Checkpoint Inhibition: Will Combination with Radiotherapy and Nanoparticle-Mediated Delivery Improve Efficacy?
Abstract
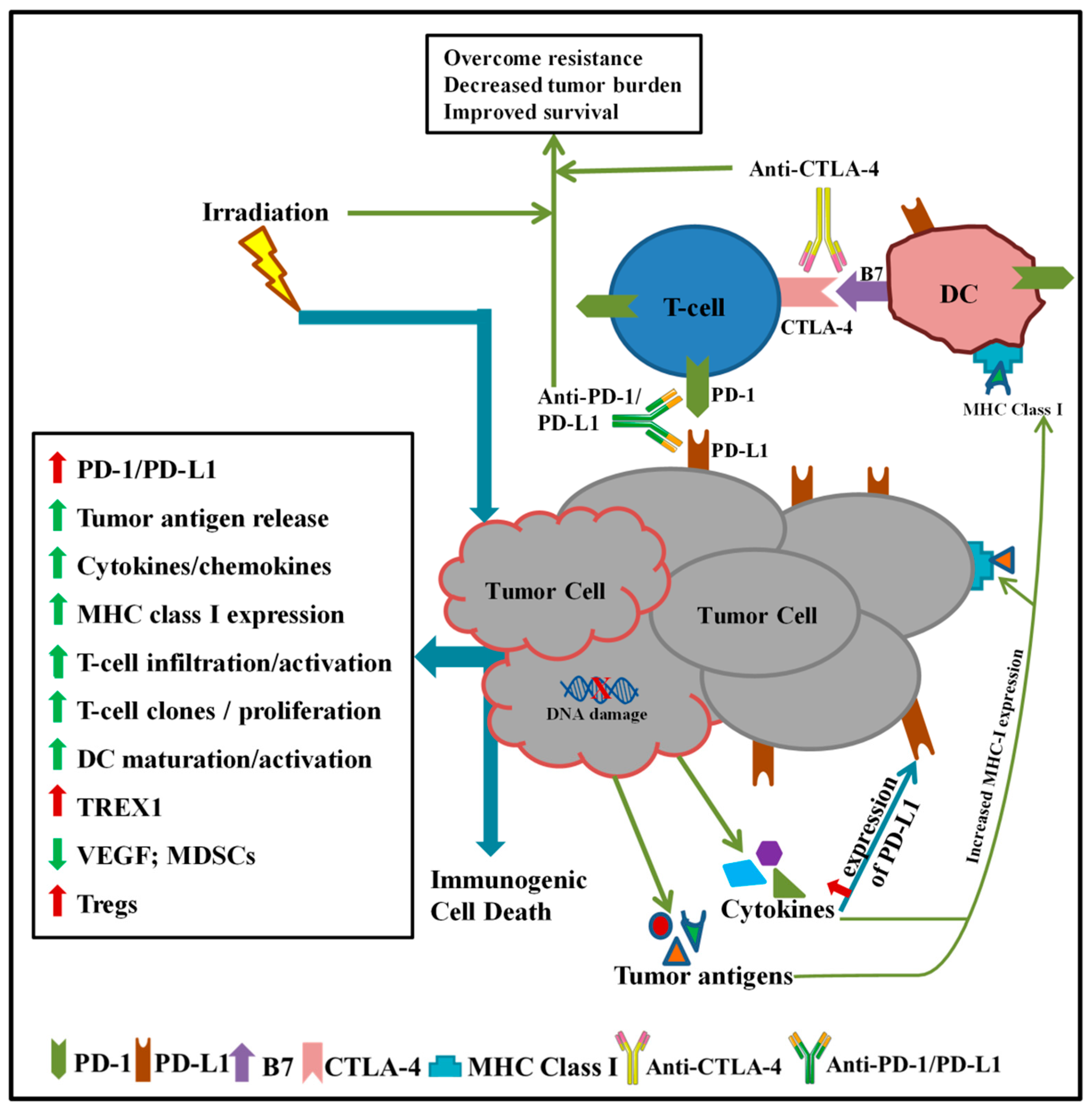
1. Introduction
2. Preclinical Studies of Radiation and Checkpoint Inhibitor Immunotherapy
3. Clinical Trials of Radiation and Checkpoint Inhibitor Immunotherapy
3.1. Melanoma
3.2. Central Nervous System
3.3. Head and Neck
3.4. Thoracic
3.4.1. Non-Small Cell Lung Cancer (NSCLC)
3.4.2. Small Cell Lung Cancer (SCLC)
3.5. Breast
3.6. Gastrointestinal
3.7. Genitourinary
3.8. Gynecologic
4. Nanoparticle and Microparticle Delivery of Checkpoint Inhibitors
4.1. Nanoparticle Delivery of Checkpoint Inhibitors
4.1.1. Polymeric and Metal Nanoparticle Delivery of Checkpoint Inhibitors
4.1.2. Liposomal Delivery of Checkpoint Inhibitors
4.2. Microparticles Delivery of Checkpoint Inhibitors
5. Conclusions
Funding
Conflicts of Interest
Abbreviations
| PD-1 | programmed death receptor 1 |
| PD-L1 | programmed death-ligand 1 |
| CTLA-4 | cytotoxic T-lymphocyte-associated protein 4 |
| CPI | checkpoint inhibition |
| TME | tumor microenvironment |
| RT | radiation therapy |
| AEs | adverse effects |
| ORR | overall response rate |
| OS | overall survival |
| DLT | dose-limiting toxicity |
| PFS | progression-free survival |
| LC | local control |
| pCR | pathologic complete response |
| TTP | time to progression |
| FFS | failure-free survival |
| DFS | disease-free survival |
| SBRT | stereotactic body radiation therapy |
| EFS | event-free survival |
| GHS | global health score |
| QoL | quality of life |
| MFS | metastasis-free survival |
| TTR | time to relapse |
References
- Iwai, Y.; Hamanishi, J.; Chamoto, K.; Honjo, T. Cancer immunotherapies targeting the PD-1 signaling pathway. J. Biomed. Sci. 2017, 24, 26. [Google Scholar] [CrossRef] [PubMed]
- Hilmi, M.; Bartholin, L.; Neuzillet, C. Immune therapies in pancreatic ductal adenocarcinoma: Where are we now? World J. Gastroenterol. 2018, 24, 2137–2151. [Google Scholar] [CrossRef] [PubMed]
- Kasamon, Y.L.; de Claro, R.A.; Wang, Y.; Shen, Y.L.; Farrell, A.T.; Pazdur, R. FDA approval summary: Nivolumab for the treatment of relapsed or progressive classical hodgkin lymphoma. Oncologist 2017, 22, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Riva, A.; Chokshi, S. Immune checkpoint receptors: Homeostatic regulators of immunity. Hepatol. Int. 2018, 2018, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Riley, J.L. PD-1 signaling in primary T cells. Immunol. Rev. 2009, 229, 114–125. [Google Scholar] [CrossRef] [PubMed]
- Keir, M.E.; Butte, M.J.; Freeman, G.J.; Sharpe, A.H. PD-1 and its ligands in tolerance and immunity. Annu. Rev. Immunol. 2008, 26, 677–704. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, R.W.; Barbie, D.A.; Flaherty, K.T. Mechanisms of resistance to immune checkpoint inhibitors. Br. J. Cancer 2018, 118, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Lamichhane, P.; Karyampudi, L.; Shreeder, B.; Krempski, J.; Bahr, D.; Daum, J.; Kalli, K.R.; Goode, E.L.; Block, M.S.; Cannon, M.J.; et al. IL10 release upon PD-1 blockade sustains immunosuppression in ovarian cancer. Cancer Res. 2017, 2017. [Google Scholar] [CrossRef] [PubMed]
- Koyama, S.; Akbay, E.A.; Li, Y.Y.; Herter-Sprie, G.S.; Buczkowski, K.A.; Richards, W.G.; Gandhi, L.; Redig, A.J.; Rodig, S.J.; Asahina, H.; et al. Adaptive resistance to therapeutic pd-1 blockade is associated with upregulation of alternative immune checkpoints. Nat. Commun. 2016, 7, 10501. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.E.; Patel, M.A.; Mangraviti, A.; Kim, E.S.; Theodros, D.; Velarde, E.; Liu, A.; Sankey, E.W.; Tam, A.; Xu, H.; et al. Combination therapy with anti-PD-1, anti-TIM-3, and focal radiation results in regression of murine gliomas. Clin. Cancer Res. 2017, 23, 124–136. [Google Scholar] [CrossRef] [PubMed]
- Dovedi, S.J.; Adlard, A.L.; Lipowska-Bhalla, G.; McKenna, C.; Jones, S.; Cheadle, E.J.; Stratford, I.J.; Poon, E.; Morrow, M.; Stewart, R.; et al. Acquired resistance to fractionated radiotherapy can be overcome by concurrent PD-L1 blockade. Cancer Res. 2014, 74, 5458–5468. [Google Scholar] [CrossRef] [PubMed]
- Deng, L.; Liang, H.; Burnette, B.; Beckett, M.; Darga, T.; Weichselbaum, R.R.; Fu, Y.-X. Irradiation and anti-PD-L1 treatment synergistically promote antitumor immunity in mice. J. Clin. Investig. 2014, 124, 687–695. [Google Scholar] [CrossRef] [PubMed]
- Sharabi, A.B.; Tran, P.T.; Lim, M.; Drake, C.G.; DeWeese, T.L. Stereotactic radiation therapy combined with immunotherapy: Augmenting the role of radiation in local and systemic treatment. Oncology 2015, 29, 331–340. [Google Scholar] [PubMed]
- Gong, X.; Li, X.; Jiang, T.; Xie, H.; Zhu, Z.; Zhou, F.; Zhou, C. Combined radiotherapy and anti-PD-L1 antibody synergistically enhances antitumor effect in non-small cell lung cancer. J. Thorac. Oncol. 2017, 12, 1085–1097. [Google Scholar] [CrossRef] [PubMed]
- Herter-Sprie, G.S.; Koyama, S.; Korideck, H.; Hai, K.; Deng, J.; Li, Y.Y.; Buczkowski, K.A.; Grant, A.K.; Ullas, S.; Rhee, K.; et al. Synergy of radiotherapy and PD-1 blockade in Kras-mutant lung cancer. JCI Insight 2016, 1, e87415. [Google Scholar] [CrossRef] [PubMed]
- Demaria, S.; Kawashima, N.; Yang, A.M.; Devitt, M.L.; Babb, J.S.; Allison, J.P.; Formenti, S.C. Immune-mediated inhibition of metastases after treatment with local radiation and CTLA-4 blockade in a mouse model of breast cancer. Clin. Cancer Res. 2005, 11, 728–734. [Google Scholar] [PubMed]
- Dewan, M.Z.; Galloway, A.E.; Kawashima, N.; Dewyngaert, J.K.; Babb, J.S.; Formenti, S.C.; Demaria, S. Fractionated but not single-dose radiotherapy induces an immune-mediated abscopal effect when combined with anti-CTLA-4 antibody. Clin. Cancer Res. 2009, 15, 5379–5388. [Google Scholar] [CrossRef] [PubMed]
- Twyman-Saint Victor, C.; Rech, A.J.; Maity, A.; Rengan, R.; Pauken, L.E.; Stelekati, E.; Benci, J.L.; Xu, B.; Dada, H.; Odorizzi, P.M.; et al. Radiation and dual checkpoint blockade activate non-redundant immune mechanisms in cancer. Nature 2015, 520, 373–377. [Google Scholar] [CrossRef] [PubMed]
- Dovedi, S.J.; Cheadle, E.J.; Popple, A.; Poon, E.; Morrow, M.; Stewart, R.; Yusko, E.; Sanders, C.; Vignali, M.; Emerson, R.; et al. Fractionated Radiation Therapy Stimulates Antitumor Immunity Mediated by Both Resident and Infiltrating Polyclonal T-cell Populations when Combined with PD-1 Blockade. Clin. Cancer Res. 2017, 23. [Google Scholar] [CrossRef] [PubMed]
- Lan, J.; Li, R.; Yin, L.-M.; Deng, L.; Gui, J.; Chen, B.-Q.; Zhou, L.; Meng, M.-B.; Huang, Q.-R.; Mo, X.-M.; et al. Targeting myeloid-derived suppressor cells and programmed death ligand 1 confers therapeutic advantage of ablative hypofractionated radiation therapy compared with conventional fractionated radiation therapy. Int. J. Radiat. Oncol. Biol. Phys. 2018, 101, 74–87. [Google Scholar] [CrossRef] [PubMed]
- Golden, E.B.; Frances, D.; Pellicciotta, I.; Demaria, S.; Barcellos-Hoff, M.H.; Formenti, S. Radiation fosters dose-dependent and chemotherapy-induced immunogenic cell death. Oncoimmunology 2014, 3, e28518. [Google Scholar] [CrossRef] [PubMed]
- Deng, L.; Liang, H.; Xu, M.; Yang, X.; Burnette, B.; Arina, A.; Li, X.-D.; Mauceri, H.; Bechett, M.; Darga, T.; et al. Sting-dependent cytosolic DNA sensing promotes radiation-induced type I interferon-dependent antitumor immunity in immunogenic tumors. Immunity 2014, 41, 843–852. [Google Scholar] [CrossRef] [PubMed]
