Coordination Properties of the Fungal Metabolite Harzianic Acid Toward Toxic Heavy Metals
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Their Analysis
2.2. Preparation of Test Solutions, Potentiometric, UV-VIS, CD, NMR, and IR Measurements
2.3. HPLC-MS Analyses
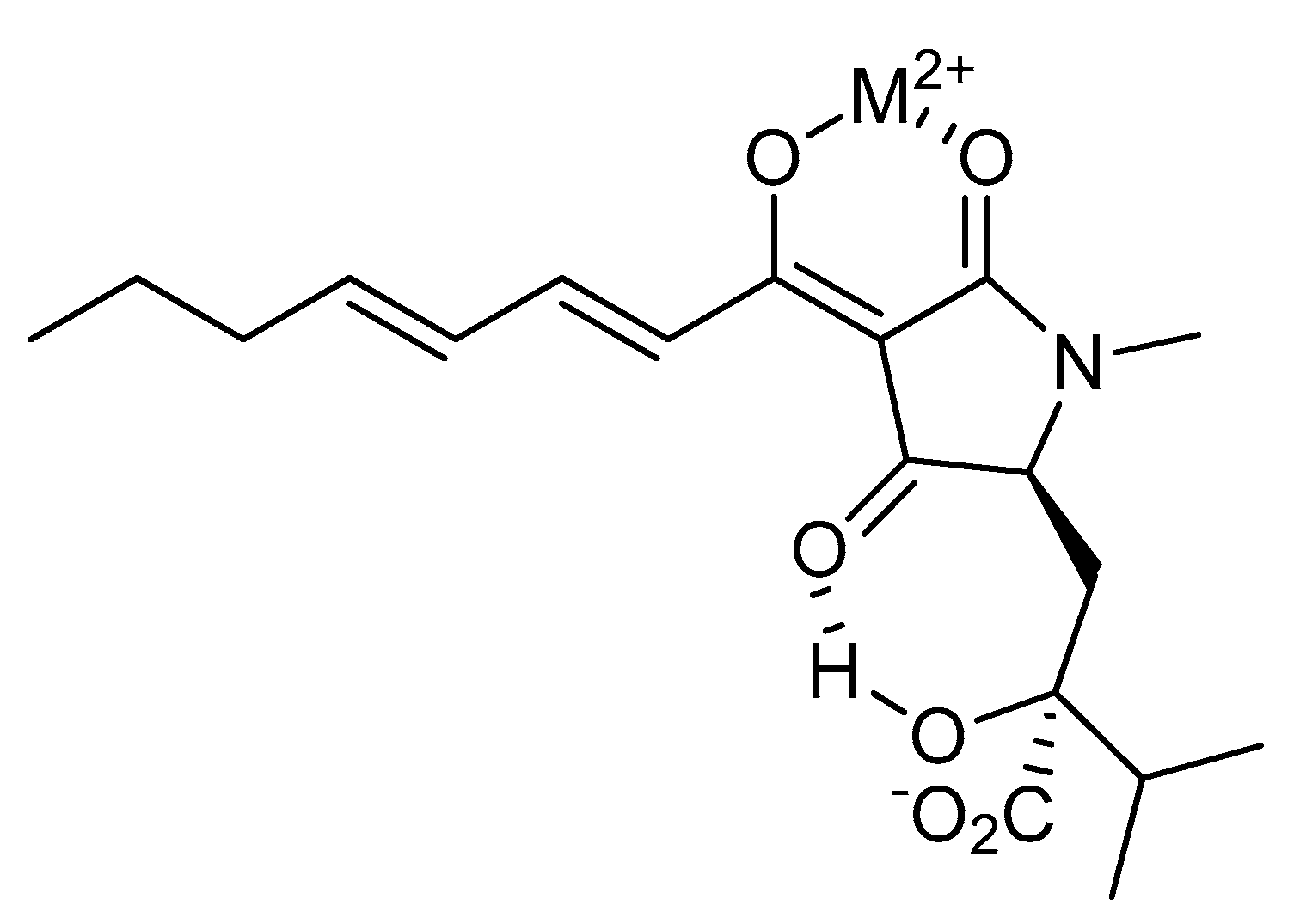
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ali, H.; Khan, E.; Ilahi, I. Environmental chemistry and ecotoxicology of hazardous heavy metals: Environmental persistence, toxicity, and bioaccumulation. J. Chem. 2019. [Google Scholar] [CrossRef]
- Singh, J.; Kalamdhad, A.S. Effects of heavy metals on soil, plants, human health and aquatic life making bricks using variety of solid waste view project anaerobic digestion. Int. J. Res. Chem. Environ. 2011, 1, 15–21. [Google Scholar]
- Jaishankar, M.; Tseten, T.; Anbalagan, N.; Mathew, B.B.; Beeregowda, K.N. Toxicity, mechanism and health effects of some heavy metals. Interdiscip. Toxicology 2014, 7, 60–72. [Google Scholar]
- Nagajyoti, P.C.; Lee, K.D.; Sreekanth, T.V.M. Heavy metals, occurrence and toxicity for plants: A review. Environ. Chem. Lett. 2010, 8, 199–216. [Google Scholar] [CrossRef]
- Pandey, G.; Madhuri, S. Heavy metals causing toxicity in humans, animals and environment. Res. J. Anim. Vet. Fish. Sci. 2014, 2, 17–23. [Google Scholar]
- Das, K.K.; Reddy, R.C.; Bagoji, I.B.; Das, S.; Bagali, S.; Mullur, L.; Khodnapur, J.P.; Biradar, M.S. Primary concept of nickel toxicity—An overview. J. Basic Clin. Physiol. Pharmacol. 2019, 30, 141–152. [Google Scholar] [CrossRef]
- Leyssens, L.; Vinck, B.; Van Der Straeten, C.; Wuyts, F.; Maes, L. Cobalt toxicity in humans—A review of the potential sources and systemic health effects. Toxicology 2017, 387, 43–56. [Google Scholar] [CrossRef]
