Sublethal Effects of Chlorantraniliprole on Spodoptera litura (Lepidoptera: Noctuidae) Moth: Implication for Attract-And-Kill Strategy
Abstract
1. Introduction
2. Materials and Methods
2.1. Insect and Insecticide
2.2. Bioassay of S. litura Moths in the Laboratory
2.3. Sublethal Effects on Reproduction
2.4. Sublethal Effects on Traits of Offspring
2.5. Data Analysis
3. Results
3.1. Insecticide Toxicity to S. litura Moths
3.2. Lethal Effects of Chlorantraniliprole on S. litura Moths
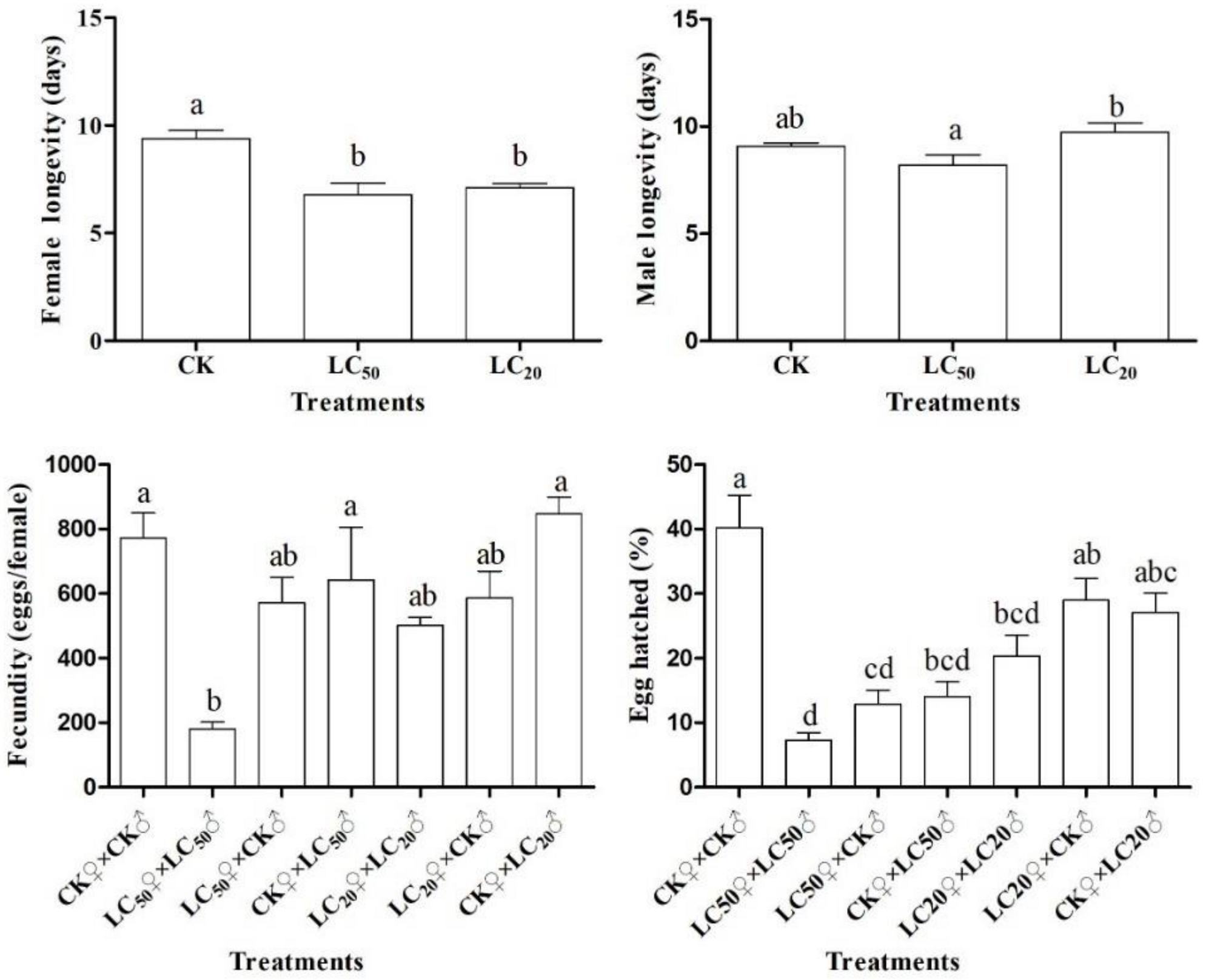
3.3. Adult Reproduction
3.4. F1 Generation Developmental Duration
3.5. Life History Parameters
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ahmad, M.; Arif, M.I.; Ahmad, M. Occurrence of insecticide resistance in field populations of Spodoptera litura (Lepidoptera: Noctuidae) in Pakistan. Crop Prot. 2007, 26, 809–817. [Google Scholar] [CrossRef]
- CABI. Invasive Species Compendium: Spodoptera litura (Taro Caterpillar). Available online: https://www.cabi.org/isc/datasheet/44520 (accessed on 12 October 2019).