- Reits, E.A.; Hodge, J.W.; Herberts, C.A.; Groothuis, T.A.; Chakraborty, M.; Wansley, E.K.; Camphausen, K.; Luiten, R.M.; de Ru, A.H.; Neijssen, J.; et al. Radiation modulates the peptide repertoire, enhances MHC class I expression, and induces successful antitumor immunotherapy. J. Exp. Med. 2006, 203, 1259–1271. [Google Scholar] [CrossRef] [PubMed]
- Ko, E.C.; Formenti, S.C. Radiotherapy and checkpoint inhibitors: A winning new combination? Ther. Adv. Med. Oncol. 2018, 10. [Google Scholar] [CrossRef] [PubMed]
- Harding, S.M.; Benci, J.L.; Irianto, J.; Discher, D.E.; Minn, A.J.; Greenberg, R.A. Mitotic progression following DNA damage enables pattern recognition within micronuclei. Nature 2017, 548, 466–470. [Google Scholar] [CrossRef] [PubMed]
- Vanpouille-Box, C.; Alard, A.; Aryankalayil, M.J.; Sarfraz, Y.; Diamond, J.M.; Schneider, R.J.; Inghirami, G.; Coleman, N.; Formenti, S.C.; Demaria, S. DNA exonuclease Trex1 regulates radiotherapy-induced tumour immunogenicity. Nat. Commun. 2017, 8, 15618. [Google Scholar] [CrossRef] [PubMed]
- Crittenden, M.R.; Zebertavage, L.; Kramer, G.; Bambina, S.; Friedman, D.; Troesch, V.; Blair, T.; Baird, J.R.; Alice, A.; Gough, M.J. Tumor cure by radiation therapy and checkpoint inhibitors depends on pre-existing immunity. Sci. Rep. 2018, 8, 7012. [Google Scholar] [CrossRef] [PubMed]
- Vonderheide, R.H. The immune revolution: A case for priming, not checkpoint. Cancer Cell 2018, 33, 563–569. [Google Scholar] [CrossRef] [PubMed]
- Demaria, S.; Coleman, C.N.; Formenti, S.C. Radiotherapy: Changing the game in immunotherapy. Trends Cancer 2016, 2, 286–294. [Google Scholar] [CrossRef] [PubMed]
- Filatenkov, A.; Baker, J.; Mueller, A.M.S.; Kenkel, J.; Ahn, G.-O.; Dutt, S.; Zhang, N.; Kohrt, H.; Jensen, K.; Dejbakhsh-Jones, S.; et al. Ablative tumor radiation can change the tumor immune cell microenvironment to induce durable complete remissions. Clin. Cancer Res. 2015, 21, 3727–3739. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.T.; Bu, L.L.; Zhao, Y.Y.; Mao, L.; Deng, W.W.; Wu, T.F.; Zhang, W.-F.; Sun, Z.J. CTLA4 blockade reduces immature myeloid cells in head and neck squamous cell carcinoma. Oncoimmunology 2016, 5, e1151594. [Google Scholar] [CrossRef] [PubMed]
- De Coana, Y.P.; Wolodarski, M.; Poschke, I.; Yoshimoto, Y.; Yang, Y.; Nyström, M.; Edbäck, U.; Brage, S.E.; Lundqvist, A.; Masucci, G.V.; et al. Ipilimumab treatment decreases monocytic MDSCs and increases CD8 effector memory T cells in long-term survivors with advanced melanoma. Oncotarget 2017, 8, 21539–21553. [Google Scholar] [CrossRef] [PubMed]
- Curran, M.A.; Montalvo, W.; Yagita, H.; Allison, J.P. PD-1 and CTLA-4 combination blockade expands infiltrating T cells and reduces regulatory T and myeloid cells within B16 melanoma tumors. Proc. Natl. Acad. Sci. USA 2010, 107, 4275–4280. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.T.; Bu, L.L.; Huang, C.F.; Zhang, W.F.; Chen, W.J.; Gutkind, J.S.; Kulkarni, A.B.; Sun, Z.-J. PD-1 blockade attenuates immunosuppressive myeloid cells due to inhibition of CD47/SIRPalpha axis in HPV negative head and neck squamous cell carcinoma. Oncotarget 2015, 6, 42067–42080. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Sun, X.; Luo, J.; Zhu, H.; Yang, X.; Guo, Q.; Song, Y.; Sun, X. Effects of radiation on T regulatory cells in normal states and cancer: Mechanisms and clinical implications. Am. J. Cancer Res. 2015, 5, 3276–3285. [Google Scholar] [PubMed]
- Wang, W.; Lau, R.; Yu, D.; Zhu, W.; Korman, A.; Weber, J. PD1 blockade reverses the suppression of melanoma antigen-specific CTL by CD4+ CD25(Hi) regulatory T cells. Int. Immunol. 2009, 21, 1065–1077. [Google Scholar] [CrossRef] [PubMed]
- Weber, J.S.; Ramakrishnan, R.; Laino, A.; Berglund, A.E.; Woods, D. Association of changes in T regulatory cells (Treg) during nivolumab treatment with melanoma outcome. J. Clin. Oncol. 2017, 35, 3031. [Google Scholar] [CrossRef]
- Simpson, T.R.; Li, F.; Montalvo-Ortiz, W.; Sepulveda, M.A.; Bergerhoff, K.; Arce, F.; Roddie, C.; Henry, J.K.; Yagita, H.; Wolchok, J.D.; et al. Fc-dependent depletion of tumor-infiltrating regulatory T cells co-defines the efficacy of anti-CTLA-4 therapy against melanoma. J. Exp. Med. 2013, 210, 1695–1710. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Subudhi, S.K.; Blando, J.; Scutti, J.; Vence, L.; Wargo, J.A.; Allison, J.P.; Ribas, A.; Sharma, P. Anti-CTLA-4 immunotherapy does not deplete FOXP3+ regulatory T cells (Tregs) in human cancers. Clin. Cancer Res. 2018. [Google Scholar] [CrossRef] [PubMed]
- Zeng, J.; See, A.P.; Phallen, J.; Jackson, C.M.; Belcaid, Z.; Ruzevick, J.; Durham, N.; Meyer, C.; Harris, T.J.; Albersiano, E.; et al. Anti-PD-1 blockade and stereotactic radiation produce long-term survival in mice with intracranial gliomas. Int. J. Radiat. Oncol. Biol. Phys. 2013, 86, 343–349. [Google Scholar] [CrossRef] [PubMed]
- Gong, J.; Le, T.Q.; Massarelli, E.; Hendifar, A.E.; Tuli, R. Radiation therapy and PD-1/PD-L1 blockade: The clinical development of an evolving anticancer combination. J. Immunother. Cancer 2018, 6, 46. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, Y.; Yasui, T.; Tamari, K.; Minami, K.; Otani, K.; Isohashi, F.; Seo, Y.; Kambe, R.; Loizumi, M.; Ogawa, K. Radiation enhanced the local and distant anti-tumor efficacy in dual immune checkpoint blockade therapy in osteosarcoma. PLoS ONE 2017, 12, e0189697. [Google Scholar] [CrossRef] [PubMed]
- Young, K.H.; Baird, J.R.; Savage, T.; Cottam, B.; Friedman, D.; Bambina, S.; Messenheimer, D.; Fox, B.; Newell, P.; Bahjat, K.S.; et al. Optimizing timing of immunotherapy improves control of tumors by hypofractionated radiation therapy. PLoS ONE 2016, 11, e0157164. [Google Scholar] [CrossRef] [PubMed]
- Niknam, S.; Barsoumian, H.B.; Schoenhals, J.E.; Jackson, H.; Yanamandra, N.; Caetano, M.S.; Li, A.; Younes, A.I.; Cadena, A.P.; Cushman, T.R.; et al. Radiation followed by OX40 stimulation drives local and abscopal antitumor effects in an anti-PD1-resistant lung tumor model. Clin. Cancer Res. 2018. [Google Scholar] [CrossRef] [PubMed]
- Messenheimer, D.J.; Jensen, S.M.; Afentoulis, M.E.; Wegmann, K.W.; Feng, Z.; Friedman, D.J.; Gough, M.J.; Urba, W.J.; Fox, B.A. Timing of PD-1 blockade is critical to effective combination immunotherapy with anti-OX40. Clin. Cancer Res. 2017, 23, 6165–6177. [Google Scholar] [CrossRef] [PubMed]
- Barker, C.A.; Postow, M.A. Combinations of radiation therapy and immunotherapy for melanoma: A review of clinical outcomes. Int. J. Radiat Oncol. Biol. Phys. 2014, 88, 986–997. [Google Scholar] [CrossRef] [PubMed]
- Hodi, F.S.; O’day, S.J.; McDermott, D.R.; Weber, R.W; Sosman, J.A.; Haanen, J.B.; Gonzalez, R.; Robert, C.; Schadendorf, D.; Hassel, J.C.; et al. Improved survival with ipilimumab in patients with advanced metastatic melanoma. N. Engl. J. Med. 2010, 263, 711–723. [Google Scholar] [CrossRef] [PubMed]
- Weber, J.S.; O’Day, S.J.; Urba, W.; Powderly, J.; Nichol, G.; Yellin, M.; Snively, J.; Hersh, E. Phase I/II study of ipilimumab for patients with metastatic melanoma. J. Clin. Oncol. 2008, 26, 5950–5956. [Google Scholar] [CrossRef] [PubMed]
- Wolchok, J.D.; Neyns, B.; Linette, G.; Negrier, S.; Lutzky, J.; Thomas, L.; Waterfirld, W.; Schadendorf, D.; Smylie, M.; Guthrie, T., Jr.; et al. Ipilimumab monotherapy in patients with pretreated advanced melanoma. A randomized, double-blind, multicenter, phase 2, dose-ranging study. Lancet Oncol. 2010, 11, 155–164. [Google Scholar] [CrossRef]
