-Fe2O3 has enhanced membrane performance significantly by increasing the PWF and improving dye retention rates of nanocomposite membranes. At 0.7 g of nanostructure content, the modified membrane (M8) achieved a PWF of 93 L/m2·h and NBB dye rejection of over 98%. The flux recovery ratio (FRR) analysis disclosed improved antifouling properties, with the M8 membrane demonstrating a 73.4% FRR. This study confirms the potential of TiO2 NWs-Fe2O3-modified membranes in enhancing water treatment processes, offering a promising solution for industrial wastewater treatment. These outstanding results highlight the potential of the novel PES-TiO2 NWs-Fe2O3 membranes for dye removal and present adequate guidance for the modification of membrane physical properties in the field of wastewater treatment.
1. Introduction
Over the past century, rapid industrialization, particularly in the textile and pigment sectors, has generated massive environmental concerns. The textile industry is a major contributor to effluent wastewater due to its high water consumption during various wet processing operations [1]. This wastewater contains a range of chemicals, including acids, alkalis, dyes, hydrogen peroxide, surfactants, dispersing agents, and metallic soaps. In terms of environmental impact, the textile industry is one of the largest water consumers globally, and the majority of its wastewater is highly contaminated [2]. On average, textile mills use approximately 200 L of water per kilogram of fabric processed daily. According to estimates by the World Bank, textile dyeing and finishing contribute to around 17–20% of global industrial wastewater [3]. Once released into aquatic systems, these dyes pose significant threats to marine life by reducing dissolved oxygen (DO) levels due to light blockage and hindrance of photochemical processes. This, in turn, leads to elevated chemical oxygen demand (COD) and biochemical oxygen demand (BOD) in affected water sources. Research reveals that COD levels in textile effluents can range between 150 and 30,000 mg/L, while BOD levels can vary between 80 and 6000 mg/L, causing serious risks to both ecosystems and human health [4]. Therefore, removing these pollutants from industrial wastewater is crucial for diminishing environmental impacts and supporting water resource sustainability and the circular economy.
Limitless research efforts have focused on developing efficient and economical methods for treating textile wastewater [5,6]. A wide spectrum of processes are available for removing contaminants from water, including physical, chemical, biological, and combined treatment methods. Globally, stricter ecological standards are being applied to every stage of textile production [7]. Physical techniques such as adsorption, ion exchange, irradiation, and filtration are widely used in wastewater treatment plants, while chemical methods like flocculation and coagulation are favored for their simplicity and cost-effectiveness despite certain limitations. In addition, biological treatments have emerged as eco-friendly approaches to dye removal, offering cost savings and optimal operating conditions [8]. Among these methods, membrane technology has shown great promise for dye removal from industrial wastewater. Membrane filtration, which separates particles from liquid solutions or gas mixtures, provides several advantages, such as low operational costs, energy efficiency, compact design, and the absence of chemical additives [9]. However, membrane fouling, caused by contaminant accumulation on the surface or within membrane pores, remains a significant challenge.
In recent years, nanocomposite membranes have gained attention in water treatment due to their ability to enhance membrane performance. These membranes, containing hydrophilic nanomaterials such as carbon nanotubes, graphene oxide, zinc oxide, silver and copper nanoparticles, and titanium dioxide (TiO2), have been shown to address the limitations of conventional membrane processes [6,10,11,12,13,14,15,16,17,18]. Incorporating nanofillers into polymeric membranes improves hydrophilicity, rejection efficiency, fouling resistance, and thermal and mechanical stability [19,20]. Moreover, combining different nanomaterials within a single nanocomposite structure has imparted promising results in various fields, including membrane technology [21,22,23,24]. Research indicates that these composite materials not only retain the properties of the individual nanomaterials but also introduce novel features not available in their counterparts. For example, Kumar et al. (2025) reported that TiO2-Fe2O3 nanocomposites demonstrated superior photocatalytic activity for decolorizing Titan Yellow (TY) and Methyl Orange (MO) dyes under UV light [25]. Similarly, another work conducted by Radwan et al. (2025) manifested that biosynthesized ZnO/CuO nanocomposites effectively removed Congo Red (CR) dye, with a removal capacity of 90.14 mg g−1 [26]. These studies suggest that nanocomposite materials are cost-effective and environmentally sustainable solutions for industrial wastewater treatment, particularly in water purification and pollution control applications.
In the current work, we present novel TiO2 NWs-Fe2O3/PES as hybrid nanocomposite membranes working towards enhancing the trade-off between separation and permeation flux with outstanding antifouling features. The TiO2 NWs-Fe2O3 hybrid nanostructure offers a synergistic advantage over individual components such as TiO2 nanoparticles NPs, TiO2 NWs, or Fe2O3 NPs as standalone nano-additives. The integration of TiO2 NWs, known for their high aspect ratio and superior photocatalytic activity, with Fe2O3, which exhibits strong adsorption affinity for anionic dyes and enhances visible-light absorption, contributes to improved pollutant degradation efficiency, enhanced dispersion within the PES matrix, and greater stability under operational conditions. Herein, NBB dye was harnessed as an organic foulant model for the separation and antifouling performance. The design and structure of these nanocomposite membranes were optimized by incorporating a wide range of the hybrid nanostructure content into the polymeric matrix. Comprehensive characterization was performed to confirm the successful modification and to assess the impact of the nanocomposite on the membrane’s surface properties, contributing to the advancement of more sustainable and efficient textile water treatment technologies.
2. Materials and Methods
2.1. Materials
Polyethersulfone (PES, UltrasonE6020P) with a molecular weight of 51 kDa was obtained from BASF, Steinheim, (Germany). Polyethylene glycol 600 was purchased from Central Drug House, (India). Dimethylformamide (DMF, purity 99.5%) and hydrochloric acid (HCl purity 37%) were purchased from Sigma-Aldrich, Steinheim, (Germany). Titanium dioxide (TiO2) (P25) with an average particle size of 21 nm, ammonium hydroxide (NH4OH, 25%), and sodium hydroxide (NaOH) were purchased from Sigma Aldrich (Budapest, Nagy Diófa u. 7, Hungary). Potassium hydroxide (KOH) was supplied by Thomasker Co. (Budapest, Cziráki u. 26 32, Hungary), while iron chloride hexahydrate (FeCl3·6H2O) was obtained from Scharlab (4034 Debrecen, Vágóhíd u. 2Hungary).
2.2. Preparation of Nanostructure
The solvothermal process was used for the synthesis of TiO2 NWs, and the impregnation technique was applied to produce the final inorganic composite as TiO2 NW-Fe2O3. More details about the synthesis process of the composite nanostructure were described in our previous work [27]. Briefly, for the preparation of TiO2 NW-Fe2O3 nanocomposites, a certain amount of FeCl3·6H2O was dissolved in a mixture of 100 mL of distilled water and 100 mL of EtOH, respectively. In the next step, TiO2 NWs were added to the solutions and stirred for 1 h, then transferred to an autoclave. The products obtained were washed to adjust the pH to 7 and then calcinated for 2 h at 500 °C using a static furnace. The load of the Fe2O3 nanoparticles in the final nanocomposite was designated to be 5 w/w%.
