A Mini-Review of Enhancing Ultrafiltration Membranes (UF) for Wastewater Treatment: Performance and Stability
Abstract
:1. Introduction
2. Polymeric Membranes
2.1. Polymer Blending
2.2. Surface Modified-Membranes
2.3. Mixed Matrix Membranes (MMM)
3. Membranes Structure and Performance
3.1. Membranes Synthesis Techniques
3.1.1. Phase Inversion Technique
3.1.2. Interfacial Polymerization Technique
3.1.3. Spray-Assisted Layer-by-Layer Technique
3.1.4. Polymer Grafting Technique
3.2. Polymer Selection and Alterations Methods
3.3. Type of the Nanoparticle (NPs) Additives
3.3.1. Carbon-Based Nanoparticles
3.3.2. Semiconductor Nanoparticles
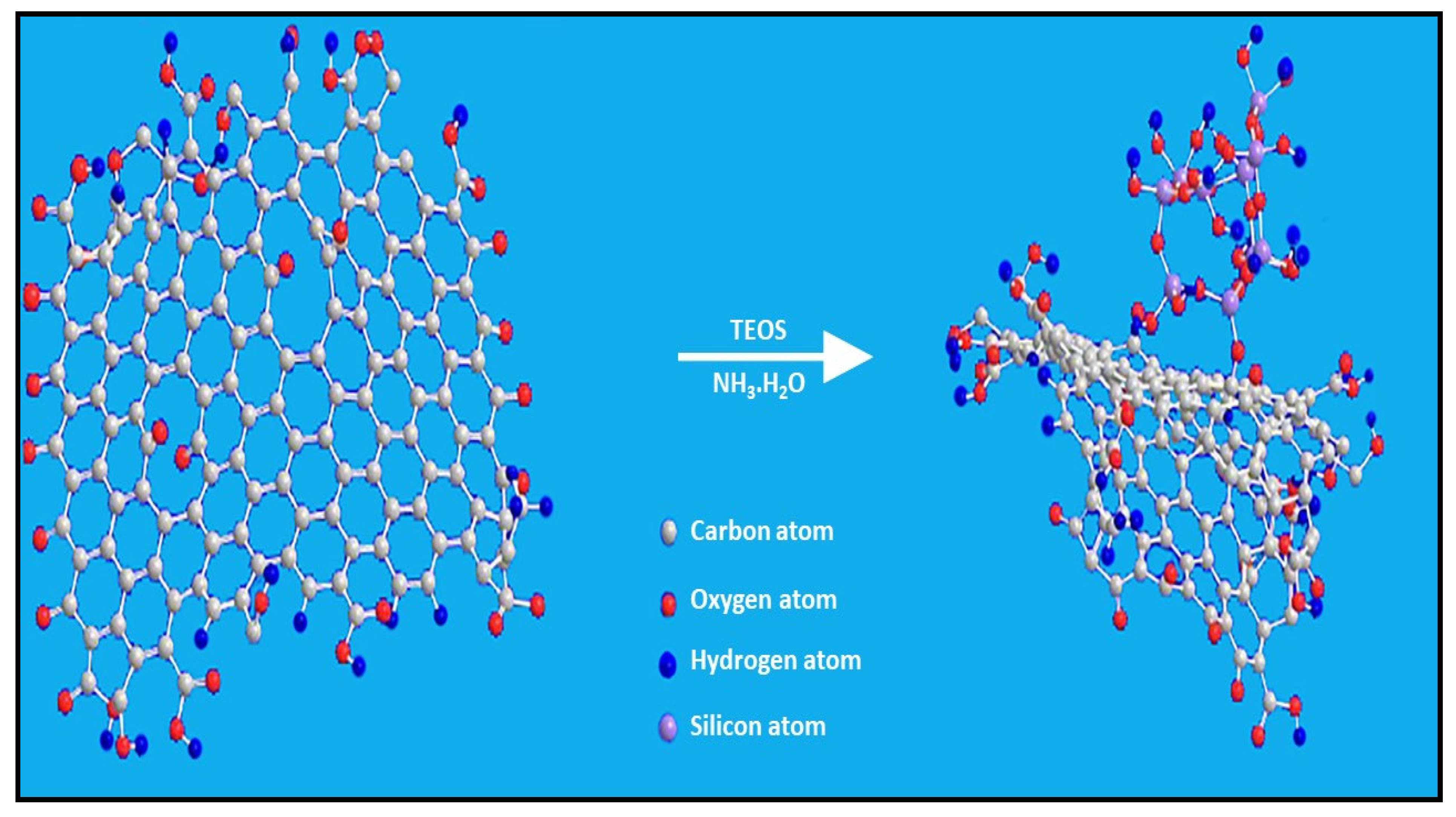
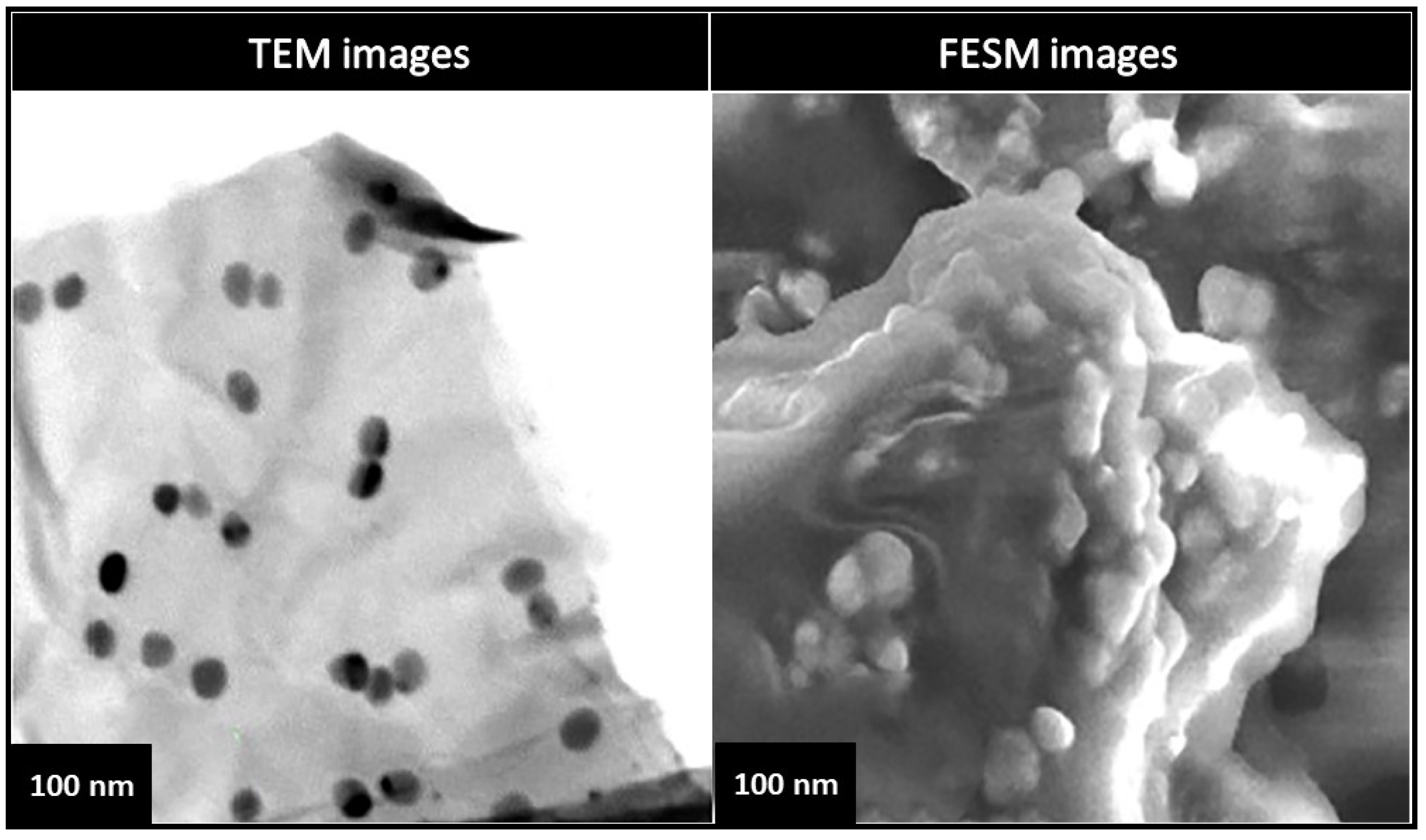
3.3.3. Ceramic Nanoparticles
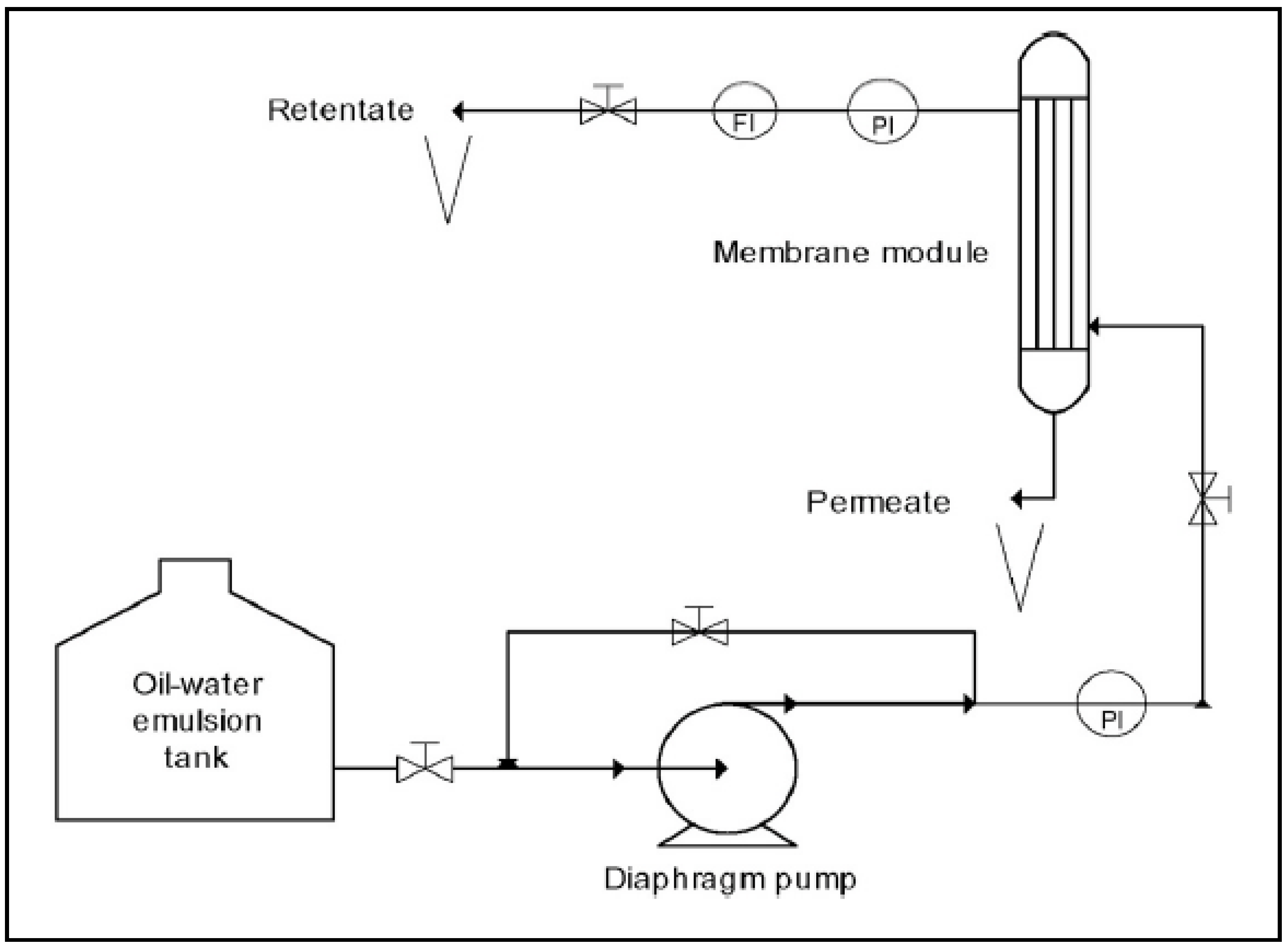
4. UF Membranes Applications in Oily Wastewater Treatment
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AFM | atomic force microscopy |
| APTES | 3-aminopropyltriethoxysilane |
| ATRP | Atom transfer radical polymerization |
| CNTs | Carbon nanotubes |
| DI | Deionized water |
| EDMA | Ethylene glycol dimethacrylate |
| EIPS | Evaporation induced phase inversion |
| FRR | Flux recovery ratio |
| F-MWCNTs | Functionalized-multiwall carbon nanotube |
| GMA | Glycidyl methacrylate |
| GO | Graphene oxide |
| HNTs | Halloysite nanotubes |
| HMO | Hydrous manganese oxide |
| HAO | Hydrous manganese oxide |
| MgO | Magnesium oxide |
| MMM | Mixed matrix membrane |
| MPD | M-phenylenediamine |
| MWCNT | Multiwall carbon nanotubes |
| NPs | Nanoparticles |
| NMP | N-methyl-2-pyrrolidone |
| NIPS | Non-solvent induced phase inversion |
| PIP | Piperazine |
| PDDA | Poly (diallyl-dimethylammonium chloride) |
| PDH | Poly (dimethylaminoethyl methacrylate-co-2-hydroxyethyl methacrylate) |
| PMMA | Poly (methyl methacrylate) |
| PNIPAAm | Poly (N-isopropylacrylamide) |
| PPEGMA | Poly (oligo ethylene glycol methacrylate) |
| PSS | Poly (sodium 4-styrenesulfonate) |
| PVA | Poly (vinylalcohol) |
| PAN | Polyacrylonitrile |
| PA | Polyamide |
| PAI-SPEEK | Polyamide imide-sulfonated poly (ether keton) |
| PANI | Polyaniline |
| PES | Polyethersulfone |
| PEI-CuNPs/PAA | Polyethyleneimine-CuNPs/poly(acrylic) acid |
| PPSU | Polyphenylsulfone |
| PSf | Polysulfone |
| PVC | Polyvinyl chloride |
| PVDF | Polyvinylidene fluoride |
| PVP | Polyvinylpirrolidone |
| R | Rejection coefficient |
| SEM | Scanning electron microscopy |
| SiO2 | Silicon dioxide |
| SSLbL | Spray-and spin-assisted layer-by-layer |
| SPPSU | Sulfonated polyphenyl sulfone |
| SPC | Sulfonated polycarbonate |
| TIPS | Thermally induced phase inversion |
| TFC | Thin film composite |
| TiO2 | Titanium dioxide |
| TMC | Trimesoyl chloride |
| UF | Ultrafiltration |
| VIPS | Vapor induced phase inversion |
| ZnO | Zinc oxide |
References
- Shakir, E. Assessment of nutrient content of raw water close to water treatment plants located in Baghdad City. Desalin. Water Treat. 2016, 57. [Google Scholar] [CrossRef]
- Rajasekhar, T.; Trinadh, M.; Veera Babu, P.; Sainath, A.V.S.; Reddy, A.V.R. Oil-water emulsion separation using ultrafiltration membranes based on novel blends of poly(vinylidene fluoride) and amphiphilic tri-block copolymer containing carboxylic acid functional group. J. Memb. Sci. 2015. [Google Scholar] [CrossRef]
