Differential Modulation of the Central and Peripheral Monoaminergic Neurochemicals by Deprenyl in Zebrafish Larvae
Abstract
1. Introduction
2. Material and Methods
2.1. Chemicals and Reagents
2.2. Fish Husbandry and Larvae Production
2.3. Experimental Protocol
2.4. Extraction and Analysis of Neurochemicals
2.5. Statistical Analysis
3. Results
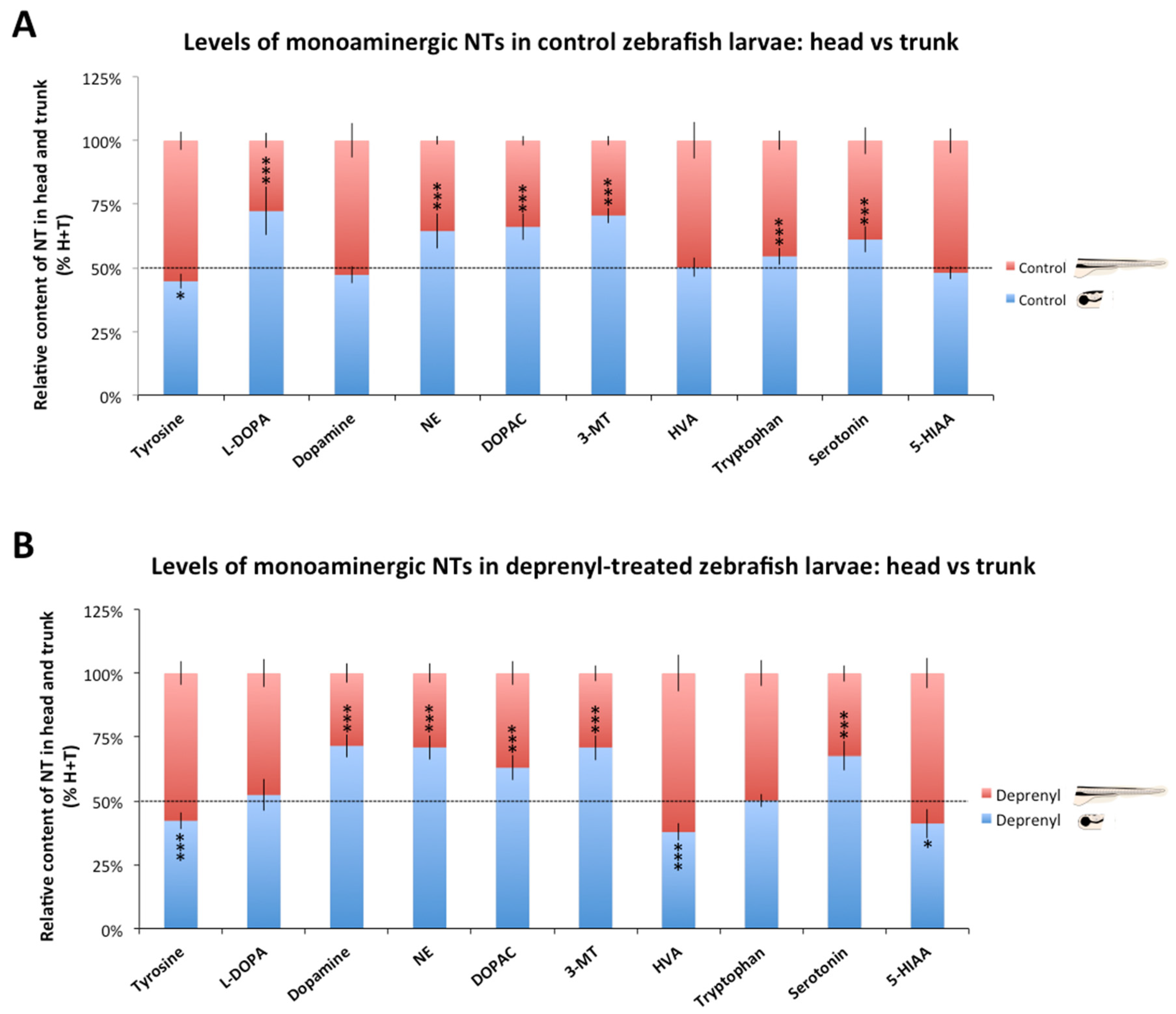
3.1. Distribution of Monoaminergic Neurochemicals between the Head and the Trunk in Control and Deprenyl-Treated Larva
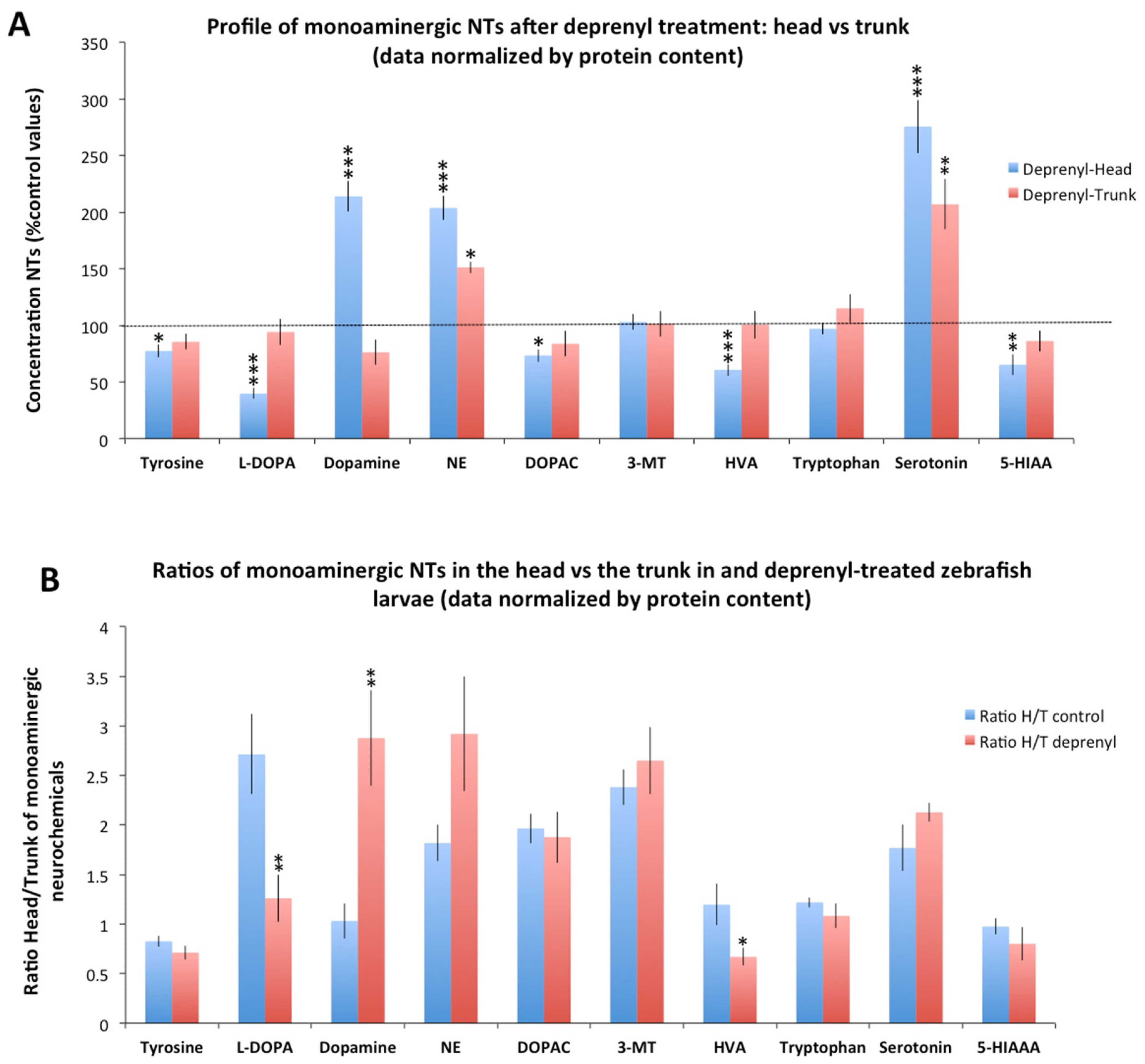
3.2. Differences in the Neurochemical Profile Obtained from the Head and the Whole Body of the Larvae after Deprenyl-Treatment
3.3. Variability of the Neurochemical Data Obtained from Heads and Whole Bodies
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Horzmann, K.; Freeman, J. Zebrafish Get Connected: Investigating Neurotransmission Targets and Alterations in Chemical Toxicity. Toxics 2016, 4, 19. [Google Scholar] [CrossRef] [PubMed]
- Rico, E.P.; Rosemberg, D.B.; Seibt, K.J.; Capiotti, K.M.; Da Silva, R.S.; Bonan, C.D. Zebrafish neurotransmitter systems as potential pharmacological and toxicological targets. Neurotoxicol. Teratol. 2011, 33, 608–617. [Google Scholar] [CrossRef]
- Boix, J.; Cauli, O. Alteration of serotonin system by polychlorinated biphenyls exposure. Neurochem. Int. 2012, 60, 809–816. [Google Scholar] [CrossRef] [PubMed]
- Sallinen, V.; Sundvik, M.; Reenilä, I.; Peitsaro, N.; Khrustalyov, D.; Anichtchik, O.; Toleikyte, G.; Kaslin, J.; Panula, P. Hyperserotonergic phenotype after monoamine oxidase inhibition in larval zebrafish. J. Neurochem. 2009, 109, 403–415. [Google Scholar] [CrossRef]
- Willhite, C.; Sharma, R.P. Acute dieldrin exposure in relation to brain monoamine oxidase activity and concentration of brain serotonin and 5-hydroxyindoleacetic acid. Toxicol. Lett. 1978, 2, 71–75. [Google Scholar] [CrossRef]