2.3. Preparation of Hybrid Nanocomposite Membranes
Pristine PES and TiO2 NWs-Fe2O3 nanocomposite membranes were synthesized via the conventional phase inversion technique. The compositions of the membranes are given in Table 1 below. In summary, 2 wt.% of PEG and predetermined concentrations of TiO2 NWs-Fe2O3 were dispersed in DMF solvent through bath sonication for 1 h. Subsequently, 20 wt.% of PES was gradually added to the suspension, and the mixture was stirred continuously for 5 h at 50 °C. The resulting casting solution was then degassed under vacuum effect to trap formed air bubbles. The dope solution was then cast onto a clean and smooth glass substrate with a 200 µm gap, harnessing an automatic casting machine (CX4mtv esstechnik, Erftstadt (Gymnich) (Germany). The membranes were immersed in a tap water coagulation bath for phase separation. Finally, the membranes were thoroughly rinsed and kept in a hydrated state for future use.

Table 1.
Casting solutions for the control PES and modified membranes with TiO2 NWs/Fe2O3.
3. Characterization
3.1. Nanocomposite Membrane and Nanostructure Characterization
Field Emission Scanning Electron Microscopy (FESEM) TESCAN-VEGA3SB, supplied by EO, Elektronen-Optik Services GmbH, Dortmund, Germany, was employed to examine the surface and cross-sectional morphology of both control and TiO2 NWs/Fe2O3 nanocomposite membranes. The samples were prepared by preparing 1 × 4 cm strips, fracturing them in liquid nitrogen, and coating them with a thin gold layer to enhance image quality. Additionally, elemental composition analysis was performed using energy dispersive X-ray spectroscopy (EDS) (Oxford Instruments, Oxford, UK) integrated with the FESEM system.
Fourier-transform infrared (FTIR) spectroscopy (Nicolet 6700 instrument, Thermo Electrons Corp., Waltham, MA, USA) was employed to identify the functional groups present in the membranes. The spectra were collected covering the spectral range from 4000 to 400 cm−1. A Single Reflection Diamond ATR accessory was used, which allowed for direct analysis without requiring any sample preparation.
The wettability of the fabricated membranes was assessed using the sessile drop technique. A 5 µL drop of deionized (DI) water was carefully deposited onto the flat surface of the membrane, and the contact angle (CA) between the water droplet and the membrane surface was measured with a contact angle analyzer (CAM 110-O4W, Tainan, Taiwan). For accuracy, at least five measurements were taken from three different samples, and the average value was reported.
The porosity of the membranes was measured through gravimetric analysis. Membrane samples were first soaked in DI water for two hours, then removed and carefully wiped with gentle tissue to eliminate excess water before weighing. After drying the samples in an oven for one hour, their dry weight was recorded. The porosity was then calculated using Equation (1). For each membrane sample, five porosity measurements were taken, and the average value was reported.
where ε represents the porosity, Wd and Ww are the dry and wet weights of the membrane, respectively, A is the membrane’s surface area (cm2), ρ is the density of water (1 g/cm3), and L is the membrane thickness (cm).
X-ray diffraction (XRD, Philips PW1730, PHILIPS, Amsterdam, The Netherlands) was used to analyze the crystalline structure of TiO2 NWs/Fe2O3. The scans were carried out over a 2-theta range of 10° to 80° with a scanning speed of 0.05° per minute, utilizing CuKα radiation (λ = 1.5406 Å).
The hydrodynamic radius of TiO2 NWs/Fe2O3 was evaluated using Dynamic Light Scattering (DLS) with a Zetasizer Nano ZS from Malvern Instruments, Malvern WR14 1XZ, U.K. A 1.5 mL suspension of the nanostructures, prepared at a defined concentration, underwent sonication prior to analysis. The measurements were conducted at room temperature, with data collected at a 173° backscatter angle.
3.2. Membrane Performance
The pure water flux (PWF) of both the unmodified and TiO2 NWs/Fe2O3-modified membranes was determined through a custom-designed cross-flow filtration system [28]. The effective membrane filtration area was 13.69 cm2. Prior to the filtration tests, each membrane sample was pre-compacted with DI water for a minimum of 30 min until the flux stabilized under a pressure of 4 bar. Filtration experiments were subsequently conducted at a constant pressure of 3 bar. To minimize experimental errors, water permeability was measured three times, and the results were averaged. The PWF (L/m2·h) was calculated using the following Equation (2) [14]:
where ΔV represents the volume of permeate collected (L), A is the effective membrane area (m2), and Δt is the permeation time (h).
In this study, textile dyes, specifically Naphthol Blue Black (NBB), were employed as model contaminants with a concentration of 25, 50, 100, and 150 mg/L. Filtration was conducted at a pressure of 1 bar, acidic condition (pH = 3), and a temperature of 25 ± 1 °C. The concentration of the dye in the solution was determined using a UV-Vis spectrophotometer, based on a calibration curve covering the relevant concentration range. The dye retention by the membranes was calculated using the following equation:
where R (%) represents the membrane rejection rate, Cp is the solute concentration in the permeate, and Cf is the solute concentration in the feed.
The flux recovery ratio (FRR) of the membranes in mg/L solutions of each textile dye (NBB) is as follows:
where J1 and J2 represent the permeate flux (L/m3·h) before and after the dye filtration, respectively.
4. Results and Discussion
4.1. Characteristics of TiO2 NWs/Fe2O3
Dynamic Light Scattering (DLS) measures the light scattering intensity to estimate the size of particles in a suspension. From this analysis, the TiO2 NWs loaded with Fe2O3 have an average particle diameter of 69 nm and a geometric standard deviation of 1.07 nm. The analysis provided in Figure 1 shows a Gaussian distribution of particle sizes. The particle size distribution falls within a narrow range of 61.3 nm to 77.3 nm, indicating a highly consistent size distribution for the nanowires. Additionally, the low polydispersity index (PDI) value of 0.005 confirms the high uniformity and mono-dispersity of the particles, signifying a highly homogeneous suspension with very little variation in particle size. This narrow distribution highlights the quality and precision of the synthesized nanowires.
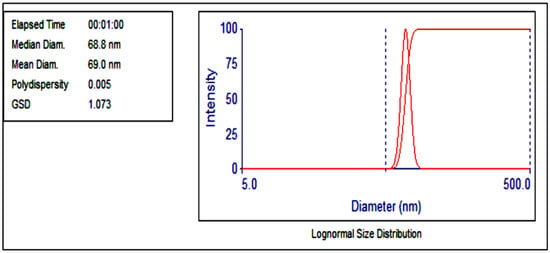
Figure 1.
Lognormal particle sizes of TiO2 NWs/Fe2O3 nanowire.
Fourier-transform infrared (FTIR) spectroscopy has been devoted to examining the composition of incorporated nanostructures. The chemical bonds and functional groups of the TiO2 NWs/Fe2O3 have all been recognized, as each functional group is represented by a wavelength. The FTIR spectra of the TiO2-Fe2O3, recorded in the range of 400–4000 cm−1, are shown in Figure 2. The peaks observed in the range of 400–800 cm−1 are ascribed to pure metal oxides and were due the presence of Ti-O and Fe-O bond vibrations, typically seen in TiO2 NW and Fe2O3 [22]. These are characteristic vibrations of TiO2 and Fe2O3, with Ti-O usually appearing around 500–700 cm−1 and Fe-O near 500 cm−1. Various peaks, particularly in the regions around 1600 cm−1 and 1400 cm−1, could correspond to the bending modes of O-H or Fe-O-H vibrations. This is typically seen in metal oxides that have some degree of adsorbed moisture. The broad peak around 3400 cm−1 is likely due to the O-H stretching vibrations, typically arising from hydroxyl groups or adsorbed water on the surface of the nanostructure [29].