- Mohammed, T.J.; Shakir, E. Effect of settling time, velocity gradient, and camp number on turbidity removal for oilfield produced water. Egypt. J. Pet. 2018, 27. [Google Scholar] [CrossRef]
- Al-Ani, F.H.; Alsalhy, Q.F.; Raheem, R.S.; Rashid, K.T.; Figoli, A. Experimental investigation of the effect of implanting tio2-nps on pvc for long-term uf membrane performance to treat refinery wastewater. Membranes 2020, 10, 77. [Google Scholar] [CrossRef] [Green Version]
- Mohammed, T.J.; Awad, E.S.; Ahmed, T.A. Oil Removal from Oilfield Produced Water, North Rumaila by Combination CoagulationFlocculation and Microfiltration Technique. Eng. Technol. J. 2019, 37, 204–208. [Google Scholar]
- Litter, M.I.; Quici, N. Photochemical advanced oxidation processes for water and wastewater treatment. Recent Patents Eng. 2010, 4, 217–241. [Google Scholar] [CrossRef]
- Ng, L.Y.; Mohammad, A.W.; Leo, C.P.; Hilal, N. Polymeric membranes incorporated with metal/metal oxide nanoparticles: A comprehensive review. Desalination 2013, 308, 15–33. [Google Scholar] [CrossRef]
- Ahmad, T.; Guria, C.; Mandal, A. A review of oily wastewater treatment using ultrafiltration membrane: A parametric study to enhance the membrane performance. J. Water Process Eng. 2020, 36, 101289. [Google Scholar] [CrossRef]
- Jalal Sadiq, A.; Shabeeb, K.M.; Khalil, B.I.; Alsalhy, Q.F. Effect of embedding MWCNT-g-GO with PVC on the performance of PVC membranes for oily wastewater treatment. Chem. Eng. Commun. 2020. [Google Scholar] [CrossRef]
- Padaki, M.; Surya Murali, R.; Abdullah, M.S.; Misdan, N.; Moslehyani, A.; Kassim, M.A.; Hilal, N.; Ismail, A.F. Membrane technology enhancement in oil-water separation. A review. Desalination 2015, 357, 197–207. [Google Scholar] [CrossRef]
- Ihsanullah Carbon nanotube membranes for water purification: Developments, challenges, and prospects for the future. Sep. Purif. Technol. 2019, 209, 307–337. [CrossRef]
- Wang, Q.; Cui, J.; Liu, S.; Gao, J.; Lang, J.; Li, C.; Yan, Y. Facile preparation of halloysite nanotube-modified polyvinylidene fluoride composite membranes for highly efficient oil/water emulsion separation. J. Mater. Sci. 2019. [Google Scholar] [CrossRef]
- Zhang, W.; Shi, Z.; Zhang, F.; Liu, X.; Jin, J.; Jiang, L. Superhydrophobic and superoleophilic PVDF membranes for effective separation of water-in-oil emulsions with high flux. Adv. Mater. 2013. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, Y.; Liu, Y.; Xu, J.; Han, Y.; Xu, X. Preparation, performances of PVDF/ZnO hybrid membranes and their applications in the removal of copper ions. Appl. Surf. Sci. 2014, 316, 333–340. [Google Scholar] [CrossRef]
- Shi, H.; He, Y.; Pan, Y.; Di, H.; Zeng, G.; Zhang, L.; Zhang, C. A modified mussel-inspired method to fabricate TiO2 decorated superhydrophilic PVDF membrane for oil/water separation. J. Memb. Sci. 2016, 506, 60–70. [Google Scholar] [CrossRef]
- Ejaz Ahmed, F.; Lalia, B.S.; Hilal, N.; Hashaikeh, R. Underwater superoleophobic cellulose/electrospun PVDF-HFP membranes for efficient oil/water separation. Desalination 2014. [Google Scholar] [CrossRef]
