Recyclable Platinum Nanocatalyst for Nitroarene Hydrogenation: Gum Acacia Polymer-Stabilized Pt Nanoparticles with TiO2 Support
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Pt@TiO2–GAP
2.3. Characterization Studies
2.4. Catalytic Hydrogenation Studies
3. Results and Discussion
3.1. Characterization of Pt@TiO2–GAP
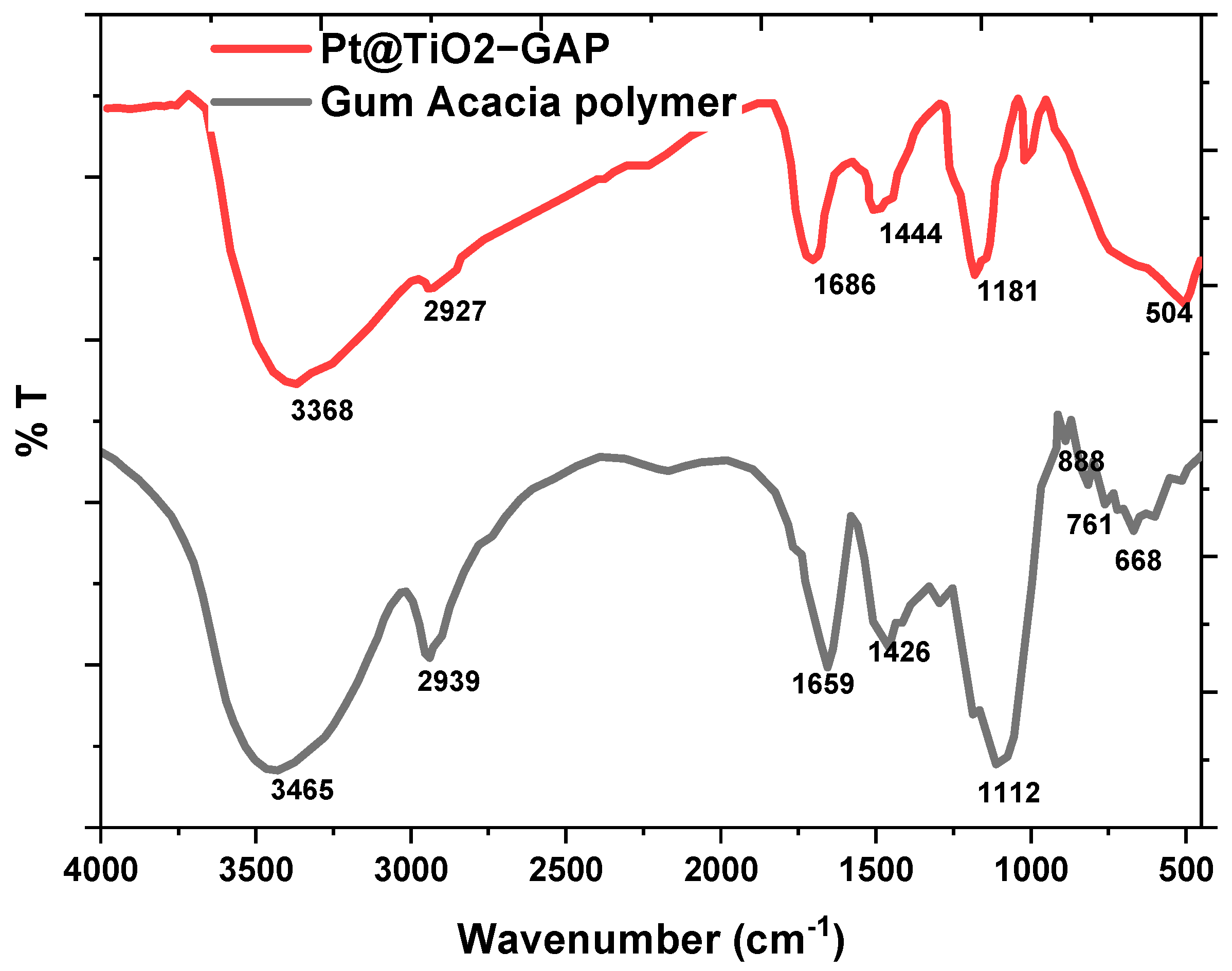
3.1.1. FT-IR and ICP-OES
3.1.2. XPS Analysis
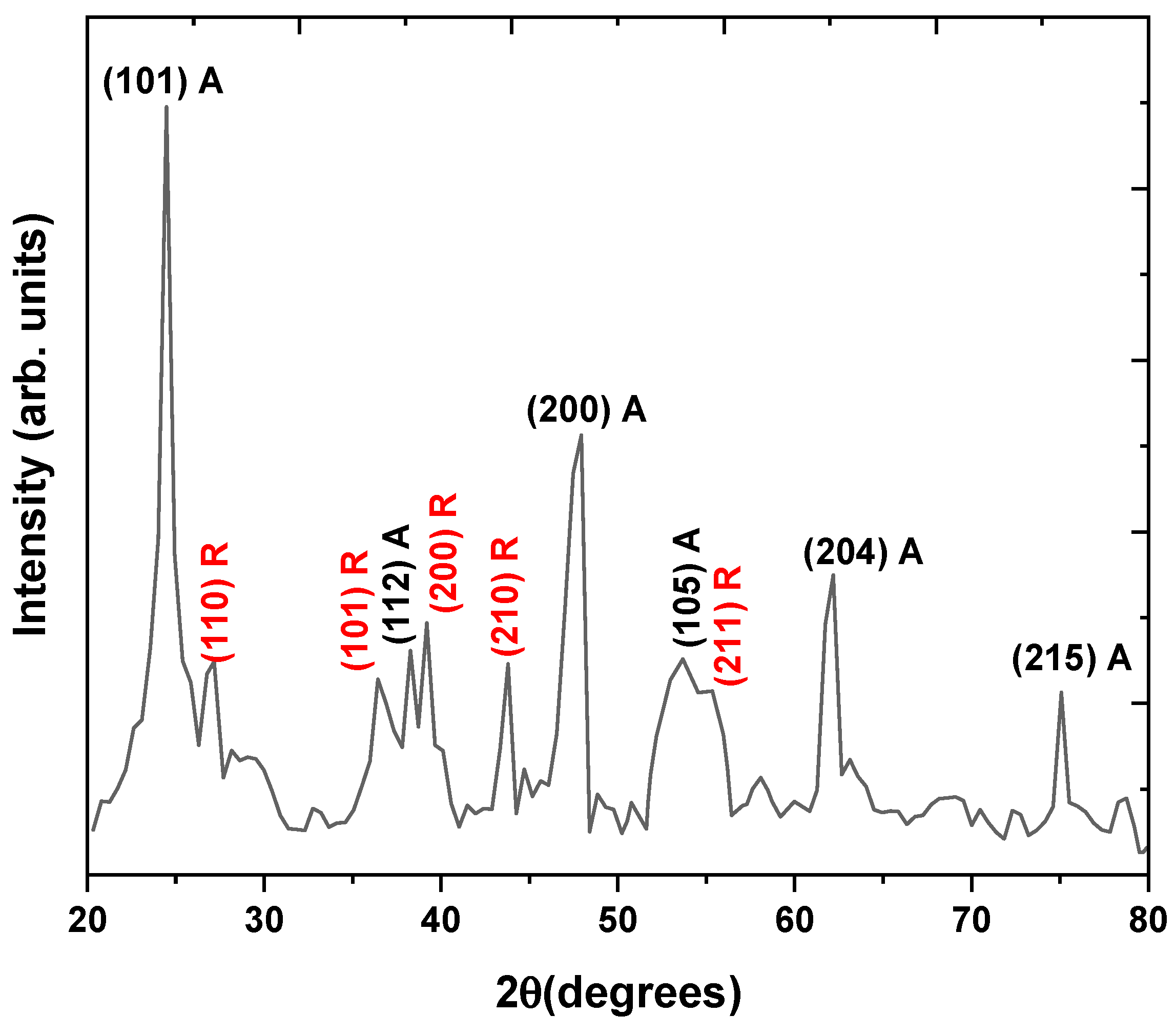
3.1.3. XRD Analysis
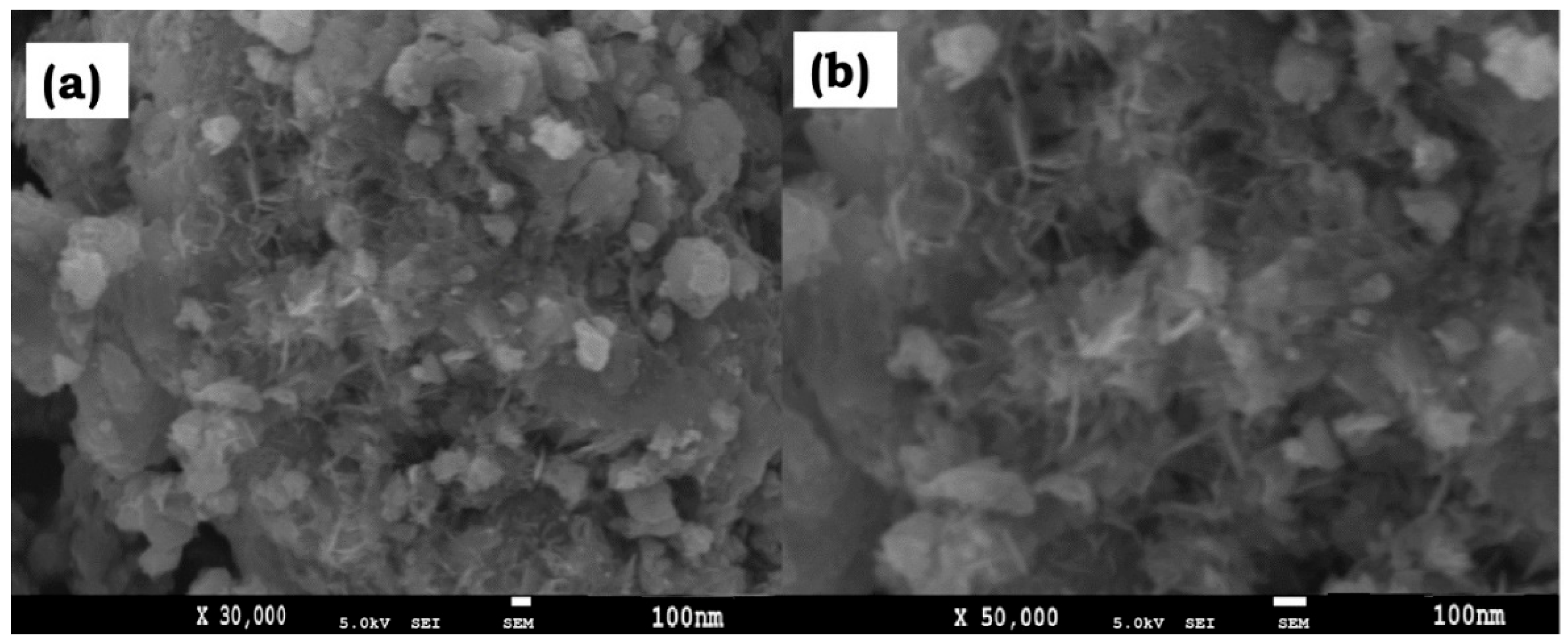
3.1.4. FE-SEM Study
3.1.5. TEM Study
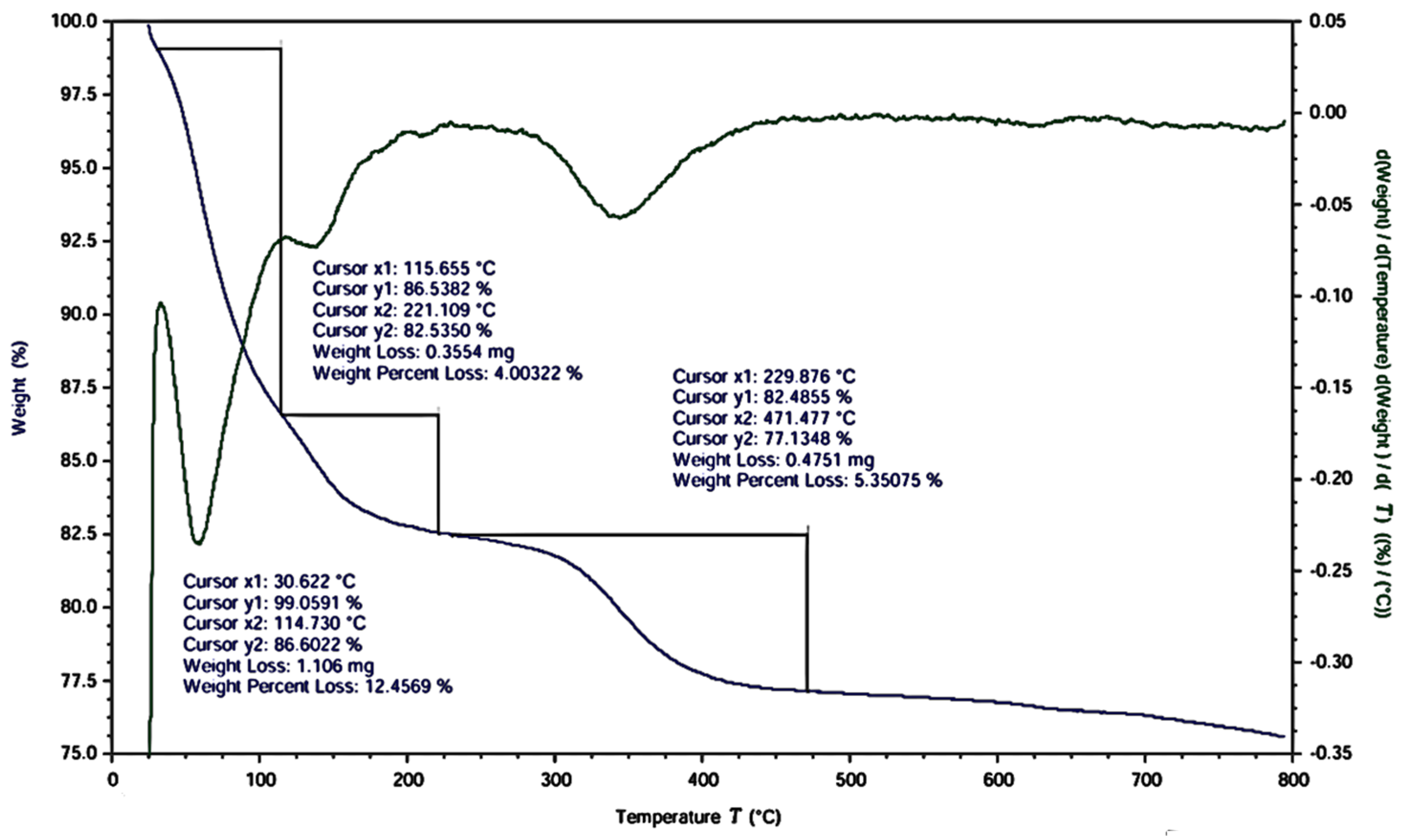
3.1.6. TGA-DTG Analysis
3.2. Catalytic Activity of Pt@TiO2–GAP
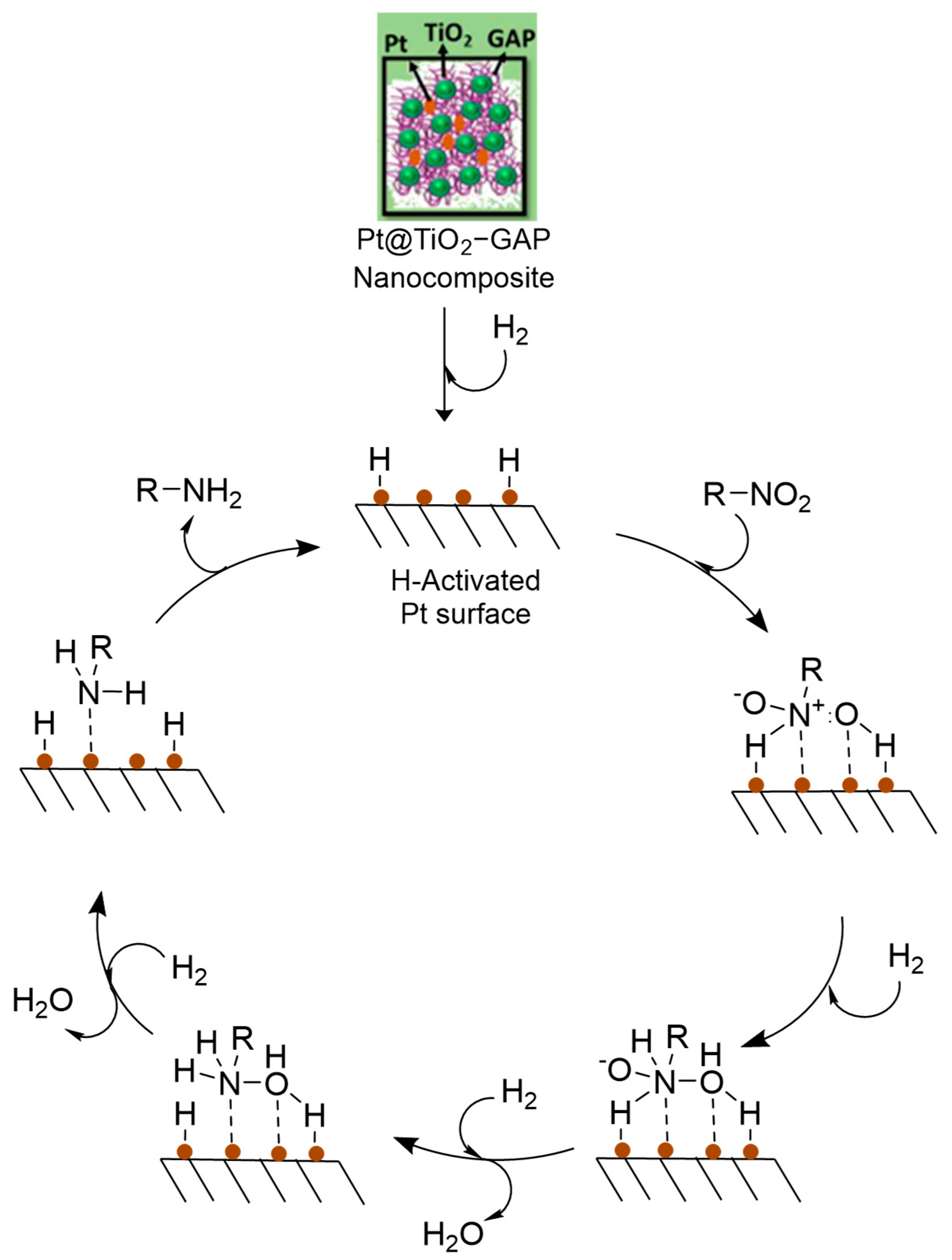
3.3. Proposed Mechanism
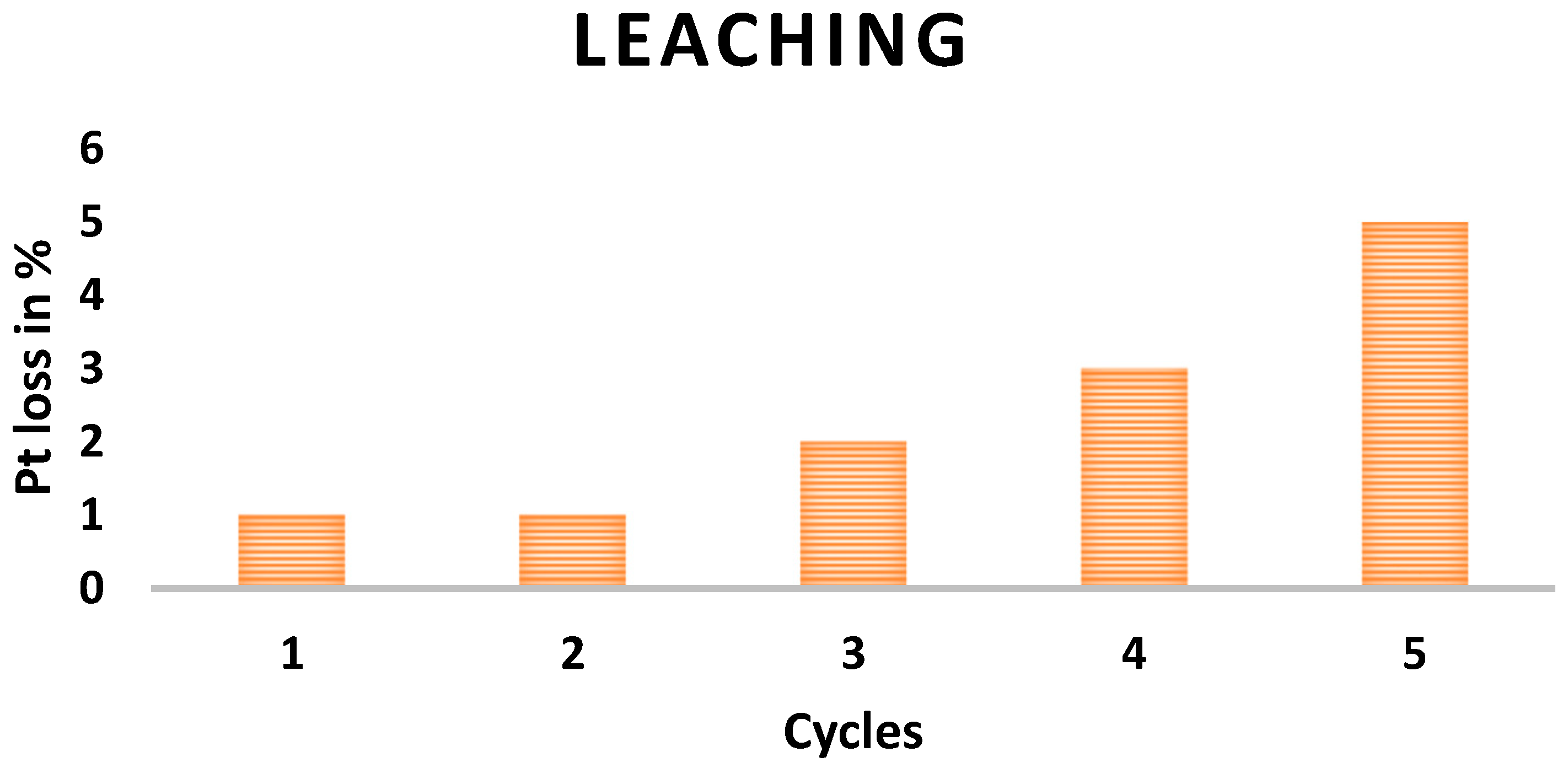
3.4. Heterogeneity and Recyclability Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gupta, N.K.; Reif, P.; Palenicek, P.; Rose, M. Toward Renewable Amines: Recent Advances in the Catalytic Amination of Biomass-Derived Oxygenates. ACS Catal. 2022, 12, 10400–10440. [Google Scholar] [CrossRef]
- Chen, S.; Ling, L.-L.; Jiang, S.-F.; Jiang, H. Selective Hydrogenation of Nitroarenes under Mild Conditions by the Optimization of Active Sites in a Well Defined Co@ NC Catalyst. Green Chem. 2020, 22, 5730–5741. [Google Scholar] [CrossRef]
- Song, J.; Huang, Z.-F.; Pan, L.; Li, K.; Zhang, X.; Wang, L.; Zou, J.-J. Review on Selective Hydrogenation of Nitroarene by Catalytic, Photocatalytic and Electrocatalytic Reactions. Appl. Catal. B Environ. 2018, 227, 386–408. [Google Scholar] [CrossRef]
- Fassbach, T.A.; Ji, J.-M.; Vorholt, A.J.; Leitner, W. Recycling of Homogeneous Catalysts—Basic Principles, Industrial Practice, and Guidelines for Experiments and Evaluation. ACS Catal. 2024, 14, 7289–7298. [Google Scholar] [CrossRef]