- Garon, E.B.; Rizvi, N.A.; Hui, R.; Leighl, N.; Balmanoukian, A.S.; Eder, J.P.; Patnaik, A.; Aggarwal, C.; Gubens, M.; Horn, L.; et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N. Engl. J. Med. 2015, 372, 2018–2028. [Google Scholar] [CrossRef] [PubMed]
- Motzer, R.J.; Escudier, B.; McDermott, D.F.; George, S.; Hammers, H.J.; Srinivas, S.; Tykodi, S.S.; Sosman, J.A.; Procopio, G.; Plimack, E.R.; et al. Nivolumab versus everolimus in advanced renal cell carcinoma. N. Engl. J. Med. 2015, 373, 1803–1813. [Google Scholar] [CrossRef] [PubMed]
- Ansell, S.M.; Lesokhin, A.M.; Borrello, I.; Halwani, A.; Scott, E.C.; Gutierrez, M.; Schuster, S.J.; Millenson, M.M.; Cattry, D.; Freeman, G.J.; et al. PD-1 blockade with nivolumab in relapsed or refractory Hodgkin’s lymphoma. N. Engl. J. Med. 2015, 372, 311–319. [Google Scholar] [CrossRef] [PubMed]
- Cohen, E.E.W.; Machiels, J.-P.H.; Harrington, K.J.; Burtness, B.; Shin, S.W.; Gause, C.K.; Swift, A.M.; Brown, H.; Perrone, A.M.; Cheng, J.D.; et al. KEYNOTE-040: A phase III randomized trial of pembrolizumab (MK-3475) versus standard treatment in patients with recurrent or metastatic head and neck cancer. J. Clin. Oncol. 2015, 33, TPS6084. [Google Scholar]
- Boutros, C.; Mateus, C.; Routier, E.; Chouaib, S.; Libenciuc, C.; Reigneau, M.; Girault, I.; Caramella, C.; Hibat, S.; Vagner, S.; et al. A dose escalation phase 1 study of radiotherapy (rt) in combination with anti-cytotoxic-t-lymphocyte-associated antigen 4 (ctla-4) monoclonal antibody ipilimumab (ipi) in patients (pts) with metastatic melanoma. Ann. Oncol. 2016, 27. [Google Scholar] [CrossRef]
- Hiniker, S.M.; Reddy, S.A.; Maecker, H.T.; Subrahmanyam, P.B.; Rosenberg-Hasson, Y.; Swetter, S.D.; Saha, S.; Shura, L.; Knox, S.J. A prospective clinical trial combining radiation therapy with systemic immunotherapy in metastatic melanoma. Int. J. Radiat. Oncol. Biol. Phys. 2016, 96, 578–588. [Google Scholar] [CrossRef] [PubMed]
- Tree, A.C.; Khoo, V.S.; Eeles, R.A.; Ahmed, M.; Dearnaley, D.P.; Hawkins, M.A.; Huddart, R.A.; Nutting, C.M.; Ostler, P.J.; van J, N. Stereotactic body radiotherapy for oligometastases. Lancet Oncol. 2013, 14, e28–e37. [Google Scholar] [CrossRef]
- Theurich, S.; Rothschild, S.I.; Hoffmann, M.; Fabri, M.; Sommer, A.; Garcia-Marquez, M.; Thelen, M.; Schill, C.; Merki, R.; Schmid, T.; et al. Local tumor treatment in combination with systemic ipilimumab immunotherapy prolongs overall survival in patients with advanced malignant melanoma. Cancer Immunol. Res. 2016, 4, 744–754. [Google Scholar] [CrossRef] [PubMed]
- Goel, A.; Kumar, R.J.; Menzel, P.L.; Abendroth, R.E.; Wei, E.K.; Minor, D. Hypofractionated radiation and ipilimumab in the management of noncranial metastatic melanoma: Long-term follow-up. Int. J. Radiat. Oncol. 2016, 96, E709. [Google Scholar] [CrossRef]
- Qin, R.; Olson, A.; Singh, B.; Thomas, S.; Wolf, S.; Bhavsar, N.A.; Hanks, B.A.; Salama, J.K.; Slama, A.K.S. Safety and efficacy of radiation therapy in advanced melanoma treated with ipilimumab. Int. J. Radiat. Oncol. Biol. Phys. 2016, 96, 72–77. [Google Scholar] [CrossRef] [PubMed]
- Boyer, M.J.; Gu, L.; Wang, X.; Kelsey, C.R.; Yoo, D.S.; Onaitis, M.W.; Dunphy, F.R.; Crawford, J.; Ready, N.E.; Salama, J.K. Toxicity of definitive and post-operative radiation following ipilimumab in non-small cell lung cancer. Lung Cancer 2016, 98, 76–78. [Google Scholar] [CrossRef] [PubMed]
- Johnson, C.B.; Suarez, E.M.; Gupta, R.; Shirai, K. Investigation into the optimal radiation dose schedule for metastatic melanoma patients receiving concurrent ipilimumab. Int. J. Radiat. Oncol. 2016, 96, E599. [Google Scholar] [CrossRef]
- Johnson, C.B.; Jagsi, R. The promise of the abscopal effect and the future of trials combining immunotherapy and radiation therapy. Int. J. Radiat. Oncol. 2016, 95, 1254–1256. [Google Scholar] [CrossRef] [PubMed]
- Weber, J.; Thompson, J.A.; Hamid, O.; Minor, D.; Amin, A.; Ron, I.; Ridolfi, R.; Assi, H.; Maraveyas, A.; Berman, D.; et al. A randomized, double-blind, placebo-controlled, phase II study comparing the tolerability and efficacy of ipilimumab administered with or without prophylactic budesonide in patients with unresectable stage iii or iv melanoma. Clin. Cancer Res. 2009, 15, 5591–5598. [Google Scholar] [CrossRef] [PubMed]
- Sundahl, N.; Wolf, K.D.; Kruse, V.; Meireson, A.; Reynders, D.; Goetghebeur, E.; Gele, M.V.; Speeckaert, R.; Hennart, B.; Brochez, L.; et al. Phase 1 Dose Escalation Trial of Ipilimumab and Stereotactic Body Radiation Therapy in Metastatic Melanoma. Int. J. Radiat. Oncol. Biol. Phys. 2018, 100, 906–915. [Google Scholar] [CrossRef] [PubMed]
- Wild, A.; Kiess, A.P.; Yang, T.J.; Weg, E.S.; Kohutek, Z.A.; Wolchok, J.D.; Postow, M.A.; Tabar, V.S.; Brennan, C.W.; Chan, T.A.; et al. Improved survival and intracranial control associated with radiation necrosis after ipilimumab and stereotactic radiosurgery for melanoma brain metastases. Int. J. Radiat. Oncol. Biol. Phys. 2017, 99, E115. [Google Scholar] [CrossRef]
- Kiess, A.P.; Wolchok, J.D.; Barker, C.A.; Postow, M.A.; Tabar, V.; Huse, J.T.; Chan, T.A.; Yamada, Y.; Beal, K. Stereotactic radiosurgery for melanoma brain metastases in patients receiving ipilimumab: Safety profile and efficacy of combined treatment. Int. J. Radiat. Oncol. 2015, 92, 368–375. [Google Scholar] [CrossRef] [PubMed]
- Knisely, J.P.S.; Yu, J.B.; Flanigan, J.; Sznol, M.; Kluger, H.M.; Chiang, V.L.S. Radiosurgery for melanoma brain metastases in the ipilimumab era and the possibility of longer survival. J. Neurosurg. 2012, 117, 227–233. [Google Scholar] [CrossRef] [PubMed]
- Anderson, E.S.; Postow, M.A.; Young, R.; Chan, T.A.; Yamada, Y.; Beal, K. Initial report on safety and lesion response of melanoma brain metastases after stereotactic radiosurgery or hypofractionated radiation therapy in patients receiving concurrent pembrolizumab. Int. J. Radiat. Oncol. 2016, 96, E132. [Google Scholar] [CrossRef] [PubMed]
- Mathew, M.; Tam, M.; Ott, P.A.; Pavlick, A.C.; Rush, S.C.; Donahue, B.R.; Golfinos, J.G.; Parker, E.C.; Huang, P.P.; Narayana, A. Ipilimumab in melanoma with limited brain metastases treated with stereotactic radiosurgery. Melanoma Res. 2013, 23, 191–195. [Google Scholar] [CrossRef] [PubMed]
- Williams, N.L.; Wuthrick, E.J.; Kim, H.; Palmer, J.D.; Garg, S.; Eldredge-Hindy, H.; Daskalakis, C.; Feeney, K.J.; Mastrangelo, M.J.; Kim, L.J.; et al. Phase 1 study of ipilimumab combined with whole brain radiation therapy or radiosurgery for melanoma patients with brain metastases. Int. J. Radiat. Oncol. Biol. Phys. 2017, 99, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Shaw, E.; Scott, C.; Souhami, L.; Dinapoli, R.; Kline, R.; Loeffler, J.; Farnan, N. Single dose radiosurgical treatment of recurrent previously irradiated primary brain tumors and brain metastases: Final report of rtog protocol 90-05. Int. J. Radiat. Oncol. 2000, 47, 291–298. [Google Scholar] [CrossRef]
- Davies, M.A.; Liu, P.; McIntyre, S.; Kim, K.B.; Papadopoulos, N.; Hwu, W.-J.; Hwu, P.; Bedikian, A. Prognostic factors for survival in melanoma patients with brain metastases. Cancer 2011, 117, 1687–1696. [Google Scholar] [CrossRef] [PubMed]
- Reardon, D.A.; Kim, T.-M.; Frenel, J.-S.; Santoro, A.; Lopez, J.; Subramaniam, D.S.; Siu, L.L.; Rodon, J.; Tamura, K.; Saraf, S.; et al. Atim-35. Results of the phase ib keynote-028 multi-cohort trial of pembrolizumab monotherapy in patients with recurrent pd-l1-positive glioblastoma multiforme (GBM). Neuro-Oncology 2016, 18, vi25–vi26. [Google Scholar] [CrossRef]