- Godt, J.; Scheidig, F.; Grosse-Siestrup, C.; Esche, V.; Brandenburg, P.; Reich, A.; Groneberg, D.A. The toxicity of cadmium and resulting hazards for human health. J. Occup. Med. Toxicol. 2006, 1, 1–6. [Google Scholar] [CrossRef]
- Flora, G.; Gupta, D.; Tiwari, A. Toxicity of lead: A review with recent updates. Interdiscip. Toxicology 2012, 5, 47–58. [Google Scholar]
- Tóth, G.; Hermann, T.; Da Silva, M.R.; Montanarella, L. Heavy metals in agricultural soils of the European Union with implications for food safety. Environ. Int. 2016, 88, 299–309. [Google Scholar] [CrossRef]
- Zhang, G.P.; Yao, H.G.; Wu, W.; Xu, M. Genotypic and environmental variation in cadmium, chromium, arsenic, nickel, and lead concentrations in rice grains. J. Zhejiang Univ. Sci. B. 2006, 7, 565–571. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yi, B.; Sun, X.; Yu, L.; Wu, L.; Liu, W.; Wang, D.; Li, Y.; Jia, R.; Yu, H.; et al. Removal and tolerance mechanism of Pb by a filamentous fungus: A case study. Chemosphere 2019, 225, 200–208. [Google Scholar] [CrossRef] [PubMed]
- Fomina, M.; Hillier, S.; Charnock, J.M.; Melville, K.; Alexander, I.J.; Gadd, G.M. Role of oxalic acid overexcretion in transformations of toxic metal minerals by Beauveria caledonica. Appl. Environ. Microbiol. 2005, 71, 371–381. [Google Scholar] [CrossRef] [PubMed]
- Arwidsson, Z.; Johansson, E.; Von Kronhelm, T.; Allard, B.; Van Hees, P. Remediation of metal contaminated soil by organic metabolites from fungi i-production of organic acids. Water Air Soil Pollut. 2010, 205, 215–226. [Google Scholar] [CrossRef]
- Machuca, A.; Pereira, G.; Aguiar, A.; Milagres, A.M.F. Metal-chelating compounds produced by ectomycorrhizal fungi collected from pine plantations. Lett. Appl. Microbiol. 2007, 44, 7–12. [Google Scholar] [CrossRef]
- Renella, G.; Landi, L.; Nannipieri, P. Degradation of low molecular weight organic acids complexed with heavy metals in soil. Geoderma 2004, 122, 311–315. [Google Scholar] [CrossRef]