- Maximino, C.; Costa, B.P.; Lima, M.G. A Review of Monoaminergic Neuropsychopharmacology in Zebrafish, 6 Years Later: Towards Paradoxes and their Solution. Curr. Psychopharmacol. 2016, 5, 96–138. [Google Scholar] [CrossRef]
- Neuhuber, W.; Wörl, J. Monoamines in the enteric nervous system. Histochem. Cell Biol. 2018, 150, 703–709. [Google Scholar] [CrossRef]
- Heredia, D.J.; Gershon, M.D.; Koh, S.D.; Corrigan, R.D.; Okamoto, T.; Smith, T.K. Important role of mucosal serotonin in colonic propulsion and peristaltic reflexes: In vitro analyses in mice lacking tryptophan hydroxylase 1. J. Physiol. 2013, 591, 5939–5957. [Google Scholar] [CrossRef] [PubMed]
- Magro, F.; Vieira-Coelho, M.A.; Fraga, S.; Serräo, M.P.; Veloso, F.T.; Ribeiro, T.; Soares-da-Silva, P. Impaired synthesis or cellular storage of norepinephrine, dopamine, and 5-hydroxytryptamine in human inflammatory bowel disease. Dig. Dis. Sci. 2002, 47, 216–224. [Google Scholar] [CrossRef]
- Sudo, N. Biogenic Amines: Signals Between Commensal Microbiota and Gut Physiology. Front. Endocrinol. 2019, 10, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Raldúa, D.; Piña, B. In vivo zebrafish assays for analyzing drug toxicity. Expert Opin. Drug Metab. Toxicol. 2014, 10, 685–697. [Google Scholar] [CrossRef]
- Faria, M.; Bedrossiantz, J.; Ramírez, J.R.R.; Mayol, M.; García, G.H.; Bellot, M.; Prats, E.; Garcia-Reyero, N.; Gómez-Canela, C.; Gómez-Oliván, L.M.; et al. Glyphosate targets fish monoaminergic systems leading to oxidative stress and anxiety. Environ. Int. 2021, 146, 106253. [Google Scholar] [CrossRef]
- Kalueff, A.V.; Echevarria, D.J.; Homechaudhuri, S.; Stewart, A.M.; Collier, A.D.; Kaluyeva, A.A.; Li, S.; Liu, Y.; Chen, P.; Wang, J.J.; et al. Zebrafish neurobehavioral phenomics for aquatic neuropharmacology and toxicology research. Aquat. Toxicol. 2016, 170, 297–309. [Google Scholar] [CrossRef]
- Faria, M.; Prats, E.; Ramírez, J.R.R.; Bellot, M.; Bedrossiantz, J.; Pagano, M.; Valls, A.; Gomez-Canela, C.; Porta, J.M.; Mestres, J.; et al. Androgenic activation, impairment of the monoaminergic system and altered behavior in zebrafish larvae exposed to environmental concentrations of fenitrothion. Sci. Total Environ. 2021, 775, 145671. [Google Scholar] [CrossRef]
