Conductivity, Viscosity, Spectroscopic Properties of Organic Sulfonic Acid solutions in Ionic Liquids
Abstract
1. Introduction
2. Materials and Methods
3. Results
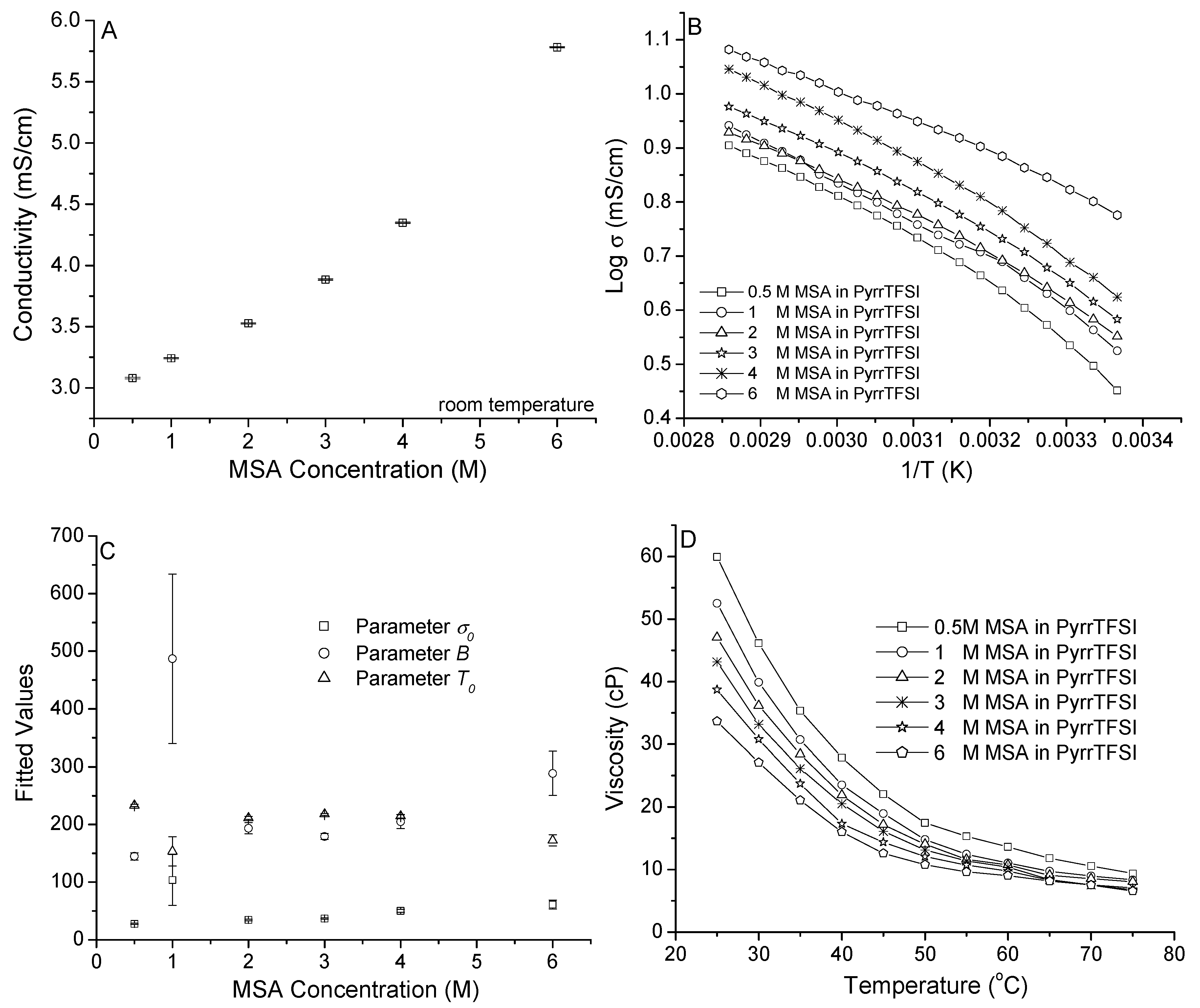
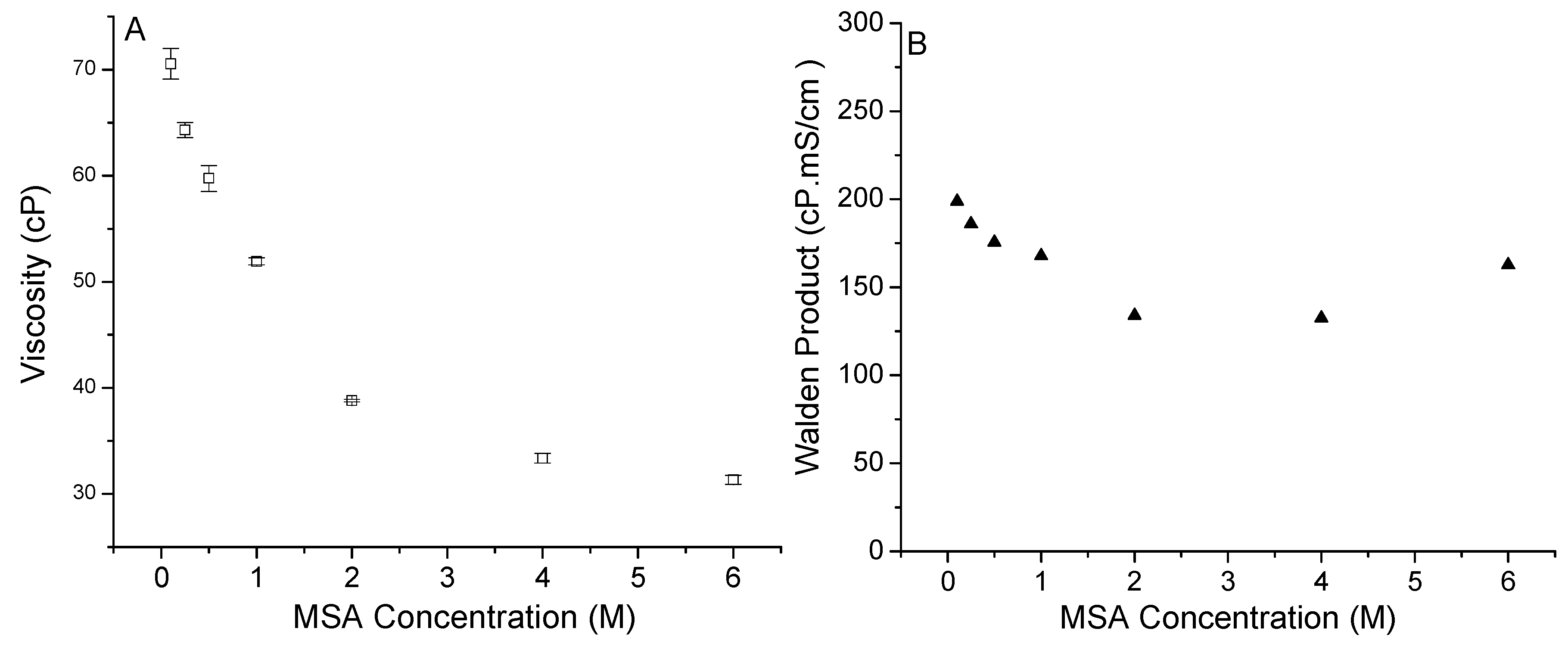
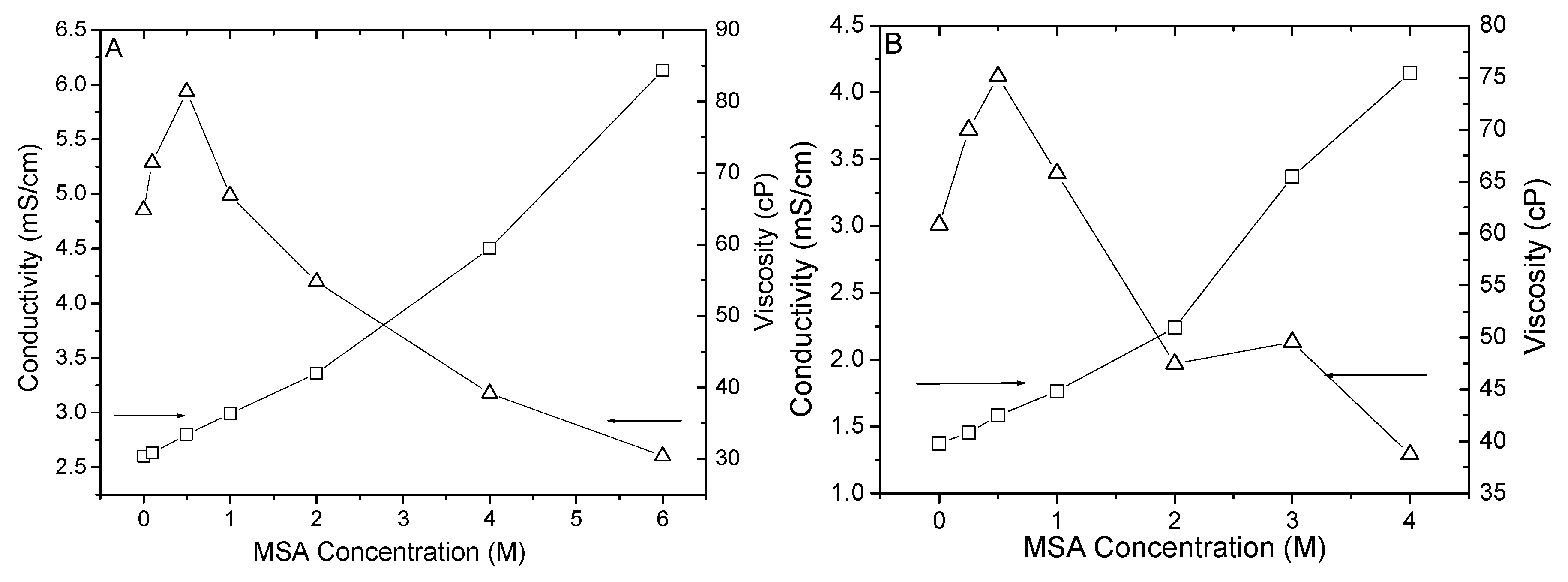
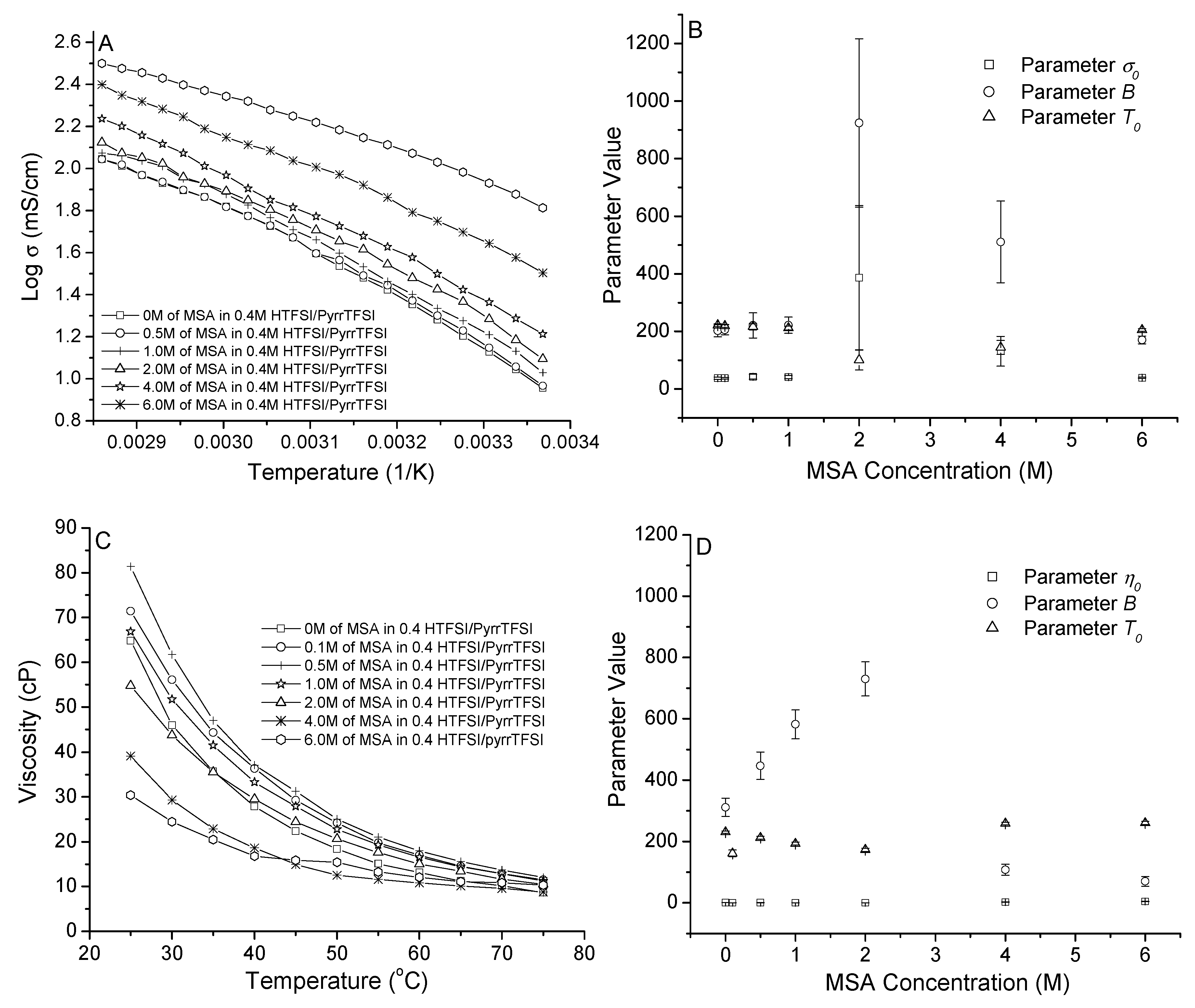
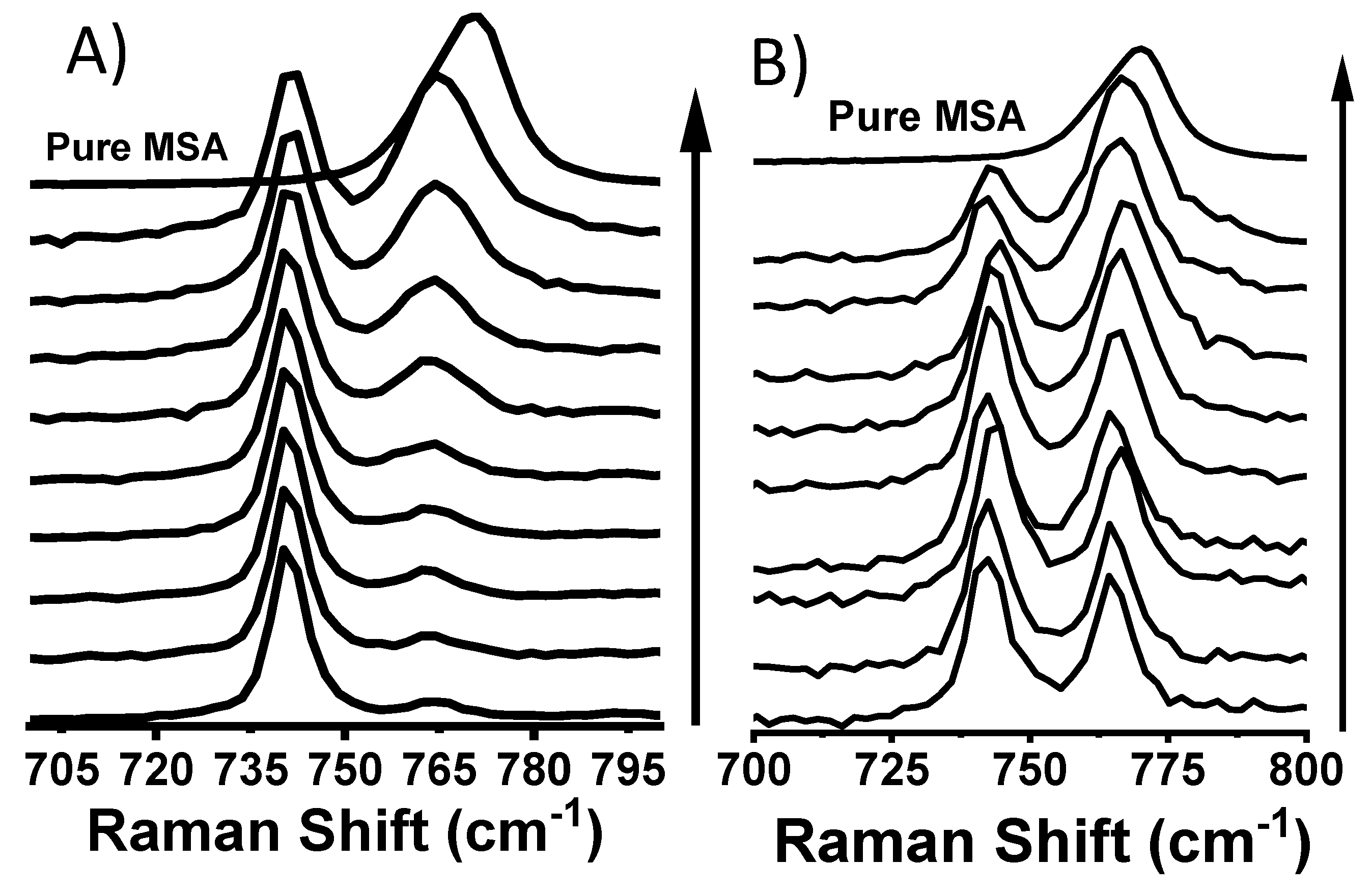
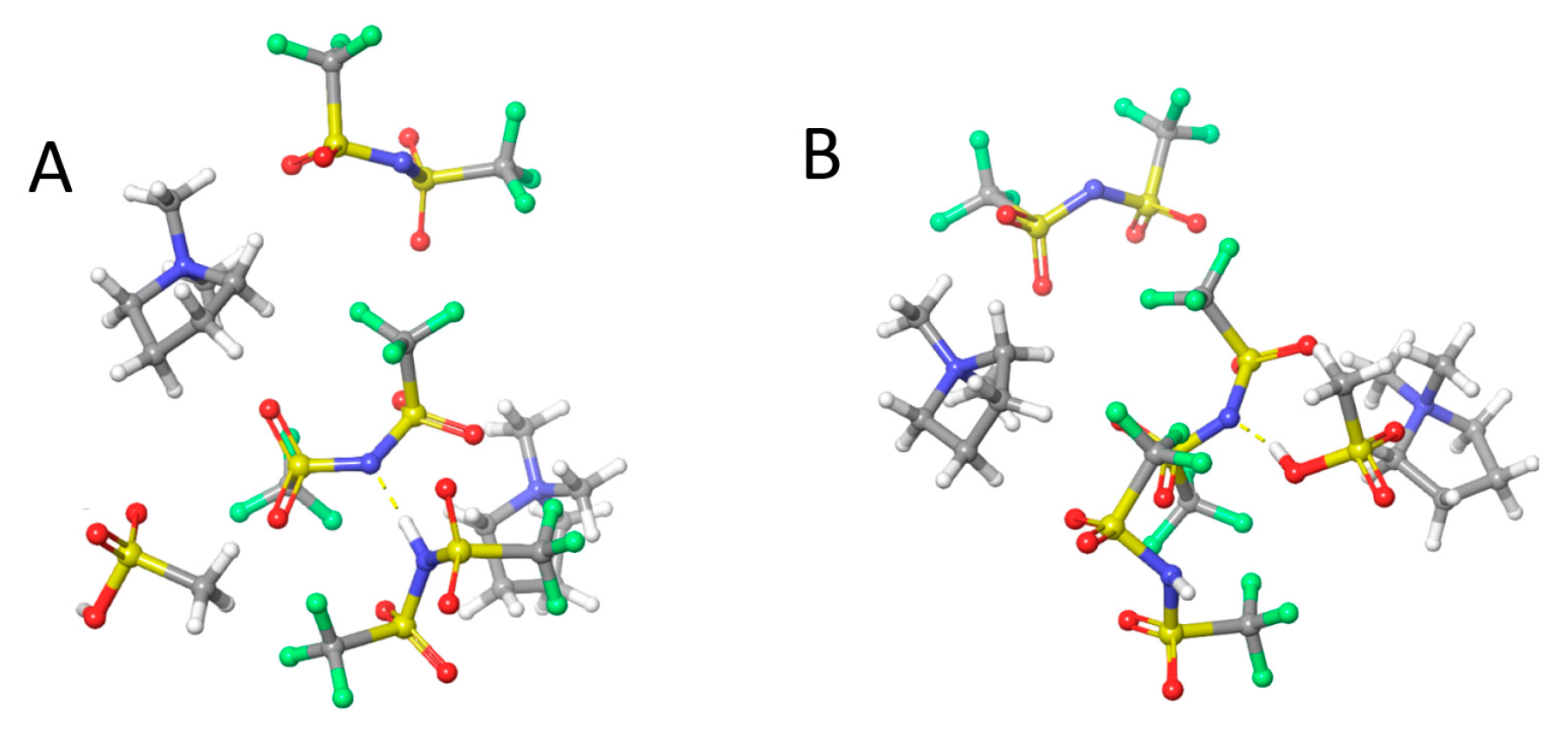
3.1. MSA in PyrrTFSI
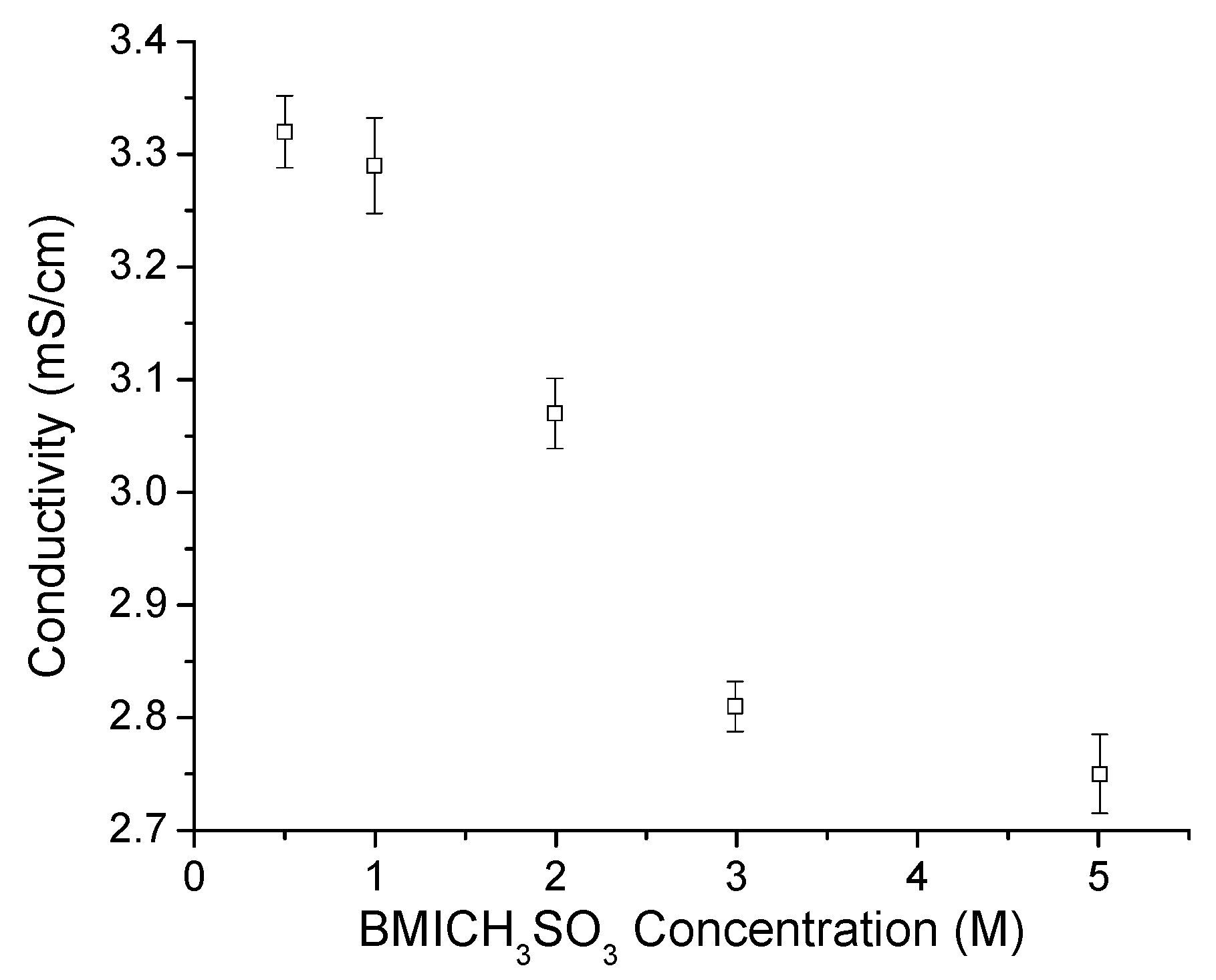
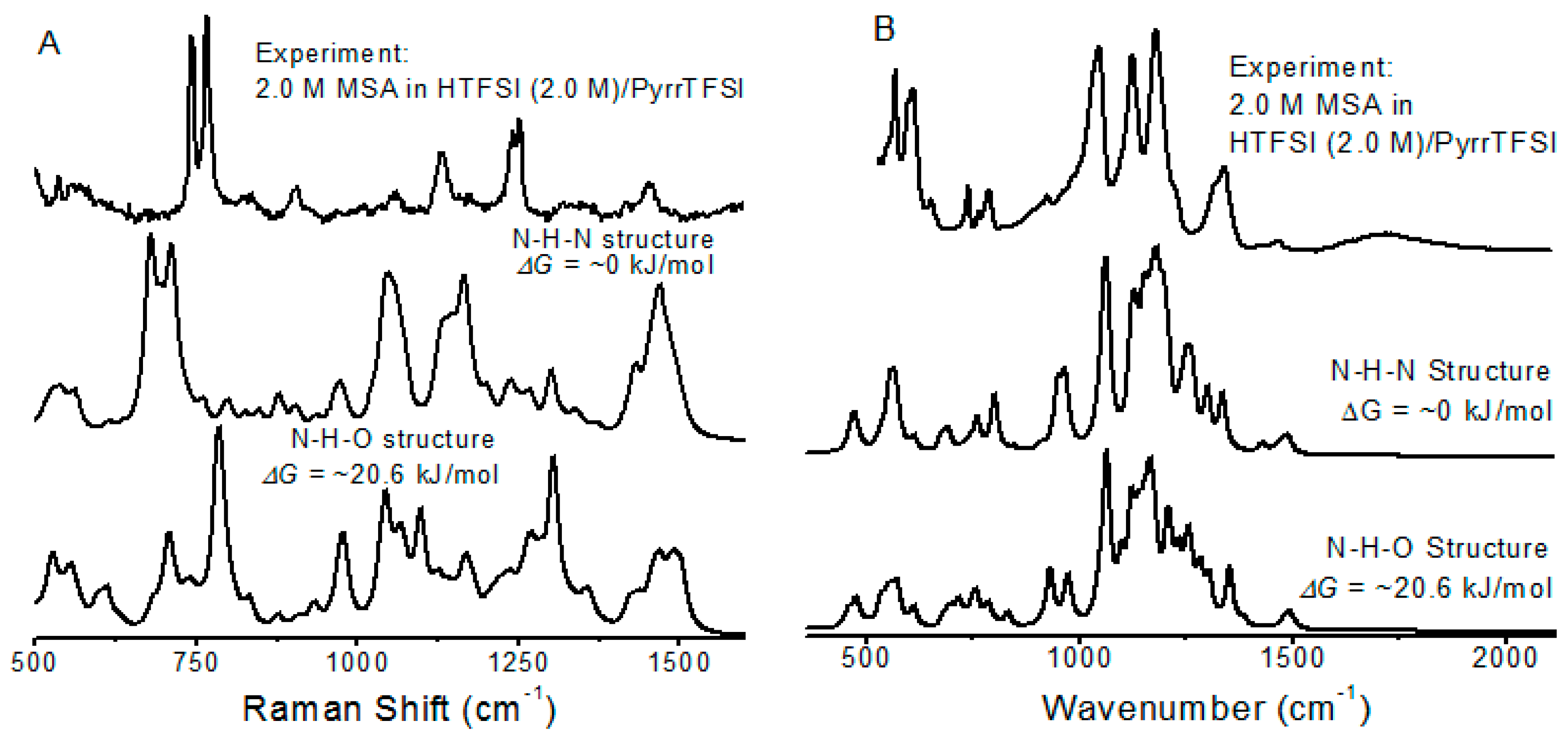
3.2. MSA in 0.4 M and 2.0 M HTFSI/PyrrTFSI
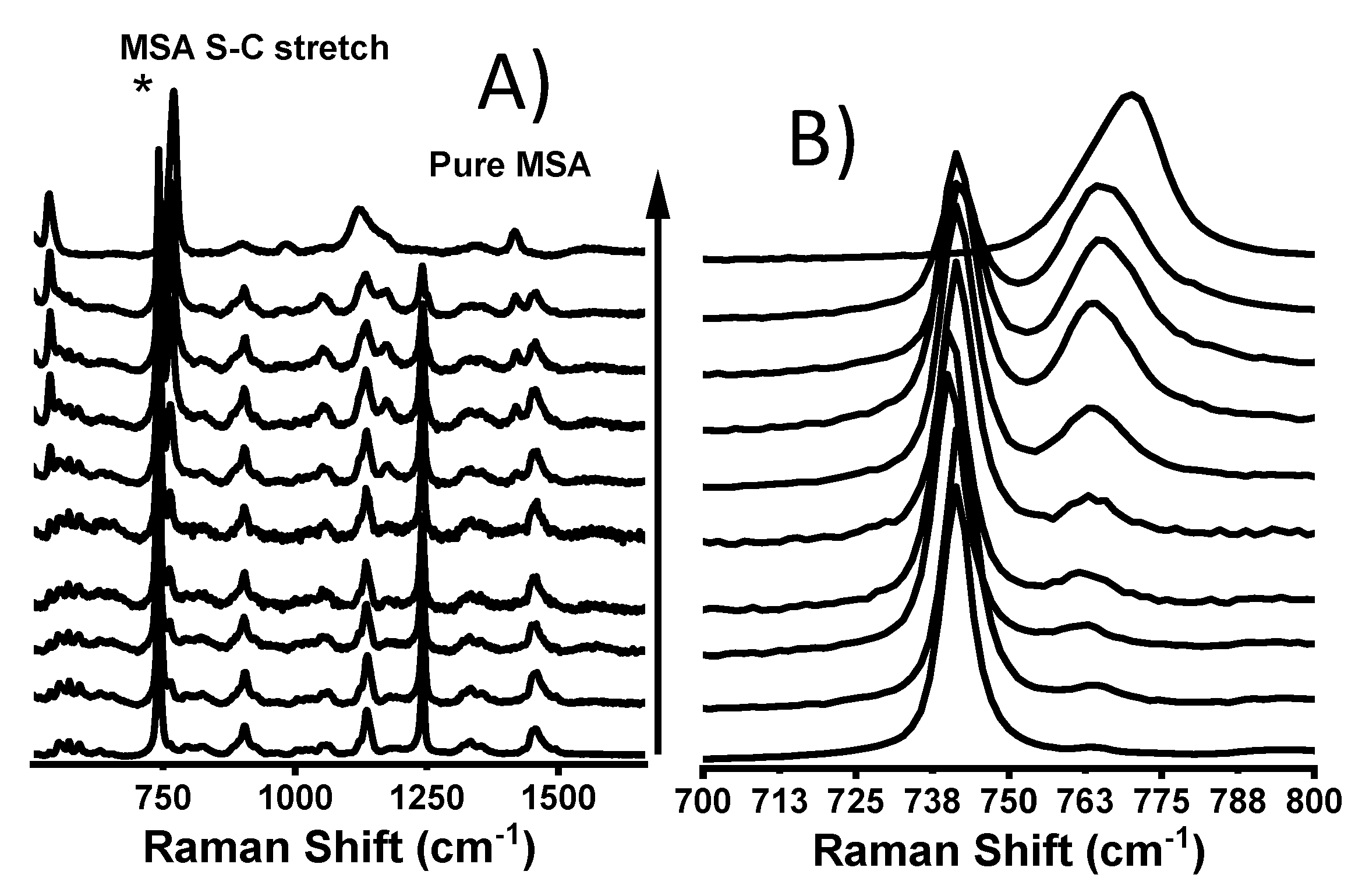
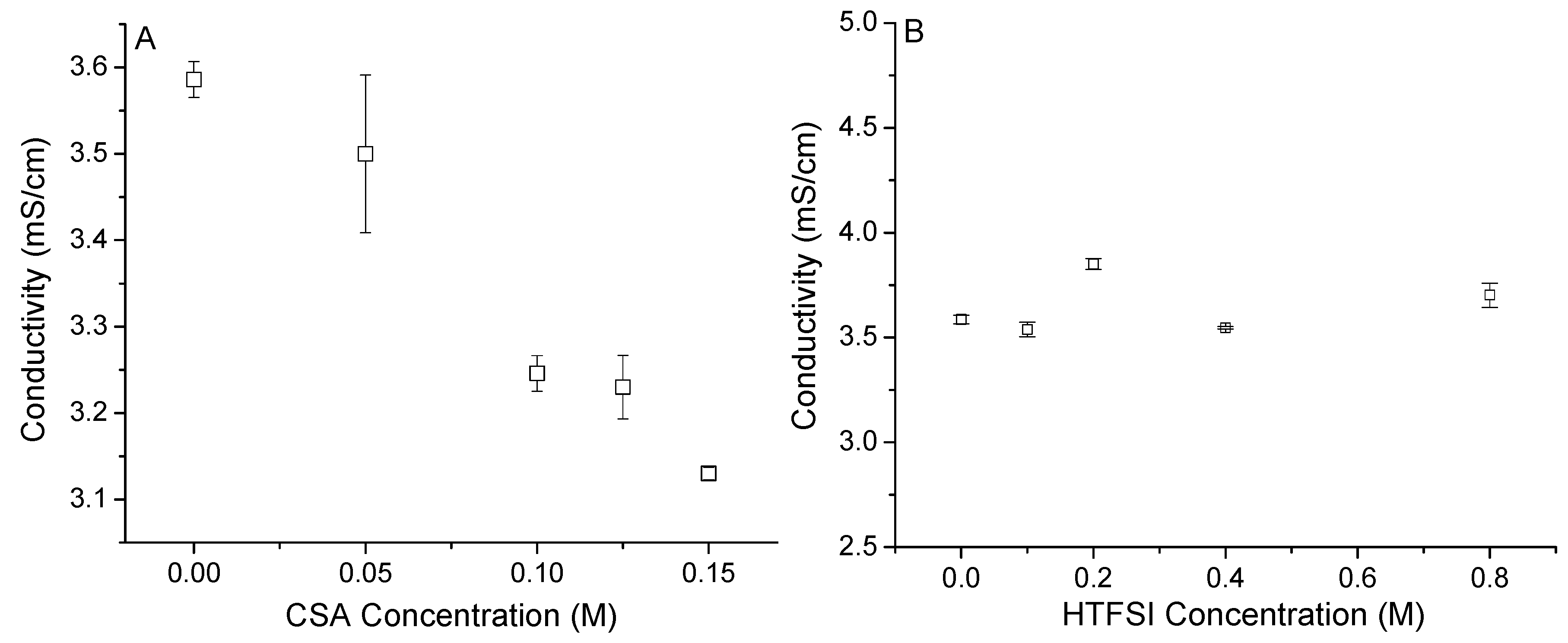
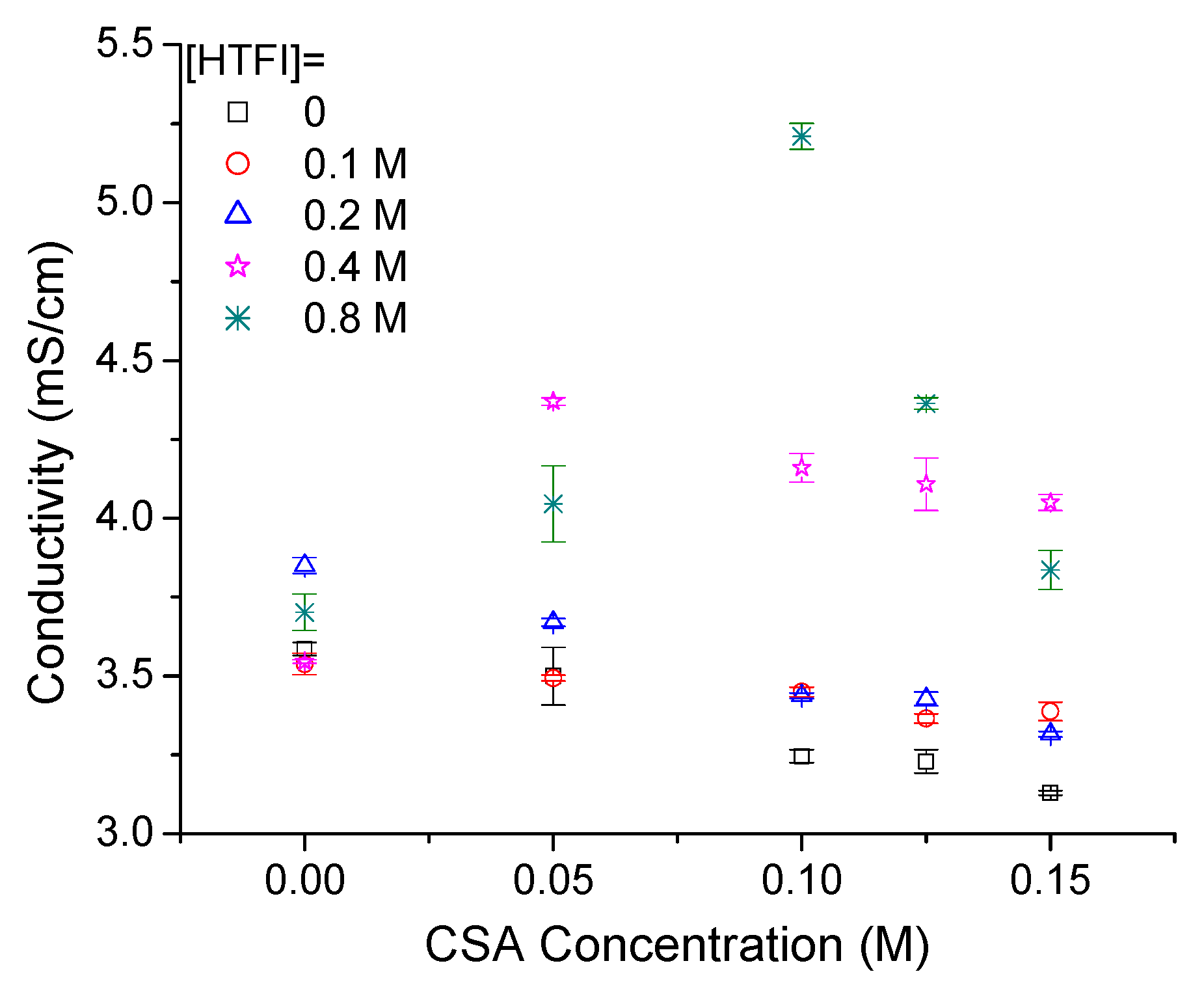
3.3. CSA in ILs and HTFSI/IL
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Rogers, R.D.; Seddon, K.R. Ionic Liquids: Industrial Applications for Green Chemistry; Oxford University Press: Washington, DC, USA, 2002. [Google Scholar]
- Amarasekara, A.S. Acidic Ionic Liquids. Chem. Rev. 2016, 116, 6133–6183. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Clifford, J.; Pham, T.T.; Almaraz, E.; Perry, F., III; Caputo, G.A.; Vaden, T.D. Conductivity, Spectroscopic, and Computational Investigation of H3O+ Solvation in Ionic Liquid BMIBF4. J. Phys. Chem. B 2013, 117, 7057–7064. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Pizio, B.S.; Vaden, T.D. Conductivity and Spectroscopic Investigation of Bis(trifluoromethanesulfonyl)imide Solution in Ionic Liquid 1-Butyl-3-methylimidazolium Bis(trifluoromethanesulfonyl)imide. J. Phys. Chem. B 2012, 116, 6553–6560. [Google Scholar] [CrossRef] [PubMed]
