Dissolution of Chitin in Deep Eutectic Solvents Composed of Imidazolium Ionic Liquids and Thiourea
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
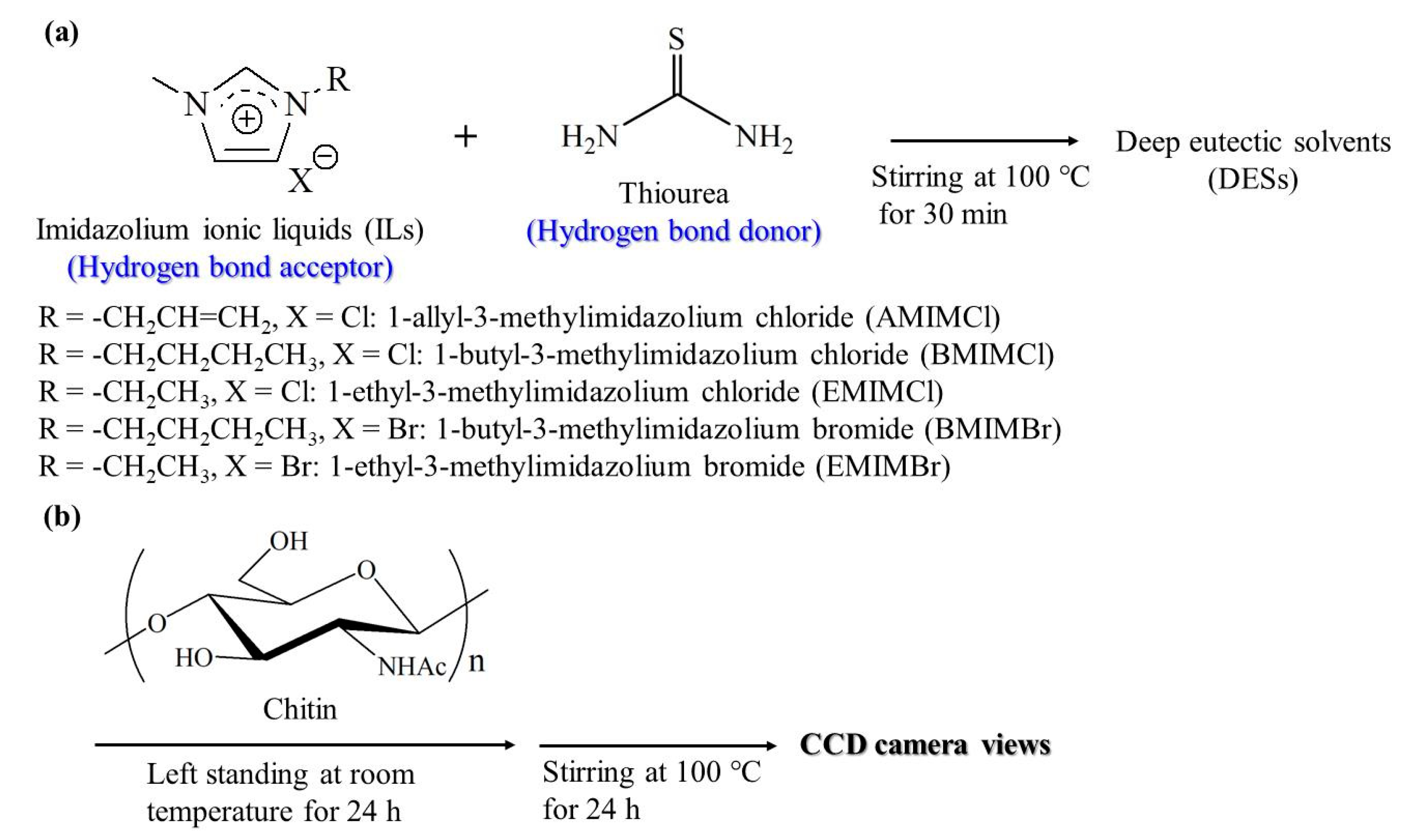
2.2. Preparation of Ionic Liquids
2.3. Preparation of DESs
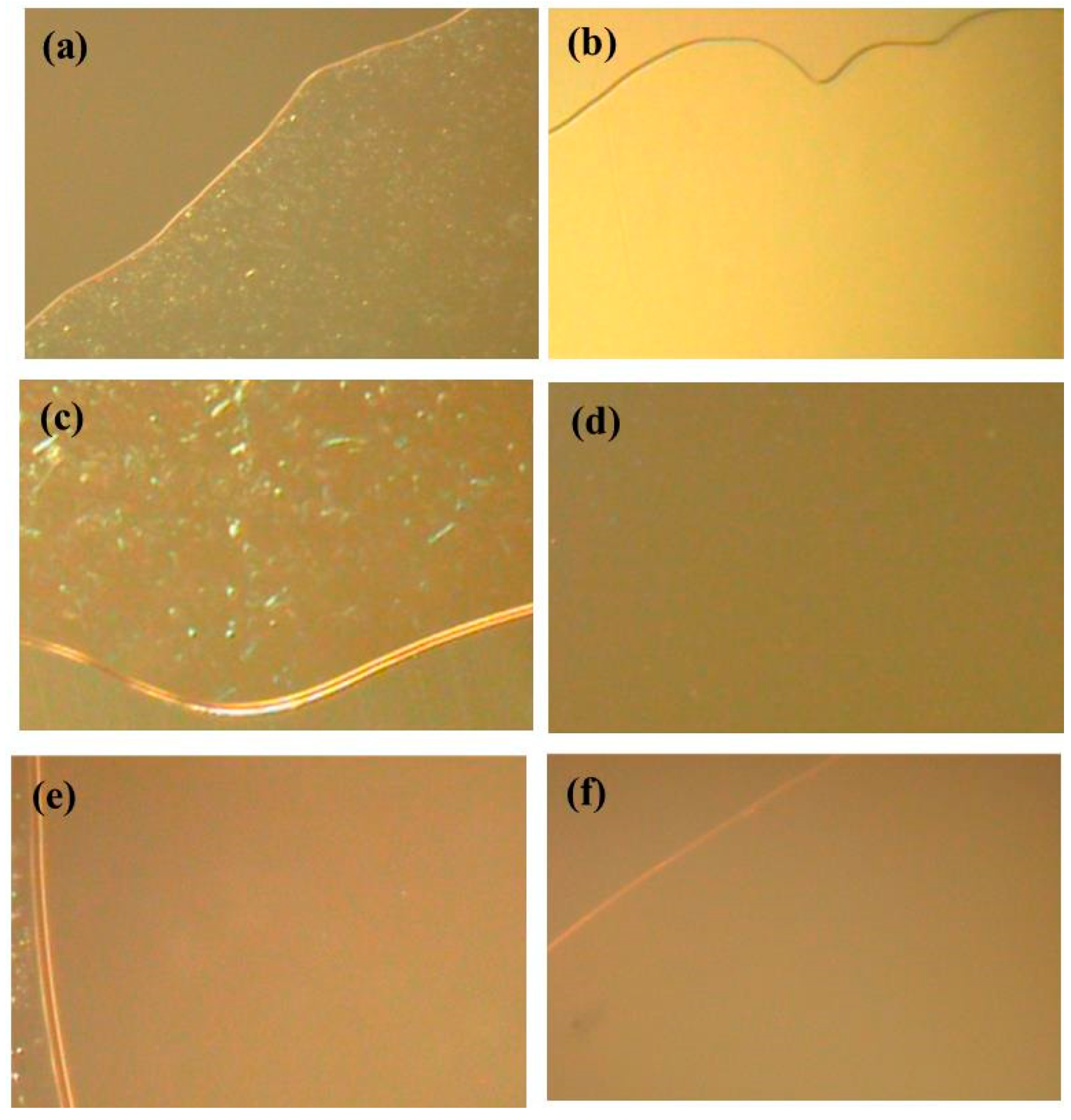
2.4. Dissolution Experiments of Chitin with DESs
2.5. Regeneration of Chitin from a Solution with a DES
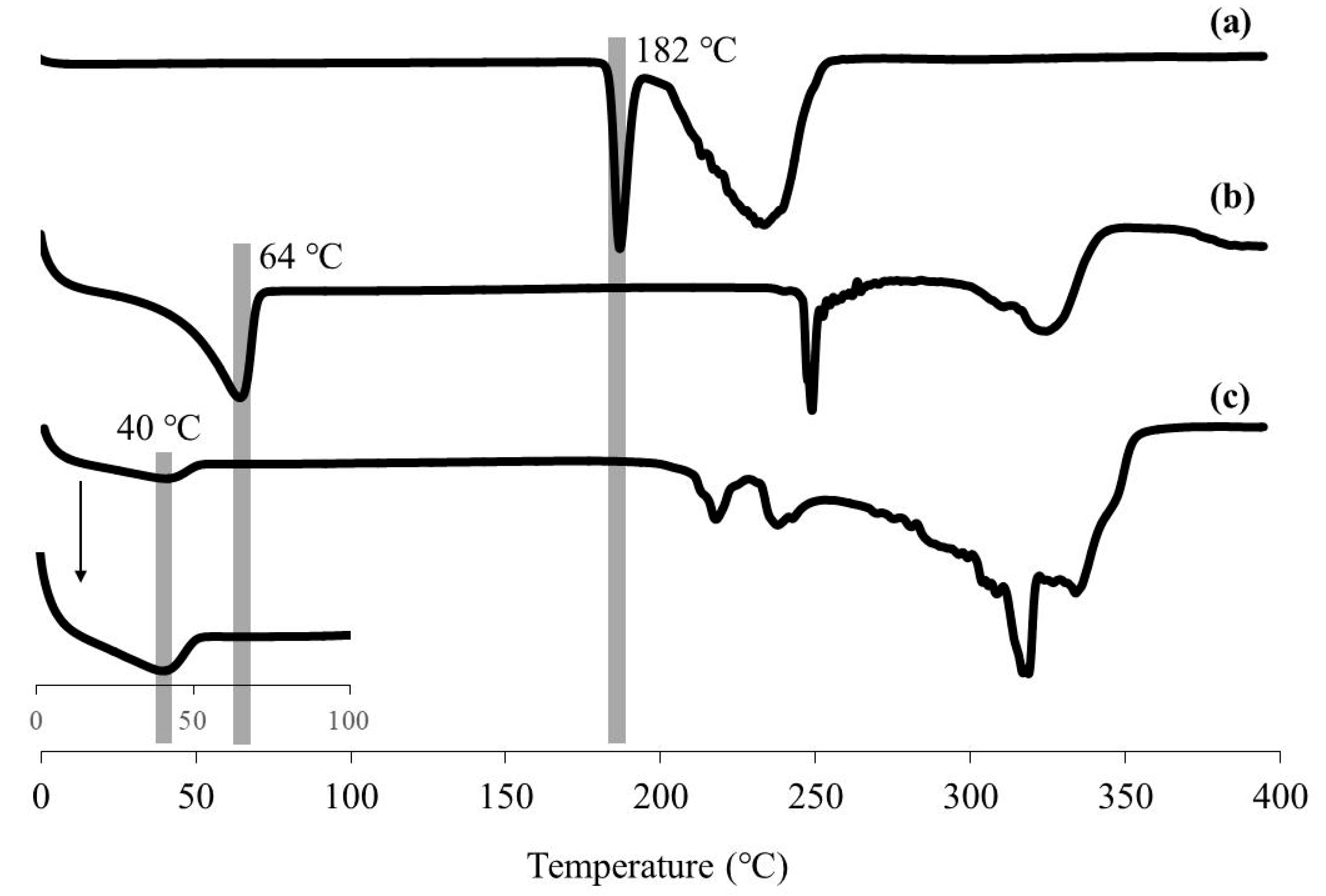
2.6. Measurements
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Liebert, T.; Heinze, T. Interaction of ionic liquids with polysaccharides 5. Solvents and reaction media for the modification of cellulose. Bioresources 2008, 3, 576–601. [Google Scholar]
- Zakrzewska, M.E.; Bogel-Łukasik, E.; Bogel-Łukasik, R. Solubility of carbohydrates in ionic liquids. Energy Fuels 2010, 24, 737–745. [Google Scholar] [CrossRef]
- Swatloski, R.P.; Spear, S.K.; Holbrey, J.D.; Rogers, R.D. Dissolution of cellose with ionic liquids. J. Am. Chem. Soc. 2002, 124, 4974–4975. [Google Scholar] [CrossRef]
- Feng, L.; Chen, Z.I. Research progress on dissolution and functional modification of cellulose in ionic liquids. J. Mol. Liq. 2008, 142, 1–5. [Google Scholar] [CrossRef]
- Pinkert, A.; Marsh, K.N.; Pang, S.S.; Staiger, M.P. Ionic liquids and their interaction with cellulose. Chem. Rev. 2009, 109, 6712–6728. [Google Scholar] [CrossRef] [PubMed]