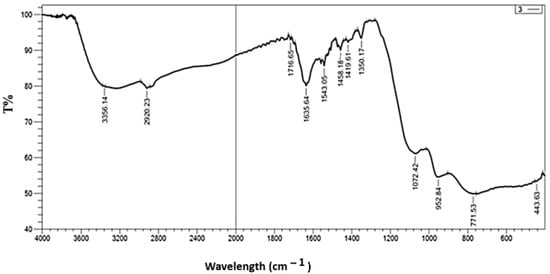
Figure 2.
FTIR spectrometer of TiO2 NWs/Fe2O3 hybrid nanostructure.
After synthesizing the TiO2 NW-Fe2O3 hybrid nanocomposites, scanning electron microscopy (SEM) was employed to analyze their surface morphology. As shown in Figure 3A, the nanocomposite exhibited a wire-like structure characterized by aggregated nanowires. Due to their small size, individual iron nanoparticles were not distinguishable in the SEM images. Nonetheless, this confirms the successful formation of the nanowire structure, with Fe2O3 likely distributed at the nanoscale.
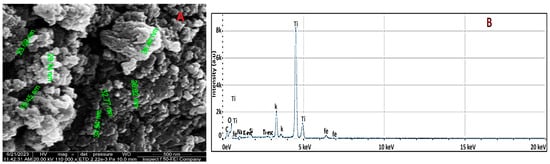
Figure 3.
TiO2 NWs/Fe2O3 as (A) morphological images by SEM and (B) elemental composition.
EDS analysis was further performed to confirm the presence of Fe2O3 nanoparticles on the surface of the TiO2 nanowire-based nanocomposite. As shown in Figure 3B, the analysis identified signals from titanium (Ti), oxygen (O), iron (Fe), and potassium (K). These signals confirmed the presence of both TiO2 and Fe2O3 in the sample. The potassium (K) peak was associated with residual KOH, which had been used in the preparation process of the TiO2 nanowires.
The X-ray diffraction (XRD) analysis provides insights into the crystalline phases and structure of TiO2 NWs loaded with Fe2O3. The XRD pattern, shown in Figure 4, reveals peaks corresponding to both TiO2 and Fe2O3 crystalline phases. The prominent diffraction peaks match well with the anatase phase of TiO2 and the rhombohedral hematite (α-Fe2O3), confirming the formation of a composite material. A notable peak near 25.2° (2θ) indicates the anatase form of TiO2 in the composite [30]. Additional peaks at higher angles, such as 37.3°, 48°, and 53.9°, correspond either to the rutile phase of TiO2 or to Fe2O3, showing the presence of both phases. Peaks between 32° and 50° further confirm the successful incorporation of the α-Fe2O3 phase into the nanowire structure [22]. Slight shifts and peak broadening suggest interactions between Fe2O3 and TiO2, indicating that Fe2O3 loading alters the crystallinity of the TiO2 without significantly affecting its overall structure. In summary, the XRD pattern demonstrates that while the TiO2 nanowires maintain their anatase phase, the incorporation of Fe2O3 introduces new peaks and results in subtle changes in the crystal structure, confirming the successful formation of the TiO2-Fe2O3 hybrid nanocomposite.
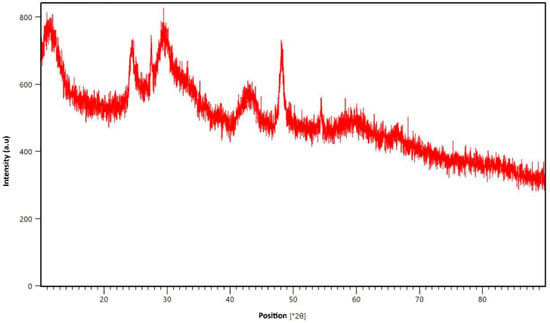
Figure 4.
XRD patterns of TiO2 NWs/Fe2O3 hybrid nanostructure.
4.2. Nanocomposite Membrane Characterization
4.2.1. Field Emission Scanning Electron Microscopy for Nanocomposite Membranes (FESEM)
Figure 5 and Figure 6 depicted the surface and cross-sectional morphologies of the membranes synthesized in the study. As displayed in Figure 5, both the TiO2 NWs-Fe2O3-PES nanocomposite membranes and the pristine PES membranes exhibit a smooth active layer, which is a typical surface characteristic of PES membranes, with no noticeable aggregates witnessed at low nanostructure content. However, with the incorporation of higher concentrations of TiO2 NWs-Fe2O3 nanostructures (≥0.3 g) into the casting solution, visible aggregates begin to appear on the membrane surface, especially at the 0.7 g loading content. These aggregates likely migrated to the surface during the phase separation process due to the higher loading content of nanostructures.
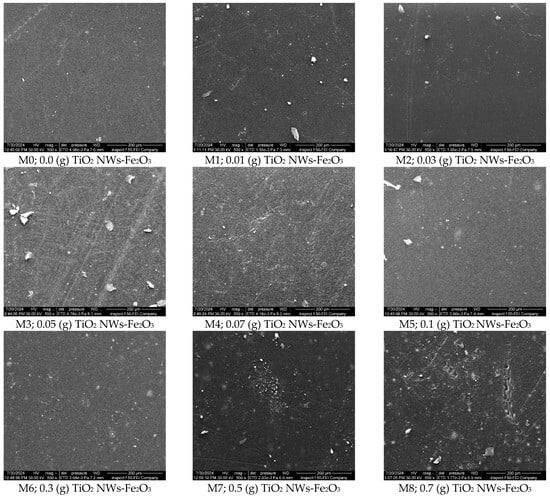
Figure 5.
FESEM of Surface images of the nanocomposite membranes.
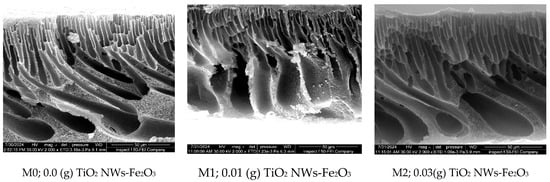
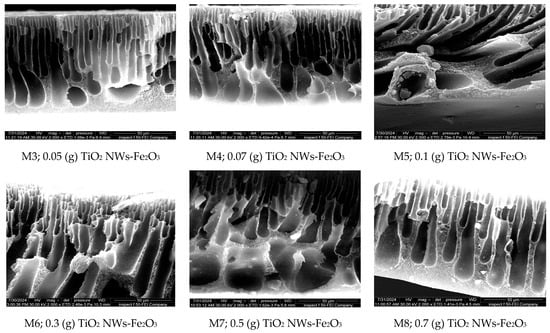
Figure 6.
FESEM of cross-section images of the nanocomposite membranes at 50 µm.
Given that membrane structure is key to determining transport mechanisms, the effect of additive content on the cross-sectional morphology of the nanocomposite membranes was imaged as well. Figure 6 disclosed that both the nascent PES and nanocomposite membranes possess a typical asymmetric structure, with a dense active layer and a porous support layer. The control PES membrane features a well-developed skin layer, supported by wide finger-like macropores at the bottom, attributed to its lower solution viscosity, which induced a faster exchange rate of nonsolvent into the polymer-lean phase compared to solvent diffusion [21].
When small amounts of nano-additives (up to 0.07 g) were incorporated into the PES matrix, the formation of finger-like macropores in the sublayer was very clear. However, as the additive content increased (0.1 g, 0.3 g, 0.5 g, and 0.7 g), narrower micropores with a finger-like structure formed, extending from the top to the bottom layer of the nanocomposite membranes. This occurs due to the increased viscosity of the casting solution, which slows the solvent–nonsolvent exchange at the surface–nonsolvent interface, resulting in a denser skin layer and a less porous structure [31]. These results align with previous studies that have reported similar structural modifications in polymeric membranes due to the presence of nanostructures [22].