- Margaretta, E.; Olmeda, C.; Yu, L. Doped Polyaniline in Bronsted Acid Ionic Liquid 1-Butyl-3-methylimidazolium bis trifluoromethyl(sulfonyl) imide/Bis trifluoromethyl(sulfonyl) imide. J. Appl. Polym. Sci. 2013, 127, 2453–2457. [Google Scholar] [CrossRef]
- Munson, K.T.; Vergara, J.; Yu, L.; Vaden, T.D. Characterization of the Bridged Proton Structure in HTFSI Acid Ionic Liquid Solutions. J. Phys. Chem. B 2015, 119, 6304–6310. [Google Scholar] [CrossRef]
- Renda, C.M.; Patel, Y.K.; Henshaw, L.R.; Munson, K.T.; Fiebig, O.C.; Tran, A.T.; Shriver, J.; Cruz, J.; Yu, L.; Vaden, T.D. Thermodynamic and conductivity properties of acetic acid EMIMOAc ionic liquid solutions. J. Mol. Liq. 2016, 216, 710–715. [Google Scholar] [CrossRef]
- Smith, M.B. Organic Chemistry: An Acid-Base Approach, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2016. [Google Scholar]
- Guthrie, J.P. Hydrolysis of esters of oxy acids: pKa values for strong acids; Brønsted relationship for attack of water at methyl; free energies of hydrolysis of esters of oxy acids; and a linear relationship between free energy of hydrolysis and pKa holding over a range of 20 pK units. Can. J. Chem. 1978, 56, 2342–2354. [Google Scholar] [CrossRef]
- Ma, X.-X.; Zhang, Q.-B.; Wei, J.; Pan, Y.; Guan, W.; Yang, J.-Z. Study on enthalpy and molar heat capacity of solution for ionic liquid C(3)mim OAc (1-propyl-3-methylimidazolium acetate). J. Chem. Thermodyn. 2013, 65, 91–94. [Google Scholar] [CrossRef]