- Gericke, M.; Fardim, P.; Heinze, T. Ionic liquids—Promising but challenging solvents for homogeneous derivatization of cellulose. Molecules 2012, 17, 7458–7502. [Google Scholar] [CrossRef]
- Isik, M.; Sardon, H.; Mecerreyes, D. Ionic liquids and cellulose: Dissolution, chemical modification and preparation of new cellulosic materials. Int. J. Mol. Sci. 2014, 15, 11922–11940. [Google Scholar] [CrossRef]
- Zhang, J.; Wu, J.; Yu, J.; Zhang, X.; He, J.; Zhang, J. Application of ionic liquids for dissolving cellulose and fabricating cellulose-based materials: State of the art and future trends. Mater. Chem. Front. 2017, 1, 1273–1290. [Google Scholar] [CrossRef]
- Hermanutz, F.; Vocht, M.P.; Panzier, N.; Buchmeiser, M.R. Processing of cellulose using ionic liquids. Macromol. Mater. Eng. 2019, 304. [Google Scholar] [CrossRef]
- Verma, C.; Mishra, A.; Chauhan, S.; Verma, P.; Srivastava, V.; Quraishi, M.A.; Ebenso, E.E. Dissolution of cellulose in ionic liquids and their mixed cosolvents: A review. Sustain. Chem. Phys. 2019, 13, 100162. [Google Scholar] [CrossRef]
- Wang, W.T.; Zhu, J.; Wang, X.L.; Huang, Y.; Wang, Y.Z. Dissolution behavior of chitin in ionic liquids. J. Macromol. Sci. B Phys. 2010, 49, 528–541. [Google Scholar] [CrossRef]