- Sankar, M.; He, Q.; Engel, R.V.; Sainna, M.A.; Logsdail, A.J.; Roldan, A.; Willock, D.J.; Agarwal, N.; Kiely, C.J.; Hutchings, G.J. Role of the Support in Gold-Containing Nanoparticles as Heterogeneous Catalysts. Chem. Rev. 2020, 120, 3890–3938. [Google Scholar] [CrossRef]
- Ndolomingo, M.J.; Bingwa, N.; Meijboom, R. Review of Supported Metal Nanoparticles: Synthesis Methodologies, Advantages and Application as Catalysts. J. Mater. Sci. 2020, 55, 6195–6241. [Google Scholar] [CrossRef]
- Lu, L.; Zheng, H.; Li, Y.; Zhou, Y.; Fang, B. Ligand-Free Synthesis of Noble Metal Nanocatalysts for Electrocatalysis. Chem. Eng. J. 2023, 451, 138668. [Google Scholar] [CrossRef]
- Bagnato, G.; Sanna, A.; Paone, E.; Catizzone, E. Recent Catalytic Advances in Hydrotreatment Processes of Pyrolysis Bio-Oil. Catalysts 2021, 11, 157. [Google Scholar] [CrossRef]
- Raghav, G.R.; Moolayadukkam, S.; Thomas, N.R.; George, G. Two-Dimensional Nanomaterials-Based Polymer Nanocomposites for Gas and Volatile Organic Compound Sensing. In Two-Dimensional Nanomaterials-Based Polymer Nanocomposites; Pandey, M., Deshmukh, K., Hussain, C.M., Eds.; Wiley: Hoboken, NJ, USA, 2024; pp. 713–741. ISBN 978-1-119-90484-7. [Google Scholar]
- Duan, X.-L.; Cao, Y.-M.; Gao, C.; Niu, S.-L.; Shen, Y.-W.; Guo, Q.; Xie, J.-J.; He, K.-Q.; Yuan, C.-G. Effective Capture and Immobilization of Hg0 from Flue Gas Using a Novel Selenium-Functionalized SrFeO3-δ/HNTs Half-Metal Composite: Adsorption, Photocatalytic Oxidation, and Mechanism. Energy Fuels 2022, 36, 12663–12676. [Google Scholar] [CrossRef]
- Bakhvalova, E.S.; Pinyukova, A.O.; Mikheev, A.V.; Demidenko, G.N.; Sulman, M.G.; Bykov, A.V.; Nikoshvili, L.Z.; Kiwi-Minsker, L. Noble Metal Nanoparticles Stabilized by Hyper-Cross-Linked Polystyrene as Effective Catalysts in Hydrogenation of Arenes. Molecules 2021, 26, 4687. [Google Scholar] [CrossRef]