- Forbes, D.C.; Weaver, K.J. Brønsted acidic ionic liquids: The dependence on water of the Fischer esterification of acetic acid and ethanol. J. Mol. Catal. A Chem. 2004, 214, 129–132. [Google Scholar] [CrossRef]
- Liu, X.; Ma, H.; Wu, Y.; Wang, C.; Yang, M.; Yan, P.; Welz-Biermann, U. Esterification of glycerol with acetic acid using double SO3H-functionalized ionic liquids as recoverable catalysts. Green Chem. 2011, 13, 697–701. [Google Scholar] [CrossRef]
- Huang, B.H.; Li, Z.J.; Wang, Y.F.; Zhang, K.; Fang, Y.X. Esterification catalyzed by Bronsted acidic ionic liquids. Acta Chim. Sin. 2008, 66, 1837–1844. [Google Scholar]
- Castanheiro, J.E.; Ramos, A.M.; Fonseca, I.M.; Vital, J. Esterification of acetic acid by isoamylic alcohol over catalytic membranes of poly(vinyl alcohol) containing sulfonic acid groups. Appl. Catal. A Gen. 2006, 311, 17–23. [Google Scholar] [CrossRef]
- Liu, S.; Xie, C.; Yu, S.; Liu, F.; Ji, K. Esterification of alpha-pinene and acetic acid using acidic ionic liquids as catalysts. Catal. Commun. 2008, 9, 1634–1638. [Google Scholar] [CrossRef]
- Cui, X.; Cai, J.; Zhang, Y.; Li, R.; Feng, T. Kinetics of Transesterification of Methyl Acetate and n-Butanol Catalyzed by Ionic Liquid. Ind. Eng. Chem. Res. 2011, 50, 11521–11527. [Google Scholar] [CrossRef]
- Tao, D.-J.; Zhang, X.-L.; Hu, N.; Li, Z.-M.; Chen, X.-S. Kinetics Study of the Esterification of Acetic Acid with Methanol using Low-Corrosive Bronsted Acidic Ionic Liquids as Catalysts. Int. J. Chem. React. Eng. 2012. [Google Scholar] [CrossRef]