4.2.2. Fourier-Transform Infrared Spectroscopy and XRD of TiO2 NWs/Fe2O3 of the Nanocomposite Membranes
The Fourier-transform infrared spectra (FTIR) is a highly prominent technique for identifying diverse functional groups on membrane surfaces and analyzing their potential molecular chemical bonds. Figure 7A showcases the FTIR spectra of the pristine PES membrane and the TiO2 NWs-Fe2O3 modified PES nanocomposite membrane, covering a range from 500 to 4000 cm−1. The FTIR spectrum confirms the chemical structure of the PES membrane, with several notable peaks observed at 1099 cm−1, 1148 cm−1, 1240 cm−1, 1337 cm−1, 1485 cm−1, and 1578 cm−1. These peaks correspond to C–O stretching, symmetric O=S=O stretching, aromatic ether, asymmetric O=S=O stretching, C=C stretching, and the benzene ring, respectively. The main characteristic peak of the PES membrane (M0) are those detected at 1485 cm−1 and 1578 cm−1. Interestingly, the TiO2 NWs-Fe2O3 nanostructure peaks were not observed in the FTIR spectra of all the nanocomposite membranes (M1 to M8), despite being visible in SEM images. This is likely due to the low concentration of nano-additives in the polymer matrix, where the polymer likely encapsulated the nanostructures. Similar observations have been reported by previous studies [22].
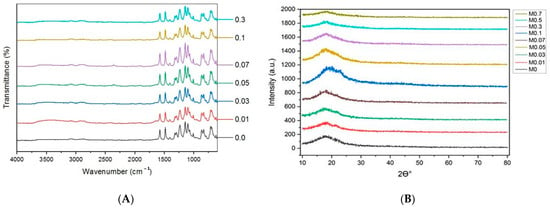
Figure 7.
(A) FTIR spectrometer of TiO2 NWs/Fe2O3 in the nanocomposite membranes. (B) XRD patterns of TiO2 NWs/Fe2O3 nanocomposite membranes.
X-ray diffraction (XRD) is a powerful, non-destructive analytical method used to examine physical properties such as phase composition, crystal structure, and orientation in various types of samples, including powders, solids, and liquids. Figure 7B presents the XRD patterns of the pristine PES membrane and TiO2 NWs-Fe2O3 modified PES nanocomposite membranes with varying amounts of nano-additives. All the membranes exhibit a prominent diffraction peak around 17.8°, which is characteristic of the PES membrane structure [32]. When the TiO2 NWs-Fe2O3 content in the nanocomposite membrane is low, the diffraction pattern remains similar to that of the pure PES membrane. However, as the concentration of nanomaterials increases up to 0.7 g, additional diffraction peaks begin to emerge at 25.2°, 37.3°, 48°, and 53.9°. These peaks correspond to the distinctive diffraction patterns of TiO2 NWs-Fe2O3, which are consistent with those observed in Figure 4. Thus, the XRD results confirm the successful incorporation of TiO2 NWs-Fe2O3 into the PES membranes.
4.2.3. EDX Analysis
The analysis of EDX was rather necessary for confirming that the elements exist in the matrix of the nanocomposite membrane. Therefore, the fabricated membranes by the current method were tested for the existence of the components of TiO2 NWs/Fe2O3. Figure 8 depicts the EDX images of the PES-TiO2 NWs/Fe2O3 membranes fabricated from different TiO2 NWs/Fe2O3 contents of the casting solution. The corresponding TiO2 element mappings confirmed scattering of TiO2 NWs/Fe2O3 among the entire membrane, as depicted in Figure 9. It can be noticed that the uniform dispersion of the TiO2 NWs/Fe2O3 in the membrane was observed with a loading of 0.5 g of the TiO2 NWs/Fe2O3. The clear observation in Figure 9 (M7) is of very slight agglomerations of TiO2 NWs/Fe2O3 in the matrix of the membrane with increasing TiO2 NWs/Fe2O3 contents in the PES casting solution. This observation is due to the homogeneity of the PES solution and excellent dispersion of TiO2 NWs/Fe2O3 in PES solution from utilizing the ultrasonic apparatus.
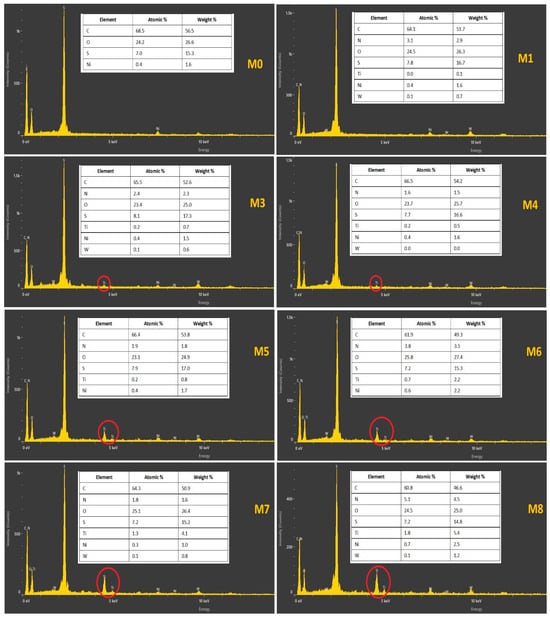
Figure 8.
EDX spectra of pristine PES and PES-TiO2 NWs/Fe2O3 at different TiO2 NWs/Fe2O3 contents in casting solution.
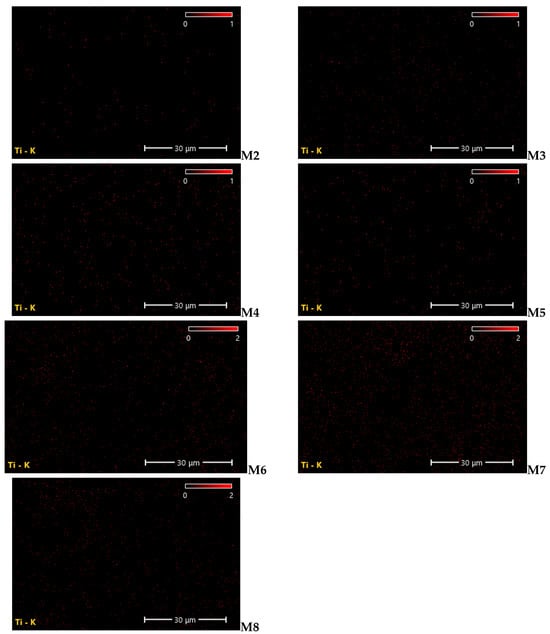
Figure 9.
EDX mapping and dispersion of the TiO2 NWs/Fe2O3nanocomposite membranes at different TiO2 NWs/Fe2O3 contents.
4.2.4. Impact of Nano-Additives Content on the Hydrophilicity of the Membranes
Contact angle (CA) measurements are a useful method for evaluating membrane hydrophilicity and drawing connections to membrane performance. Figure 10 presents the CA values of membranes prepared with varying amounts of TiO2 NWs-Fe2O3. The pristine PES membrane displayed the highest CA of 65.35°, compared to the lower CA value measured for the different nanocomposite membranes. Overall, the incorporation of TiO2 NWs-Fe2O3 into the casting solution improved the hydrophilicity of the PES membranes, with the CA changing based on the amount of TiO2 NWsFe2O3 added. As the nanostructure content increased, the CA of the nanocomposite membranes decreased, showing improved hydrophilicity, and reaching the minimal value of 49.13° for the M8 membrane, which was prepared with 0.7 g of nanostructure. Meanwhile, although some nanoaggregates have been observed at the surface of the M8 membrane, the hydrophilicity characteristics are still better than other modified membranes. This confirms that the hydrophilic nature of TiO2 NWs/Fe2O3 was successfully imparted to the fabricated membranes surface.