- Su, J.Y.; Lai, T.C.; Li, J. Susceptibility of field populations of Spodoptera litura (Fabricius) (Lepidoptera: Noctuidae) in China to chlorantraniliprole and the activities of detoxification enzymes. Crop Prot. 2012, 42, 217–222. [Google Scholar] [CrossRef]
- Huang, Q.; Wang, X.G.; Yao, X.G.; Gong, C.W.; Shen, L.T. Effects of bistrifluron resistance on the biological traits of Spodoptera litura (Fab.) (Noctuidae: Lepidoptera). Ecotoxicology 2019, 28, 323–332. [Google Scholar] [CrossRef]
- Wu, R.Z. A survey on the peanut noctuids in Kwangtung province. Acta Entomol. Sin. 1977, 20, 445–450. [Google Scholar]
- Yao, W.H. Biology characteristics of Prodenia litura. Entomol. J. East China 2005, 14, 122–127. [Google Scholar]
- Qin, H.G.; Ye, Z.H. Occurrence and Management of the Common Cutworm, Spodoptera Litura in China; Agricultural Science and Technology Press: Beijing, China, 2007. [Google Scholar]
- Armes, N.J.; Wightman, J.A.; Jadhav, D.R.; Rao, G.V.R. Status of insecticide resistance in Spodoptera litura in Andhra Pradesh, India. Pest Manag. Sci. 2015, 50, 240–248. [Google Scholar] [CrossRef]
- Zhang, P.; Gao, M.; Mu, W.; Zhou, C.; Li, X.H. Resistant levels of Spodoptera exigua to eight various insecticides in Shandong, China. J. Pestic. Sci. 2014, 39, 7–13. [Google Scholar] [CrossRef]
- Gregg, P.C.; Del Socorro, A.P.; Landolt, P.J. Advances in attract-and-kill for agricultural pests: Beyond pheromones. Annu. Rev. Entomol. 2018, 63, 453–470. [Google Scholar] [CrossRef]
- Su, J.W.; Fan, W.M.; Wang, H.T.; Xuan, W.J.; Sheng, C.F. Technology system for adult controlling of pest insect. Entoml. Knowl. 2001, 38, 405–409. [Google Scholar]
- Lu, Y.F.; Kan, H.L.; Li, L.L.; Zhuang, Q.Y.; Men, X.Y.; Guo, W.X.; Yu, Y. Preliminary evaluation of the monitoring and trapping efficacy of biological food attractant on Noctuidae adults in peanut fields in Junan county, Shandong province. Plant Prot. 2020, 46, 248–253. [Google Scholar]
- Ma, X.F.; Yao, G.X.; Li, J.J. Control effect of tobacco-leaf moth attractant against Lepidopterous pests in tobacco field. J. Anhui Agri. Sci. 2013, 41, 6697–6698. [Google Scholar]
- Wang, Y.H.; Liu, M.J.; Zhang, L.; Zhang, Z.R. New technology of monitoring and control of Lepidoptera pests in soybean fields. J. Anhui Agri. Sci. 2016, 44, 149–151. [Google Scholar]