- Fraga-Dubreuil, J.; Bourahla, K.; Rahmouni, M.; Bazureau, J.P.; Hamelin, J. Catalysed esterifications in room temperature ionic liquids with acidic counteranion as recyclable reaction media. Catal. Commun. 2002, 3, 185–190. [Google Scholar] [CrossRef]
- Cole, A.C.; Jensen, J.L.; Ntai, I.; Tran, K.L.T.; Weaver, K.J.; Forbes, D.C.; Davis, J.H. Novel Brønsted Acidic Ionic Liquids and Their Use as Dual Solvent−Catalysts. J. Am. Chem. Soc. 2002, 124, 5962–5963. [Google Scholar] [CrossRef]
- Irimescu, R.; Kato, K. Lipase-catalyzed enantioselective reaction of amines with carboxylic acids under reduced pressure in non-solvent system and in ionic liquids. Tetrahedron Lett. 2004, 45, 523–525. [Google Scholar] [CrossRef]
- Greaves, T.L.; Drummond, C.J. Protic Ionic Liquids: Properties and Applications. Chem. Rev. 2007, 108, 206–237. [Google Scholar] [CrossRef]
- Joseph, T.; Sahoo, S.; Halligudi, S.B. Bronsted acidic ionic liquids: A green, efficient and reusable catalyst system and reaction medium for Fischer esterification. J. Mol. Catal. A Chem. 2005, 234, 107–110. [Google Scholar] [CrossRef]
- Vafaeezadeh, M.; Alinezhad, H. Bronsted acidic ionic liquids: Green catalysts for essential organic reactions. J. Mol. Liq. 2016, 218, 95–105. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Y.T.; Liu, T.; Tao, D.J. Facile synthesis of fructone from ethyl acetoacetate and ethylene glycol catalyzed by SO3H-functionalized Bronsted acidic ionic liquids. RSC Adv. 2014, 4, 22520–22525. [Google Scholar] [CrossRef]
- Ghorbani, M.; Noura, S.; Oftadeh, M.; Gholami, E.; Zolfigol, M.A. Novel ionic liquid 2-Eim HSO4 as a dual catalytic-solvent system for preparation of hexahydroquinolines under green conditions. RSC Adv. 2015, 5, 55303–55312. [Google Scholar] [CrossRef]