- Seiwert, T.Y.; Burtness, B.; Mehra, R.; Weiss, J.; Berger, R.; Eder, J.P.; Heath, K.; McClanahan, T.; Lunceford, J.; Gause, C.; et al. Safety and clinical activity of pembrolizumab for treatment of recurrent or metastatic squamous cell carcinoma of the head and neck (keynote-012): An open-label, multicentre, phase 1B trial. Lancet Oncol. 2016, 17, 956–965. [Google Scholar] [CrossRef]
- Reck, M.; Rodriguez-Abreu, D.; Robinson, A.G.; Hui, R.; Csőszi, T.; Fülöp, A.; Gottfried, M.; Peled, N.; Tafreshi, A.; Cuffe, S.; et al. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N. Engl. J. Med. 2016, 375, 1823–1833. [Google Scholar] [CrossRef] [PubMed]
- Onishi, H.; Araki, T.; Shirato, H.; Nagata, Y.; Hiraoka, M.; Gomi, K.; Yamashita, T.; Niibe, Y.; Karasawa, K.; Hayakawa, K.; et al. Stereotactic hypofractionated high-dose irradiation for stage i nonsmall cell lung carcinoma. Cancer 2004, 101, 1623–1631. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.; Welsh, J.W.; de Groot, P.; Massarelli, E.; Chang, J.Y.; Hess, K.R.; Basu, S.; Curran, M.A.; Cabanillas, M.E.; Subbiah, V.; et al. Ipilimumab with Stereotactic Ablative Radiation Therapy: Phase I Results and Immunologic Correlates from Peripheral T Cells. Clin. Cancer Res. 2017, 23, 1388. [Google Scholar] [CrossRef] [PubMed]
- Theelen, W.; Lalezari, F.; Vries, J.; Langen, J.D.; Aerts, J.; Monkhorst, K.; Baas, P. Randomized phase II study of pembrolizumab after stereotactic body radiotherapy (SBRT) versus pembrolizumab alone in patients with advanced non-small cell lung cancer: The PEMBRO-RT study. J. Clin. Oncol. 2018, 36, 9023. [Google Scholar] [CrossRef]
- Oze, I.; Hotta, K.; Kiura, K.; Ochi, N.; Takigawa, N.; Fujiwara, Y.; Tabata, M.; Tanimoto, M. Twenty-seven years of phase iii trials for patients with extensive disease small-cell lung cancer: Disappointing results. PLoS ONE 2009, 4, e7835. [Google Scholar] [CrossRef] [PubMed]
- Antonia, S.J.; López-Martin, J.A.; Bendell, J.; Ott, P.A.; Taylor, M.; Eder, J.P.; Jäger, D.; Pietanza, M.C.; Le, D.T.; de Braud, F.; et al. Nivolumab alone and nivolumab plus ipilimumab in recurrent small-cell lung cancer (checkmate 032): A multicentre, open-label, phase 1/2 trial. Lancet Oncol. 2016, 17, 883–895. [Google Scholar] [CrossRef]
- Reck, M.; Luft, A.; Szczesna, A.; Havel, L.; Kim, S.-W.; Akerley, W.; Pietanza, M.C.; Wu, Y.; Zielinski, C.; Thomas, M.; et al. Phase iii randomized trial of ipilimumab plus etoposide and platinum versus placebo plus etoposide and platinum in extensive-stage small-cell lung cancer. J. Clin. Oncol. 2016, 34, 3740–3748. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.I.; Ho, A.Y.; McArther, H.L. Combined Radiation Therapy and Immune Checkpoint Blockade Therapy for Breast Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2017, 99, 153. [Google Scholar] [CrossRef] [PubMed]
- Stanton, S.E.; Adams, S.; Disis, M.L. Variation in the incidence and magnitude of tumor-infiltrating lymphocytes in breast cancer subtypes: A systematic review. JAMA Oncol. 2016, 2, 1354. [Google Scholar] [CrossRef] [PubMed]
- Adams, S.; Schmid, P.; Rugo, H.S.; Winer, E.P.; Loirat, D.; Awada, A.; Cescon, D.W.; Iwata, H.; Campone, M.; Nanda, R.; et al. Phase 2 study of pembrolizumab (pembro) monotherapy for previously treated metastatic triple-negative breast cancer (mTNBC): KEYNOTE-086 cohort A. J. Clin. Oncol. 2017, 35, S1008. [Google Scholar] [CrossRef]
- McArthur, H.L.; Barker, A.; Gucalp, A.; Lebron-Zapata, L.; Wen, Y.H.; Phung, A.; Rodine, M.; Arnold, B.; Zhang, Z.; Ho, A. A single-arm, phase II study assessing the efficacy of pembrolizumab (pembro) plus radiotherapy (RT) in metastatic triple negative breast cancer (mTNBC). JCO 2018, 35, S14. [Google Scholar] [CrossRef]
- Doi, T.; Piha-Paul, S.A.; Jalal, S.I.; Mai-Dang, H.; Saraf, S.; Koshiji, M.; Csiki, I.; Bennouna, J. Updated results for the advanced esophageal carcinoma cohort of the phase ib keynote-028 study of pembrolizumab (mk-3475). J. Clin. Oncol. 2016, 34, 7. [Google Scholar] [CrossRef]
- Muro, K.; Chung, H.C.; Shankaran, V.; Geva, R.; Catenacci, D.; Gupta, S.; Eder, J.P.; Golan, T.; Le, D.T.; Burtness, B.; et al. Pembrolizumab for patients with PD-L1-positive advanced gastric cancer (KEYNOTE-012): A multicentre, open-label, phase 1b trial. Lancet Oncol. 2016, 17, 717. [Google Scholar] [CrossRef]
- Royal, R.E.; Levy, C.; Turner, K.; Mathur, A.; Hughes, M.; Kammula, U.S.; Sherry, R.M.; Topalian, S.L.; Yang, J.C.; Lowy, I.; et al. Phase 2 trial of single agent ipilimumab (anti-ctla-4) for locally advanced or metastatic pancreatic adenocarcinoma. J. Immunother. 2010, 33, 828–833. [Google Scholar] [CrossRef] [PubMed]
- Kwon, E.D.; Drake, C.G.; Scher, H.I.; Fizazi, K.; Bossi, A.; van den Eertwegh, A.J.M.; Krainer, M.; Houede, N.; Santos, R.; Mahammedi, H.; et al. Ipilimumab versus placebo after radiotherapy in patients with metastatic castration-resistant prostate cancer that had progressed after docetaxel chemotherapy (ca184-043): A multicentre, randomised, double-blind, phase 3 trial. Lancet Oncol. 2014, 15, 700–712. [Google Scholar] [CrossRef]
- Slovin, S.F.; Higano, C.S.; Hamid, O.; Tejwani, S.; Harzstark, A.; Alumkal, J.J.; Scher, H.I.; Chin, K.; Gagnier, P.; McHenry, M.B.; et al. Ipilimumab alone or in combination with radiotherapy in metastatic castration-resistant prostate cancer: Results from an open-label, multicenter phase I/II study. Ann. Oncol. 2013, 24, 1813. [Google Scholar] [CrossRef] [PubMed]
- Motzer, R.J.; Tannir, N.M.; McDermott, D.F.; Fronter, O.A.; Melichar, B.; Choueiri, T.K.; PLimack, E.R.; Barthélémy, P.; Porta, C.; George, S.; et al. Nivolumab plus Ipilimumab versus Sunitinib in Advanced Renal-Cell Carcinoma. N. Engl. J. Med. 2018, 378, 1277–1290. [Google Scholar] [CrossRef] [PubMed]
- Swaminath, A.; Chu, W. Stereotactic body radiotherapy for the treatment of medically inoperable primary renal cell carcinoma: Current evidence and future directions. Can. Urol. Assoc. J. 2015, 9, 275. [Google Scholar] [CrossRef] [PubMed]
- Morales, A.; Eidinger, D.; Bruce, A.W. Intracavitary Bacillus Calmette-Guerin in the treatment of superficial bladder tumors. J. Urol. 1976, 116, 180–183. [Google Scholar] [CrossRef]
- Rosenberg, J.E.; Hoffman-Censits, J.; Powles, T.; van der Heijden, M.S.; Balar, A.V.; Necchi, A.; Dawson, N.; O’Donnell, P.H.; Balmanoukian, A.; Loriot, Y.; et al. Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: A single-arm, multicentre, phase 2 trial. Lancet 2016, 387, 1909–1920. [Google Scholar] [CrossRef]
- Hollebecque, A.; Meyer, T.; Moore, K.N.; Machiels, J.-P.H.; De Greve, J.; López-Picazo, J.M.; Oaknin, A.; Kerger, J.N.; Boni, V.; Evans, T.R.J.; et al. An open-label, multicohort, phase I/II study of nivolumab in patients with virus-associated tumors (CheckMate 358): Efficacy and safety in recurrent or metastatic (R/M) cervical, vaginal, and vulvar cancers. JCO 2017, 35, S5504. [Google Scholar] [CrossRef]
- Ott, P.A.; Bang, Y.J.; Berton-Rigaud, D.; Elez, E.; Pishvaian, M.J.; Rugo, H.S.; Puzanov, I.; Mehnert, J.M.; Aung, K.L.; Lopez, J.; et al. Safety and Antitumor Activity of Pembrolizumab in Advanced Programmed Death Ligand 1–Positive Endometrial Cancer: Results from the KEYNOTE-028 Study. JCO 2017, 35, 2535–2541. [Google Scholar] [CrossRef] [PubMed]
- Almeida, J.P.; Figueroa, E.R.; Drezek, R.A. Gold nanoparticle mediated cancer immunotherapy. Nanomedicine 2014, 10, 503–514. [Google Scholar] [CrossRef] [PubMed]