- Jaryal, V.B.; Villa, A.; Gupta, N. Metal-Free Carbon-Based Nanomaterials: Insights from Synthesis to Applications in Sustainable Catalysis. ACS Sustain. Chem. Eng. 2023, 11, 14841–14865. [Google Scholar] [CrossRef]
- Chouhan, D.; Mandal, P. Applications of Chitosan and Chitosan Based Metallic Nanoparticles in Agrosciences—A Review. Int. J. Biol. Macromol. 2021, 166, 1554–1569. [Google Scholar] [CrossRef]
- Biliuta, G.; Coseri, S. Cellulose: A Ubiquitous Platform for Ecofriendly Metal Nanoparticles Preparation. Coord. Chem. Rev. 2019, 383, 155–173. [Google Scholar] [CrossRef]
- Qiu, C.; Hu, Y.; Jin, Z.; McClements, D.J.; Qin, Y.; Xu, X.; Wang, J. A Review of Green Techniques for the Synthesis of Size-Controlled Starch-Based Nanoparticles and Their Applications as Nanodelivery Systems. Trends Food Sci. Technol. 2019, 92, 138–151. [Google Scholar] [CrossRef]
- Figueiredo, P.; Lintinen, K.; Hirvonen, J.T.; Kostiainen, M.A.; Santos, H.A. Properties and Chemical Modifications of Lignin: Towards Lignin-Based Nanomaterials for Biomedical Applications. Prog. Mater. Sci. 2018, 93, 233–269. [Google Scholar] [CrossRef]
- Sangeetha, J.; Shettar, A.K.; Thangadurai, D. Nanomaterials from Biomass: An Update. In Handbook of Nanomaterials and Nanocomposites for Energy and Environmental Applications; Kharissova, O.V., Torres-Martínez, L.M., Kharisov, B.I., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 1005–1022. ISBN 978-3-030-36267-6. [Google Scholar]
- Farooq, M.; Ihsan, J.; Saeed, S.; Haleem, A.; Siddiq, M. Highly Versatile Gum Acacia Based Swellable Microgels Encapsulating Cobalt Nanoparticles; An Approach to Rapid and Recoverable Environmental Nano-Catalysis. J. Inorg. Organomet. Polym. Mater. 2021, 31, 2030–2042. [Google Scholar] [CrossRef]