- Peng, Q.H.; Mahmood, K.; Wu, Y.; Wang, L.L.; Liang, Y.Y.; Shen, J.N.; Liu, Z.P. A facile route to realize the copolymerization of L-lactic acid and epsilon-caprolactone: Sulfonic acid-functionalized Bronsted acidic ionic liquids as both solvents and catalysts. Green Chem. 2014, 16, 2234–2241. [Google Scholar] [CrossRef]
- Noda, A.; Susan, M.A.B.H.; Kudo, K.; Mitsushima, S.; Hayamizu, K.; Watanabe, M. Brønsted Acid−Base Ionic Liquids as Proton-Conducting Nonaqueous Electrolytes. J. Phys. Chem. B 2003, 107, 4024–4033. [Google Scholar] [CrossRef]
- Nakamoto, H.; Watanabe, M. Bronsted acid-base ionic liquids for fuel cell electrolytes. Chem. Commun. 2007. [Google Scholar] [CrossRef]
- Lee, S.Y.; Yasuda, T.; Watanabe, M. Fabrication of protic ionic liquid/sulfonated polyimide composite membranes for non-humidified fuel cells. J. Power Sources 2010, 195, 5909–5914. [Google Scholar] [CrossRef]
- Diao, H.B.; Yan, F.; Qiu, L.H.; Lu, J.M.; Lu, X.H.; Lin, B.C.; Li, Q.; Shang, S.M.; Liu, W.M.; Liu, J.G. High Performance Cross-Linked Poly(2-acrylamido-2-methylpropanesulfonic acid)-Based Proton Exchange Membranes for Fuel Cells. Macromolecules 2010, 43, 6398–6405. [Google Scholar] [CrossRef]
- Schmidt, C.; Gluck, T.; Schmidt-Naake, G. Modification of Nafion membranes by impregnation with ionic liquids. Chem. Eng. Technol. 2008, 31, 13–22. [Google Scholar] [CrossRef]
- Fuller, J.; Breda, A.C.; Carlin, R.T. Ionic Liquid-Polymer Gel Electrolytes. J. Electrochem. Soc. 1997, 144, L67–L70. [Google Scholar] [CrossRef]