- Gohari, R.J.; Halakoo, E.; Lau, W.J.; Kassim, M.A.; Matsuura, T.; Ismail, A.F. Novel polyethersulfone (PES)/hydrous manganese dioxide (HMO) mixed matrix membranes with improved anti-fouling properties for oily wastewater treatment process. RSC Adv. 2014, 4, 17587–17596. [Google Scholar] [CrossRef]
- Salahi, A.; Mohammadi, T.; Mosayebi Behbahani, R.; Hemmati, M. Asymmetric polyethersulfone ultrafiltration membranes for oily wastewater treatment: Synthesis, characterization, ANFIS modeling, and performance. J. Environ. Chem. Eng. 2015. [Google Scholar] [CrossRef]
- Li, J.; Wang, Q.; Deng, L.; Kou, X.; Tang, Q.; Hu, Y. Fabrication and characterization of carbon nanotubes-based porous composite forward osmosis membrane: Flux performance, separation mechanism, and potential application. J. Memb. Sci. 2020. [Google Scholar] [CrossRef]
- Alkindy, M.B.; Naddeo, V.; Banat, F.; Hasan, S.W. Synthesis of polyethersulfone (PES)/GO-SiO2 mixed matrix membranes for oily wastewater treatment. Water Sci. Technol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Zarghami, S.; Mohammadi, T.; Sadrzadeh, M.; Van der Bruggen, B. Bio-inspired anchoring of amino-functionalized multi-wall carbon nanotubes (N-MWCNTs) onto PES membrane using polydopamine for oily wastewater treatment. Sci. Total Environ. 2020. [Google Scholar] [CrossRef]
- Chakrabarty, B.; Ghoshal, A.K.; Purkait, M.K. Ultrafiltration of stable oil-in-water emulsion by polysulfone membrane. J. Memb. Sci. 2008. [Google Scholar] [CrossRef]
- Ahmad, A.L.; Majid, M.A.; Ooi, B.S. Functionalized PSf/SiO2 nanocomposite membrane for oil-in-water emulsion separation. Desalination 2011, 268, 266–269. [Google Scholar] [CrossRef]
- Saadati, J.; Pakizeh, M. Separation of oil/water emulsion using a new PSf/pebax/F-MWCNT nanocomposite membrane. J. Taiwan Inst. Chem. Eng. 2017, 71, 265–276. [Google Scholar] [CrossRef]
- Modi, A.; Bellare, J. Efficiently improved oil/water separation using high flux and superior antifouling polysulfone hollow fiber membranes modified with functionalized carbon nanotubes/graphene oxide nanohybrid. J. Environ. Chem. Eng. 2019. [Google Scholar] [CrossRef]
- Ahmad, T.; Guria, C.; Mandal, A. Optimal synthesis of high fouling-resistant PVC-based ultrafiltration membranes with tunable surface pore size distribution and ultralow water contact angle for the treatment of oily wastewater. Sep. Purif. Technol. 2021, 257, 117829. [Google Scholar] [CrossRef]
- Salahi, A.; Abbasi, M.; Mohammadi, T. Permeate flux decline during UF of oily wastewater: Experimental and modeling. Desalination 2010. [Google Scholar] [CrossRef]
- Zhang, F.; Gao, S.; Zhu, Y.; Jin, J. Alkaline-induced superhydrophilic/underwater superoleophobic polyacrylonitrile membranes with ultralow oil-adhesion for high-efficient oil/water separation. J. Memb. Sci. 2016. [Google Scholar] [CrossRef]
- Alsalhy, Q.F. Hollow fiber ultrafiltration membranes prepared from blends of poly (vinyl chloride) and polystyrene. Desalination 2012. [Google Scholar] [CrossRef]
- Yuan, T.; Meng, J.; Hao, T.; Wang, Z.; Zhang, Y. A scalable method toward superhydrophilic and underwater superoleophobic PVDF membranes for effective oil/water emulsion separation. ACS Appl. Mater. Interfaces 2015. [Google Scholar] [CrossRef] [PubMed]