- Ousmanova, D.; Parker, W. Fungal generation of organic acids for removal of lead from contaminated soil. Water Air Soil Pollut. 2007, 179, 365–380. [Google Scholar] [CrossRef]
- Salvatore, M.M.; Nicoletti, R.; Salvatore, F.; Naviglio, D.; Andolfi, A. GC–MS approaches for the screening of metabolites produced by marine-derived Aspergillus. Mar. Chem. 2018, 206, 19–33. [Google Scholar] [CrossRef]
- Das, A.; Prasad, R.; Srivastava, A.; Giang, P.H.; Bhatnagar, K.; Varma, A. Fungal siderophores: Structure, functions and regolation. In Microbial Siderophores; Springer: Berlin/Heidelberg, Germany, 2007; pp. 1–42. ISBN 978-3-540-71159-9. [Google Scholar]
- Schalk, I.J.; Hannauer, M.; Braud, A. New roles for bacterial siderophores in metal transport and tolerance. Environ. Microbiol. 2011, 13, 2844–2854. [Google Scholar] [CrossRef]
- Ahmed, E.; Holmström, S.J.M. Siderophores in environmental research: Roles and applications. Microb. Biotechnol. 2014, 7, 196–208. [Google Scholar] [CrossRef]
- Khan, A.G.; Kuek, C.; Chaudhry, T.M.; Khoo, C.S.; Hayes, W.J. Role of plants, mycorrhizae and phytochelators in heavy metal contaminated land remediation. Chemosphere 2000, 41, 197–207. [Google Scholar] [CrossRef]
- Schwab, A.P.; Zhu, D.S.; Banks, M.K. Influence of organic acids on the transport of heavy metals in soil. Chemosphere 2008, 72, 986–994. [Google Scholar] [CrossRef]
- Woo, S.L.; Pepe, O. Microbial consortia: Promising probiotics as plant biostimulants for sustainable agriculture. Front. Plant Sci. 2018, 9, 7–12. [Google Scholar] [CrossRef]
- Vinale, F.; Sivasithamparam, K.; Ghisalberti, E.L.; Marra, R.; Woo, S.L.; Lorito, M. Trichoderma-plant-pathogen interactions. Soil Biol. Biochem. 2008, 40, 1–10. [Google Scholar] [CrossRef]
- Harman, G.E.; Howell, C.R.; Viterbo, A.; Chet, I.; Lorito, M. Trichoderma species—Opportunistic, avirulent plant symbionts. Nat. Rev. Microbiol. 2004, 2, 43–56. [Google Scholar] [CrossRef]