- Robertson, N.J.; Kostalik, H.A.; Clark, T.J.; Mutolo, P.F.; AbruñA, H.C.D.; Coates, G.W. Tunable High Performance Cross-Linked Alkaline Anion Exchange Membranes for Fuel Cell Applications. J. Am. Chem. Soc. 2010, 132, 3400–3404. [Google Scholar] [CrossRef] [PubMed]
- Mcintosh, S.; Gorte, R.J. Direct Hydrocarbon Solid Oxide Fuel Cells. Chem. Rev. 2004, 104, 4845–4866. [Google Scholar] [CrossRef] [PubMed]
- Whittingham, M.S.; Zawodzinski, T. Introduction: Batteries and Fuel Cells. Chem. Rev. 2004, 104, 4243–4244. [Google Scholar] [CrossRef] [PubMed]
- Winter, M.; Brodd, R.J. What Are Batteries, Fuel Cells, and Supercapacitors? Chem. Rev. 2004, 104, 4245–4270. [Google Scholar] [CrossRef] [PubMed]
- Mehta, V.; Cooper, J.S. Review and analysis of PEM fuel cell design and manufacturing. J. Power Sources 2003, 114, 32–53. [Google Scholar] [CrossRef]
- Aricò, A.S.; Srinivasan, S.; Antonucci, V. DMFCs: From Fundamental Aspects to Technology Development. Fuel Cells 2001, 1, 133–161. [Google Scholar] [CrossRef]
- Dupont, D.; Raiguel, S.; Binnemans, K. Sulfonic acid functionalized ionic liquids for dissolution of metal oxides and solvent extraction of metal ions. Chem. Commun. 2015, 51, 9006–9009. [Google Scholar] [CrossRef]
- Dupont, D.; Renders, E.; Binnemans, K. Alkylsulfuric acid ionic liquids: A promising class of strongly acidic room-temperature ionic liquids. Chem. Commun. 2016, 52, 4640–4643. [Google Scholar] [CrossRef]
