Comparison of EI-GC-MS/MS, APCI-LC-MS/MS, and ESI-LC-MS/MS for the Simultaneous Analysis of Nine Nitrosamines Eluted from Synthetic Resins into Artificial Saliva and Health Risk Assessment
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Instrumentation and Apparatus
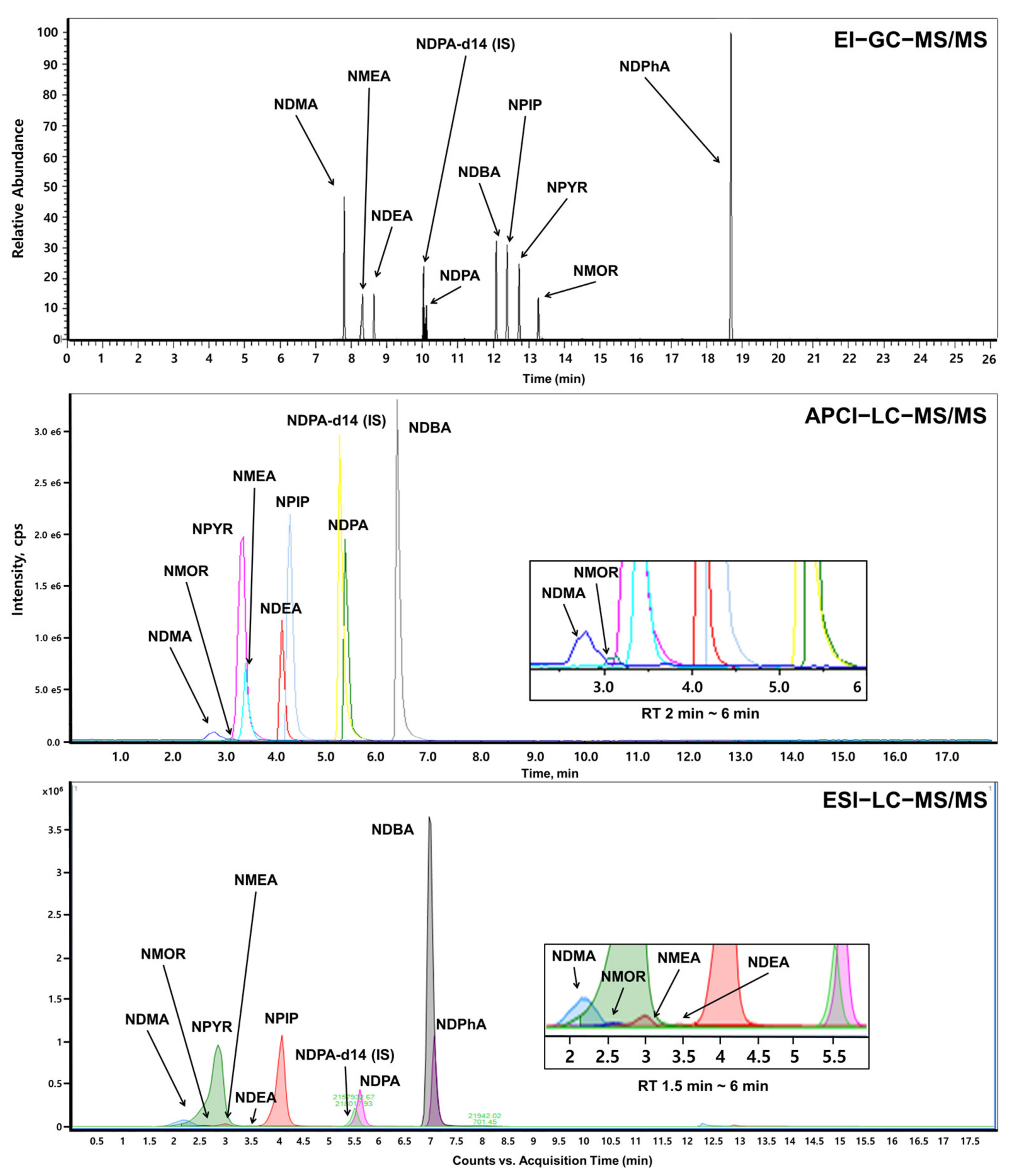
2.2.1. EI-GC-MS/MS
2.2.2. LC-MS/MS
APCI-LC-MS/MS
ESI-LC-MS/MS
2.3. Samples
2.4. Preparation of the Standard Solutions
2.4.1. Standard Solution
2.4.2. Internal Standard Solution
2.5. Preparation of the Sample
2.5.1. Preparation of Artificial Saliva
2.5.2. Extraction of Nitrosamines
2.5.3. Pretreatment of Artificial Saliva
2.6. Method Validation
2.7. Health Risk Assessment
2.7.1. Exposure Assessment
2.7.2. Non-Carcinogenic Risk Assessment: Margin of Exposure (MOE)
2.7.3. Carcinogenic Risk Assessment
3. Results and Discussion
3.1. Optimization of GC and LC Conditions for the Simultaneous Analysis of Nine Nitrosamines in Artificial Saliva
3.2. Method Validation of GC-MS/MS and LC-MS/MS for the Analysis of Nine Nitrosamines in Artificial Saliva
3.2.1. Linearity
3.2.2. LOD and LOQ
3.2.3. Recovery (Accuracy)
3.2.4. Precision
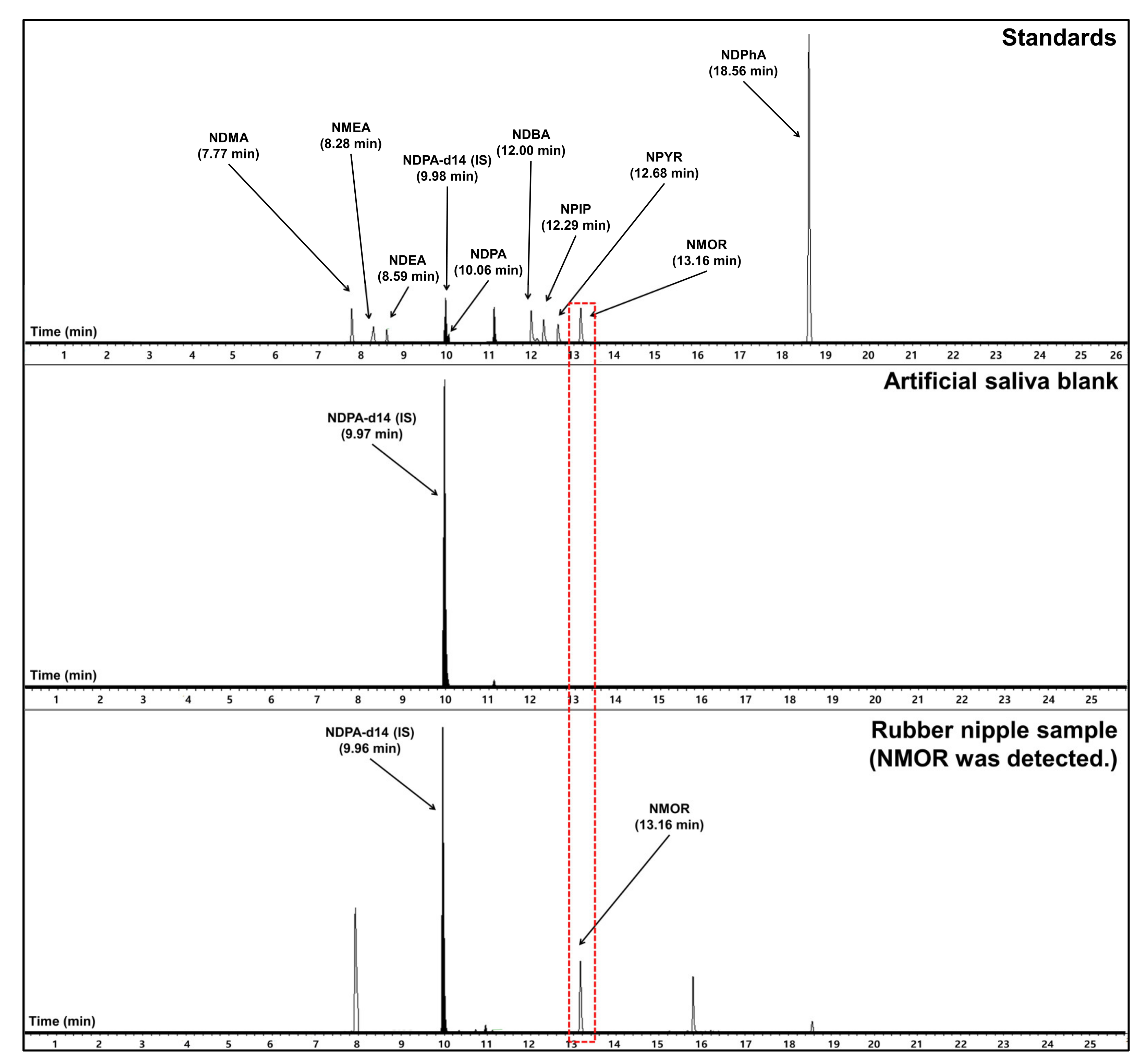
3.3. Measurement of Nitrosamines Eluted from Synthetic Resin into Artificial Saliva with EI-GC-MS/MS