- Santos-Fandila, A.; Vázquez, E.; Barranco, A.; Zafra-Gómez, A.; Navalón, A.; Rueda, R.; Ramírez, M. Analysis of 17 neurotransmitters, metabolites and precursors in zebrafish through the life cycle using ultrahigh performance liquid chromatography-tandem mass spectrometry. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2015, 1001, 191–201. [Google Scholar] [CrossRef]
- Tufi, S.; Leonards, P.; Lamoree, M.; De Boer, J.; Legler, J.; Legradi, J. Changes in Neurotransmitter Profiles during Early Zebrafish (Danio rerio) Development and after Pesticide Exposure. Environ. Sci. Technol. 2016, 50, 3222–3230. [Google Scholar] [CrossRef] [PubMed]
- Aragon, A.; Legradi, J.; Ballesteros-Gómez, A.; Legler, J.; van Velzen, M.; de Boer, J.; Leonards, P. Determination of monoamine neurotransmitters in zebrafish (Danio rerio) by gas chromatography coupled to mass spectrometry with a two-step derivatization. Anal. Bioanal. Chem. 2017, 409, 2931–2939. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Canela, C.; Tornero-Cañadas, D.; Prats, E.; Piña, B.; Tauler, R.; Raldúa, D. Comprehensive characterization of neurochemicals in three zebrafish chemical models of human acute organophosphorus poisoning using liquid chromatography-tandem mass spectrometry. Anal. Bioanal. Chem. 2018, 410, 1735–1748. [Google Scholar] [CrossRef] [PubMed]
- Prats, E.; Gómez-Canela, C.; Ben-Lulu, S.; Ziv, T.; Padrós, F.; Tornero, D.; Garcia-Reyero, N.; Tauler, R.; Admon, A.; Raldúa, D. Modelling acrylamide acute neurotoxicity in zebrafish larvae. Sci. Rep. 2017, 7. [Google Scholar] [CrossRef]
- Chen, Y.C.; Semenova, S.; Rozov, S.; Sundvik, M.; Bonkowsky, J.L.; Panula, P. A novel developmental role for dopaminergic signaling to specify hypothalamic neurotransmitter identity. J. Biol. Chem. 2016, 291, 21880–21892. [Google Scholar] [CrossRef] [PubMed]