- Jaworska, M.M.; Kozlecki, T.; Gorak, A. Review of the application of ionic liquids as solvents for chitin. J. Polym. Eng. 2012, 32, 67–69. [Google Scholar] [CrossRef]
- Kadokawa, J. Ionic liquid as useful media for dissolution, derivatization, and nanomaterial processing of chitin. Green Sustain. Chem. 2013, 3, 19–25. [Google Scholar] [CrossRef]
- Kadokawa, J. Fabrication of nanostructured and microstructured chitin materials through gelation with suitable dispersion media. RSC Adv. 2015, 5, 12736–12746. [Google Scholar] [CrossRef]
- Silva, S.S.; Mano, J.F.; Reis, R.L. Ionic liquids in the processing and chemical modification of chitin and chitosan for biomedical applications. Green Chem. 2017, 19, 1208–1220. [Google Scholar] [CrossRef]
- Sikorski, P.; Hori, R.; Wada, M. Revisit of α-chitin crystal structure using high resolution X-ray diffraction data. Biomacromolecules 2009, 10, 1100–1105. [Google Scholar] [CrossRef]
- Singh, S.K. Solubility of lignin and chitin in ionic liquids and their biomedical applications. Int. J. Biol. Macromol. 2019, 132, 265–277. [Google Scholar] [CrossRef]
- Wu, Y.; Sasaki, T.; Irie, S.; Sakurai, K. A novel biomass-ionic liquid platform for the utilization of native chitin. Polymer 2008, 49, 2321–2327. [Google Scholar] [CrossRef]