- Shan, W.D.; Yang, Q.W.; Su, B.G.; Bao, Z.B.; Ren, Q.L.; Xing, H.B. Proton Microenvironment and Interfacial Structure of Sulfonic-Acid-Functionalized Ionic Liquids. J. Phys. Chem. C 2015, 119, 20379–20388. [Google Scholar] [CrossRef]
- Andriola, A.; Singh, K.; Lewis, J.; Yu, L. Conductivity, Viscosity, and Dissolution Enthalpy of LiNTF2 in Ionic Liquid BMINTF2. J. Phys. Chem. B 2010, 114, 11709–11714. [Google Scholar] [CrossRef]
- Tian, Y.; Meng, X.; Duan, J.-Y.; Shi, L. A Novel Application of Methanesulfonic Acid as Catalyst for the Alkylation of Olefins with Aromatics. Ind. Eng. Chem. Res. 2012, 51, 13627–13631. [Google Scholar] [CrossRef]
- Gazeau-Bureau, S.; Delcroix, D.; Martín-Vaca, B.; Bourissou, D.; Navarro, C.; Magnet, S. Organo-Catalyzed ROP of ε-Caprolactone: Methanesulfonic Acid Competes with Trifluoromethanesulfonic Acid. Macromolecules 2008, 41, 3782–3784. [Google Scholar] [CrossRef]
- Gernon, D.M.; Wu, M.; Buszta, T.; Janney, P. Environmental benefits of methanesulfonic acid. Comparative properties and advantages. Green Chem. 1999, 1, 127–140. [Google Scholar] [CrossRef]
- Tran, A.T.; Lam, P.H.; Miller, A.; Walczyk, D.J.; Tomlin, J.; Vaden, T.D.; Yu, L. Proton transfer and esterification reactions in EMIMOAc-based acidic ionic liquids. RSC Adv. 2017, 7, 18333–18339. [Google Scholar] [CrossRef]
- Lam, P.H.; Tran, A.T.; Walczyk, D.J.; Miller, A.M.; Yu, L. Conductivity, viscosity, and thermodynamic properties of propylene carbonate solutions in ionic liquids. J. Mol. Liq. 2017, 246, 215–220. [Google Scholar] [CrossRef]
- Kuan, W.-F.; Remy, R.; Mackay, M.E.; Epps, T.H., III. Controlled ionic conductivity via tapered block polymer electrolytes. RSC Adv. 2015, 5, 12597–12604. [Google Scholar] [CrossRef]