- Athinarayanan, J.; Periasamy, V.S.; Alshatwi, A.A. Ultrasonic-Assisted Synthesis and Cytocompatibility Assessment of TiO2/SiO2 Nanoparticles-Impregnated Gum Arabic Nanocomposite: Edible Coating of Dates for Shelf-Life Extension. Polymers 2025, 17, 161. [Google Scholar] [CrossRef] [PubMed]
- Sreedhar, B.; Devi, D.K.; Neetha, A.S.; Kumar, V.P.; Chary, K.V.R. Green Synthesis of Gum-Acacia Assisted Gold-Hydroxyapatite Nanostructures–Characterization and Catalytic Activity. Mater. Chem. Phys. 2015, 153, 23–31. [Google Scholar] [CrossRef]
- Hassanzadeh-Afruzi, F.; Maleki, A.; Zare, E.N. Novel Eco-Friendly Acacia Gum-Grafted-Polyamidoxime@copper Ferrite Nanocatalyst for Synthesis of Pyrazolopyridine Derivatives. J. Nanostruct. Chem. 2023, 13, 451–462. [Google Scholar] [CrossRef]
- Sreedhar, B.; Devi, D.K.; Yada, D. Selective Hydrogenation of Nitroarenes Using Gum Acacia Supported Pt Colloid an Effective Reusable Catalyst in Aqueous Medium. Catal. Commun. 2011, 12, 1009–1014. [Google Scholar] [CrossRef]
- Wiles, C.; Watts, P.; Haswell, S.J. Clean and Selective Oxidation of Aromatic Alcohols Using Silica-Supported Jones’ Reagent in a Pressure-Driven Flow Reactor. Tetrahedron Lett. 2006, 47, 5261–5264. [Google Scholar] [CrossRef]
- Lee, S.; Jørgensen, M.; Hartwig, J.F. Palladium-Catalyzed Synthesis of Arylamines from Aryl Halides and Lithium Bis(Trimethylsilyl)Amide as an Ammonia Equivalent. Org. Lett. 2001, 3, 2729–2732. [Google Scholar] [CrossRef] [PubMed]
- Shen, Q.; Hartwig, J.F. Palladium-Catalyzed Coupling of Ammonia and Lithium Amide with Aryl Halides. J. Am. Chem. Soc. 2006, 128, 10028–10029. [Google Scholar] [CrossRef]
- Lee, D.-Y.; Hartwig, J.F. Zinc Trimethylsilylamide as a Mild Ammonia Equivalent and Base for the Amination of Aryl Halides and Triflates. Org. Lett. 2005, 7, 1169–1172. [Google Scholar] [CrossRef]