- Qin, Y.; Lu, X.M.; Sun, N.; Rogers, R.D. Dissolution or extraction of crustacean shells using ionic liquids to obtain high molecular weight purified chitin and direct production of chitin films and fibers. Green Chem. 2010, 12, 968–971. [Google Scholar] [CrossRef]
- Walther, P.; Ota, A.; Müller, A.; Hermanutz, F.; Gähr, F.; Buchmeiser, M.R. Chitin foils and coatings prepared from ionic liquids. Macromol. Mater. Eng. 2016, 301, 1337–1344. [Google Scholar] [CrossRef]
- Prasad, K.; Murakami, M.; Kaneko, Y.; Takada, A.; Nakamura, Y.; Kadokawa, J. Weak gel of chitin with ionic liquid, 1-allyl-3-methylimidazolium bromide. Int. J. Biol. Macromol. 2009, 45, 221–225. [Google Scholar] [CrossRef] [PubMed]
- Mine, S.; Izawa, H.; Kaneko, Y.; Kadokawa, J. Acetylation of a-chitin in ionic liquids. Carbohydr. Res. 2009, 344, 2263–2265. [Google Scholar] [CrossRef] [PubMed]
- Kadokawa, J.; Takegawa, A.; Mine, S.; Prasad, K. Preparation of chitin nanowhiskers using an ionic liquid and their composite materials with poly(vinyl alcohol). Carbohydr. Polym. 2011, 84, 1408–1412. [Google Scholar] [CrossRef]
- Tajiri, R.; Setoguchi, T.; Wakizono, S.; Yamamoto, K.; Kadokawa, J. Preparation of self-assembled chitin nanofibers by regeneration from ion gels using calcium halide · dihydrate/methanol solutions. J. Biobased Mater. Bioenergy 2013, 7, 655–659. [Google Scholar] [CrossRef]