- Kong, D.S.; Sun, M.H.; Zhao, Y.L.; Xu, L.; Hui, X.H.; Qu, M.J.; Lu, X.T. Control effect and benefit analysis of sex attractant and biological food attractant on cotton bollworm in peanut field. Shandong Agric. Sci. 2016, 48, 102–105. [Google Scholar]
- Gregg, P.C.; Del Socorro, A.P.; Henderson, G.S. Development of a synthetic plant volatile-based attracticide for female noctuid moth. II. Bioassays of synthetic plant volatiles as attractants for the adults of the cotton bollworm, Helicoverpa armigera (Hübner) (Lepidoptera: Noctuidae). Aust. J. Entomol. 2010, 49, 31–39. [Google Scholar] [CrossRef]
- Farré, M.; Fernandez, J.; Paez, M.; Granada, L.; Barba, L.; Gutierrez, H.M.; Pulgarin, C.; Barceló, D. Analysis and toxicity of methomyl and ametryn after biodegradation. Anal. Bioanal. Chem. 2002, 373, 704–709. [Google Scholar] [CrossRef]
- Liu, D.; Jia, Z.Q.; Peng, Y.C.; Sheng, C.W.; Tang, T.; Xu, L.; Han, Z.J.; Zhao, C.Q. Toxicity and sublethal effects of fluralaner of Spodoptera litura Fabricius (Lepidoptera: Noctuidae). Pestic. Biochem. Physiol. 2018, 152, 8–16. [Google Scholar] [CrossRef]
- Maqsood, S.; Sabri, M.A.; Ali, A.; Abbas, M.; Aziz, A. Comparative toxicity of some insecticides against army worm, Spodoptera litura L. (Lepidoptera: Noctuidae) under laboratory conditions. J. Entomol. Zool. Stud. 2016, 5, 770–773. [Google Scholar]
- Jameel, M.; Jamal, K. Efficacy of sub lethal concentration of flubendiamide against larvae of Spodoptera litura (Fab.) (Lepidoptera: Noctuidae). J. Entomol. Zool. Stud. 2017, 5, 670–674. [Google Scholar]
- Lutz, A.L.; Bertolaccini, I.; Scotta, R.R.; Curis, M.C.; Favaro, M.A.; Fernandez, L.N.; Sánchez, D.E. Lethal and sublethal effects of chlorantraniliprole on Spodoptera cosmioides (Lepidoptera: Noctuidae). Pest Manag. Sci. 2018, 74, 2817–2821. [Google Scholar] [CrossRef]
- Desneux, N.; Decourtye, A.; Delpuech, J.M. The sublethal effects of pesticides on beneficial arthropods. Annu. Rev. Entomol. 2007, 52, 81–106. [Google Scholar] [CrossRef]
- Liang, G.M.; Wu, K.M.; Yu, H.K.; Li, K.K.; Feng, X.; Guo, Y.Y. Changes of inheritance mode and fitness in Helicoverpa armigera (Hübner) (Lepidoptera: Noctuidae) along with its resistance evolution to Cry1Ac toxin. J. Invertebr. Pathol. 2008, 97, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Gu, S.H.; Wu, K.M.; Guo, Y.Y.; Pickett, J.A.; Field, L.M.; Zhou, J.J.; Zhang, Y.J. Identification of genes expressed in the sex pheromone gland of the black cutworm Agrotis ipsilon with putative roles in sex pheromone biosynthesis and transport. BMC Genom. 2013, 14, 636. [Google Scholar] [CrossRef] [PubMed]