- Manickam, A.; Selvakumaran, D.; Narendran, K.; Razack, S.A.; Selvakumar, S.; Krishnamurthy, B. Fabrication of Gum Acacia Protected Zinc Oxide Nanoparticles for UV Assisted Photocatalysis of Methyl Green Textile Dye. Chem. Phys. Lett. 2022, 800, 139662. [Google Scholar] [CrossRef]
- Devi, D.K.; Pratap, S.V.; Haritha, R.; Sivudu, K.S.; Radhika, P.; Sreedhar, B. Gum Acacia as a Facile Reducing, Stabilizing, and Templating Agent for Palladium Nanoparticles. J. Appl. Polym. Sci. 2011, 121, 1765–1773. [Google Scholar] [CrossRef]
- Haghighi, F.H.; Mercurio, M.; Cerra, S.; Salamone, T.A.; Bianymotlagh, R.; Palocci, C.; Spica, V.R.; Fratoddi, I. Surface Modification of TiO2 Nanoparticles with Organic Molecules and Their Biological Applications. J. Mater. Chem. B 2023, 11, 2334–2366. [Google Scholar] [CrossRef]
- Edirisooriya, E.T.; Senanayake, P.S.; Xu, P.; Wang, H. Recyclability and Regeneration of Au/TiO2 Nanocomposite and Pt/TiO2 Atom-Nanocomposite Catalysts in Photo-Reforming Plastics for Hydrogen Production. J. Environ. Chem. Eng. 2025, 13, 116467. [Google Scholar] [CrossRef]
- Mohan, Y.M.; Raju, K.M.; Sambasivudu, K.; Singh, S.; Sreedhar, B. Preparation of Acacia-stabilized Silver Nanoparticles: A Green Approach. J. Appl. Polym. Sci. 2007, 106, 3375–3381. [Google Scholar] [CrossRef]
- Kim, S.; Muhammad, R.; Schuetzenduebe, P.; Kalidindi, S.B.; Schütz, G.; Oh, H.; Son, K. Hybrids of Pd Nanoparticles and Metal–Organic Frameworks for Enhanced Magnetism. J. Phys. Chem. Lett. 2021, 12, 4742–4748. [Google Scholar] [CrossRef]
- Khane, Y.; Albukhaty, S.; Sulaiman, G.M.; Fennich, F.; Bensalah, B.; Hafsi, Z.; Aouf, M.; Amar, Z.H.; Aouf, D.; Al-Kuraishy, H.M.; et al. Fabrication, Characterization and Application of Biocompatible Nanocomposites: A Review. Eur. Polym. J. 2024, 214, 113187. [Google Scholar] [CrossRef]