- Hirayama, H.; Yoshida, J.; Yamamoto, K.; Kadokawa, J. Facile acylation of α-chitin in ionic liquid. Carbohydr. Polym. 2018, 200, 567–571. [Google Scholar] [CrossRef] [PubMed]
- Kadokawa, J.; Kawano, A.; Yamamoto, K. Fabrication of Semi-crystalline Film by Hexanoylation on Self-assembled Chitin Nanofibers. ChemistrySelect 2019, 4, 797–801. [Google Scholar] [CrossRef]
- Sharma, M.; Mukesh, C.; Mondal, D.; Prasad, K. Dissolution of α-chitin in deep eutectic solvents. RSC Adv. 2013, 3, 18149–18155. [Google Scholar] [CrossRef]
- Mukesh, C.; Mondal, D.; Sharma, M.; Prasad, K. Choline chloride-thiourea, a deep eutectic solvent for the production of chitin nanofibers. Carbohydr. Polym. 2014, 103, 466–471. [Google Scholar] [CrossRef]
- Zhu, P.; Gu, Z.; Hong, S.; Lian, H. One-pot production of chitin with high purity from lobster shells using choline chloride-malonic acid deep eutectic solvent. Carbohydr. Polym. 2017, 177, 217–223. [Google Scholar] [CrossRef]
- Hong, S.; Yuan, Y.; Yang, Q.; Zhu, P.; Lian, H. Versatile acid base sustainable solvent for fast extraction of various molecular weight chitin from lobster shell. Carbohydr. Polym. 2018, 201, 211–217. [Google Scholar] [CrossRef]