- Ismail, N.H.; Salleh, W.N.W.; Ismail, A.F.; Hasbullah, H.; Yusof, N.; Aziz, F.; Jaafar, J. Hydrophilic polymer-based membrane for oily wastewater treatment: A review. Sep. Purif. Technol. 2020, 233, 116007. [Google Scholar] [CrossRef]
- Masuelli, M.; Marchese, J.; Ochoa, N.A. SPC/PVDF membranes for emulsified oily wastewater treatment. J. Memb. Sci. 2009. [Google Scholar] [CrossRef]
- Zhu, Y.; Xie, W.; Zhang, F.; Xing, T.; Jin, J. Superhydrophilic In-Situ-Cross-Linked Zwitterionic Polyelectrolyte/PVDF-Blend Membrane for Highly Efficient Oil/Water Emulsion Separation. ACS Appl. Mater. Interfaces 2017. [Google Scholar] [CrossRef] [PubMed]
- Johari, A.; Razmjouei, M.; Mansourizadeh, A.; Emadzadeh, D. Fabrication of blend hydrophilic polyamide imide (Torlon®)-sulfonated poly (ether ether ketone) hollow fiber membranes for oily wastewater treatment. Polym. Test. 2020. [Google Scholar] [CrossRef]
- Rajaeian, B.; Heitz, A.; Tade, M.O.; Liu, S. Improved separation and antifouling performance of PVA thin film nanocomposite membranes incorporated with carboxylated TiO2 nanoparticles. J. Memb. Sci. 2015. [Google Scholar] [CrossRef]
- Wandera, D.; Wickramasinghe, S.R.; Husson, S.M. Modification and characterization of ultrafiltration membranes for treatment of produced water. J. Memb. Sci. 2011. [Google Scholar] [CrossRef]
- Masuelli, M.A.; Grasselli, M.; Marchese, J.; Ochoa, N.A. Preparation, structural and functional characterization of modified porous PVDF membranes by γ-irradiation. J. Memb. Sci. 2012. [Google Scholar] [CrossRef]
- Yuliwati, E.; Ismail, A.F. Effect of additives concentration on the surface properties and performance of PVDF ultrafiltration membranes for refinery produced wastewater treatment. Desalination 2011. [Google Scholar] [CrossRef]
- Ong, C.S.; Lau, W.J.; Goh, P.S.; Ismail, A.F. Preparation and characterization of PVDF-TiO2 composite membranes blended with different Mw of PVP for oily wastewater treatment using submerged membrane system. J. Teknol. Sci. Eng. 2014. [Google Scholar] [CrossRef] [Green Version]
- Ong, C.S.; Lau, W.J.; Goh, P.S.; Ng, B.C.; Ismail, A.F. Preparation and characterization of PVDF–PVP–TiO2 composite hollow fiber membranes for oily wastewater treatment using submerged membrane system. Desalin. Water Treat. 2015. [Google Scholar] [CrossRef]
- Yi, X.S.; Yu, S.L.; Shi, W.X.; Sun, N.; Jin, L.M.; Wang, S.; Zhang, B.; Ma, C.; Sun, L.P. The influence of important factors on ultrafiltration of oil/water emulsion using PVDF membrane modified by nano-sized TiO2/Al2O3. Desalination 2011. [Google Scholar] [CrossRef]
- Shen, J.N.; Yu, C.C.; Ruan, H.M.; Gao, C.J.; Van der Bruggen, B. Preparation and characterization of thin-film nanocomposite membranes embedded with poly(methyl methacrylate) hydrophobic modified multiwalled carbon nanotubes by interfacial polymerization. J. Memb. Sci. 2013. [Google Scholar] [CrossRef]
- Marquez, J.A.D.; Ang, M.B.M.Y.; Doma, B.T.; Huang, S.H.; Tsai, H.A.; Lee, K.R.; Lai, J.Y. Application of cosolvent-assisted interfacial polymerization technique to fabricate thin-film composite polyamide pervaporation membranes with PVDF hollow fiber as support. J. Memb. Sci. 2018. [Google Scholar] [CrossRef]