- Velpurisiva, P.; Gad, A.; Piel, B.; Jadia, R.; Rai, P. Nanoparticle Design Strategies for Effective Cancer Immunotherapy. J. Biomed. 2017, 2, 64–77. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Trase, I.; Ren, M.; Duval, K.; Guo, X.; Chen, Z. Design of Nanoparticle-Based Carriers for Targeted Drug Delivery. J. Nanomater. 2016, 2016, 1087250. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, A.; Rao, P.; Natarajan, S.; Goldman, A.; Sabbisetti, V.S.; Khater, Y.; Korimerla, N.; Chandrasekar, V.; Mashelkar, R.A.; Sengupta, S. Reporter nanoparticle that monitors its anticancer efficacy in real time. Proc. Natl. Acad. Sci. USA 2016, 113, E2104–E2113. [Google Scholar] [CrossRef] [PubMed]
- Kosmides, A.K.; Sidhom, J.-W.; Fraser, A.; Bessell, C.A.; Schneck, J.P. Dual Targeting Nanoparticle Stimulates the Immune System to Inhibit Tumor Growth. ACS Nano 2017, 11, 5417–5429. [Google Scholar] [CrossRef] [PubMed]
- Shao, K.; Singha, S.; Clemente-Casares, X.; Tsai, S.; Yang, Y.; Santamaria, P. Nanoparticle-based immunotherapy for cancer. ACS Nano 2015, 9, 16–30. [Google Scholar] [CrossRef] [PubMed]
- Grimaldi, A.M.; Incoronato, M.; Salvatore, M.; Soricelli, A. Nanoparticle-based strategies for cancer immunotherapy and immunodiagnostics. Nanomedicine 2017, 12, 2349–2365. [Google Scholar] [CrossRef] [PubMed]
- Saleh, T.; Shojaosadati, S.A. Multifunctional nanoparticles for cancer immunotherapy. Hum. Vaccin. Immunother. 2016, 12, 1863–1875. [Google Scholar] [CrossRef] [PubMed]
- Toy, R.; Roy, K. Engineering nanoparticles to overcome barriers to immunotherapy. Bioeng. Transl. Med. 2016, 1, 47–62. [Google Scholar] [CrossRef] [PubMed]
- Lamichhane, N.; Udayakumar, T.S.; D’Souza, W.D.; Simone, C.B., II; Raghavan, S.R.; Polf, J.; Mahmood, J. Liposomes: Clinical Applications and Potential for Image-Guided Drug Delivery. Molecules 2018, 23, 288. [Google Scholar] [CrossRef] [PubMed]
- Akbarzadeh, A.; Rezaei-Sadabady, R.; Davaran, S.; Joo, S.W.; Zarghami, N.; Hanifehpour, Y.; Samiei, M.; Kouhi, M.; Nejati-Koshki, K. Liposome: Classification, preparation, and applications. Nanoscale Res. Lett. 2013, 8, 102. [Google Scholar] [CrossRef] [PubMed]
- Swierczewska, M.; Lee, K.C.; Lee, S. What is the future of PEGylated therapies? Expert Opin. Emerg. Drugs 2015, 20, 531–536. [Google Scholar] [CrossRef] [PubMed]
- Veronese, F.M.; Mero, A. The impact of PEGylation on biological therapies. BioDrugs 2008, 22, 315–329. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Liang, X.; Li, Y.; Sun, T.; Jin, Z.; Xue, H.; Tian, J. Nuclear and Fluorescent Labeled PD-1-Liposome-DOX-(64)Cu/IRDye800CW Allows Improved Breast Tumor Targeted Imaging and Therapy. Mol. Pharm. 2017, 14, 3978–3986. [Google Scholar] [CrossRef] [PubMed]
- Nikpoor, A.R.; Tavakkol-Afshari, J.; Sadri, K.; Jalali, S.A.; Jaafari, M.R. Improved tumor accumulation and therapeutic efficacy of CTLA-4-blocking antibody using liposome-encapsulated antibody: In vitro and in vivo studies. Nanomedicine 2017, 13, 2671–2682. [Google Scholar] [CrossRef] [PubMed]
- Nikpoor, A.R.; Tavakkol-Afshari, J.; Gholizadeh, Z.; Sadri, K.; Babaei, M.H.; Chamani, J.; Badiee, A.; Jalali, S.A.; Jaafari, M.R. Nanoliposome-mediated targeting of antibodies to tumors: IVIG antibodies as a model. Int. J. Pharm. 2015, 495, 162–170. [Google Scholar] [CrossRef] [PubMed]
- La-Beck, N.M.; Gabizon, A.A. Nanoparticle Interactions with the Immune System: Clinical Implications for Liposome-Based Cancer Chemotherapy. Front. Immunol. 2017, 8, 416. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.M.; Scott, M.D. Current and future applications of immunological attenuation via pegylation of cells and tissue. BioDrugs 2001, 15, 833–847. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Liang, X.; Li, Y.; Sun, T.; Xue, H.; Jin, Z.; Tian, J. Liposomal nanohybrid cerasomes targeted to PD-L1 enable dual-modality imaging and improve antitumor treatments. Cancer Lett. 2018, 414, 230–238. [Google Scholar] [CrossRef] [PubMed]
- Rahimian, S.; Fransen, M.F.; Kleinovink, J.W.; Amidi, M.; Ossendorp, F.; Hennink, W.E. Polymeric microparticles for sustained and local delivery of antiCD40 and antiCTLA-4 in immunotherapy of cancer. Biomaterials 2015, 61, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Lei, C.; Liu, P.; Chen, B.; Mao, Y.; Engelmann, H.; Shin, Y.; Jaffar, J.; Hellstrom, I.; Liu, J.; Hellstrom, K.E. Local release of highly loaded antibodies from functionalized nanoporous support for cancer immunotherapy. J. Am. Chem. Soc. 2010, 132, 6906–6907. [Google Scholar] [CrossRef] [PubMed]
| NCT Number | Phase | Title | Condition(s) | Systemic Therapy | Radiation Therapy | Outcome Measures |
|---|---|---|---|---|---|---|
| NCT01449279 | 2 | A Pilot Study of Ipilimumab in Subjects with Stage IV Melanoma Receiving Palliative Radiation Therapy | Melanoma | Ipilimumab | RT to 1–2 sites | Primary: AEs Secondary: ORR, OS, duration of response |
| NCT03354962 | 1/2 | Induction of Immune-mediated aBscOpal Effect thrOugh STEreotactic Radiation Therapy in Metastatic Melanoma Patients Treated by PD-1 + CTLA-4 Inhibitors (BOOSTER MELANOMA) | Melanoma | Nivolumab + ipilimumab | SBRT | Primary: DLT, abscopal effect, PFS Secondary: safety, PFS, pattern of response in irradiated vs. non-irradiated lesions. |
| NCT03601455 | 2 | Phase II Study of Radiation Therapy and Anti-PD-L1 Checkpoint Inhibitor (Durvalumab) with or without Anti-CTLA-4 Inhibition (Tremelimumab) in Patients with Unresectable, Locally Advanced, or Metastatic Urothelial Bladder Cancer That Are Ineligible or Refusing Chemotherapy | Bladder Cancer Stage IVA-IVB | Arm 1: Durvalumab + EBRT Arm 2: Durvalumab + tremelimumab + EBRT | EBRT | Primary: AEs, PFS Secondary: LC, pCR, ORR, abscopal response, duration of response, OS |
| NCT02254772 | 1/2 | A Phase I/II Study of Intratumoral Injection of SD-101, an Immunostimulatory CpG, and Intratumoral Injection of Ipilimumab, an Anti-CTLA-4 Monoclonal Antibody, in Combination with Local Radiation in Low-Grade B-Cell Lymphomas | Extranodal Marginal Zone B-Cell Lymphoma of Mucosa-Associated Lymphoid Tissue Nodal Marginal Zone B-Cell Lymphoma Recurrent Grade 1/2 Follicular Lymphoma Recurrent Marginal Zone Lymphoma Recurrent Small Lymphocytic Lymphoma Splenic Marginal Zone Lymphoma | TLR9 agonist SD-101 via intratumoral injections and ipilimumab via intratumoral injection + EBRT | Low dose RT to 1 site of disease | Primary: DLT Secondary: tumor response, TTP |
| NCT02115139 | 2 | A Multicenter, Single Arm, Phase 2 Clinical Study on the Combination of Radiation Therapy and Ipilimumab, for the Treatment of Patients with Melanoma and Brain Metastases Actual Study Start Date: 4 April 2014 Actual Primary Completion Date: 31 December 2016 Estimated Study Completion Date: August 2018 | Melanoma with Brain Metastases | Ipilimumab + RT | Whole-brain radiotherapy (WBRT) 30 Gy in 10 fractions | Primary: 1 year survival Secondary: PFS, PFS, OS, ORR, AEs |
| NCT02843165 | 2 | Randomized Phase II Study of Checkpoint Blockade Immunotherapy Combined with Stereotactic Body Radiation Therapy in Advanced Metastatic Disease | Metastatic Cancer | Checkpoint blockade immunotherapy ± SBRT | SBRT: 28.5 Gy in 3 fractions of 9.5 Gy | Primary: ORR Secondary: safety/toxicity, PFS, OS, rate of stable disease, change in antitumor response |