- Sadia, S.I.; Shishir, K.H.; Ahmed, S.; Aidid, A.R.; Islam, M.; Rana, M.; Al-Reza, S.M.; Alam, A. Crystallographic Biography on Nanocrystalline Phase of Polymorphs Titanium Dioxide (TiO2): A Perspective Static Review. S. Afr. J. Chem. Eng. 2024, 50, 51–64. [Google Scholar] [CrossRef]
- Liu, Z.; Yang, Z.; Zhang, H.; Wang, S.; Liu, Q.; Zhao, Z.; Bao, X.; Yuan, P. Crystal Facet Engineering of Pd/TiO2 to Boost the Activity and Selectivity for Nitroarenes Hydrogenation. Chem. Eng. J. 2025, 503, 158337. [Google Scholar] [CrossRef]
- Vamsi Krishna, B.; Durga Lakshmi, B.; Tirupathi Rao, P.; Ramachandra, R.K. Structural, Optical, and Antioxidant Properties of Biocompatible CuO-MgO Nanocomposites. Results Mater. 2025, 26, 100695. [Google Scholar] [CrossRef]
- Kogularasu, S.; Lee, Y.; Sriram, B.; Wang, S.; George, M.; Chang-Chien, G.; Sheu, J. Unlocking Catalytic Potential: Exploring the Impact of Thermal Treatment on Enhanced Electrocatalysis of Nanomaterials. Angew. Chem. 2024, 136, e202311806. [Google Scholar] [CrossRef]
- Jeon, D.; Sagong, M.; Kim, M.S.; Nam, J.S.; Park, H.; Kim, I. Electrospun Carbon Nanofibers for Clean Energy Applications: A Comprehensive Review. EcoMat 2025, 7, e12517. [Google Scholar] [CrossRef]
- Zhang, S.; Liu, J.; Shang, C.; Hu, M.; Zhou, H.; Hu, X.; Yang, W.; Duan, E.; Wang, Z.; Xu, J. Enhanced Catalysis of Au/TiO2 for Transfer Hydrogenation of Unsaturated Nitro Compounds by Surface Engineering. Appl. Surf. Sci. 2024, 654, 159503. [Google Scholar] [CrossRef]
- Prasanna; Usha, K.M. Highly Recyclable Palladium Ion Substituted TiO2 as the Versatile Ligand-Free Catalyst for the Selective Oxidation of Alcohols and the Reduction of Nitroarenes. J. Chem. Sci. 2022, 134, 96. [Google Scholar] [CrossRef]
- Yamashita, T.; Tomiyama, M.; Noguchi, D.; Okabe, Y. Fine Control of TiO2-and Pt/TiO2-Catalyzed Sequential Photoreduction and Reductive N-Alkylation of Nitroarenes by Connecting Different Types of Microflow Reactors. Bull. Chem. Soc. Jpn. 2023, 96, 724–730. [Google Scholar] [CrossRef]