- Ma, W.; Soroush, A.; Van Anh Luong, T.; Brennan, G.; Rahaman, M.S.; Asadishad, B.; Tufenkji, N. Spray- and spin-assisted layer-by-layer assembly of copper nanoparticles on thin-film composite reverse osmosis membrane for biofouling mitigation. Water Res. 2016. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Son, M.; Chakraborty, S.; Bhattacharjee, C.; Choi, H. Fabrication of ultra-thin polyelectrolyte/carbon nanotube membrane by spray-assisted layer-by-layer technique: Characterization and its anti-protein fouling properties for water treatment. Desalin. Water Treat. 2013, 51, 6194–6200. [Google Scholar] [CrossRef]
- Yang, X.; He, Y.; Zeng, G.; Chen, X.; Shi, H.; Qing, D.; Li, F.; Chen, Q. Bio-inspired method for preparation of multiwall carbon nanotubes decorated superhydrophilic poly(vinylidene fluoride) membrane for oil/water emulsion separation. Chem. Eng. J. 2017. [Google Scholar] [CrossRef]
- Zhu, Z.; Jiang, J.; Wang, X.; Huo, X.; Xu, Y.; Li, Q.; Wang, L. Improving the hydrophilic and antifouling properties of polyvinylidene fluoride membrane by incorporation of novel nanohybrid GO@SiO2 particles. Chem. Eng. J. 2017. [Google Scholar] [CrossRef]
- Aerts, P.; Genné, I.; Leysen, R.; Jacobs, P.A.; Vankelecom, I.F.J. The role of the nature of the casting substrate on the properties of membranes prepared via immersion precipitation. J. Memb. Sci. 2006. [Google Scholar] [CrossRef]
- Wu, H.; Tang, B.; Wu, P. Novel ultrafiltration membranes prepared from a multi-walled carbon nanotubes/polymer composite. J. Memb. Sci. 2010, 362, 374–383. [Google Scholar] [CrossRef]
- Mansourizadeh, A.; Ismail, A.F.; Abdullah, M.S.; Ng, B.C. Preparation of polyvinylidene fluoride hollow fiber membranes for CO2 absorption using phase-inversion promoter additives. J. Memb. Sci. 2010. [Google Scholar] [CrossRef]
- Sakinah, A.M.M.; Ismail, A.F.; Illias, R.M.; Zularisam, A.W.; Hassan, O.; Matsuura, T. Cyclodextrin production in hollow fiber membrane reactor system: Effect of substrate preparation. Sep. Purif. Technol. 2008. [Google Scholar] [CrossRef]
- Kim, J.; Van der Bruggen, B. The use of nanoparticles in polymeric and ceramic membrane structures: Review of manufacturing procedures and performance improvement for water treatment. Environ. Pollut. 2010, 158, 2335–2349. [Google Scholar] [CrossRef]
- Wu, Z.; Zhang, C.; Peng, K.; Wang, Q.; Wang, Z. Hydrophilic/underwater superoleophobic graphene oxide membrane intercalated by TiO 2 nanotubes for oil/water separation. Front. Environ. Sci. Eng. 2018, 12, 15. [Google Scholar] [CrossRef]
- Khan, I.; Saeed, K.; Khan, I. Nanoparticles: Properties, applications and toxicities. Arab. J. Chem. 2019, 12, 908–931. [Google Scholar] [CrossRef]
- Moslehyani, A.; Ismail, A.F.; Othman, M.H.D.; Matsuura, T. Design and performance study of hybrid photocatalytic reactor-PVDF/MWCNT nanocomposite membrane system for treatment of petroleum refinery wastewater. Desalination 2015, 363, 99–111. [Google Scholar] [CrossRef]
- Alsalhy, Q.F.; Ali, J.M.; Abbas, A.A.; Rashed, A.; van der Bruggen, B.; Balta, S. Enhancement of poly(phenyl sulfone) membranes with ZnO nanoparticles. Desalin. Water Treat. 2013. [Google Scholar] [CrossRef]