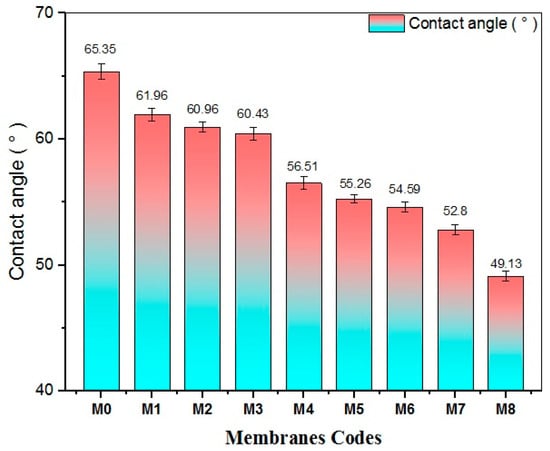
Figure 10.
The effect of nanostructure content on the contact angle measurements of the membrane.
4.2.5. Impact of Nano-Additives Content on the Porosity of the Membranes
Porosity plays a crucial impact in determining the performance of membranes. Figure 11 displayed the porosity measurements of both pristine PES and modified nanocomposite membranes. The pristine PES membrane exhibited the lowest porosity value compared to other nanocomposite membranes. Herein, as the concentration of TiO2 NWs-Fe2O3 increased, the porosity of the membranes also increased, reaching a peak value of 82.7% at 0.5 g of nanostructure. However, with the further impregnation of nanomaterials, the porosity began to diminish, dropping to 69.3% for the M8 nanocomposite membrane, which has been prepared with 0.7 g of nanostructure. Despite this decline, the porosity of M8 was still higher than that of the pristine PES membrane, which revealed a porosity value of 64.5%.
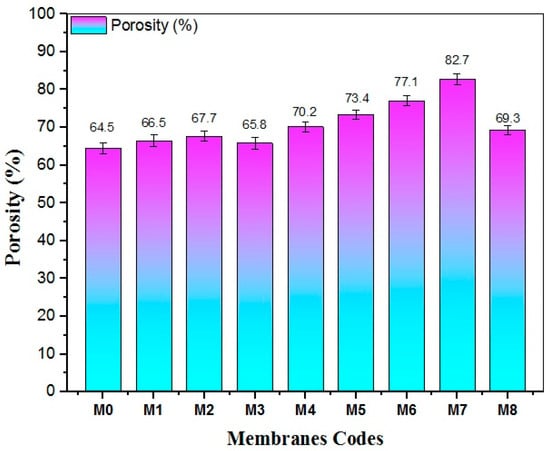
Figure 11.
The effect of TiO2 NWs-Fe2O3 content on the porosity of the PES membranes.
This proportional increase in membrane porosity measurements with additive content was attributed to the hydrophilic nature of TiO2 NWs-Fe2O3, which enhanced the thermodynamic instability of the casting solution, as demonstrated by hydrophilicity measurements earlier. However, beyond the 0.5 g loading, the slight reduction in porosity observed in the M8 membrane is due to the deposition of TiO2 NWs-Fe2O3 aggregates within the membrane structure, which may have blocked some of the pores.
4.3. Membrane Filtration Experiment Results
Figure 12 illustrates the pure water flux (PWF) of both pristine PES membranes and the nanocomposite membranes incorporating disparate amounts of TiO2 NWs-Fe2O3. A clear trend of improved permeability is witnessed across all nanocomposite membranes as the nanostructure content increases. Comparing to the control PES membrane which revealed the lowest PWF, the incorporation of a small amount (0.01 g) of TiO2 NWs-Fe2O3 into the polymer matrix resulted in a noticeable increase in permeability, reaching 60 L/m2·h. Notably, the nanocomposite membrane with 0.7 g of TiO2 NWs-Fe2O3 (M8) demonstrated the highest PWF, rising from 58 L/m2·h in the unmodified PES membrane (M0) to 93 L/m2·h. This significant improvement in flux can be ascribed to the concurrent enhancement in surface features of the nanocomposite membranes, mainly increased hydrophilicity and porosity.
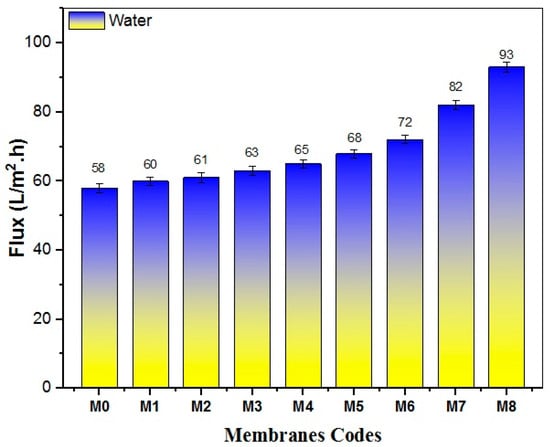
Figure 12.
The pure water flux of pristine PES and modified PES Membranes.
An NBB dye solute permeation test was also performed using four solute concentrations (25, 50, 100, and 150 mg/L) as shown in Figure 13. The flux of all membranes declined by almost one third of its flux when filtrating 25 mg/L NBB. Similarly, increasing the NBB feed solution concentration caused further decline in the solute permeate flux. This further decline is associated with the higher compacted layer of dye molecules at the surface of the membranes forming additional resistance to the flux passage.
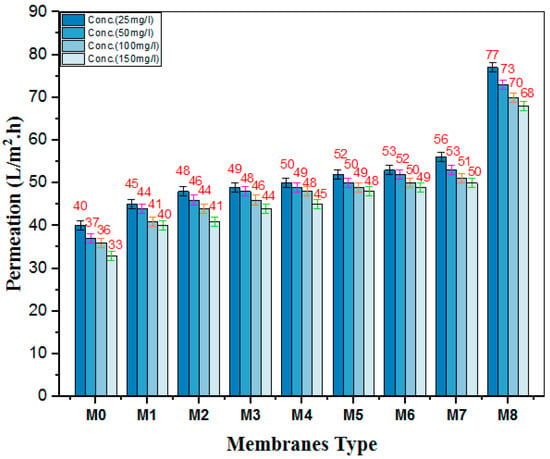
Figure 13.
Solute flux of different concentrations of NBB dye by nanocomposite membranes.
Figure 14 illustrates the retention performance of the membranes against four different concentrations of NBB dye (25, 50, 100, and 150 mg/L). The control PES membrane exhibited the lowest dye removal efficiency, ranging from 81% to 86.5%, compared to the TiO2 NWs-Fe2O3-modified membranes. The incorporation of nano-additives into the casting solution significantly improved the removal rates, with the nanocomposite membranes achieving retention rates of over 98%.
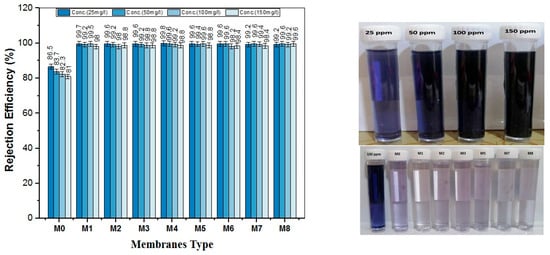
Figure 14.