- Buske, C.; Gerlai, R. Early embryonic ethanol exposure impairs shoaling and the dopaminergic and serotoninergic systems in adult zebrafish. Neurotoxicol. Teratol. 2011, 33, 698–707. [Google Scholar] [CrossRef]
- Varga, Z.K.; Pejtsik, D.; Biró, L.; Zsigmond, Á.; Varga, M.; Tóth, B.; Salamon, V.; Annus, T.; Mikics, É.; Aliczki, M. Conserved serotonergic background of experience-dependent behavioral responsiveness in zebrafish (Danio rerio). J. Neurosci. 2020, 40, 4551–4564. [Google Scholar] [CrossRef]
- Faria, M.; Prats, E.; Novoa-Luna, K.A.; Bedrossiantz, J.; Gómez-Canela, C.; Gómez-Oliván, L.M.; Raldúa, D. Development of a vibrational startle response assay for screening environmental pollutants and drugs impairing predator avoidance. Sci. Total Environ. 2019, 650, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Maes, J.; Verlooy, L.; Buenafe, O.E.; de Witte, P.A.M.; Esguerra, C.V.; Crawford, A.D. Evaluation of 14 Organic Solvents and Carriers for Screening Applications in Zebrafish Embryos and Larvae. PLoS ONE 2012, 7, e43850. [Google Scholar] [CrossRef] [PubMed]
- Vliet, S.M.; Ho, T.C.; Volz, D.C. Behavioral screening of the LOPAC1280 library in zebrafish embryos. Toxicol. Appl. Pharmacol. 2017, 329, 241–248. [Google Scholar] [CrossRef]
- Fleming, A.; Diekmann, H.; Goldsmith, P. Functional Characterisation of the Maturation of the Blood-Brain Barrier in Larval Zebrafish. PLoS ONE 2013, 8, e77548. [Google Scholar] [CrossRef]
- Mayol-Cabré, M.; Prats, E.; Raldúa, D.; Gómez-Canela, C. Characterization of monoaminergic neurochemicals in the different brain regions of adult zebrafish. Sci. Total Environ. 2020, 745, 141205. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Kislyuk, S.; Van den Bosch, W.; Adams, E.; de Witte, P.; Cabooter, D. Development of a sensitive and quantitative capillary LC-UV method to study the uptake of pharmaceuticals in zebrafish brain. Anal. Bioanal. Chem. 2018, 410, 2751–2764. [Google Scholar] [CrossRef]