- Huang, W.-C.; Zhao, D.; Guo, N.; Xue, C.; Mao, X. Green and facile production of chitin from crustacean shells using a natural deep eutectic solvent. J. Agric. Food Chem. 2018, 66, 11897–11901. [Google Scholar] [CrossRef] [PubMed]
- Saravana, P.S.; Ho, T.C.; Chae, S.J.; Cho, Y.J.; Park, J.S.; Lee, H.J.; Chun, B.S. Deep eutectic solvent-based extraction and fabrication of chitin films from crustacean waste. Carbohydr. Polym. 2018, 195, 622–630. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.; Yuan, Y.; Yang, Q.; Chen, L.; Deng, J.; Chen, W.; Lian, H.; Mota-Morales, J.D.; Liimatainen, H. Choline chloride-zinc chloride deep eutectic solvent mediated preparation of partial O-acetylation of chitin nanocrystal in one step reaction. Carbohydr. Polym. 2019, 220, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Cao, S.L.; Gu, W.M.; Ou-Yang, W.D.; Chen, D.C.; Yang, B.Y.; Lai, L.H.; Wu, Y.D.; Liu, Y.J.; Zhu, J.; Chen, W.J.; et al. Preparation, characterization and application of rod-like chitin nanocrystal by using p-toluenesulfonic acid/choline chloride deep eutectic solvent as a hydrolytic media. Carbohydr. Polym. 2019, 213, 304–310. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Huang, W.C.; Guo, N.; Zhang, S.; Xue, C.; Mao, X. Two-step separation of chitin from shrimp shells using citric acid and deep eutectic solvents with the assistance of microwave. Polymers 2019, 11, 409. [Google Scholar] [CrossRef]