- Ma, J.; Mao, X.; Hu, C.; Wang, X.; Gong, W.; Liu, D.; Long, R.; Du, A.; Zhao, H.; Xiong, Y. Highly Efficient Iron-Based Catalyst for Light-Driven Selective Hydrogenation of Nitroarenes. J. Am. Chem. Soc. 2024, 146, 970–978. [Google Scholar] [CrossRef]
- Qiu, B.; Deng, Y.; Li, Q.; Shen, B.; Xing, M.; Zhang, J. Rational Design of a Unique Ternary Structure for Highly Photocatalytic Nitrobenzene Reduction. J. Phys. Chem. C 2016, 120, 12125–12131. [Google Scholar] [CrossRef]
- Warkad, I.R.; Gaikwad, R.P.; Chaudhari, D.S.; Pham, H.N.; Datye, A.K.; Gawande, M.B. Titanium Dioxide Supported Ru Nanoparticles as Efficient Catalysts for Microwave-Assisted Sustainable Organic Transformations. ACS Sustain. Chem. Eng. 2024, 12, 15077–15088. [Google Scholar] [CrossRef]
- Bilgicli, H.G.; Burhan, H.; Diler, F.; Cellat, K.; Kuyuldar, E.; Zengin, M.; Sen, F. Composites of Palladium Nanoparticles and Graphene Oxide as a Highly Active and Reusable Catalyst for the Hydrogenation of Nitroarenes. Microporous Mesoporous Mater. 2020, 296, 110014. [Google Scholar] [CrossRef]



| Entry | Substrate | Product | Time (h) | Yield (%) |
|---|---|---|---|---|
| 1 | 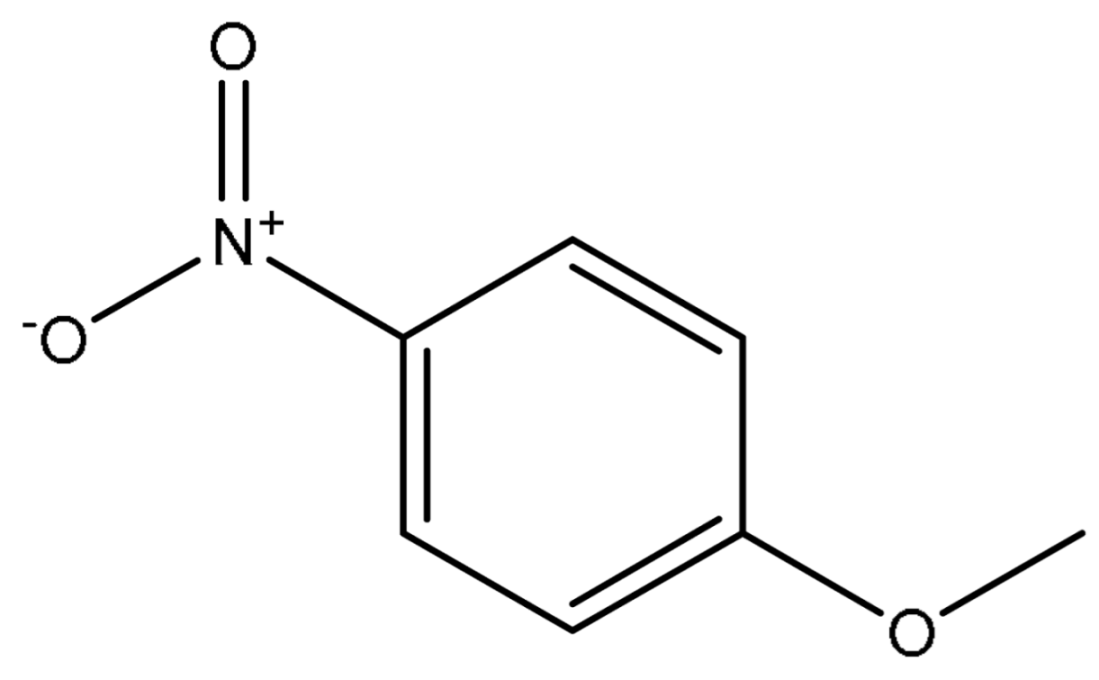 |  | 2 | 99 |
| 2 |  |  | 3 | 97 |
| 3 |  | 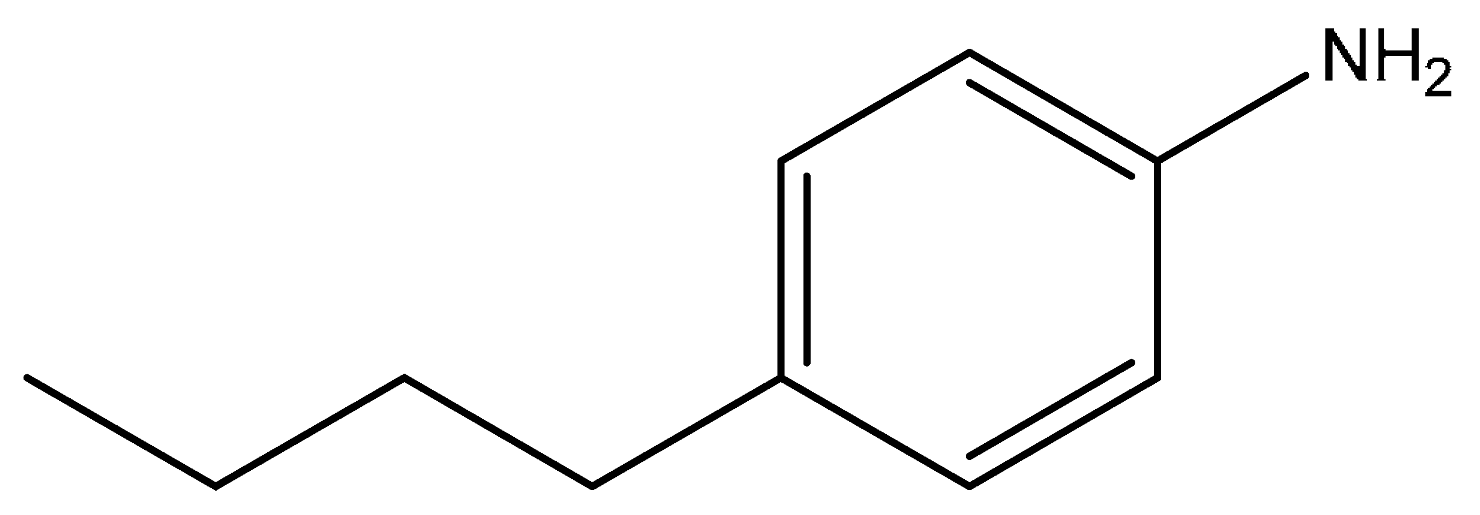 | 2 | 99 |
| 4 |  |  | 2 | 98 |
| 5 |  | 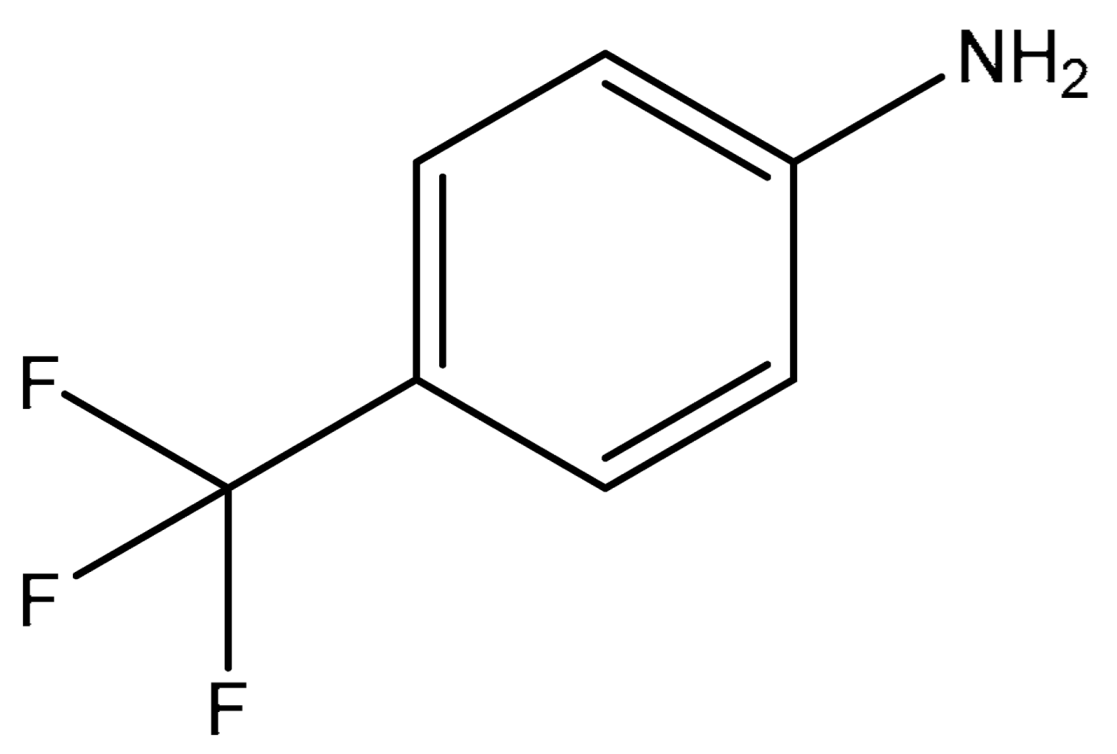 | 3 | 94 |
| Cycle (% Yield) | |||||||
|---|---|---|---|---|---|---|---|
| Entry | Catalyst | 1st | 2nd | 3rd | 4th | 5th | Avg. % Yield |
| 1 | Pt@TiO2–GAP | 99 | 99 | 98 | 97 | 95 | 97.6 |
| Catalyst | Substrate | % Yield | Time (min) | Reaction Conditions | TOF (min−1) | Ref. |
|---|---|---|---|---|---|---|
| Au/TiO2@PVA | 1-methyl-4-nitrobenzene | 97 | 300 | MeOH, RT, H2 (1 atm) | 0.166 | [39] |