- Kredics, L.; Hatvani, L.; Naeimi, S.; Körmöczi, P.; Manczinger, L.; Vágvölgyi, C.; Druzhinina, I. Biodiversity of the Genus Hypocrea/Trichoderma in Different Habitats; Gupta, V.K., Schmoll, M., Herrera-Estrella, A., Upadhyay, R.S., Druzhinina, I., Tuohy, M.G., Eds.; Elsevier: Oxford, UK, 2014; pp. 3–24. ISBN 978-0-444-59576-8. [Google Scholar]
- Nicoletti, R.; Vinale, F. Bioactive compounds from marine-derived Aspergillus, Penicillium, Talaromyces and Trichoderma Species. Mar. Drugs 2018, 16, 408. [Google Scholar] [CrossRef]
- Marra, R.; Nicoletti, R.; Pagano, E.; DellaGreca, M.; Salvatore, M.M.M.M.; Borrelli, F.; Lombardi, N.; Vinale, F.; Woo, S.L.S.L.; Andolfi, A. Inhibitory effect of trichodermanone C, a sorbicillinoid produced by Trichoderma citrinoviride associated to the green alga Cladophora sp., on nitrite production in LPS-stimulated macrophages. Nat. Prod. Res. 2019, 33, 3389–3397. [Google Scholar] [CrossRef]
- Salvatore, M.M.; Nicoletti, R.; DellaGreca, M.; Andolfi, A. Occurrence and properties of thiosilvatins. Mar. Drugs 2019, 17, 664. [Google Scholar] [CrossRef]
- Vinale, F.; Sivasithamparam, K.; Ghisalberti, E.L.; Ruocco, M.; Woo, S.; Lorito, M. Trichoderma secondary metabolites that affect plant metabolism. Nat. Prod. Commun. 2012, 7, 1545–1550. [Google Scholar] [CrossRef]
- Vinale, F.; Sivasithamparam, K. Beneficial effects of Trichoderma secondary metabolites on crops. Phyther. Res. 2020, 2835–2842. [Google Scholar] [CrossRef]
- Vinale, F.; Nicoletti, R.; Borrelli, F.; Mangoni, A.; Parisi, O.A.; Marra, R.; Lombardi, N.; Lacatena, F.; Grauso, L.; Finizio, S.; et al. Co-culture of plant beneficial microbes as source of bioactive metabolites. Sci. Rep. 2017, 7, 1–12. [Google Scholar] [CrossRef]