- Smith, E.L.; Abbott, A.P.; Ryder, K.S. Deep eutectic solvents (DESs) and their applications. Chem. Rev. 2014, 114, 11060–11082. [Google Scholar] [CrossRef]
- Zdanowicz, M.; Spychaj, T.; Maka, H. Imidazole-based deep eutectic solvents for starch dissolution and plasticization. Carbohydr. Polym. 2016, 140, 416–423. [Google Scholar] [CrossRef]
- Bonhôte, P.; Dias, A.-P.; Papageorgiou, N.; Kalyanasundaram, K.; Grätzel, M. Hydrophobic, highly conductive ambient-temperature molten salts. Inorg. Chem. 1996, 35, 1168–1178. [Google Scholar] [CrossRef]
- Bradley, A.E.; Hardacre, C.; Holbrey, J.D.; Johnston, S.; McMath, S.E.J.; Nieuwenhuyzen, M. Small-angle X-ray scattering studies of liquid crystalline 1-alkyl-3-methylimidazolium salts. Chem. Mater. 2002, 14, 629–635. [Google Scholar] [CrossRef]
- Bochek, A.M.; Murav’Ev, A.A.; Novoselov, N.P.; Zaborski, M.; Zabivalova, N.M.; Petrova, V.A.; Vlasova, E.N.; Volchek, B.Z.; Lavrent’Ev, V.K. Specific features of cellulose and chitin dissolution in ionic liquids of varied structure and the structural organization of regenerated polysaccharides. Russ. J. Appl. Chem. 2012, 85, 1718–1725. [Google Scholar] [CrossRef]
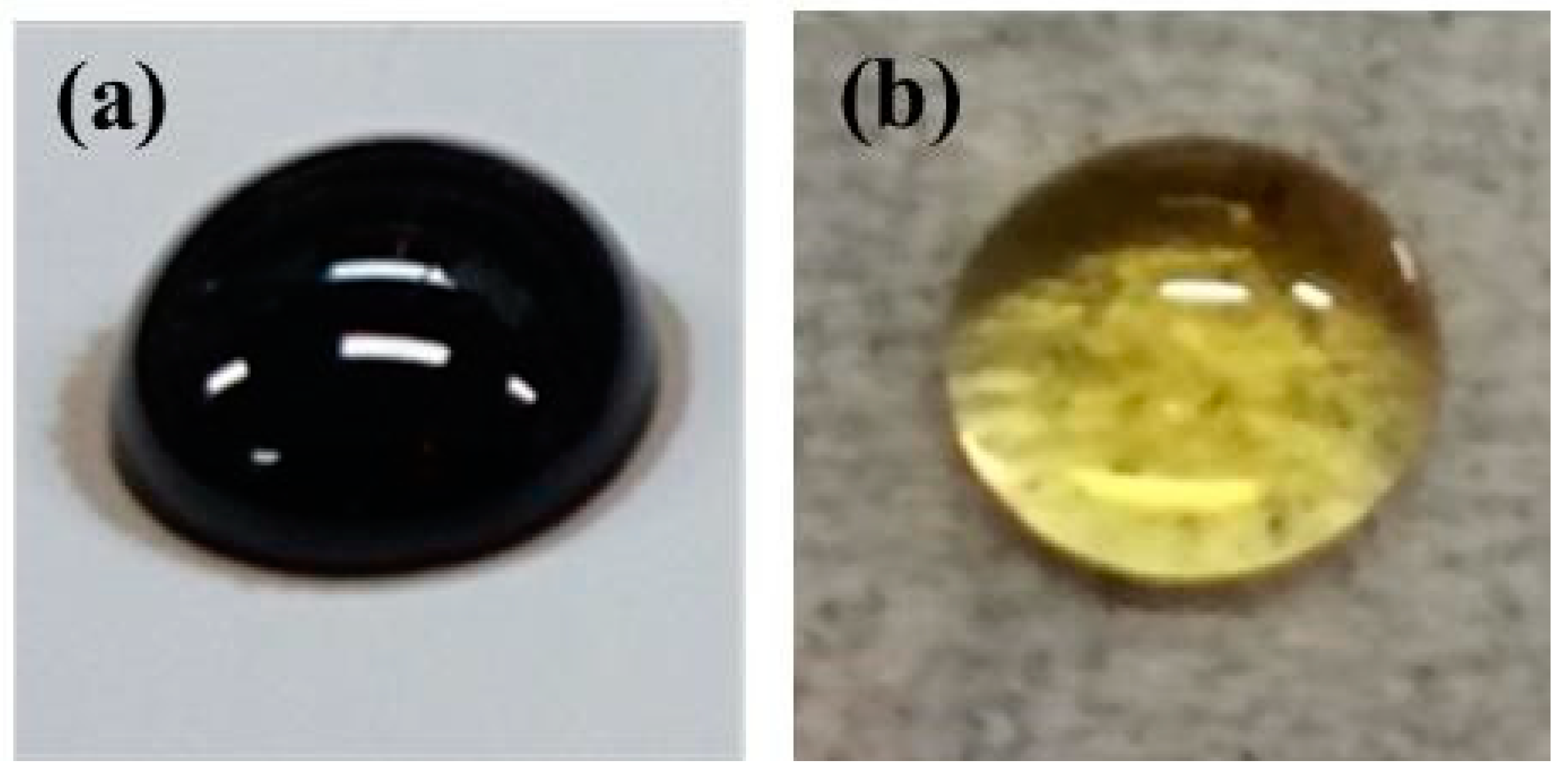