- Umecky, T.; Saito, Y.; Okumura, Y.; Maeda, S.; Sakai, T. Ionization Condition of Lithium Ionic Liquid Electrolytes under the Solvation Effect of Liquid and Solid Solvents. J. Phys. Chem. B 2008, 112, 3357–3364. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tran, A.T.; Tomlin, J.; Lam, P.H.; Stinger, B.L.; Miller, A.D.; Walczyk, D.J.; Cruz, O.; Vaden, T.D.; Yu, L. Conductivity, Viscosity, Spectroscopic Properties of Organic Sulfonic Acid solutions in Ionic Liquids. ChemEngineering 2019, 3, 81. https://doi.org/10.3390/chemengineering3040081
Tran AT, Tomlin J, Lam PH, Stinger BL, Miller AD, Walczyk DJ, Cruz O, Vaden TD, Yu L. Conductivity, Viscosity, Spectroscopic Properties of Organic Sulfonic Acid solutions in Ionic Liquids. ChemEngineering. 2019; 3(4):81. https://doi.org/10.3390/chemengineering3040081
Chicago/Turabian StyleTran, Anh T., Jay Tomlin, Phuoc H. Lam, Brittany L. Stinger, Alexandra D. Miller, Dustin J. Walczyk, Omar Cruz, Timothy D. Vaden, and Lei Yu. 2019. "Conductivity, Viscosity, Spectroscopic Properties of Organic Sulfonic Acid solutions in Ionic Liquids" ChemEngineering 3, no. 4: 81. https://doi.org/10.3390/chemengineering3040081
APA StyleTran, A. T., Tomlin, J., Lam, P. H., Stinger, B. L., Miller, A. D., Walczyk, D. J., Cruz, O., Vaden, T. D., & Yu, L. (2019). Conductivity, Viscosity, Spectroscopic Properties of Organic Sulfonic Acid solutions in Ionic Liquids. ChemEngineering, 3(4), 81. https://doi.org/10.3390/chemengineering3040081