- Vinale, F.; Nigro, M.; Sivasithamparam, K.; Flematti, G.; Ghisalberti, E.L.; Ruocco, M.; Varlese, R.; Marra, R.; Lanzuise, S.; Eid, A.; et al. Harzianic acid: A novel siderophore from Trichoderma harzianum. FEMS Microbiol. Lett. 2013, 347, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Vinale, F.; Flematti, G.; Sivasithamparam, K.; Lorito, M.; Marra, R.; Skelton, B.W.; Ghisalberti, E.L. Harzianic acid, an antifungal and plant growth promoting metabolite from Trichoderma harzianum. J. Nat. Prod. 2009, 72, 2032–2035. [Google Scholar] [CrossRef] [PubMed]
- Manganiello, G.; Sacco, A.; Ercolano, M.R.; Vinale, F.; Lanzuise, S.; Pascale, A.; Napolitano, M.; Lombardi, N.; Lorito, M.; Woo, S.L. Modulation of tomato response to rhizoctonia solani by Trichoderma harzianum and its secondary metabolite harzianic acid. Front. Microbiol. 2018, 9, 1966. [Google Scholar] [CrossRef] [PubMed]
- De Filippis, A.; Nocera, F.P.; Tafuri, S.; Ciani, F.; Staropoli, A.; Comite, E.; Bottiglieri, A.; Gioia, L.; Lorito, M.; Woo, S.L.; et al. Antimicrobial activity of harzianic acid against Staphylococcus pseudintermedius. Nat. Prod. Res. 2020, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Healy, A.R.; Vinale, F.; Lorito, M.; Westwood, N.J. Total synthesis and biological evaluation of the tetramic acid based natural product harzianic acid and its stereoisomers. Org. Lett. 2015, 17, 692–695. [Google Scholar] [CrossRef] [PubMed]
- Mo, X.; Li, Q.; Ju, J. Naturally occurring tetramic acid products: Isolation, structure elucidation and biological activity. RSC Adv. 2014, 4, 50566–50593. [Google Scholar] [CrossRef]
- De Tommaso, G.; Salvatore, M.M.; Nicoletti, R.; DellaGreca, M.; Vinale, F.; Bottiglieri, A.; Staropoli, A.; Salvatore, F.; Lorito, M.; Iuliano, M.; et al. Bivalent metal-chelating properties of harzianic acid produced by Trichoderma pleuroticola associated to the gastropod Melarhaphe neritoides. Molecules 2020, 25, 2147. [Google Scholar] [CrossRef]