Rejection of different concentrations of NBB dye by nanocomposite membranes.
Notably, all nanocomposite membranes (M1 to M8) showed nearly complete retention of NBB dye compared to M0 membrane. This enhanced retention can be attributed to enhanced hydrophilic nature and structural change induced by the nanostructures impregnated into the polymeric matrix. Besides that, the rapid fouling behavior at the initial stage of filtration, particularly at higher dye concentrations, could also contribute in all membrane sample retention potentials. Since the NBB dye molecules are much smaller than the average pore size of the membranes, they easily infiltrated the pores, leading to swift clogging. This process resulted in the formation of a “cake layer” on the surface of the membranes, as seen in Figure 15. The cake layer effectively functioned as a barrier, preventing further dye molecules from passing through the membrane. As a result, this layer significantly contributed to the high retention efficiency observed in the nanocomposite membranes.
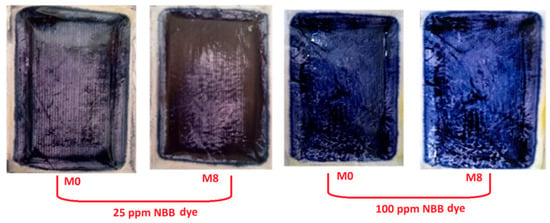
Figure 15.
Fouling of M0 and M8 with 25 and 100 mg/L NBB dye concentration.
The antifouling properties of the membranes were assessed harnessing the flux recovery ratio (FRR), which is a crucial indicator of irreversible fouling. The FRR was determined by measuring the pure water flux of the membranes both before and after performing NBB dye filtration tests. Membrane fouling typically occurs due to solute accumulation after filtration, and the antifouling performance of the membranes was determined by their FRR values. Higher FRR values, combined with relatively high NBB dye retention, suggest effective antifouling behavior. According to Figure 16A, the unmodified PES membrane demonstrated the lowest FRR, at 57%. In contrast, the incorporation of TiO2 NWs-Fe2O3 nanostructures into the PES matrix significantly improved the FRR of the membranes. Notably, the M8 nanocomposite membrane exhibited an FRR of 73.4%, reflecting a substantial enhancement in antifouling performance.
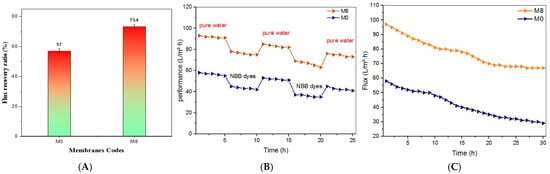
Figure 16.
(A) The flux recovery ratio of M0 and M8, for 100 mg/L NBB dye solution. (B) Time-dependent flux of pure water and NBB dye solution for M0 and M8 membranes. (C) Stability operation for M0 and M8 membranes.
To assess the long-time operation of the best membrane, three cycles of extended filtration experiments were performed for pristine PES (M0) and M8 membranes, as depicted in Figure 16B. This test aimed to assess the longevity and stability of the synthesized membranes during 25 h of operational time. Throughout each cycle, the pure water flux for 5 h of the operation was measured, assessing the decrease in permeation flux using an NBB dye solution for another 5 h, and then measuring the permeation flux of pure water again for 5 h following a 5 h backwashing process. The permeation flux of both membranes exhibited a gradual decline throughout the continuous filtration of the NBB dye solution after 3 cycles. After the third cycle of NBB dye filtration, the permeation flux of M0 and M8 decreased from 58 to 43 L/m2·h and from 93 to 77 L/m2·h, respectively. This reduction is attributed to the buildup of NBB dye molecules on the membrane surface following long-time filtration [33]. The pristine PES membrane gives a practical exhibition of a remarkable and predominantly irreversible reduction in flux compared to the M8 modified membrane. The obtained differences in fouling trend highlight the pivotal effect of surface characteristics of the membrane, including porosity, pore size, and hydrophilicity, on the capabilities of membrane antifouling.
Figure 16C depicts the influence of TiO2 NWs-Fe2O3 in the polymer solution on the stability of the PES-TiO2 NWs-Fe2O3 membranes in the removal of the NBB dye system. The stability of the membrane is obviously assessed by the membrane permeance. From Figure 16C we notice that for the permeance of the membrane fabricated from pristine PES, a gradual decline in permeance performance over time was obtained, attributed to the NBB dye adsorbing onto the membrane surface. The permeance of the best membrane fabricated from 0.7 g of TiO2 NWs-Fe2O3 in PES solution began to be more stable after 18 h of the operation. The purpose of TiO2 NWs-Fe2O3 in the PES matrix was to destroy the NBB dye layer formed at the surface of the nanocomposite membrane and prevent the accumulation and adsorption of NBB molecules at the membrane throughout the stability operation. This observation depicts that the fouling was more reversible with the embedded TiO2 NWs-Fe2O3. This may confirm our hypothesis that the embedded TiO2 NWs-Fe2O3 in PES solution leads to more stability of the membrane permeance. In general, these findings focus on the prospective of the PES-TiO2 NWs-Fe2O3 membrane for efficient removal of dyes along with its significant stability under the operating conditions.
4.4. Mechanism of Dye Separation
Important information about dye removal from water can be obtained by carefully examining the interaction and transport mechanisms of PES and TiO2 NWs-Fe2O3 PES membranes. As seen in Figure 17A, the PES polymer chain links with NBB dye via H-bonding, electrostatic interactions, and π-π stacking. As such, sulfone (-SO2-) and ether (-O-) groups found in PES can receive H-bonds. PES and NBB can interact more easily because of the hydroxyl (-OH) and sulfonic acid (-SO3H) groups in NBB, which can donate H-bonds. Moreover, because of its -SO3− groups, which can interact with positively charged sites on PES, NBB is an anionic dye; however, because PES is typically neutral and hydrophobic, these interactions are weak. Aromatic rings found in both PES and NBB have the potential to cause π-π stacking interactions. In dye separation procedures, these interactions are important. As was covered in previous sections, the hydrophilicity and porosity of the membrane have a major impact on dye rejection. These elements efficiently inhibit dye molecules while facilitating the transit of water molecules. Also, the TiO2 NWs-Fe2O3 PES nanocomposite links with NBB dye via H-bonding, electrostatic interactions, and π-π stacking, as shown in Figure 17B. The -OH and -SO3H groups of NBB can form H-bonds with PES (-SO2- and -O-), TiO2 (-OH groups on surface), and Fe2O3 (-OH groups on surface). When it comes to dye adsorption on the nanocomposite membrane, this is crucial.
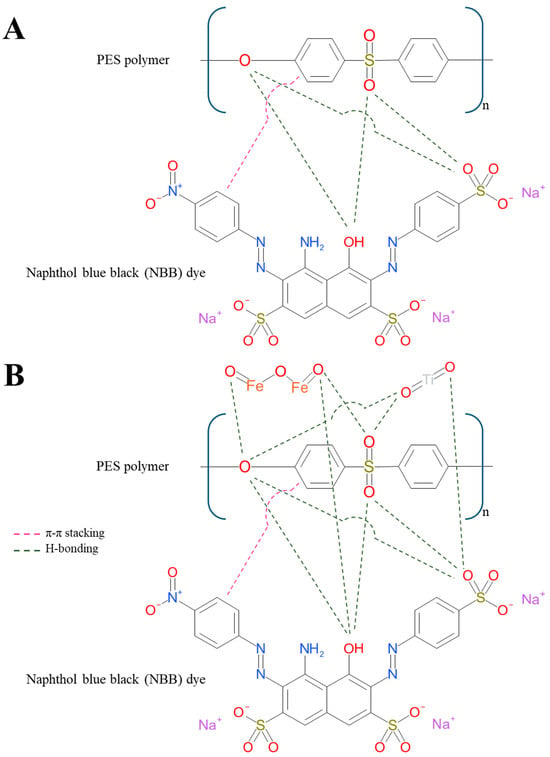
Figure 17.