| NCT02107755 | 2 | A Phase 2 Study Using Stereotactic Ablative Radiation Therapy and Ipilimumab in Patients with Oligometastatic Melanoma | Liver Metastases Lung Metastases Recurrent/Metastatic Melanoma Melanoma Metastatic to Brain | Ipilimumab RT ipilimumab | Stereotactic radiosurgery | Primary: PFS Secondary: AEs, ORR, LF, OS |
| NCT03426657 | 2 | First-Line Treatment of Locally Advanced HNSCC with Double Checkpoint Blockade and Radiotherapy Dependent on Intratumoral CD8+ T-Cell Infiltration | Locally Advanced Head and Neck Squamous Cell Carcinoma | Durvalumab + tremelimumab + RT | 35 × 2.0/1.8/1.6 Gy | Primary: DLT Secondary: PFS, pCR, OS |
| NCT02701400 | 2 | A Randomized Study of Tremelimumab Plus Durvalumab Combination with or without Radiation in Relapsed Small Cell Lung Cancer | Recurrent Small Cell Lung Carcinoma | Tremelimumab & durvalumab ± RT | SBRT | Primary: PFS, ORR Secondary: Immune-related objective response rate, OS |
| NCT01970527 | 2 | RADVAX: A Stratified Phase II Dose Escalation Trial of Stereotactic Body Radiotherapy Followed by Ipilimumab in Metastatic Melanoma | Recurrent/Metastatic Melanoma | SBRT → ipilimumab | SBRT 3 fractions | Primary: Immune-related clinical response. Immune-related PFS, late toxicity, OS Secondary: Lymphocyte activation/analysis, T-cell response |
| NCT02888743 | 2 | A Phase 2 Study of MEDI4736 (Durvalumab) and Tremelimumab Alone or in Combination with High- or Low-Dose Radiation in Metastatic Colorectal and NSCLC | Metastatic Non-Small Cell Lung Cancer Colorectal Cancer Stage IVA/IVB | Tremelimumab + durvalumab ± RT | High-dose daily RT Low-dose BID RT | Primary: ORR Secondary: PFS, PS, AEs, LC, abscopal responses |
| NCT03437200 | 2 | Phase II Trial in Inoperable Esophageal Cancer Evaluating the Feasibility of the Combination of Definitive Chemoradiation with the Immune Checkpoint Blockers Nivolumab ± Ipilimumab | Inoperable Esophageal Cancer | Chemoradiation + nivolumab ± ipilimumab | RT: 50 Gy in 25 fractions of 2 Gy | Primary: PFS Secondary: Best overall response, pattern of progression, FFS, OS |
| NCT03522584 | 1/2 | Recurrent/Metastatic Head and Neck Squamous Cell Carcinoma | Durvalumab (MEDI4376), Tremelimumab, and Palliative Hypofractionated Radiation (SBRT) in Patients with Recurrent/Metastatic Squamous Cell Carcinomas of the Head and Neck Previously Treated with Immune Checkpoint Inhibitors | Tremelimumab + durvalumab + SBRT | SBRT over 3 fractions | Primary: AEs Secondary: ORR, PFS, OS |
| NCT03604978 | 1/2 | Grade II, III, or Recurrent Meningioma | A Phase I/II Study of Nivolumab Plus or Minus Ipilimumab in Combination with Multi-Fraction Stereotactic Radiosurgery for Recurrent High-Grade Radiation-Relapsed Meningioma | Nivolumab + radiosurgery ± ipilimumab | Multi-fraction stereotactic radiosurgery | Primary: MTD, AEs, ORR Secondary: PFS, OS, changes of peripheral T cells |
| NCT03604991 | 2/3 | A Phase II/III Study of Peri-Operative Nivolumab and Ipilimumab in Patients with Locoregional Esophageal and Gastroesophageal Junction Adenocarcinoma | Adenocarcinoma of the Esophagus or Gastroesophageal Junction Stage I–IIIA | Arm 1: carboplatin, paclitaxel, radiation therapy Arm 2: carboplatin, paclitaxel, radiation therapy, nivolumab Arm 3: nivolumab Arm 4: nivolumab, ipilimumab | Radiation therapy once a week | Primary: pCR, DFS Secondary: AEs, OS, DFS |
| NCT03618134 | Ib/II | Phase Ib/II Trial of Stereotactic Body Radiotherapy (SBRT) in Combination with Immunotherapy Prior to Transoral Robotic Surgery (TORS) for Human Papillomavirus Positive (HPV+) Squamous Cell Carcinoma of the Head and Neck (SCCHN) | HPV-Mediated (p16-Positive) Oropharyngeal Carcinoma Stages I–III | SBRT, durvalumab, TORS, neck dissection ± tremelimumab | SBRT in 5 fractions | Primary: AEs, PFS Secondary: OS, LC, DF, LRC |
| NCT02868632 | 1 | A Phase I Study of Immune Checkpoint Inhibition (Anti-CTLA-4 and/or Anti-PD-L1) in Combination with Radiation Therapy in Patients with Unresectable and Non-Metastatic Pancreatic Cancer | Pancreatic Cancer | Arm1: MEDI4736 + SBRT Arm 2: Tremelimumab + SBRT Arm 3: MEDI4736 + Tremelimumab + SBRT | SBRT: 30 Gy in 5 fractions of 6 Gy | Primary: OS Secondary: PFS, response |
| NCT03275597 | 1 | Comprehensive Stereotactic Body Radiotherapy (SBRT) to All Sites of Oligometastatic Non-Small Cell Lung Cancer (NSCLC) Combined with Durvalumab (MEDI4736) and Tremelimumab Dual Immune Checkpoint Inhibition | Metastatic Non-Small Cell Lung Cancer | SBRT followed by Durvalumab + tremelimumab | SBRT to all sites of disease. 30–50 Gy in 5 fractions | Primary: safety Secondary: PFS, OS |
| NCT03509584 | 1 | Phase I Multicenter Trial Combining Nivolumab, Alone or with Ipilimumab, Plus Hypofractionated Radiotherapy for Pretreated Advanced Stage Non-Small Cell Lung Cancer Patients | Non-Small Cell Lung Cancer | RT + nivolumab ± ipilimumab | SBRT: 8 Gy × 3 | |
| NCT01935921 | 1 | A Phase Ib Trial of Concurrent Cetuximab (ERBITUX®) and Intensity Modulated Radiotherapy (IMRT) with Ipilimumab (YERVOY®) in Locally Advanced Head and Neck Cancer | Hypopharyngeal Squamous Cell Carcinoma Stage III–IVB Laryngeal Squamous Cell Carcinoma Stage III–IVB Oropharyngeal Squamous Cell Carcinoma Stage III–IVB (AJCC v7) | Cetuximab, RT, and ipilimumab | IMRT | Primary: DLT Secondary: clinical response, PFS, T-cell phenotypes, T regulatory cell counts, Myeloid-derived suppressor cell, HPV status |
| NCT03477864 | 1 | R2810-ONC-16XX: A Phase 1 Neoadjuvant Study of Stereotactic Body Radiation Therapy with Systemic REGN2810 and Intraprostatic Ipilimumab, Alone or in Combination, in Patients with Locally Advanced Prostate Cancer Prior to Radical Prostatectomy | Prostate Cancer Stage II–IVB | Arm A: REGN2810, SBRT, surgery Arm B: ipilimumab, SBRT, surgery Arm C: REGN2810, ipilimumab, SBRT, surgery | SBRT for 4 fractions | Primary: AEs Secondary: pathologic response rate. PSA PFS, radiographic PFS |
| NCT03507699 | 1 | Combination Treatment of Nivolumab, Ipilimumab, Intratumoral CMP-001 and Radiosurgery for Liver Metastases in Colorectal Carcinoma | Colorectal Cancer with Liver Metastases | Nivolumab +Ipilimumab + CMP-001 (TLR9 agonist) ± RT | SBRT: 21 Gy in three fractions to one liver metastasis | Primary: DLT Secondary: response rat, PFS |
| NCT01711515 | 1 | A Phase I Trial of Sequential Ipilimumab After Chemoradiation for the Primary Treatment of Patients with Locally Advanced Cervical Cancer Stages IB2/IIA With Positive Para-Aortic Lymph Nodes Only and Stage IIB/IIIB/IVA with Positive Lymph Nodes | Cervical Cancer Stage IB–IVA | Cisplatin, radiation therapy, and ipilimumab | EBRT followed by intracavitary brachytherapy | Primary: DLT Secondary: Response rate, PFS, OS, location of recurrence, chronic toxicities |
| NCT Number | Phase | Title | Condition(s) | Systemic Therapy | Radiation Therapy | Outcome Measures |
|---|---|---|---|---|---|---|
| NCT03040999 | 3 | Study of Pembrolizumab (MK-3475) or Placebo with Chemoradiation in Participants with Locally Advanced Head and Neck Squamous Cell Carcinoma (MK-3475-412/KEYNOTE-412) | Oropharyngeal Cancer (Independent of p16) Larynx/Hypopharynx Unresectable Oral Cavity Cancer | Arm 1: priming dose of Pembro before CRT. 2 cycles with RT along with 3 cycles of CDDP. 14 cycles of pembro maintenance Arm 2: placebo delivered at same schedule as pembro above | Accelerated or standard fractionation RT | Primary: EFS Secondary: OS, AEs, treatment discontinuations due to AEs, GHS/QoL, swallowing, speech and pain symptoms |