- Meloun, M.; Havel, J.; Högfeldt, E. Computation of Solution Equilibria a Guide to Methods in Potentiometry, Extraction and Spectrophotometry; John Wiley & Sons: New York, NY, USA, 1988; ISBN 0-470-20975-5. [Google Scholar]
- Gans, P.; Sabatini, A.; Vacca, A. Investigation of equilibria in solution. Determination of equilibrium constants with the HYPERQUAD suite of programs. Talanta 1996, 43, 1739–1753. [Google Scholar] [CrossRef]
- Ohtaki, H.; Tanaka, N. Ionic equilibria in mixed solvents. VI. Dissociation constants of aliphatic diamines in water-methanol solutions. J. Phys. Chem. 1971, 75, 90–92. [Google Scholar] [CrossRef]
- Matsui, H.; Ohtaki, H. Ionic equilibria in mixed solvents. XII. Hydrolysis of cadmium (II) ion in dioxane–water and methanol–water mixtures. Bull. Chem. Soc. Jpn. 1977, 50. [Google Scholar] [CrossRef]
- Baes, C.F.; Mesmer, R.S. The hydrolysis of cations. In Berichte der Bunsengesellschaft für Physikalische Chemie; John Wiley & Sons: New York, NY, USA, 1977; pp. 245–246. [Google Scholar]
- Martell, A..; Smith, R.M. Critical Stability Constants; Springer: New York, NY, USA, 1989; ISBN 978-1-4615-6766-0. [Google Scholar]
- Kaufmann, G.F.; Sartorio, R.; Lee, S.H.; Rogers, C.J.; Meijler, M.M.; Moss, J.A.; Clapham, B.; Brogan, A.P.; Dickerson, T.J.; Janda, K.D. Revisiting quorum sensing: Discovery of additional chemical and biological functions for 3-oxo-N-acylhomoserine lactones. Proc. Natl. Acad. Sci. USA 2005, 102, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Zaghouani, M.; Nay, B. 3-Acylated tetramic and tetronic acids as natural metal binders: Myth or reality? Nat. Prod. Rep. 2016, 33, 540–548. [Google Scholar] [CrossRef] [PubMed]
- Markopoulou, O.; Markopoulos, J.; Nicholls, D. Synthesis of 3-butanoyl- and 3-benzoyl-4-hydroxy-3-pyrrolin-2-ones and their complexes with metal ions. J. Inorg. Biochem. 1990, 39, 307–316. [Google Scholar] [CrossRef]