- SPSS Incorporation. SPSS 13.0 for the Windows; SPSS Inc.: Chicago, IL, USA, 2006. [Google Scholar]
- Chi, H.; You, M.S.; Atlihan, R.; Smith, C.L.; Kavousi, A.; Özgökçe, M.S.; Liu, T.X. Age-stage, two-sex life table: An introduction to theory, data analysis, and application. Entomol. Gen. 2020, 40, 102–124. [Google Scholar] [CrossRef]
- Huang, Y.B.; Chi, H. Life tables of Bactrocera cucurbitae (Diptera: Tephritidae): With an invalidation of the jackknife technique. J. Appl. Entomol. 2013, 137, 327–339. [Google Scholar] [CrossRef]
- Swift, M.L. GraphPad prism, data analysis, and scientific graphing. J. Chem. Inf. Comp. Sci. 1997, 37, 411–412. [Google Scholar] [CrossRef]
- Mensah, R.K.; Gregg, P.C.; Del Socorro, A.P.; Moore, C.J.; Hawes, A.J.; Watts, N. Integrated pest management in cotton: Exploiting behaviour-modifying (semiochemical) compounds for managing cotton pests. Crop Pasture Sci. 2013, 64, 763–773. [Google Scholar] [CrossRef]
- Gregg, P.C.; Del Socorro, A.P.; Hawes, A.J.; Binns, M.R. Developing bisexual attract-and-kill for polyphagous insects: Ecological rationale versus pragmatics. J. Chem. Ecol. 2016, 42, 666–675. [Google Scholar] [CrossRef] [PubMed]
- Saeed, Q.; Saleem, M.A.; Ahmad, M. Toxicity of Some Commonly Used Synthetic Insecticides against Spodoptera exigua (Fab) (Lepidoptera: Noctuidae). Pak. J. Zool. 2012, 44, 1197–1201. [Google Scholar]
- Khan, R.A.; Rashid, M.; Wang, D.; Zhang, Y.L. Lethal and sublethal effects of cantharidin on the life history traits and population parameters of Helicoverpa armigera (Hübner) (Lepidoptera: Noctuidae). Pest Manag. Sci. 2014, 70, 39–45. [Google Scholar] [CrossRef]
- Han, W.S.; Zhang, S.F.; Shen, F.Y.; Liu, M.; Ren, C.C.; Gao, X.W. Residual toxicity and sublethal effects of chlorantraniliprole on Plutella xylostella (Lepidoptera: Plutellidae). Pest Manag. Sci. 2012, 68, 1184–1190. [Google Scholar] [CrossRef]
- Dong, J.; Wang, K.; Li, Y.; Wang, S.L. Lethal and sublethal effects of cyantraniliprole on Helicoverpa assulta (Lepidoptera: Noctuidae). Pestic. Biochem. Physiol. 2017, 136, 58–63. [Google Scholar] [CrossRef]