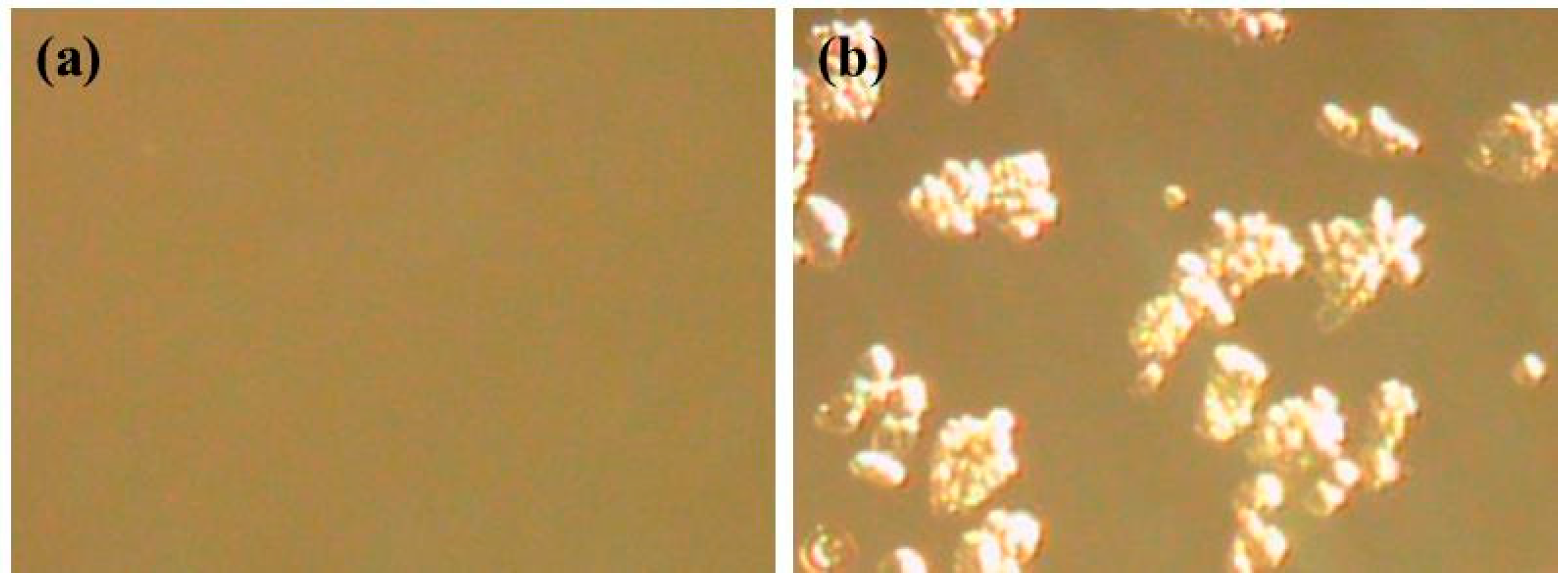
| Entry | IL in a DES (Molar ratio of IL to Thiourea) | Maximum Concentration of Chitin for Dissolved (wt %) b |
|---|---|---|
| 1 | AMIMCl (1:0.5) | 5 |
| 2 | BMIMCl (1:0.5) | ~0 |
| 3 | EMIMCl (1:0.3) | 2 |
| 4 | BMIMBr (1:0.5) | 5 |
| 5 | EMIMBr (1:0.5) | 2 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Idenoue, S.; Yamamoto, K.; Kadokawa, J.-i. Dissolution of Chitin in Deep Eutectic Solvents Composed of Imidazolium Ionic Liquids and Thiourea. ChemEngineering 2019, 3, 90. https://doi.org/10.3390/chemengineering3040090
Idenoue S, Yamamoto K, Kadokawa J-i. Dissolution of Chitin in Deep Eutectic Solvents Composed of Imidazolium Ionic Liquids and Thiourea. ChemEngineering. 2019; 3(4):90. https://doi.org/10.3390/chemengineering3040090
Chicago/Turabian StyleIdenoue, Satoshi, Kazuya Yamamoto, and Jun-ichi Kadokawa. 2019. "Dissolution of Chitin in Deep Eutectic Solvents Composed of Imidazolium Ionic Liquids and Thiourea" ChemEngineering 3, no. 4: 90. https://doi.org/10.3390/chemengineering3040090
APA StyleIdenoue, S., Yamamoto, K., & Kadokawa, J.-i. (2019). Dissolution of Chitin in Deep Eutectic Solvents Composed of Imidazolium Ionic Liquids and Thiourea. ChemEngineering, 3(4), 90. https://doi.org/10.3390/chemengineering3040090