- Heaton, B.T.; Jacob, C.; Sampanthar, J.T. Rhodium carbonyl complexes containing pyridine; crystal structure of an unusual octahedral rhodium(I) complex [Rh2(μ-CO)3Cl2(py)4]. J. Chem. Soc. Dalt. Trans. 1998, 2, 1403–1410. [Google Scholar] [CrossRef]
- Burgess, J.; Rangel, M. Hydroxypyranones, hydroxypyridinones, and their complexes. In Advances in Inorganic Chemistry; Accademic Press: London, UK, 2008; pp. 167–243. ISBN 978-0-12-373977-3. [Google Scholar]
- Al Alousi, A.S.; Shehata, M.R.; Shoukry, M.M.; Hassan, S.A.; Mahmoud, N. Coordination properties of dehydroacetic acid—Binary and ternary complexes. J. Coord. Chem. 2008, 61, 1906–1916. [Google Scholar] [CrossRef]
- Pinto, I.S.S.; Neto, I.F.F.; Soares, H.M.V.M. Biodegradable chelating agents for industrial, domestic, and agricultural applications—A review. Environ. Sci. Pollut. Res. 2014, 21, 11893–11906. [Google Scholar] [CrossRef]
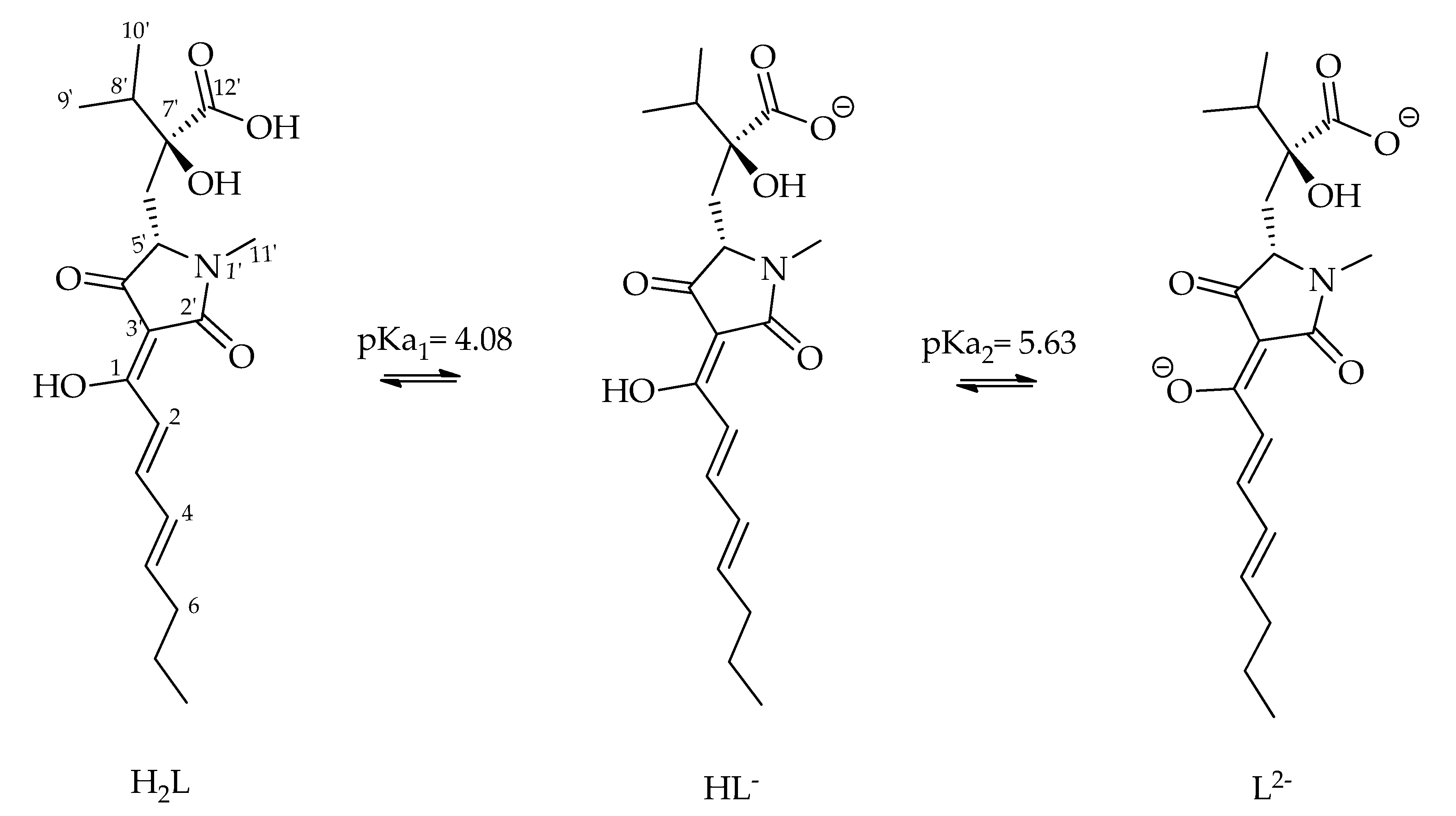

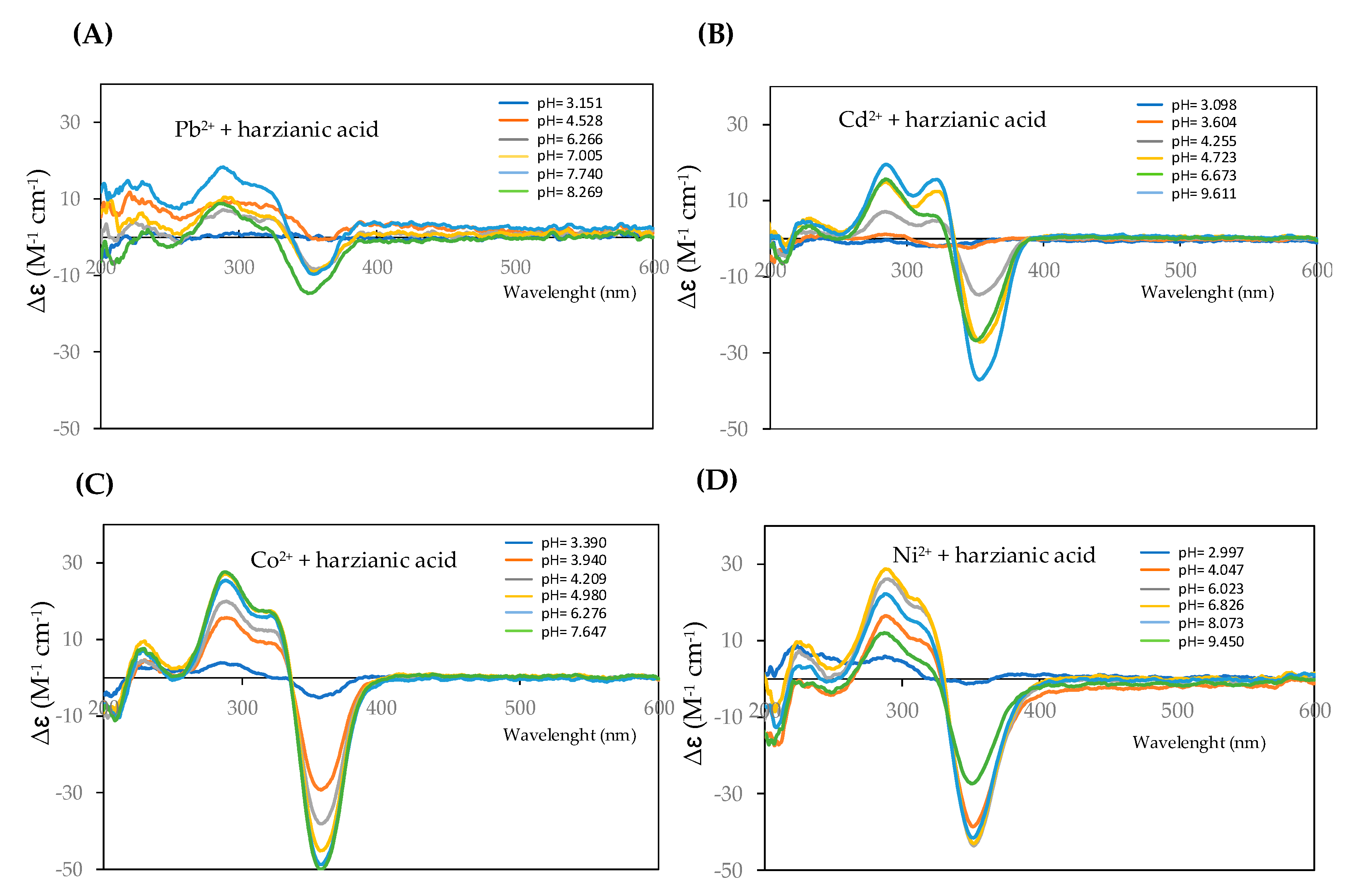
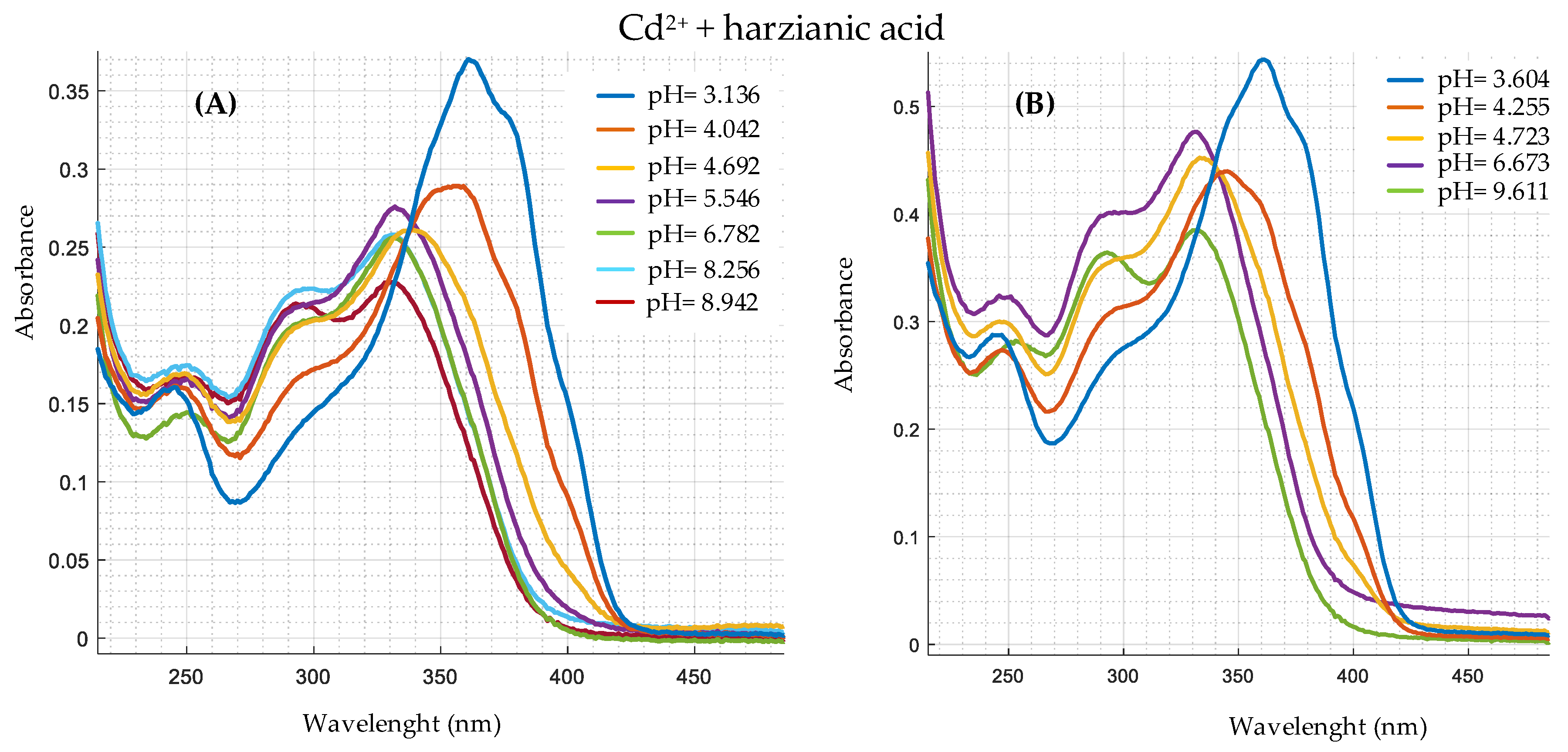

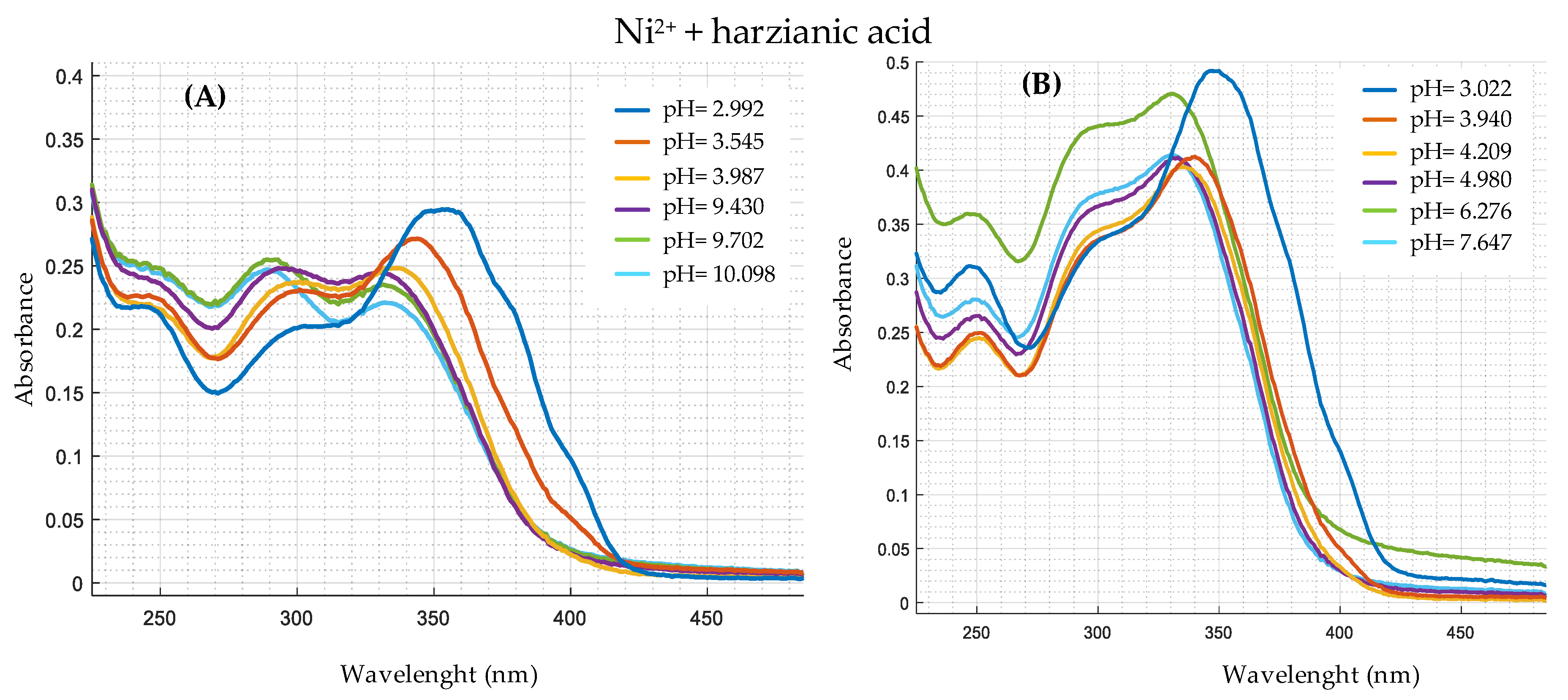
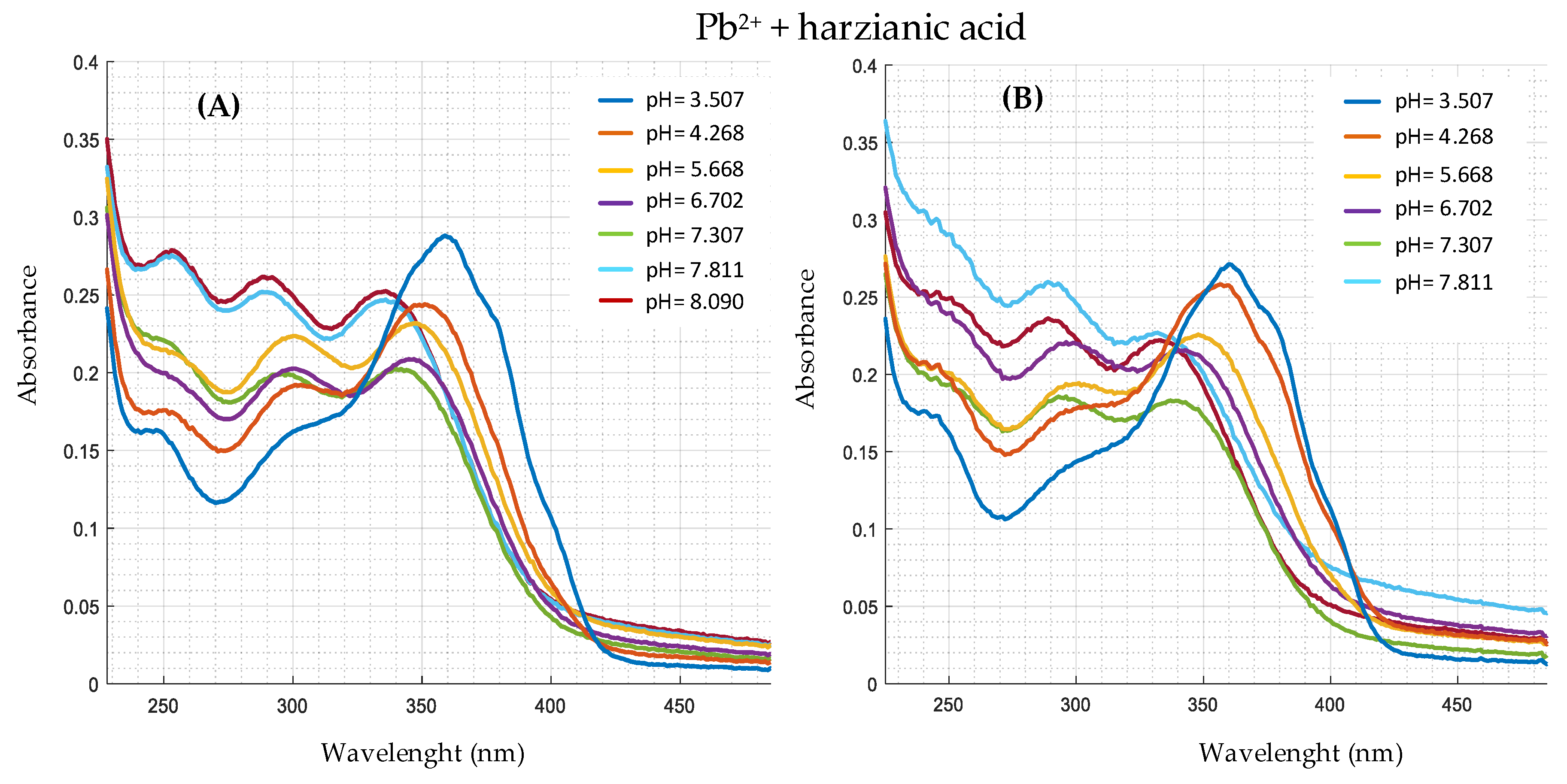

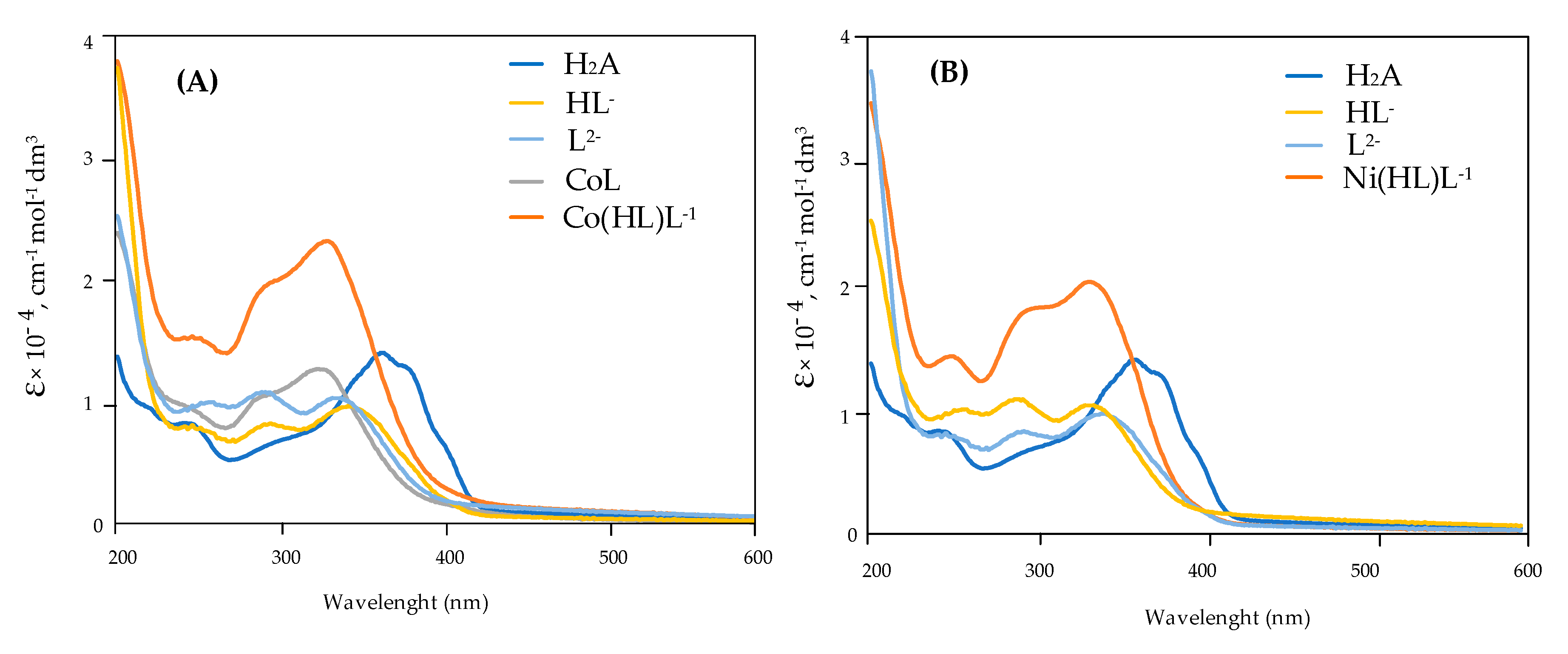
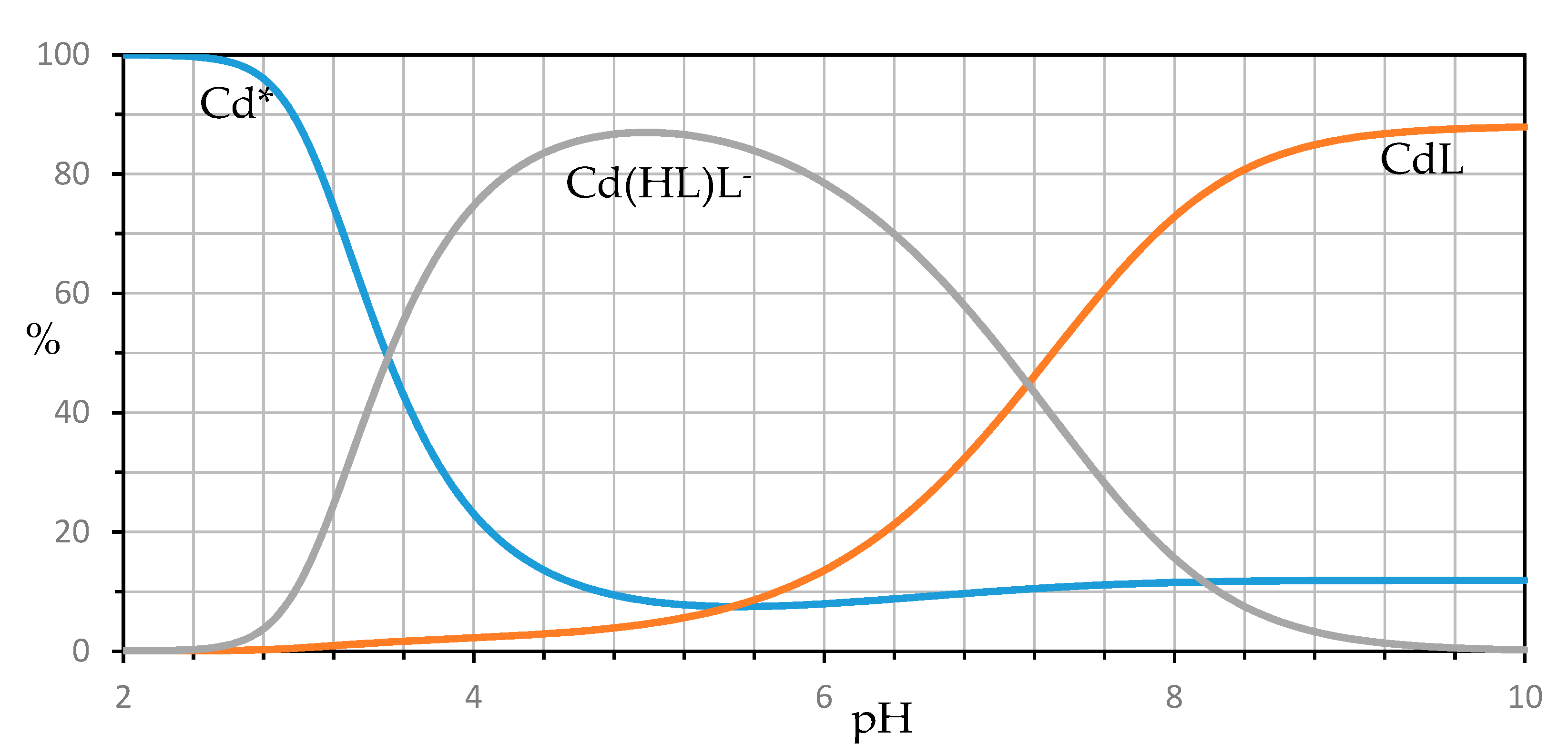
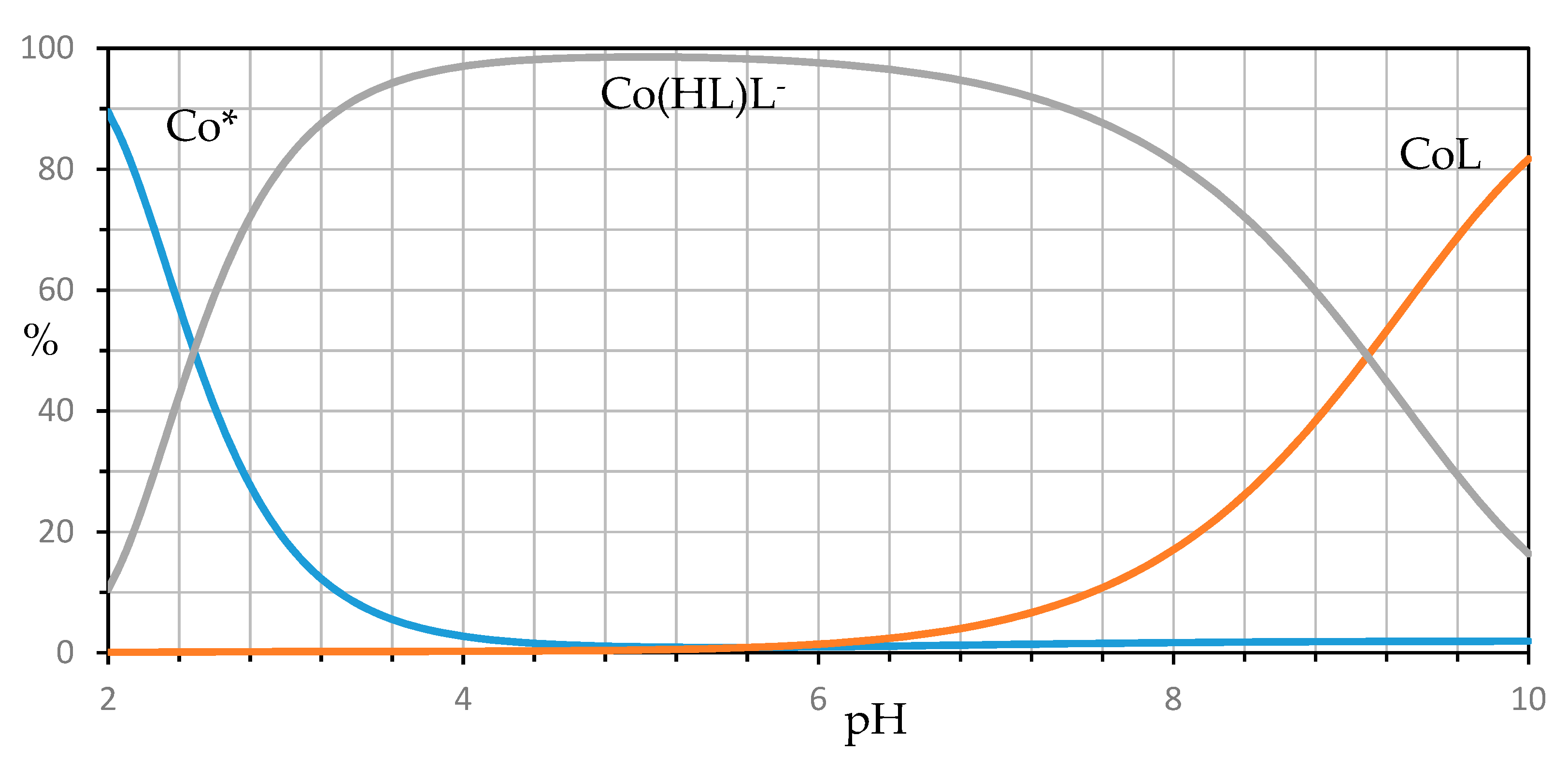
| Ion | Experimental Mass | Formula | Exact Mass |
|---|---|---|---|
| Harzianic Acid + Cd(ClO4)2 | |||
| [H2L + H]+ | 366.1915 | C19H28NO6 | 366.1917 |
| [H2L + Cd + ClO4]+ | 578.0335 | C19H27NO10CdCl | 578.0331 |
| [2H2L − H + Cd]+ | 843.2624 | C38H53N2O12Cd | 843.2632 |
| Harzianic Acid + CoCl2 | |||
| [H2L + H]+ | 366.1913 | C19H28NO6 | 366.1917 |
| [H2L + Na]+ | 388.1721 | C19H27NO6Na | 388.1736 |
| [2H2L − H + Co]+ | 788.2910 | C38H53N2O12Co | 788.2931 |
| Harzianic Acid + NiCl2 | |||
| [H2L + H]+ | 366.1910 | C19H28NO6 | 366.1917 |
| [H2L + Na]+ | 388.1762 | C19H27NO6Na | 388.1736 |
| [2H2L − H + Ni]+ | 787.2938 | C38H53N2O12Ni | 787.2952 |