- Lahm, G.P.; Selby, T.P.; Freudenberger, J.H.; Stevenson, T.M.; Myers, B.J.; Seburyamo, G.; Cordova, D. Insecticidal anthranilic diamides: A new class of potent ryanodine receptor activators. Bioorgan. Med. Chem. 2005, 15, 4898–4906. [Google Scholar] [CrossRef] [PubMed]
- Xie, S.H.; Liang, Y.P.; Lin, Z.F.; Li, H.; Ji, X.C. The toxicity and control efficiency of 9 insecticides to Spodoptera litura. Plant Prot. 2010, 36, 175–177. [Google Scholar]
- Leonova, I.N.; Slynko, N.M. Comparative study of insecticide susceptibility and activities of detoxifying enzymes in larvae and adults of cotton bollworm. Insect Biochem. Physiol. 1996, 32, 157–172. [Google Scholar] [CrossRef]
- Muthusamy, R.; Vishnupriya, M.; Shivakumar, M.S. Biochemical mechanism of chlorantraniliprole resistance in Spodoptera litura (Fab) (Lepidoptera: Noctuidae). J. Asia-Pac. Entomol. 2014, 17, 865–869. [Google Scholar] [CrossRef]
- Carpenter, J.E.; Chandler, L.D. Effects of Sublethal Doses of Two Insect Growth Regulators on Helicoverpa zea (Lepidoptera: Noctuidae) reproduction. J. Entomol. Sci. 1994, 29, 428–435. [Google Scholar] [CrossRef]
- Biddinger, D.; Hull, L.; Huang, H.; McPheron, B.; Loyer, M. Sublethal effects of chronic exposure to tebufenozide on the development, survival, and reproduction of the tufted apple bud moth (Lepidoptera: Tortricidae). J. Econ. Entomol. 2006, 99, 834–842. [Google Scholar] [CrossRef]
- López, J.D., Jr.; Latheef, M.A.; Hoffmann, W.C. Mortality and reproductive effects of ingested spinosad on adult bollworms. Pest Manag. Sci. 2011, 67, 220–225. [Google Scholar] [CrossRef]
- Quan, L.F.; Qiu, G.S.; Sun, L.N.; Li, Y.Y.; Yan, W.T.; Yue, Q.; Zhang, H.J. Sublethal effects of bate-cypermethrin on Carposina sasakii Walsingham (Lepidoptera: Carposinidae). Acta Entomol. Sin. 2017, 60, 799–808. [Google Scholar]
- Dixon, A.F.G. Parthenogenetic reproduction and the rate of increase in aphids. In Aphids. Their Biology, Natural Enemies and Control; Minks, A.K., Harrewijn, P., Eds.; Elsevier: Amsterdam, The Netherlands, 1987; Volume A, pp. 269–287. [Google Scholar]
- Stark, J.D.; Tanigoshi, L.; Bounfour, M.; Antonelli, A. Reproductive potential, its influence on the susceptibility of the species to pesticides. Ecotoxicol. Environ. Saf. 1997, 37, 273–279. [Google Scholar] [CrossRef]