Mechanism of NBB dye separation from water by (A) PES and (B) TiO2 NWs/Fe2O3 PES membranes.
5. Conclusions
The current work presented a novel endeavor to design a novel nanocomposite membrane harnessing the hybrid nanostructure of TiO2 NWs-Fe2O3. The content of nano-additives were varied to probe their influence of surface and structural characteristics. The incorporation of TiO2 NWs-Fe2O3 into PES membranes has induced significant improvements in water flux, hydrophilicity, and dye retention efficiency. Membranes modified with 0.7 g of nanostructures demonstrated optimal performance, achieving high permeability (93 L/m2·h) and a dye removal efficiency exceeding 98%. The increased porosity and hydrophilicity due to nanostructure addition were key factors contributing to enhanced membrane performance. However, higher nanostructure concentrations led to diminished permeability, likely due to pore blockage induced by nanostructure aggregations. Furthermore, the nanocomposite membranes manifested superior antifouling properties, as indicated by the flux recovery ratio (FRR) results. These findings suggest that TiO2 NWs-Fe2O3-modified PES membranes offer a viable and efficient solution for dye removal from industrial effluents, contributing to flourishing sustainable water treatment technologies. These promising results could stimulate further research in utilizing such hybrid materials for crafting functional membranes for different water treatment applications. Future work could focus on optimizing the membrane structure and nanomaterial ratios to enhance selectivity and long-term stability under real wastewater conditions. Additionally, exploring the photocatalytic regeneration of the membranes may offer a sustainable approach for repeated dye removal cycles.
Author Contributions
Conceptualization, M.M.H., Q.F.A. and M.M.E.-H.; methodology, Q.F.A., M.M.E.-H. and M.G.A.; software, M.M.H.; validation, Q.F.A., M.M.E.-H., M.M.H. and M.G.A.; formal analysis, M.M.H. and Q.F.A.; investigation, Q.F.A. and M.M.H.; writing—original draft preparation, Q.F.A., M.M.E.-H., M.G.A. and M.M.H.; writing—review and editing, Q.F.A., M.M.E.-H., M.G.A. and M.M.H.; supervision, Q.F.A., M.M.E.-H. and M.G.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Data is contained within the article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Al-Timimi, D.A.H.; Alsalhy, Q.F.; AbdulRazak, A.A.; Shehab, M.A.; Németh, Z.; Hernadi, K. Optimum operating parameters for PES nanocomposite membranes for mebeverine hydrochloride remova. J. Mater. Res. Technol. 2023, 24, 6779–6790. [Google Scholar] [CrossRef]
- Grassi, M.; Kaykioglu, G.; Belgiorno, V.; Lofrano, G. Removal of Emerging Contaminants from Water and Wastewater by Adsorption Process. In Emerging Compounds Removal from Wastewater; Lofrano, G., Ed.; SpringerBriefs in Molecular Science; Springer: Dordrecht, The Netherlands, 2012; pp. 15–37. [Google Scholar] [CrossRef]
- Holkar, C.R.; Jadhav, A.J.; Pinjari, D.V.; Mahamuni, N.M.; Pandit, A.B. A critical review on textile wastewater treatments: Possible approaches. J. Environ. Manag. 2016, 182, 351–366. Available online: https://www.sciencedirect.com/science/article/pii/S0301479716305266 (accessed on 24 January 2025). [CrossRef] [PubMed]
- Yaseen, D.A.; Scholz, M. Textile dye wastewater characteristics and constituents of synthetic effluents: A critical review. Int. J. Environ. Sci. Technol. 2019, 16, 1193–1226. [Google Scholar] [CrossRef]
- Al-Arajia, D.D.; Al-Ania, F.H.; Alsalhy, Q.F. The permeation and Separation Characteristics of Polymeric Membranes Incorporated with Nanoparticles for Dye Removal and Interaction Mechanisms between Polymer and Nanoparticles: A Mini Review. Eng. Technol. J. 2022, 40, 1399–1411. [Google Scholar] [CrossRef]
- Al-Araji, D.D.; Al-Ani, F.H.; Alsalhy, Q.F. Modification of polyethersulfone membranes by Polyethyleneimine (PEI) grafted Silica nanoparticles and their application for textile wastewater treatment. Environ. Technol. 2023, 44, 3033–3049. [Google Scholar] [CrossRef]
- Siddique, K.; Rizwan, M.; Shahid, M.J.; Ali, S.; Ahmad, R.; Rizvi, H. Textile Wastewater Treatment Options: A Critical Review. In Enhancing Cleanup of Environmental Pollutants; Anjum, N.A., Gill, S.S., Tuteja, N., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 183–207. [Google Scholar] [CrossRef]
- Gosavi, V.D.; Sharma, S. A general review on various treatment methods for textile wastewater. J. Environ. Sci. Comput. Sci. Eng. Technol. 2014, 3, 29–39. Available online: https://www.academia.edu/download/32836851/GV3I_3.pdf (accessed on 24 January 2025).