- Li, Z.-K.; Lang, W.-Z.; Miao, W.; Yan, X.; Guo, Y.-J. Preparation and properties of PVDF/SiO2@ GO nanohybrid membranes via thermally induced phase separation method. J. Memb. Sci. 2016, 511, 151–161. [Google Scholar] [CrossRef]
- Yuliwati, E.; Ismail, A.F.; Matsuura, T.; Kassim, M.A.; Abdullah, M.S. Effect of modified pvdf hollow fiber submerged ultrafiltration membrane for refinery wastewater treatment. Desalination 2011. [Google Scholar] [CrossRef]
- Liu, M.; Li, J.; Guo, Z. Polyaniline coated membranes for effective separation of oil-in-water emulsions. J. Colloid Interface Sci. 2016. [Google Scholar] [CrossRef]
- Arumugham, T.; Kaleekkal, N.J.; Rana, D.; Doraiswamy, M. Separation of oil/water emulsions using nano MgO anchored hybrid ultrafiltration membranes for environmental abatement. J. Appl. Polym. Sci. 2016, 133. [Google Scholar] [CrossRef]
- Jamshidi Gohari, R.; Korminouri, F.; Lau, W.J.; Ismail, A.F.; Matsuura, T.; Chowdhury, M.N.K.; Halakoo, E.; Jamshidi Gohari, M.S. A novel super-hydrophilic PSf/HAO nanocomposite ultrafiltration membrane for efficient separation of oil/water emulsion. Sep. Purif. Technol. 2015. [Google Scholar] [CrossRef]
- Kumar, S.; Nandi, B.K.; Guria, C.; Mandal, A. Oil removal from produced water by ultrafiltration using polysulfone membrane. Brazilian J. Chem. Eng. 2017. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, T.; Guria, C.; Mandal, A. Synthesis, characterization and performance studies of mixed-matrix poly (vinyl chloride)-bentonite ultrafiltration membrane for the treatment of saline oily wastewater. Process Saf. Environ. Prot. 2018, 116, 703–717. [Google Scholar] [CrossRef]
- Panda, S.R.; Bhandaru, N.; Mukherjee, R.; De, S. Ultrafiltration of oily waste water: Contribution of surface roughness in membrane properties and fouling characteristics of polyacrylonitrile membranes. Can. J. Chem. Eng. 2015. [Google Scholar] [CrossRef]
- Kallem, P.; Othman, I.; Ouda, M.; Hasan, S.W.; AlNashef, I.; Banat, F. Polyethersulfone hybrid ultrafiltration membranes fabricated with polydopamine modified ZnFe2O4 nanocomposites: Applications in humic acid removal and oil/water emulsion separation. Process Saf. Environ. Prot. 2021, 148, 813–824. [Google Scholar] [CrossRef]
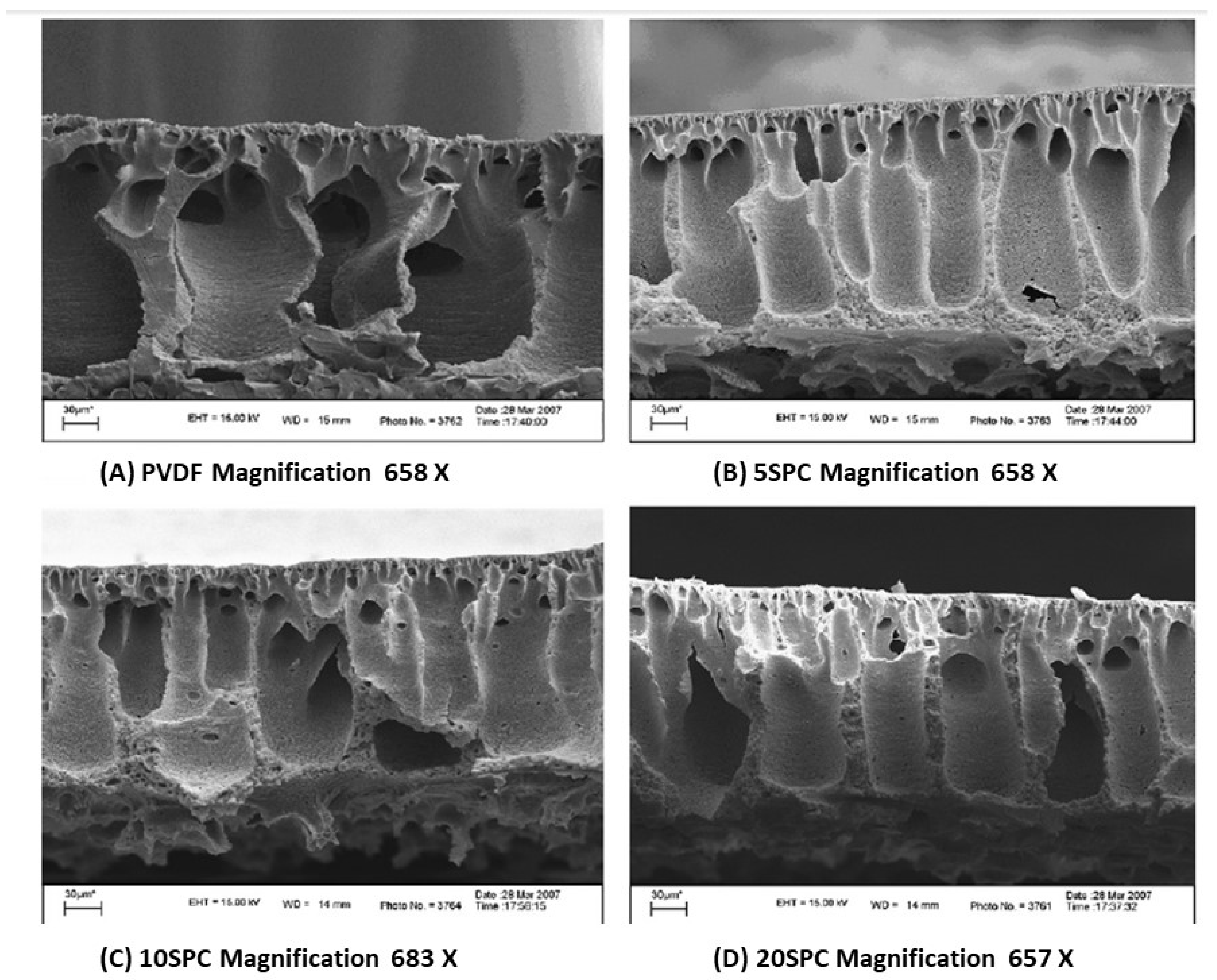
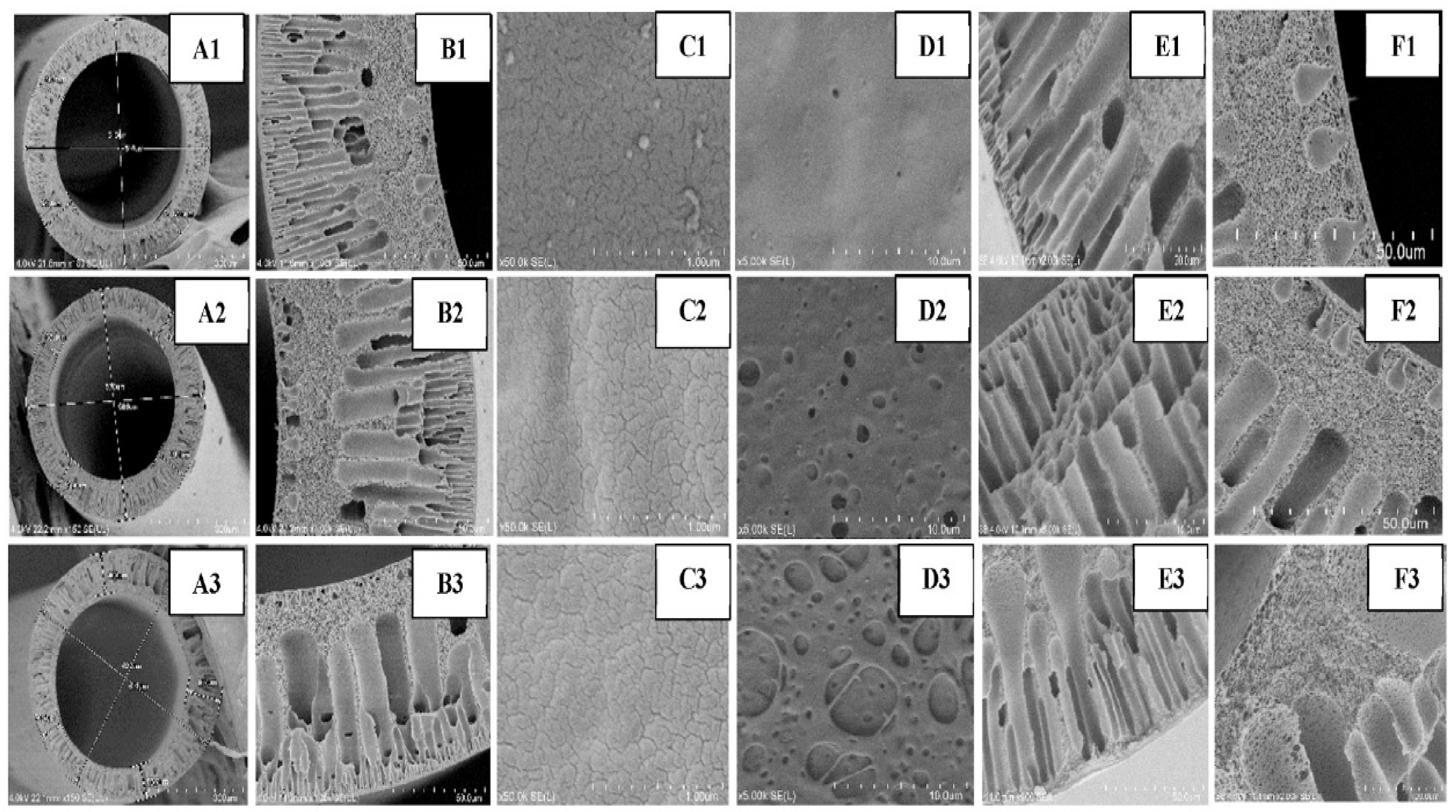
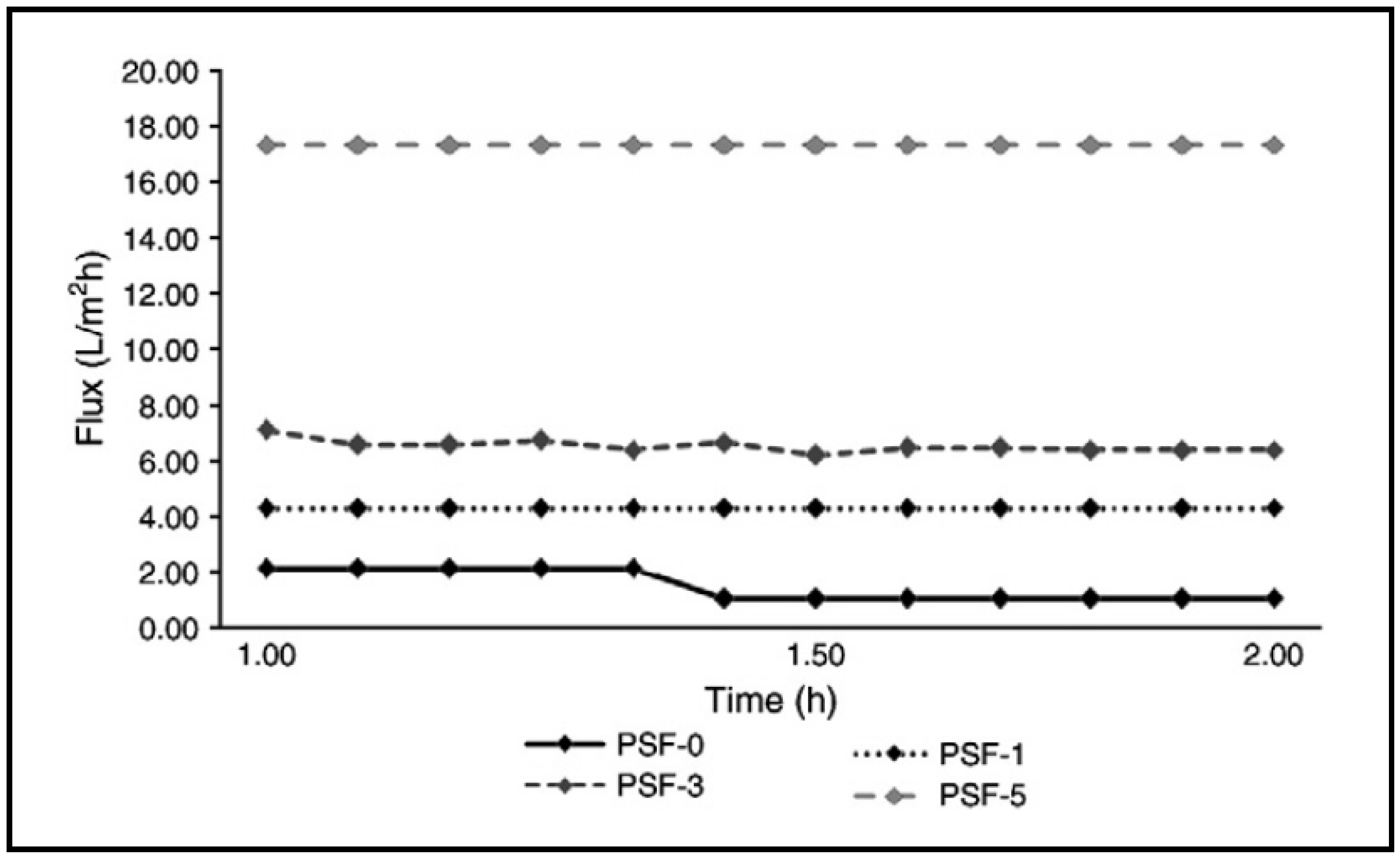
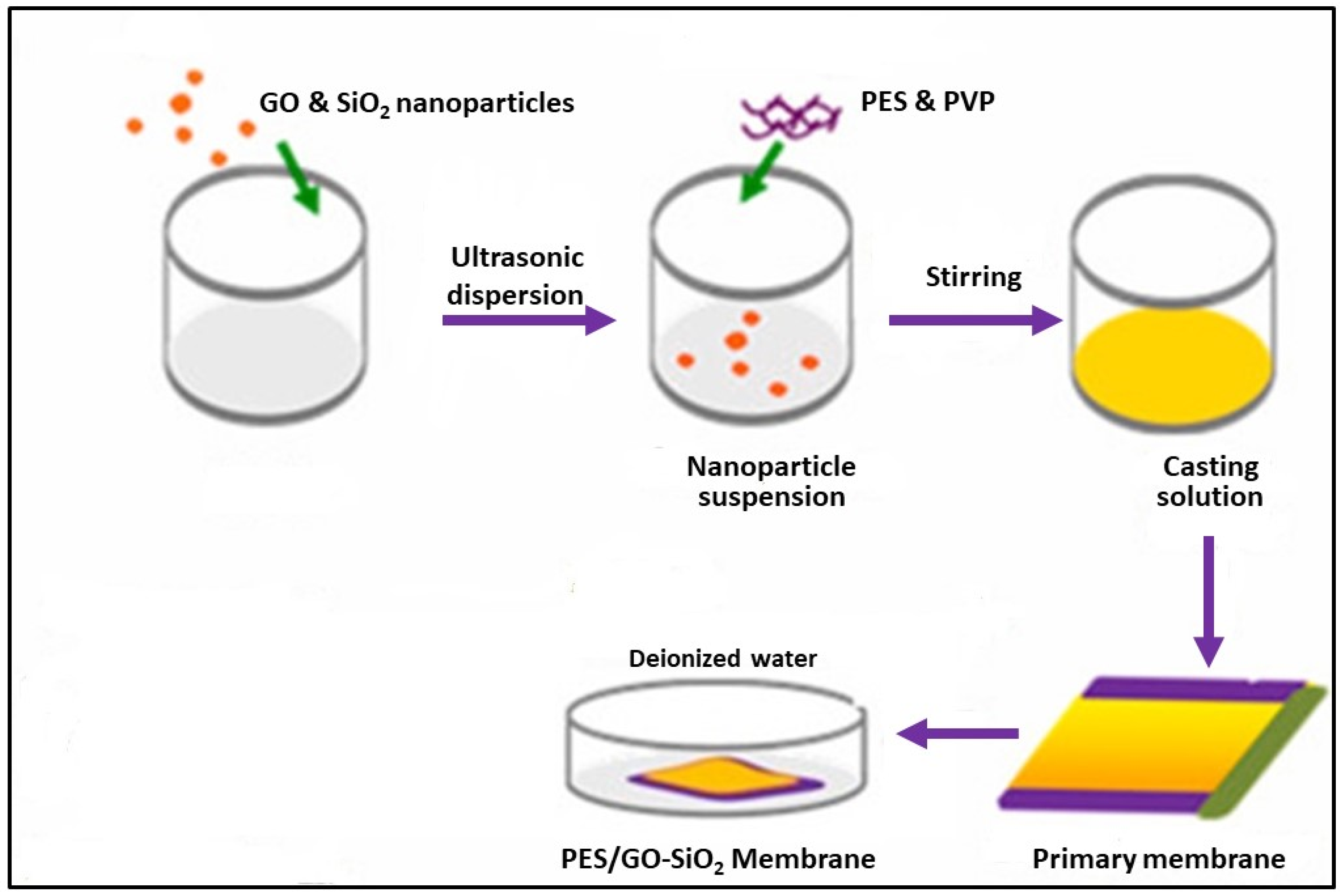
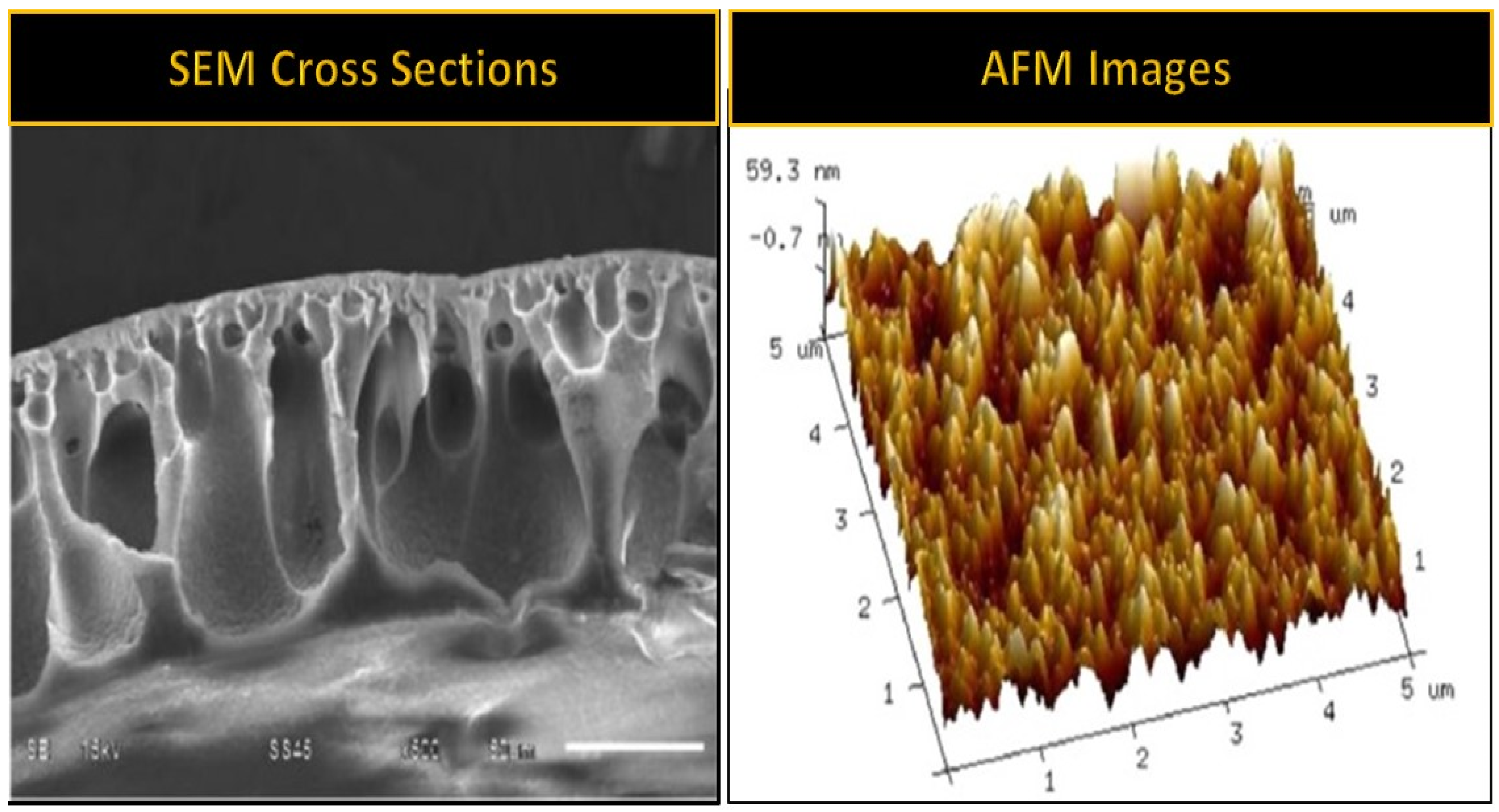
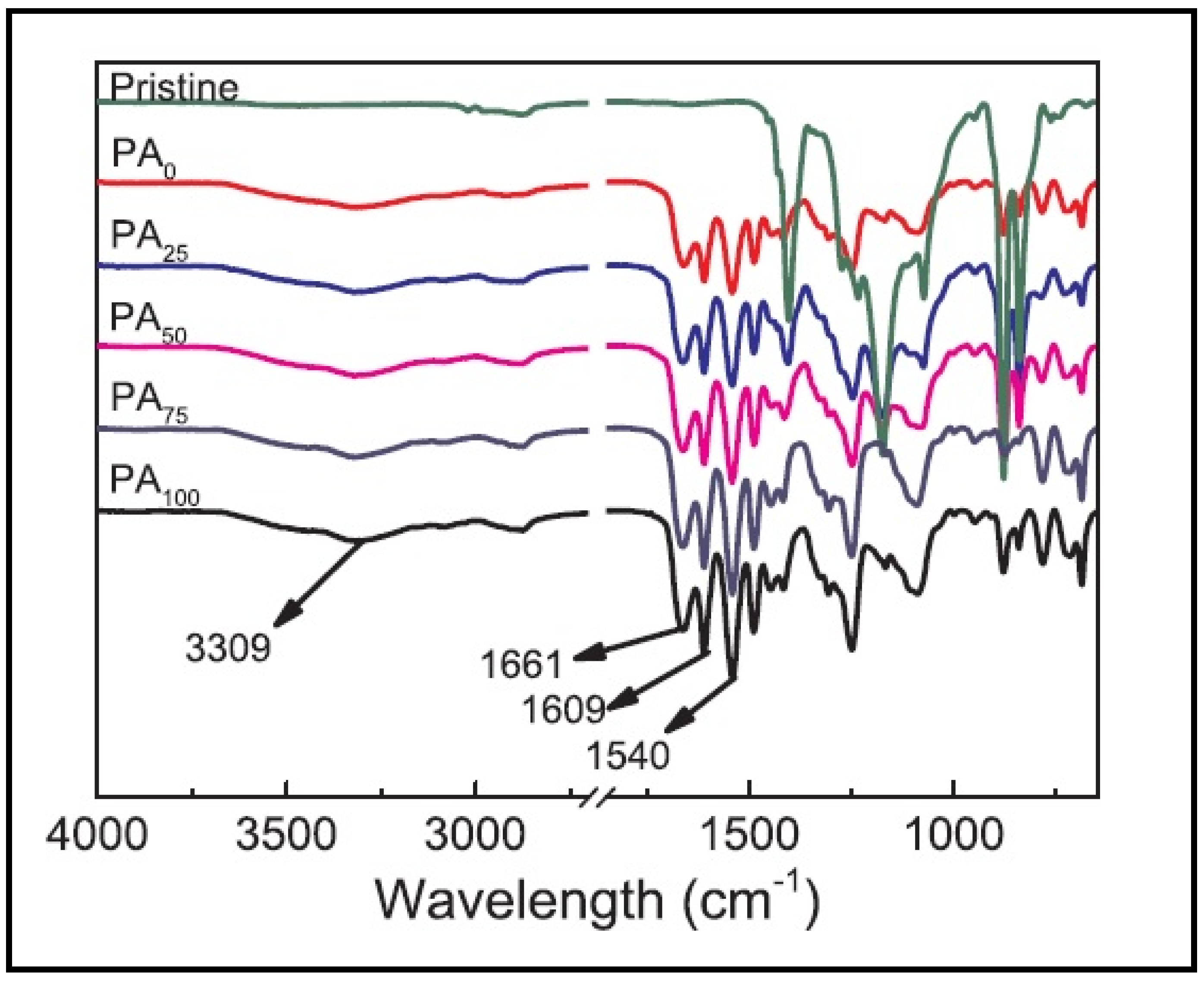
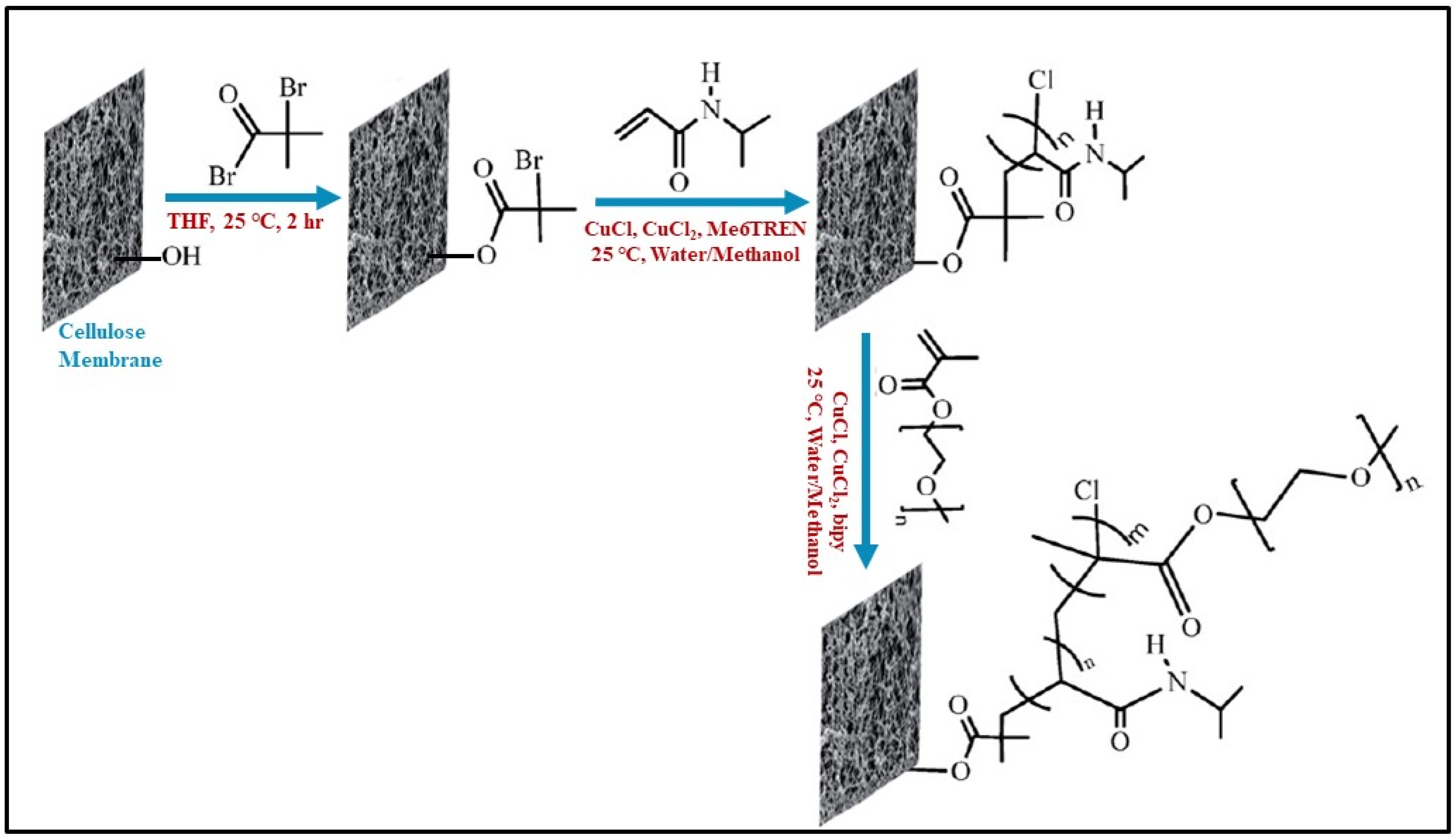
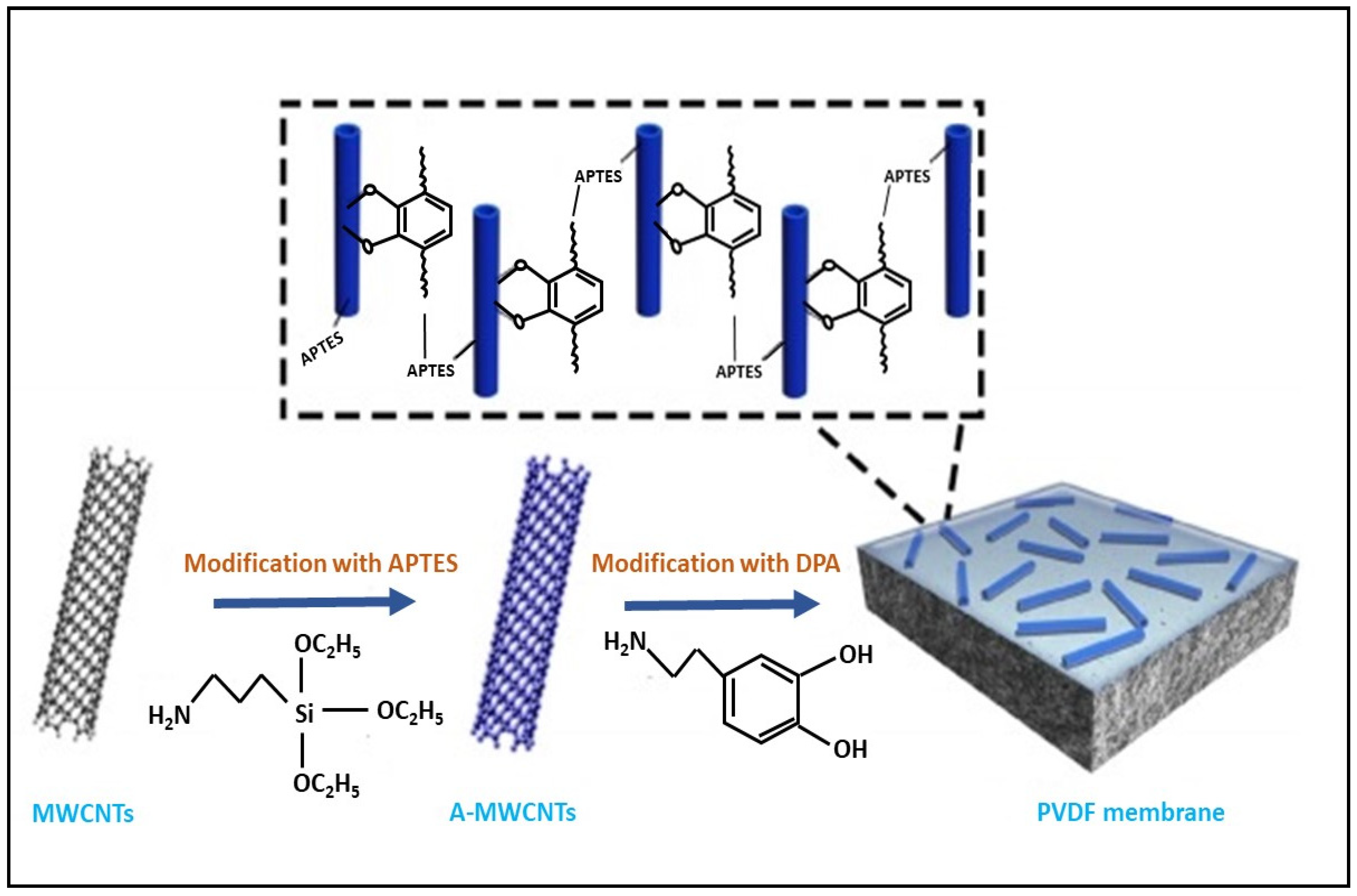
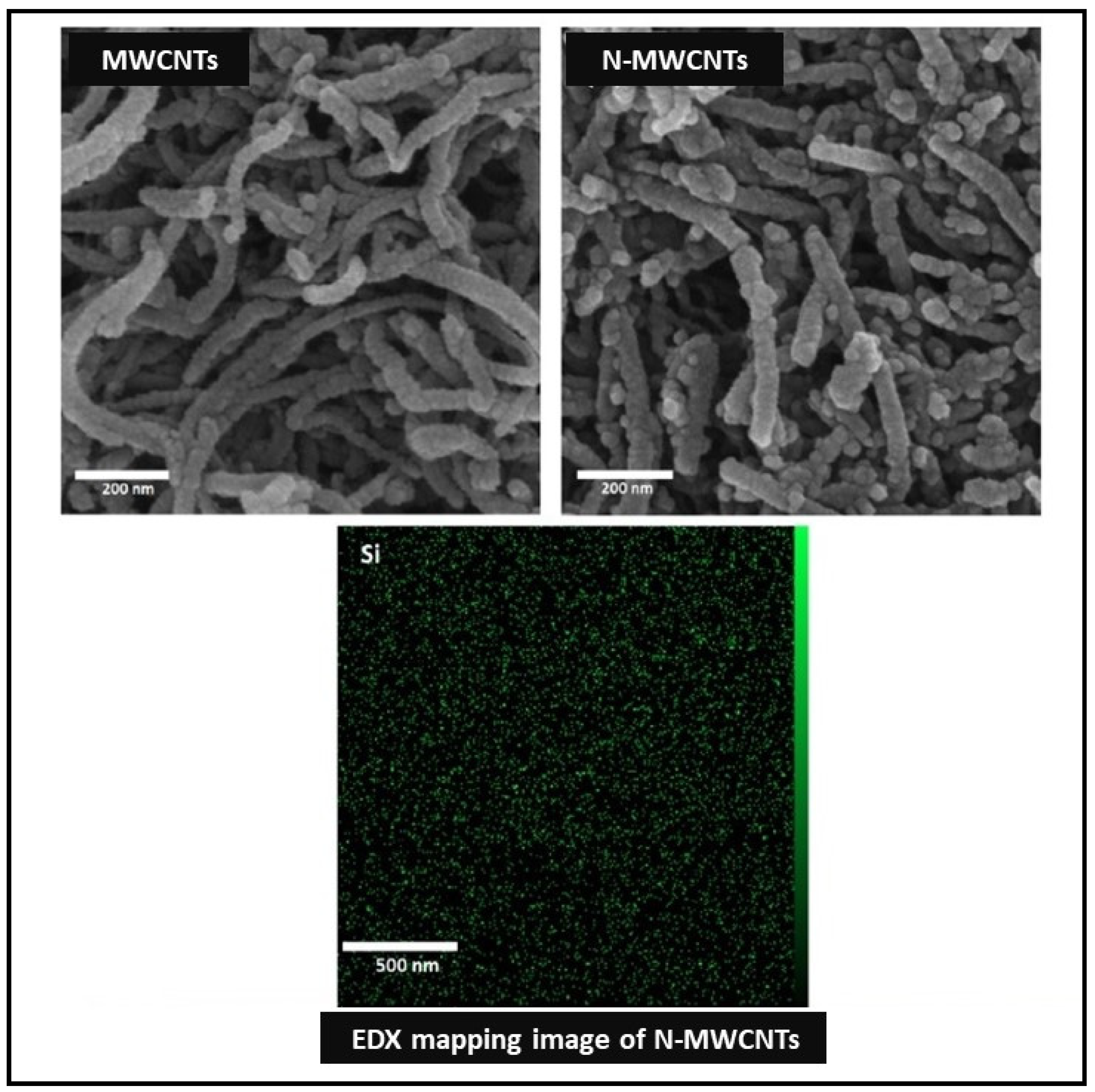

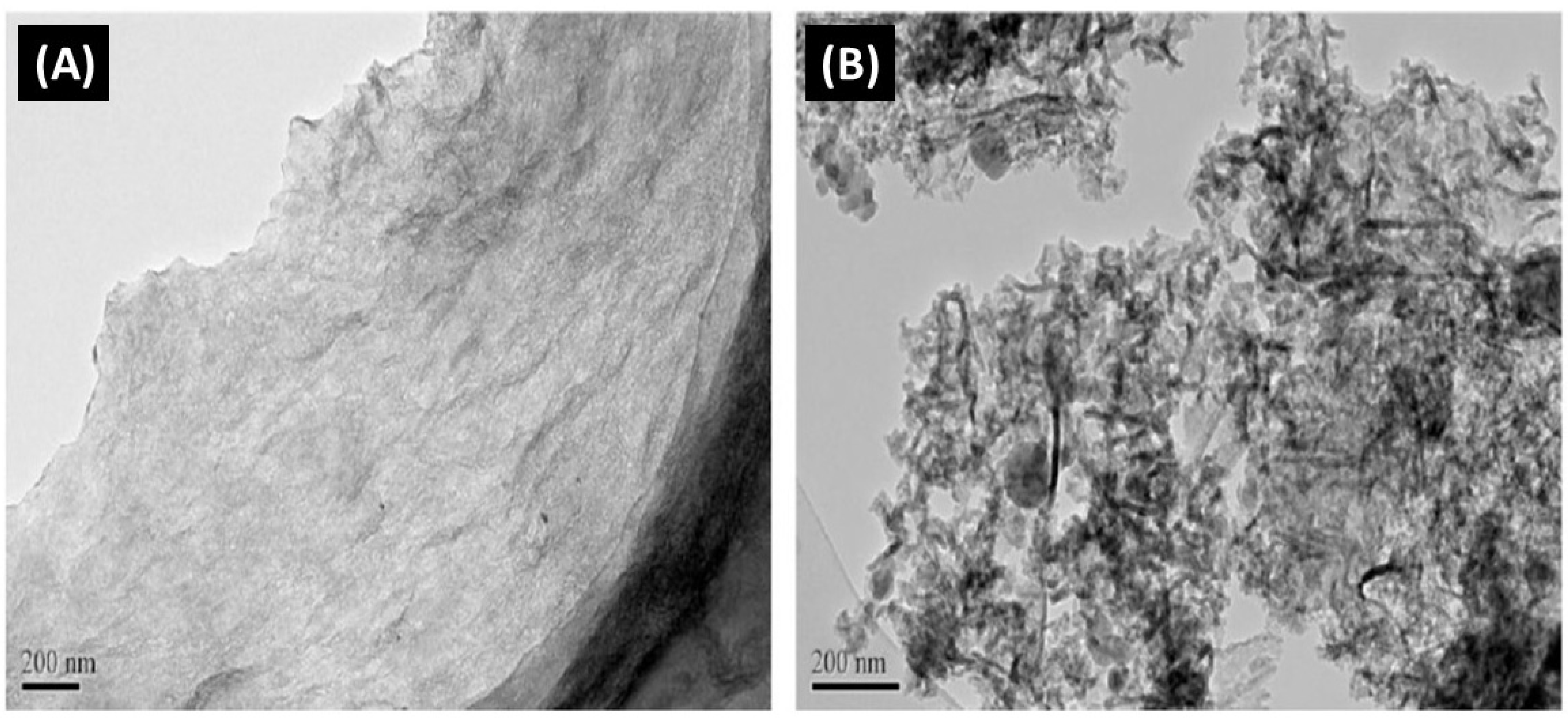
| Membrane | Fabrication Method | Properties | Operating Condition | Performance | Ref. | |||
|---|---|---|---|---|---|---|---|---|
| Pore Size, nm | Porosity, % | Contact Angle ° | Oil Rejection % | Water Flux (LMH) | ||||
| PVDF/SPC | Polymer-blending | 46.06–35.89 | - | - | pressures = 20–100 kPa pH = 7 | 96.63 | - | [32] |
| PVDF/Zwitterionic Polyelectrolyte | Polymer-blending | - | - | - | - | 98.00 | 6350.00 | [33] |
| hydrophilic (PAI)-sulfonated poly (ether ether keton) | Polymer-blending | 81.00 | 79.00% | 58° | Operating time: 5 h, pressure: 400.00 kPa, temperature: 25 °C | 95.00 | - | [34] |