| NCT02992912 | 2 | A Phase II Study to Assess the Efficacy of the Anti-PD-L1 Antibody Atezolizumab (MPDL3280A) Administered with Stereotactic Ablative Radiotherapy (SABR) in Patients with Metastatic Tumours | Metastatic Tumors | Atezolizumab 1200 mg every 3 weeks | Hypofractionated SABR: 45 Gy in 3 fractions of 15 Gy | PFS |
| NCT03115801 | 2 | A Phase II Randomized Controlled Trial of Programmed Death-1/Programmed Death Ligand-1(PD-1/PD-L1) Axis Blockade Versus PD-1/PD-L1 Axis Blockade Plus Radiotherapy in Metastatic Genitourinary (Renal/Urothelial) Malignancies | Metastatic Renal Cell Carcinoma Metastatic Urothelial Carcinoma | Arm 1: Nivolumab or atezolizumab alone Arm 2: Nivolumab or atezolizumab + radiation | 30 Gy in 3 fractions of 10 Gy | Primary outcome: best overall response rate Secondary: PFS, toxicity, OS |
| NCT03087864 | 2 | PD-L1 Targeting in Resectable Oesophageal Cancer: A Phase II Feasibility Study of Atezolizumab and Chemoradiation | Resectable Esophageal Cancer Stages II–III | Carboplatin + paclitaxel + atezolizumab + radiation | 23 fractions of 1.8 Gy | Primary: feasibility Secondary: toxicity, postoperative complications. Pathologic response, relationship between gut microbiota composition with response and toxicity |
| NCT03220854 | 2 | Phase 2 Clinical Trial of Stereotactic Radiotherapy and PD-1 or PD-L1 Inhibiting Therapy for Treatment of Advanced Solid Tumors Progression on PD-1 or PD-L1 Inhibiting Therapy | Advanced Solid Tumors | Commercially available PD-1 or PD-L1 inhibitor + radiation | SBRT: 18–60 Gy in 3–5 fractions | Primary: OS, PFS per RECIST/RANO Secondary: OS, PFS per irRC |
| NCT02866747 | 1/2 | A Phase I/II Multicenter Trial Evaluating the Association of Hypofractionated Stereotactic Radiation Therapy and the Anti-Programmed Death-Ligand 1 (PD-L1) Durvalumab (Medi4736) for Patients with Recurrent Glioblastoma (STERIMGLI) | Glioblastoma | Arm 1: hFSRT Arm 2: hFSRT + Durvalumab | 24 Gy in 3 fractions of 8 Gy | Primary: dose-limiting toxicities, PFS Secondary: intracranial PFS, OS, safety/tolerability, QOL, neurologic/neurocognitive functions |
| NCT03474094 | 2 | A European, Multicenter, Randomized, Open-label, Phase II Trial Aiming to Assess the Clinical and Biological Activity of an Anti-PD-L1 (Atezolizumab) in Operable Localized Soft Tissue Sarcomas Patients to be Treated with Radiotherapy | Soft Tissue Sarcoma | Arm 1: RT → atezolizumab → surgery Arm 2: Atezolizumab → surgery → RT Arm 3: RT → surgery → atezolizumab | 50 Gy in 25 fractions of 2 Gy | Primary: pathologic response Secondary: PCR, at least 50% necrosis, % residual viable cells, ORR, tumor volume change, LRR at 1 year, TTR, DFS, immune cell infiltration, adverse events, amputation rates |
| NCT03446547 | 2 | Ablative STEreotactic RadiOtherapy wIth Durvalumab (MEDI4736). An Open Label Randomized Phase II Trial with Durvalumab Following Stereotactic Body Radiotherapy (SBRT) in Patients with Stage I Non-Small Cell Lung Cancer (NSCLC) | Stage I NSCLC | Arm 1: SBRT Arm 2: SBRT → durvalumab | Primary: TTP Secondary: OS, LC, QoL, TTP by PD-L1 expression, | |
| NCT03212469 | 1/2 | A Phase I/II Study Evaluating the Safety and Clinical Activity of Anti-PD-L1 (Durvalumab [MEDI4736]) + Anti CTLA-4 (Tremelimumab) Antibodies Administrated in Combination with Stereotactic Body Radiotherapy (SBRT) in Patients with Metastatic Squamous Cell Carcinoma of Head and Neck, Lung, Oesophagus, Cervix, Vagina, Vulva, or Anus | Head and Neck Squamous Cell Carcinoma, Lung Cancer, Esophageal Cancer | Durvalumab + tremelimumab + SBRT at C1D15 → Durvalumab | Primary: DLT | |
| NCT03421652 | 2 | Phase II Trial of Concurrent Nivolumab in Urothelial Bladder Cancer with Radiation Therapy in Localized/Locally Advanced Disease for Chemotherapy Ineligible Patients [NUTRA] | Stage II–IV Bladder Urothelial Carcinoma | Nivolumab + RT | Radiation therapy on weeks 1, 3, 5, 7, and 9. | Primary: PFS Secondary: adverse events, ORR, MFS, OS, QOL, PD-1, and PD-L1 expression, Th1/Th2 cytokine ratio |
| NCT02311361 | 1/2 | A Pilot Study of Immune Checkpoint Inhibition (Durvalumab with or without Tremelimumab) in Combination with Radiation Therapy in Patients with Unresectable Pancreatic Cancer | Pancreatic Cancer | Tremelimumab/durvalum or both + RT | SBRT: 8 Gy × 1 of 5 Gy × 5 | Primary: safety Secondary: plasma pharmacokinetic, OS, ORR, PFS |
| NCT02968940 | 2 | A Phase II, Open-Label, Single Arm, Multicenter Study of Avelumab with Hypofractionated Radiation in Adult Subjects with Transformed IDH Mutant Glioblastoma | Glioblastoma | Avelumab 10 mg/kg every 2 weeks + RT | 30 Gy in 5 fractions of 6 Gy | Primary: safety, PFS Secondary: OS, median PFS, ORR, duration of response |
| NCT02913417 | 1/2 | A Feasibility Study of Sequential Hepatic Internal Radiation and Systemic Ipilimumab and Nivolumab in Patients with Uveal Melanoma Metastatic to Liver | Uveal Melanoma | Yttrium 90 + ipilimumab 3 mg/kg every 3 weeks × 4 + nivolumab 1 mg/kg every 3 weeks × 4 then nivolumab 3 mg/kg every 2 weeks until progression or 3 years | SIR-Spheres Yttrium 90 | Primary: safety/tolerability Secondary: clinical efficacy, immunologic changes, correlation of tissue markers and response to immunotherapy, tumor melanin |
| NCT03407144 | 2 | An Open-label, Uncontrolled, Multicenter Phase II Trial of MK-3475 (Pembrolizumab) in Children and Young Adults with Newly Diagnosed Classical Hodgkin Lymphoma with Inadequate (Slow Early) Response to Frontline Chemotherapy (KEYNOTE 667) | Hodgkin Lymphoma | Arm 1: ABVD (doxorubicin, bleomycin, vinblastine and dacarbazine) induction pembrolizumab + AVD chemotherapy (doxorubicin, vinblastine, dacarbazine) × 2 followed by RT Arm 2: OEPA (vincristine, etoposide/etopophos, prednisone/prednisolone and doxorubicin) induction pembrolizumab + COPDAC-28 chemotherapy (cyclophosphamide, vincristine, prednisone/prednisolone, dacarbazine) × 4 RT if PET response | 21 Gy with boosts to 30 Gy for PET-avid sites | Primary: ORR Secondary: rate of negative PET, EFS, OS, frequency of RT, AE |
| NCT03116529 | 1/2 | Neoadjuvant Anti-PD-L1 (Durvalumab/MEDI4736) Plus Anti-CTLA-4 (Tremelimumab) and Radiation for High Risk Soft-Tissue Sarcoma | Soft Tissue sarcoma | Durvalumab 1500 mg + tremelimumab 75 mg every 4 weeks × 3 concurrent with RT followed by surgery followed by maintainence Durvalumab until disease progression | 50 Gy in 25–28 fractions of 1.8–2.0 Gy/fraction. Tumors > 10 cm receive a single 15 Gy fraction of high-dose spatially fractionated (GRID) radiation therapy within 1–3 days prior to radiation therapy | Primary: toxicity, pathologic response Secondary: OS, DSS, RFS, radiologic response |
| NCT02530502 | 1/2 | Phase I/II Trial of Radiation Therapy Plus Temozolomide with MK-3475 in Patients with Newly Diagnosed Glioblastoma (GBM) Study Start Date: October 2015 Estimated Primary Completion Date: March 2019 Estimated Study Completion Date: November 2020 | Glioblastoma | RT with concurrent temozolomide + pembrolizumab followed by temozolomide and pembrolizumab × 6 or until disease progression or unacceptable toxicities | focal RT | Primary: MTD Secondary: PFS Tertiary: PD-1/PD-L1 expression and T-cell infiltration. Correlate MGMT status with outcome |
| NCT03469713 | 2 | Nivolumab Plus Stereotactic Body Radiotherapy (SBRT) in II and III Line of Patients with Metastatic Renal Cell Carcinoma (mRCC) | Metastatic Renal Cancer | Nivolumab + RT followed by nivolumab for responders until PD or toxicities | 30 Gy in 3 fractions of 10 Gy to a metastatic disease site | Primary: ORR Secondary: PFS, OS, ORR of irradiated and non-irradiated metastases, AEs |