- Njagi, J.; Ball, M.; Best, M.; Wallace, K.N.; Andreescu, S. Electrochemical quantification of serotonin in the live embryonic zebrafish intestine. Anal. Chem. 2010, 82, 1822–1830. [Google Scholar] [CrossRef]
- Roach, G.; Heath Wallace, R.; Cameron, A.; Emrah Ozel, R.; Hongay, C.F.; Baral, R.; Andreescu, S.; Wallace, K.N. Loss of ascl1a prevents secretory cell differentiation within the zebrafish intestinal epithelium resulting in a loss of distal intestinal motility. Dev. Biol. 2013, 376, 171–186. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Anichtchik, O.; Sallinen, V.; Peitsaro, N.; Panula, P. Distinct structure and activity of monoamine oxidase in the brain of zebrafish (Danio rerio). J. Comp. Neurol. 2006, 498, 593–610. [Google Scholar] [CrossRef] [PubMed]
- Aldeco, M.; Arslan, B.K.; Edmondson, D.E. Catalytic and inhibitor binding properties of zebrafish monoamine oxidase (zMAO): Comparisons with human MAO A and MAO B. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2011, 159, 78–83. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bellot, M.; Bartolomé, H.; Faria, M.; Gómez-Canela, C.; Raldúa, D. Differential Modulation of the Central and Peripheral Monoaminergic Neurochemicals by Deprenyl in Zebrafish Larvae. Toxics 2021, 9, 116. https://doi.org/10.3390/toxics9060116
Bellot M, Bartolomé H, Faria M, Gómez-Canela C, Raldúa D. Differential Modulation of the Central and Peripheral Monoaminergic Neurochemicals by Deprenyl in Zebrafish Larvae. Toxics. 2021; 9(6):116. https://doi.org/10.3390/toxics9060116
Chicago/Turabian StyleBellot, Marina, Helena Bartolomé, Melissa Faria, Cristian Gómez-Canela, and Demetrio Raldúa. 2021. "Differential Modulation of the Central and Peripheral Monoaminergic Neurochemicals by Deprenyl in Zebrafish Larvae" Toxics 9, no. 6: 116. https://doi.org/10.3390/toxics9060116
APA StyleBellot, M., Bartolomé, H., Faria, M., Gómez-Canela, C., & Raldúa, D. (2021). Differential Modulation of the Central and Peripheral Monoaminergic Neurochemicals by Deprenyl in Zebrafish Larvae. Toxics, 9(6), 116. https://doi.org/10.3390/toxics9060116