- Walthall, W.K.; Stark, J.D. A comparison of acute mortality and population growth rate as endpoints of toxicological effect. Ecotoxicol. Environ. Saf. 1997, 37, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Forbes, V.E.; Calow, P. Is the per capita rate of increase a good measure of population-level effects in ecotoxicology? Environ. Toxicol. Chem. 1999, 18, 1544–1556. [Google Scholar] [CrossRef]
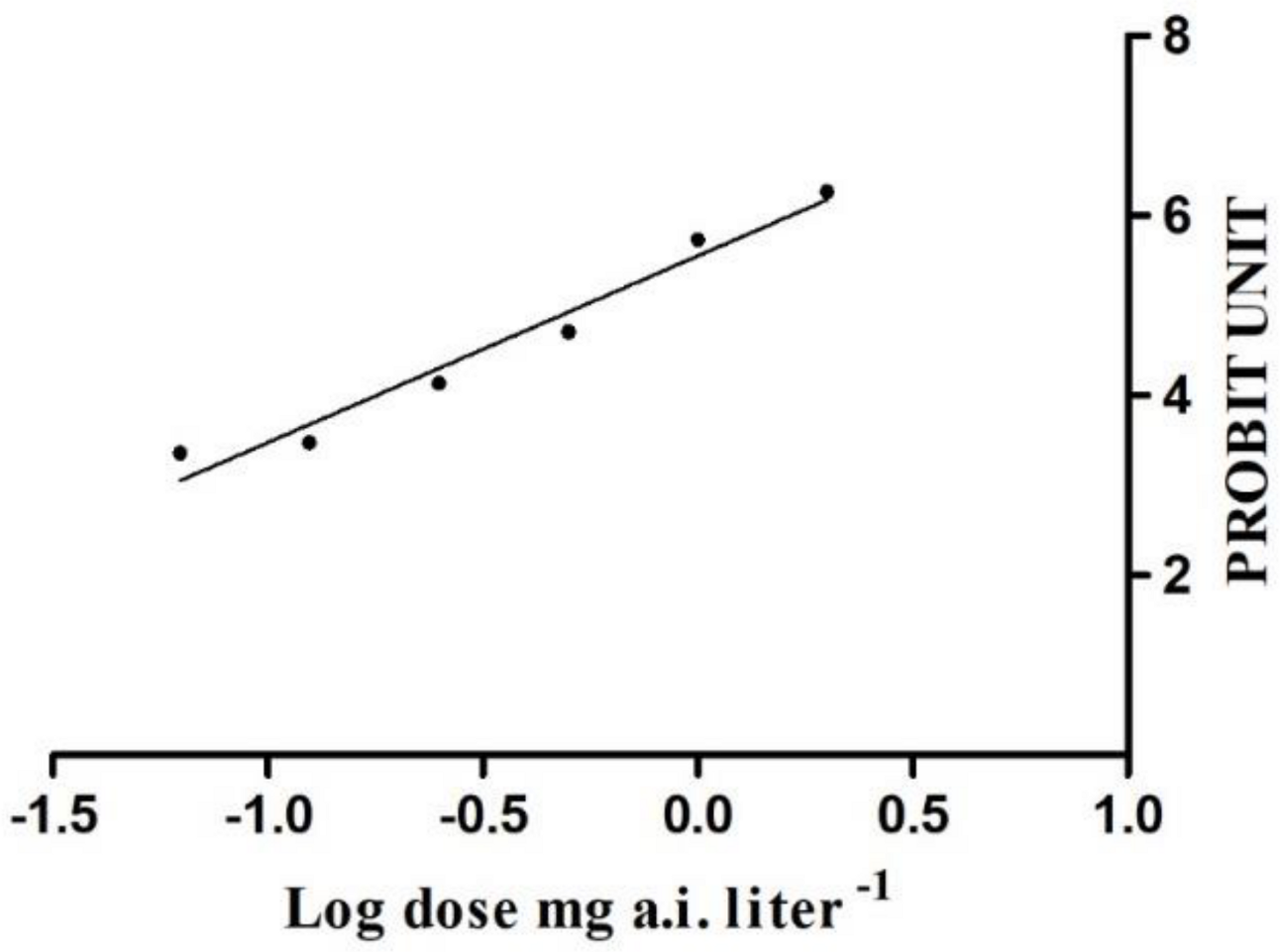
| Insecticide | Slope ± SE | LC50 (mg a.i. Liter−1) | 95% Fiducial Limits | R2 (df) | p |
|---|---|---|---|---|---|
| Chlorantraniliprole | 2.126 ± 0.170 | 0.56 | 0.48 ~ 0.67 | 20.506 (16) | 0.1983 |
| Flubendiamide | 2.392 ± 0.237 | 3.85 | 3.25 ~ 4.53 | 6.314 (13) | 0.934 |
| Emamectin benzoate | 1.962 ± 0.179 | 6.03 | 5.01 ~ 7.23 | 7.535 (16) | 0.9615 |
| Fenpropathrin | 2.153 ± 0.226 | 7.31 | 6.12 ~ 8.88 | 4.187 (13) | 0.989 |
| Chlorpyrifos | 1.814 ± 0.170 | 13.29 | 10.88 ~ 16.09 | 4.106 (16) | 0.9987 |
| Fenvalerate | 1.714 ± 0.206 | 16.57 | 13.02 ~ 20.47 | 5.275 (13) | 0.9686 |
| Indoxacarb | 2.224 ± 0.231 | 17.36 | 14.42 ~ 20.63 | 8.300 (13) | 0.8234 |
| Lambda-cyhalothrin | 1.691 ± 0.163 | 28.12 | 22.94 ~ 34.48 | 7.432 (16) | 0.964 |
| Beta cypermethrin | 1.681 ± 0.162 | 33.83 | 23.34 ~ 51.08 | 44.476 (16) | 0.0002 |
| Thiodicarb | 2.362 ± 0.235 | 41.16 | 34.72 ~ 48.54 | 5.284 (13) | 0.9684 |
| Avermectin | >100 | ||||
| Spinetoram | >100 | ||||
| Spinosad | >100 | ||||
| Carbosulfan | >100 | ||||
| Chlorfenapyr | >100 |
| Treatment | Development | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1st Instar (Days) | 2nd Instar (Days) | 3rd Instar (Days) | 4th Instar (Days) | 5th Instar (Days) | 6th Instar (Days) | Pupa Weight (mg) | Adult Emergence (%) | Larval Mortality (%) | |
| CK♀ × CK♂ | 3.36 ± 0.07 d | 2.69 ± 0.08 d | 2.95 ± 0.10 b | 3.00 ± 0.11 bc | 3.12 ± 0.12 cd | 3.86 ± 0.15 a | 452.86 ± 10.58 ab | 85.09 ± 0.78 a | 24.00 ± 2.00 cd |
| LC50♀ × LC50♂ | 3.46 ± 0.14 cd | 2.99 ± 0.09 cd | 3.00 ± 0.16 b | 3.37 ± 0.07 ab | 3.14 ± 0.18 c | 4.07 ± 0.18 a | 411.90 ± 16.41 b | 65.50 ± 1.17 b | 61.33 ± 1.76 a |
| LC50♀ × CK♂ | 5.14 ± 0.16 a | 3.14 ± 0.01 bc | 3.04 ± 0.06 b | 2.49 ± 0.05 c | 2.18 ± 0.02 d | 1.91 ± 0.10 c | 503.88 ± 14.90 a | 88.14 ± 3.77 a | 38.67 ± 4.06 bc |
| CK♀ × LC50♂ | 3.52 ± 0.15 bcd | 3.39 ± 0.11 ab | 4.72 ± 0.44 a | 3.67 ± 0.27 a | 3.14 ± 0.16 c | 2.76 ± 0.18 b | 418.02 ± 12.37 b | 77.51 ± 2.92 ab | 44.67 ± 4.06 ab |