| NCT03283943 | 1 | Phase I (Safety Assessment) of Durvalumab (MEDI4736) with Focal Sensitizing Radiotherapy in Platinum Resistant Ovarian, Primary Peritoneal or Fallopian Tube Epithelial Carcinoma | Ovarian Cancer, Primary Peritoneal Carcinoma, Fallopian Tube Cancer | Durvalumab + RT | Focal sensitizing radiotherapy: Starting dose level of 24 Gy in 4 fractions of 6 Gy and may be escalated to 32 Gy in 4 fractions of 8 Gy | Primary: MTD Secondary: ORR, Ca-125 response rate, immune-related response rate |
| NCT02400814 | 1 | Pilot Study of MPDL3280A Plus Stereotactic Ablative Radiotherapy (SAR) in Stage IV Non-Small Cell Lung Cancer | Stage IV Non-Small Cell Lung Cancer | Arm 1: Concurrent MPDL3280A (anti-PD-L1, every 3 weeks) + SBRT Arm 2: MPDL3280A followed by concurrent SBRT starting on 3rd course Arm 3: SBRT followed by MPDL3280A | SBRT | Primary: AEs, response rate using irRECIST, PFS |
| NCT02837263 | 1 | Pembrolizumab in Combination with Stereotactic Body Radiotherapy for Liver Metastatic Colorectal Cancer | Colorectal Cancer Stage IVA/IVB | SBRT followed by single cycle of pre-operative pembrolizumab followed by surgery to remove all known sites of metastatic disease; followed by pembrolizumab alone | SBRT 40–60 Gy in 5 fractions | Primary: 1 year recurrence rate Secondary: time to recurrence, DFS, OS |
| NCT02735239 | 1/2 | Phase 1/2 Study of Anti-PD-L1 in Combination with Chemo (Radio)Therapy for Oesophageal Cancer | Oesophageal Cancer | Arm 1: Durvalumab + standard of care chemotherapy Arm 2: Durvalumab + tremelimumab + standard of care chemotherapy Arm 3: Recommended combination of doses from Cohort A1 or A2 Arm 4: durvalumab, + surgery + standard of care chemotherapy Arm 5: Durvalumab + surgery + standard of care chemotherapy + radiotherapy | Primary: AEs, dose-limiting toxicity, change in baseline laboratory evaluations Secondary: Tumor response, PFS, OS, 1 year survival | |
| NCT02621398 | 1 | Moving PD-1 Blockade with Pembrolizumab into Concurrent Chemoradiation for Locally Advanced Non-Small Cell Lung Cancer | Non-Small Cell Lung Cancer Stages II–IIIB | Paclitaxel + carboplatin + pembrolizumab + RT | 3DCRT or IMRT | Primary: MTD and DLT Secondary: ORR, MFS, OS, PFS |
| NCT02608385 | 1 | Phase I Study of PD-1 Blockade by Pembrolizumab With Stereotactic Body Radiotherapy in Advanced Solid Tumors | Solid Tumors | RT followed by Pembrolizumab | SBRT | Primary: recommended SBRT dose Secondary: AEs, response rate, PFS, OS, LC |
| NCT02444741 | 1/2 | Phase I/II Trial of MK-3475 and Hypofractionated Stereotactic Radiation Therapy in Patients with Non-Small Cell Lung Cancer (NSCLC) | Lung Cancer | RT + Pembrolizumab | SBRT) to a total dose of 50 Gy in 12.5 Gy fractions (4 fractions total). Wide-field radiation therapy (WFRT) delivered at 45 Gy in 15 daily fractions | Primary: MTD Secondary: PFS |
| NCT02696993 | 1/2 | Phase I/II Trial of Nivolumab with Radiation or Nivolumab and Ipilimumab with Radiation for the Treatment of Intracranial Metastases from Non-Small Cell Lung Cancer | Metastatic Brain Cancer | Nivolumab +RT ± ipilimumab | SRS: physician prescribed dose; WBRT: 30 Gy in 10 fractions | Primary: recommended dose Secondary: intracranial PFS, neurocognitive changes |
| NCT03050554 | 1/2 | Phase I/II Study of the Safety, Tolerability, and Efficacy of Stereotactic Body Radiation Therapy (SBRT) Combined with Concurrent and Adjuvant Avelumab for Definitive Management of Early Stage Non-Small Cell Lung Cancer (NSCLC) | Early Stage Non-Small Cell Lung Cancer | Avelumab + RT | SBRT: 12 Gy × 4 fractions or 10 Gy × 5 fractions (4–5 radiation doses given over 10–12 days every other day) | Primary: safety/tolerability Secondary: LRC, OS |
| NCT02658097 | 2 | A Phase II Trial of Pembrolizumab Sequentially Following Single Fraction Non-Ablative Radiation to One of the Target Lesions, in Previously Treated Patients with Stage IV NSCLC | Stage IV Non-Small Cell Lung Cancer | Pembrolizumab ± RT | 8 Gy × 1 fraction | Primary: RECIST response Secondary: PFS, PS, LC |
| NCT02434081 | 2 | A Phase II Trial Evaluating the Safety and Efficacy of the Addition of Concurrent Anti-PD-1 Nivolumab to Standard First-Line Chemotherapy and Radiotherapy in Locally Advanced Stage IIIA/B Non-Small Cell Lung Carcinoma | Non-small Cell Lung Cancer Stage III | Nivolumab concurrent with standard chemoradiotherapy | EBRT | Primary: ≥grade 3 pneumonitis Secondary: PFS, time to pneumonitis, ORR, TTF |
| NCT02831933 | 2 | ENSIGN: Phase II Window of Opportunity Trial of Stereotactic Body Radiation Therapy and In Situ Gene Therapy Followed by Nivolumab in Metastatic Squamous or Non-Squamous Non-Small Cell Lung Carcinoma and Metastatic Uveal Melanoma | Lung Cancer | Nivolumab + ADV/HSV-tk intratumoral injection + Valacyclovir + RT | 30 gray (Gy; 6 Gy × 5 fractions) | ORR, OS, PFS, AEs |
| NCT Number | Phase | Title | Condition(s) | Systemic Therapy | Radiation Therapy | Outcome Measures |
|---|---|---|---|---|---|---|
| NCT01862900 | 1/2 | Phase I/II Study of Stereotactic Body Radiation Therapy to Metastatic Lesions in the Liver or Lung in Combination with Monoclonal Antibody to OX40 (MEDI6469) in Patients with Progressive Metastatic Breast Cancer After Systemic Therapy | Breast Cancer Metastatic to Lung/Liver | SBRT → MEDI6469 | SBRT: Cohort 1: 15 Gy (central tumors 10 Gy) Cohort 2: 20 Gy (central tumors 15 Gy) Cohort 3: 20 Gy × 2 (central tumors 15 Gy × 2). | Primary: DLT Secondary: response rate in both irradiated and non-irradiated tumors |
| NCT01303705 | 1/2 | Phase Ib Study of Monoclonal Antibody to OX40, Cyclophosphamide (CTX) and Radiation in Patients with Progressive Metastatic Prostate Cancer After Systemic Therapy | Metastatic Prostate Cancer | Anti-OX40 + cyclophosphamide (300 mg, 600 mg, or 900 mg) + RT | 8.0 Gy in 1 fraction to a maximum of three bone metastatic deposits | Primary: MTD Secondary: immune and clinical responses |
| NCT03410901 | 1 | Intratumoral Injection of SD-101, an Immunostimulatory CpG, in Combination with BMS-986178 and Local Radiation in Low-Grade B-Cell Lymphomas | Follicular Lymphoma Grade 1–3a Lymphoplasmacytic Lymphoma Mantle Cell Lymphoma Marginal Zone Lymphoma Small Lymphocytic Lymphoma | Radiation therapy + SD-101 + BMS-986178 | Radiation therapy on days 1–2 | Primary: DLT Secondary: ORR, PFS |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lamichhane, P.; Amin, N.P.; Agarwal, M.; Lamichhane, N. Checkpoint Inhibition: Will Combination with Radiotherapy and Nanoparticle-Mediated Delivery Improve Efficacy? Medicines 2018, 5, 114. https://doi.org/10.3390/medicines5040114
Lamichhane P, Amin NP, Agarwal M, Lamichhane N. Checkpoint Inhibition: Will Combination with Radiotherapy and Nanoparticle-Mediated Delivery Improve Efficacy? Medicines. 2018; 5(4):114. https://doi.org/10.3390/medicines5040114
Chicago/Turabian StyleLamichhane, Purushottam, Neha P. Amin, Manuj Agarwal, and Narottam Lamichhane. 2018. "Checkpoint Inhibition: Will Combination with Radiotherapy and Nanoparticle-Mediated Delivery Improve Efficacy?" Medicines 5, no. 4: 114. https://doi.org/10.3390/medicines5040114
APA StyleLamichhane, P., Amin, N. P., Agarwal, M., & Lamichhane, N. (2018). Checkpoint Inhibition: Will Combination with Radiotherapy and Nanoparticle-Mediated Delivery Improve Efficacy? Medicines, 5(4), 114. https://doi.org/10.3390/medicines5040114