| Pd/TiO2 | p-nitrophenol | 81 | 360 | MeOH, RT, H2 (1 atm) | 0.507 | [40] |
| Pt/TiO2@glass film | Nitrobenzene | 87 | 30 | RT, H2 (1 atm) | 0.631 | [41] |
| Fe3O4@TiO2 | p-nitroanisole | 90 | 150 | EtOH, RT, H2 (1 atm) | 0.143 | [42] |
| Pt/TiO2@RGO | Nitrobenzene | 95 | 1200 | MeOH, RT, H2 (1 atm) | 0.309 | [43] |
| Ru/TiO2 | p-chloro-nitrobenzene | 76 | 120 | MeOH, RT, H2 (1 atm) | 28.45 | [44] |
| Pd/RGO | p-methoxy nitrobenzene | 99 | 300 | EtOH\H2O, RT, H2 (1 atm) | 0.943 | [45] |
| Pt/TiO2@GAP | p-methoxy nitrobenzene | 99 | 120 | MeOH, RT, H2 (1 atm) | 0.362 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prakash, S.; Ponnusamy, S.; Rangaraman, J.; Nakkala, K.; Balla, P. Recyclable Platinum Nanocatalyst for Nitroarene Hydrogenation: Gum Acacia Polymer-Stabilized Pt Nanoparticles with TiO2 Support. ChemEngineering 2025, 9, 81. https://doi.org/10.3390/chemengineering9040081
Prakash S, Ponnusamy S, Rangaraman J, Nakkala K, Balla P. Recyclable Platinum Nanocatalyst for Nitroarene Hydrogenation: Gum Acacia Polymer-Stabilized Pt Nanoparticles with TiO2 Support. ChemEngineering. 2025; 9(4):81. https://doi.org/10.3390/chemengineering9040081
Chicago/Turabian StylePrakash, Supriya, Selvakumar Ponnusamy, Jagadeeswari Rangaraman, Kundana Nakkala, and Putrakumar Balla. 2025. "Recyclable Platinum Nanocatalyst for Nitroarene Hydrogenation: Gum Acacia Polymer-Stabilized Pt Nanoparticles with TiO2 Support" ChemEngineering 9, no. 4: 81. https://doi.org/10.3390/chemengineering9040081
APA StylePrakash, S., Ponnusamy, S., Rangaraman, J., Nakkala, K., & Balla, P. (2025). Recyclable Platinum Nanocatalyst for Nitroarene Hydrogenation: Gum Acacia Polymer-Stabilized Pt Nanoparticles with TiO2 Support. ChemEngineering, 9(4), 81. https://doi.org/10.3390/chemengineering9040081