| LC20♀ × LC20♂ | 4.09 ± 0.13 b | 3.42 ± 0.08 ab | 2.40 ± 0.06 b | 3.23 ± 0.07 ab | 4.84 ± 0.22 b | 2.15 ± 0.01 c | 413.47 ± 18.77 b | 73.11 ± 8.63 ab | 15.33 ± 8.35 d |
| LC20♀ × CK♂ | 3.84 ± 0.06 bcd | 3.48 ± 0.03 ab | 2.28 ± 0.02 b | 3.22 ± 0.01 ab | 5.27 ± 0.22 b | 1.99 ± 0.01 c | 397.45 ± 4.47 b | 82.56 ± 1.17 ab | 23.33 ± 2.40 cd |
| CK♀ × LC20♂ | 3.99 ± 0.20 bc | 3.57 ± 0.10 a | 2.53 ± 0.06 b | 3.40 ± 0.09 ab | 6.49 ± 0.28 a | 1.98 ± 0.05 c | 421.74 ± 5.48 b | 88.79 ± 1.91 a | 16.00 ± 3.06 d |
| Treatments | Net Reproductive Rate (R0) | Intrinsic Rate of Increase (r) | Finite Rate of Increase (λ) | Mean Generation Time (T) |
|---|---|---|---|---|
| CK♀ × CK♂ | 137.43 ± 22.43 a | 0.18 ± 0.01 a | 1.20 ± 0.01 a | 27.35 ± 0.54 d |
| LC50♀ × LC50♂ | 2.44 ± 0.58 c | 0.03 ± 0.01 c | 1.03 ± 0.01 c | 28.72 ± 0.47 cd |
| LC50♀ × CK♂ | 47.99 ± 17.23 b | 0.14 ± 0.02 b | 1.15 ± 0.02 b | 27.99 ± 0.54 d |
| CK♀ × LC50♂ | 88.68 ± 29.43 ab | 0.14 ± 0.01 b | 1.15 ± 0.01 b | 31.39 ± 0.99 ab |
| LC20♀ × LC20♂ | 110.58 ± 31.51 ab | 0.15 ± 0.01 b | 1.16 ± 0.01 b | 31.02 ± 0.58 ab |
| LC20♀ × CK♂ | 123.39 ± 28.81 a | 0.16 ± 0.01 ab | 1.17 ± 0.01 ab | 30.02 ± 0.50 bc |
| CK♀ × LC20♂ | 140.63 ± 29.46 a | 0.15 ± 0.01 b | 1.16 ± 0.01 b | 32.47 ± 0.57 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kong, F.; Song, Y.; Zhang, Q.; Wang, Z.; Liu, Y. Sublethal Effects of Chlorantraniliprole on Spodoptera litura (Lepidoptera: Noctuidae) Moth: Implication for Attract-And-Kill Strategy. Toxics 2021, 9, 20. https://doi.org/10.3390/toxics9020020
Kong F, Song Y, Zhang Q, Wang Z, Liu Y. Sublethal Effects of Chlorantraniliprole on Spodoptera litura (Lepidoptera: Noctuidae) Moth: Implication for Attract-And-Kill Strategy. Toxics. 2021; 9(2):20. https://doi.org/10.3390/toxics9020020
Chicago/Turabian StyleKong, Fanfang, Yaqin Song, Qian Zhang, Zhongyue Wang, and Yongqiang Liu. 2021. "Sublethal Effects of Chlorantraniliprole on Spodoptera litura (Lepidoptera: Noctuidae) Moth: Implication for Attract-And-Kill Strategy" Toxics 9, no. 2: 20. https://doi.org/10.3390/toxics9020020
APA StyleKong, F., Song, Y., Zhang, Q., Wang, Z., & Liu, Y. (2021). Sublethal Effects of Chlorantraniliprole on Spodoptera litura (Lepidoptera: Noctuidae) Moth: Implication for Attract-And-Kill Strategy. Toxics, 9(2), 20. https://doi.org/10.3390/toxics9020020