| Harzianic acid + Pb(ClO4)2 | |||
| [H2L + H]+ | 366.1912 | C19H28NO6 | 366.1917 |
| [H2L − H + Pb]+ | 572.1507 | C19H26NO6Pb | 572.1526 |
| [2H2L − H + Pb]+ | 937.3326 | C38H53N2O12Pb | 937.3365 |
| M2+ | Reaction | Log (Formation Constant) ± 3σ |
|---|---|---|
| Cd2+ | Cd2+ + L2− ⇌ CdL | 3.82 ± 0.19 |
| Cd2+ + HL− + L2− ⇌ Cd(HL)L− | 9.13 ± 0.23 | |
| Co2+ | Co2+ + L2− ⇌ CoL | 4.70 ± 0.12 |
| Co2+ + HL− + L2−⇌ Co(HL)L− | 11.93 ± 0.06 | |
| Ni2+ | Ni2+ + HL− + L2− ⇌ Ni(HL)L− | 11.85 ± 0.12 |
| Pb2+ | Pb2+ + L2− ⇌ PbL | 4.25 ± 0.5 |
| Pb2+ + HL− + L2− ⇌ Pb(HL)L− | 10.77 ± 0.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tommaso, G.D.; Salvatore, M.M.; Nicoletti, R.; DellaGreca, M.; Vinale, F.; Staropoli, A.; Salvatore, F.; Lorito, M.; Iuliano, M.; Andolfi, A. Coordination Properties of the Fungal Metabolite Harzianic Acid Toward Toxic Heavy Metals. Toxics 2021, 9, 19. https://doi.org/10.3390/toxics9020019
Tommaso GD, Salvatore MM, Nicoletti R, DellaGreca M, Vinale F, Staropoli A, Salvatore F, Lorito M, Iuliano M, Andolfi A. Coordination Properties of the Fungal Metabolite Harzianic Acid Toward Toxic Heavy Metals. Toxics. 2021; 9(2):19. https://doi.org/10.3390/toxics9020019
Chicago/Turabian StyleTommaso, Gaetano De, Maria Michela Salvatore, Rosario Nicoletti, Marina DellaGreca, Francesco Vinale, Alessia Staropoli, Francesco Salvatore, Matteo Lorito, Mauro Iuliano, and Anna Andolfi. 2021. "Coordination Properties of the Fungal Metabolite Harzianic Acid Toward Toxic Heavy Metals" Toxics 9, no. 2: 19. https://doi.org/10.3390/toxics9020019
APA StyleTommaso, G. D., Salvatore, M. M., Nicoletti, R., DellaGreca, M., Vinale, F., Staropoli, A., Salvatore, F., Lorito, M., Iuliano, M., & Andolfi, A. (2021). Coordination Properties of the Fungal Metabolite Harzianic Acid Toward Toxic Heavy Metals. Toxics, 9(2), 19. https://doi.org/10.3390/toxics9020019