| PSf/PEG/PVP | Polymer-blending | 3.00–3.88 | - | - | Press. = 68.90–137.90 kPa pH = 5–8 | 90.00 | - | [22] |
| PAN | Surface modified | - | 71.7–79.6 | - | - | 85.00 | 2270.00 | [28] |
| PVDF/PVA/TiO2 | Surface modified (coating) | 1.95–3.68 | 83.00 | 46.05–57.07° | - | 91.50 | - | [35] |
| PNIPAAm/PPEGMA | Surface modified (grafting) | - | - | - | 97.00 | - | [36] | |
| PVDF | Surface modified (grafting) | - | - | - | 98.00 | - | [37] | |
| PSf/SiO2 | MMM | 36.21–127.20 | - | - | - | - | 17.32 | [23] |
| PVDF/LiCl·H2O/SiO2 | MMM | 14.93–34.05 | 63.26–85.41 | - | pressure = 0.10 MPa | 62.56–98.83 | 82.50 | [38] |
| PVDF/PVP/TiO2 | MMM | 94.30–104.40 | 84.10–88.60 | 68.40–75.70° | Temperature: 25 °C | 99.70 | 70.48 | [39] |
| PVDF/PVP | MMM | - | - | - | - | 99.70 | 70.48 | [40] |
| Membrane | PVDF (%) | SPC (%) | R (%) |
|---|---|---|---|
| PVDF | 100 | - | 95.46 |
| 5-SPC | 95 | 5 | 96.66 |
| 10-SPC | 90 | 10 | 96.71 |
| 20-SPC | 80 | 20 | 96.63 |
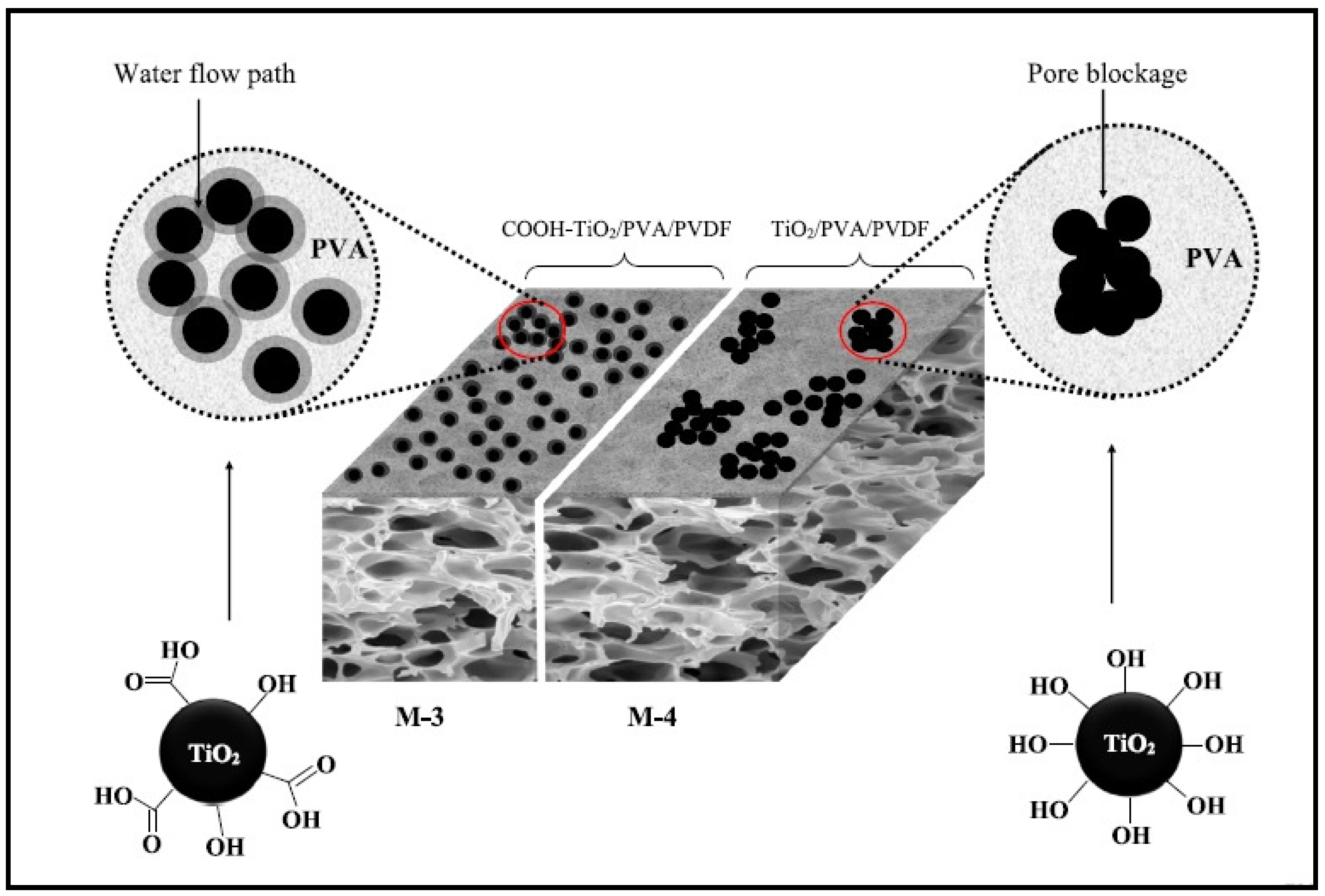
| Membrane | PVDF (wt.%) | PVA (wt.%) | PVP (wt.%) | TiO2-COOH (wt.%) | Pure TiO2 (wt.%) |
|---|---|---|---|---|---|
| PVDF | 15 | - | 2 | - | - |
| M-1 | 15 | 1 | 2 | - | - |
| M-2 | 15 | 1 | 2 | 0.5 | - |
| M-3 | 15 | 1 | 2 | 1 | - |
| M-4 | 15 | 1 | 2 | - | 1 |
| Membrane Type | Synthesis Technique | Water Flux (LMH) | Removal Efficiency % | Ref. |
|---|---|---|---|---|
| PVC/MWCNT-g-GO | phase inversion | 254.00 | COD rejection 60.00–88.90 | [9] |
| PNIPAAm)-block (PPEGMA) | polymer grafting | - | Rejection > 97.00 | [36] |
| PES/GO-SiO2 MMM | phase inversion | - | Oil rejection 38.00 | [20] |
| PVDF/GO@SiO2/PVP | phase inversion | 1232.00 | Rejection rate 78.50 | [47] |
| PMMA–MWCNTs composites | interfacial polymerization | - | Na2SO4 rejection > 99.00 | [42] |
| Thin-film composite PA/PVDF | interfacial polymerization | 1654.98 | - | [43] |
| CuNP-functionalized membrane | Spray-assisted layer-by-layer | - | - | [44] |
| PES/F-MWCNTs membrane | Spray-assisted layer-by-layer | - | - | [45] |
| A-MWCNTs/PVDF membrane | polymer grafting | 900.00 | Oil rejection > 99.00 | [46] |
| Monomer Solution | Monomer Concentration | Immersion Time | Cosolvent | Cosolvent Concentration |
|---|---|---|---|---|
| Aqueous-phase MPD | 2.00 wt.% | 5 | Acetone | 0, 25, 50, 75, 100 |
| Organic-phase TMC | 0.50 wt.% | 2 | None | N/A |
| Polymer | Method | Additive Type | Additive Conc. | Properties | Performance | Ref. | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Thickness, μm | Contact Angle ° | Pore Size, nm | Porosity % | Rejection | Water Flux, LMH | |||||
| PVC/MWCNT-g-GO | Phase inversion | MWCNT-g-GO | 0.06, 0.12, and 0.22 wt.% | - | 13.9–34 | 194–259 | 81.4 | COD rejection 88.9% | 254.00 at 0.12 wt.% | [9] |
| PVDF/GO@SiO2/PVP | Immersion precipitation | GO@SiO2 | 0.30 wt.% | 100.00 | - | - | - | - | 1.23 | [47] |
| PVDF MF membrane | - | GO/TiO2 | GO 20.00 μg | - | 62.00–162.00 | - | - | Oil rejection 70.2% | 531.00 | [53] |
| PSf/pebax | Added | F-MWCNTs | 0.50, 1, and 2.00 wt.% | 0.75 | 42.50–55.10 | - | - | Oil rejection 98.63% at 0.5 wt% | 230.00 at 0.50% | [24] |
| PES/PDA/N-MWCNTs membranes | Coating | N-MWCNTs | 0.01 and 0.05 wt.% | - | 30.20–38.70 | 12.77 | - | Oil rejection 99% | 90.85 | [21] |