- Ng, L.Y.; Mohammad, A.W.; Leo, C.P.; Hilal, N. Polymeric membranes incorporated with metal/metal oxide nanoparticles: A comprehensive review. Desalination 2013, 308, 15–33. Available online: https://www.sciencedirect.com/science/article/pii/S0011916410008611 (accessed on 24 January 2025). [CrossRef]
- Sadiq, A.J.; Awad, E.S.; Shabeeb, K.M.; Khalil, B.I.; Al-Jubouri, S.M.; Sabirova, T.M.; Tretyakova, N.A.; Majdi, H.S.; Alsalhy, Q.F.; Braihi, A.J. Comparative study of embedded functionalised MWCNTs and GO in Ultrafiltration (UF) PVC membrane: Interaction mechanisms and performance. International. J. Environ. Anal. Chem. 2023, 103, 415–436. [Google Scholar] [CrossRef]
- Abood, T.W.; Shabeeb, K.M.; Alzubaydi, A.B.; Goh, P.S.; Ismail, A.F.; Zrelli, A.; Alsalhy, Q.F. Effect of MXene Ti3C2 on the PVDF ultrafiltration membrane properties and performance. Eng. Technol. J. 2024, 42, 754–767. [Google Scholar] [CrossRef]
- Ghadhban, M.Y.; Rashid, K.T.; Abdulrazak, A.A.; Meskher, H.; Benguerba, Y.; Al-Sarraj, E.A.Y.; Alsalhy, Q.F. Optimal operational conditions of PLA/PBAT mixed matrix membrane for the treatment of oily wastewater. Eng. Technol. J. 2024, 42, 1179–1192. [Google Scholar] [CrossRef]
- Ali, A.M.; Rashid, K.T.; Yahya, A.A.; Majdi, H.S.; Salih, I.K.; Yusoh, K.; Alsalhy, Q.F.; AbdulRazak, A.A.; Figoli, A. Fabrication of gum arabic-graphene (Gga) modified polyphenylsulfone (ppsu) mixed matrix membranes: A systematic evaluation study for ultrafiltration (uf) applications. Membranes 2021, 11, 542. Available online: https://www.mdpi.com/2077-0375/11/7/542 (accessed on 10 August 2024). [CrossRef]
- Ibraheem, B.M.; Al-Timimi, D.A.H.; Abdullah, S.N.; Majdi, H.S.; Alsalhy, Q.F. Decoration of Polyethersulfone Membranes with Zinc Oxide Nanoparticles for Efficient Treatment of Food Dyes. Chem. Eng. Res. Des. 2025, 218, 312–327. [Google Scholar] [CrossRef]
- Al-Ansary, H.K.; Al-Ani, F.H.; Hernadi, K.; Alsalhy, Q.F. Optimization of PPSU Membranes with ZnO Nanoparticles: A Morphological and Performance Evaluation. Desalination Water Treat. 2025, 321, 101062. [Google Scholar] [CrossRef]
- Shawket, A.N.; Ali, N.S.; Alsalhy, Q.F. Systematic study for a comprehensive evaluation of PPSU modified with ZnO for ultrafiltration membranes: Morphological characteristics and performance. Desalination Water Treat. 2023, 284, 27–38. [Google Scholar] [CrossRef]
- Abbas, T.K.; Rashid, K.T.; Al-Saady, S.; Alsarayrehd, A.A.; Figoli, A.; AlSalhy, Q.F. Decontamination of aqueous nuclear waste via pressure-driven membrane application–A short review. Eng. Technol. J. 2023, 41, 1152–1174. [Google Scholar] [CrossRef]
- Abbas, T.K.; Ibrahim, Z.H.; Al-Juboori, R.A.; Nafae, T.M.; Al-Mashhadani, A.H.; Fal, M.; Alotaibi, A.M.; Alsalhy, Q.F. A modified zeolite (Na2SO4 @zeolite NaA) as a novel adsorbent for radium-226,228 from acidic radioactive wastewater: Synthesis, characterization and testing. J. Environ. Chem. Eng. 2024, 12, 112197. [Google Scholar] [CrossRef]
- Al Aani, S.; Wright, C.J.; Atieh, M.A.; Hilal, N. Engineering nanocomposite membranes: Addressing current challenges and future opportunities. Desalination 2017, 401, 1–15. Available online: https://www.sciencedirect.com/science/article/pii/S001191641630995X (accessed on 24 January 2025). [CrossRef]
- Alsalhy, Q.F.; Al-Ani, F.H.; Al-Najar, A.E. A new Sponge-GAC-Sponge membrane module for submerged membrane bioreactor use in hospital wastewater treatment. Biochem. Eng. J. 2018, 133, 130–139. Available online: https://www.sciencedirect.com/science/article/pii/S1369703X18300585 (accessed on 2 July 2024). [CrossRef]
- Al Aani, S.; Gomez, V.; Wright, C.J.; Hilal, N. Fabrication of antibacterial mixed matrix nanocomposite membranes using hybrid nanostructure of silver coated multi-walled carbon nanotubes. Chem. Eng. J. 2017, 326, 721–736. Available online: https://www.sciencedirect.com/science/article/pii/S1385894717309804 (accessed on 24 January 2025). [CrossRef]
- Al Aani, S.; Haroutounian, A.; Wright, C.J.; Hilal, N. Thin Film Nanocomposite (TFN) membranes modified with polydopamine coated metals/carbon-nanostructures for desalination applications. Desalination 2018, 427, 60–74. Available online: https://www.sciencedirect.com/science/article/pii/S0011916417319793 (accessed on 24 January 2025). [CrossRef]
- Al-Maliki, R.M.; Alsalhy, Q.F.; Al-Jubouri, S.; AbdulRazak, A.A.; Shehab, M.A.; Németh, Z.; Hernadi, K.; Majdi, H.S. Enhanced antifouling in flat-sheet polyphenylsulfone membranes incorporating graphene oxide–tungsten oxide for ultrafiltration applications. Membranes 2023, 13, 269. Available online: https://www.mdpi.com/2077-0375/13/3/269 (accessed on 2 June 2024). [CrossRef]
- Alanezi, A.A.; Abdallah, H.; Shalaby, M.S.; Aljumaily, M.M.; Alsalhy, Q.F.; Shaban, M.; Nemeth, Z.; Hernadi, K. Super-antifouling PES nanocomposite membrane encapsulated silica nanoparticles and coated nano-Ag/polyvinyl alcohol layer. Alex. Eng. J. 2024, 91, 103–114. [Google Scholar] [CrossRef]
- Kumar, M.A.; Abebe, B.; Nagaswarupa, H.P.; Murthy, H.A.; Ravikumar, C.R.; Sabir, F.K. Enhanced photocatalytic and electrochemical performance of TiO2-Fe2O3 nanocomposite: Its applications in dye decolorization and as supercapacitors. Sci. Rep. 2020, 10, 1249. Available online: https://www.nature.com/articles/s41598-020-58110-7 (accessed on 24 January 2025). [CrossRef] [PubMed]
- Radwan, A.; Mohamed, S.; Khalil, M.M.; El-Sewify, I.M. Effective adsorption of fluorescent congo red azo dye from aqueous solution by green synthesized nanosphere ZnO/CuO composite using propolis as bee byproduct extract. Sci. Rep. 2024, 14, 9061. Available online: https://www.nature.com/articles/s41598-024-58306-1 (accessed on 24 January 2025). [CrossRef] [PubMed]
- Shehab, M.A.; Szőri-Dorogházi, E.; Szabó, S.; Valsesia, A.; Chauhan, T.; Koós, T.; Muránszky, G.; Szabó, T.; Hernadi, K.; Németh, Z. Virus and bacterial removal ability of TiO2 nanowire-based self-supported hybrid membranes. Arab. J. Chem. 2023, 16, 104388. Available online: https://www.sciencedirect.com/science/article/pii/S1878535222007043 (accessed on 24 January 2025). [CrossRef]
- Huang, J.H.; Cheng, X.Q.; Wu, Y.D.; Zhang, Y.Q.; Li, S.W.; Lau, C.H.; Shao, L. Critical operation factors and proposed testing protocol of nanofiltration membranes for developing advanced membrane materials. Adv. Compos. Hybrid Mater. 2021, 4, 1092–1101. [Google Scholar] [CrossRef]
- Shehab, M.A.; Németh, Z.; Mertinger, V. Design of Nanowire-Based Hybrid Structures for Wastewater Treatment. Available online: http://193.6.1.94:9080/JaDoX_Portlets/documents/document_45624_section_43998.pdf (accessed on 24 January 2025).
- Mansour, H.; Omri, K.; Bargougui, R.; Ammar, S. Novel α-Fe2O3/TiO2 nanocomposites with enhanced photocatalytic activity. Appl. Phys. A 2020, 126, 151. [Google Scholar] [CrossRef]
- Al Aani, S.; Wright, C.J.; Hilal, N. Investigation of UF membranes fouling and potentials as pre-treatment step in desalination and surface water applications. Desalination 2018, 432, 115–127. Available online: https://www.sciencedirect.com/science/article/pii/S0011916417326772 (accessed on 24 January 2025). [CrossRef]
- Zhang, S.; Yuan, H.; Wang, C.; Liu, X.; Lu, J. Antifouling performance enhancement of polyethersulfone ultrafiltration membrane through increasing charge-loading capacity over Prussian blue nanoparticles. J. Appl. Polym. Sci. 2020, 137, 49410. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Y.; Wang, X.; Zhang, Z.; Li, S.; Hu, Y.; Gong, G. Construction of robust one-dimensional nanowire-regulated graphene oxide membranes for efficient dye/salt separation. J. Membr. Sci. 2023, 686, 122027. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).