| PVDF/MWCNTs | - | MWCNTs | 200.00 | 20.00–60.00 | 54.02–89.36 | - | 700.00 | [55] | ||
| PSf hollow fiber membranes | Embedded | CNTs/GO | 1.00 wt.% | - | - | - | - | Oil rejection 98.7 ± 1.2% | 487.90 ± 25.40 | [25] |
| PPSU/ZnO-NPs | Phase inversion | ZnO-NPs | 0.03 wt.% | - | - | - | - | - | 76.00–107.00 | [56] |
| PVDF/SiO2/GO | Thermally induced phase separation (TIPS) method | SiO2@GO | 1.20 wt.% | - | 50–95 | - | - | - | 679.10 | [57] |
| Polymer | Additives | Preparation Technique | Oil Concentration (ppm) | Membrane Characterization | Performance | Reference | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Qi % | Contact Angle | Mean Pore Size (nm) | Flu x(L/m2 h) | Oil Rejection (%) | Flux Recovery Ratio (FRR) (%) | |||||
| PVDF | TBC | RAFT | 1000.00 | - | 71.00° | - | 60.50 | 99.00 | 67.00–78.00 | [2] |
| PVC | TiO2-NPs | - | 40.41 | 79.50 | 62.50° | 77.00 | 116.00 | 96.30 | 89.90 | [4] |
| PVDF | LiCl·H2O/SiO2 | MMM | - | 85.41 | 50.00° | 34.05 | 82.50 | 98.83 | 81.70 | [38] |
| PVDF | LiCl·H2O/TiO2 | - | 17.00 | 85.41 | 47.33° | - | 82.50 | 98.80 | 98.83 | [58] |
| PANI/PVDF | PANI | dilute polymerization | - | - | - | 25.00 | 3000.00 | - | - | [59] |
| PPSU | SPPSU/MgO | NIPS | 1000.00 | 65.70 | 48.90° | 24.00 | 234.00 | 99.00 | 94.90 | [60] |
| PSf | PVP/HAO | NIPS | 100.00–1000.00 | 87.20 | 8.00° | 48.98 | 1194.00 | ≈100.00 | 67.00 | [61] |
| PSf | PVP | NIPS | 100.00–400.00 | 37.00 | 60.00° | 90.00 | >90.00 | - | [62] | |
| PVC | Bentonite | NIPS | 200.00 | 78.64 | 55.10° | 118.90 | 412.00 | 97.00 | 81.97 | [63] |
| PES | PVP/HMO | NIPS | 100.00–1000.00 | 87.90 | 16.40° | 76.40 | 573.20 | 100.00 | 75.40 | [17] |
| PAN | PEG | NIPS | 100.00–1000.00 | - | - | - | 60.00 | 90.00 | - | [64] |
| PES | PDA@ZnFe2O4NCs | NIPS | 500.00 | - | 52.00° | 69.00 | ∼687.00 | - | ∼82.50 | [65] |
| PVC | PAN/PF127/bentonite blended | Single-step phase inversion | 200.00 | - | 0.00° | 89.00 | 790.12 ± 40.15 | 97.25 ± 1.35 | 82.90 | [26] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Awad, E.S.; Sabirova, T.M.; Tretyakova, N.A.; Alsalhy, Q.F.; Figoli, A.; Salih, I.K. A Mini-Review of Enhancing Ultrafiltration Membranes (UF) for Wastewater Treatment: Performance and Stability. ChemEngineering 2021, 5, 34. https://doi.org/10.3390/chemengineering5030034
Awad ES, Sabirova TM, Tretyakova NA, Alsalhy QF, Figoli A, Salih IK. A Mini-Review of Enhancing Ultrafiltration Membranes (UF) for Wastewater Treatment: Performance and Stability. ChemEngineering. 2021; 5(3):34. https://doi.org/10.3390/chemengineering5030034
Chicago/Turabian StyleAwad, Eman Sh., Tamara M. Sabirova, Natalia A. Tretyakova, Qusay F. Alsalhy, Alberto Figoli, and Issam K. Salih. 2021. "A Mini-Review of Enhancing Ultrafiltration Membranes (UF) for Wastewater Treatment: Performance and Stability" ChemEngineering 5, no. 3: 34. https://doi.org/10.3390/chemengineering5030034
APA StyleAwad, E. S., Sabirova, T. M., Tretyakova, N. A., Alsalhy, Q. F., Figoli, A., & Salih, I. K. (2021). A Mini-Review of Enhancing Ultrafiltration Membranes (UF) for Wastewater Treatment: Performance and Stability. ChemEngineering, 5(3), 34. https://doi.org/10.3390/chemengineering5030034