3.3.1. Classification by Product Type
3.3.2. Classification by Nitrosamines
3.3.3. Comparison of the Results with Those of Previous Studies
3.4. Risk Assessment
3.4.1. Non-Carcinogenic Risk Assessment
3.4.2. Carcinogenic Risk Assessment
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kubacki, S.J.; Havery, D.C.; Fazio, T. Volatile N-nitrosamines in polish malt and beer. Food Addit. Contam. 1989, 6, 29–33. [Google Scholar] [CrossRef] [PubMed]
- Sung, J.H.; Kwak, I.S.; Park, S.K.; Kim, H.I.; Lim, H.S.; Park, H.J.; Kim, S.H. Liquid chromatography-tandem mass spectrometry determination of N-nitrosamines released from rubber or elastomer teats and soothers. Food Addit. Contam. Part A 2010, 27, 1745–1754. [Google Scholar] [CrossRef] [PubMed]
- IARC (International Agency for Research on Cancer). Monographs on the Evaluation of Carcinogenic Risks to Humans; IARC: Lyon, France, 2016; Volumes 1–15. [Google Scholar]
- Fajen, J.M.; Carson, G.A.; Rounbehler, D.P.; Fan, T.Y.; Vita, R.; Goff, U.E.; Wolf, M.H.; Edwards, G.S.; Fine, D.H.; Reinhold, V.; et al. N-nitrosamines in the rubber and tire industry. Science 1979, 205, 1262–1264. [Google Scholar] [CrossRef] [PubMed]
- Van Bruggen, M.; van Putten, E.M.; Janssen, P.J.C.M. Nitrosamines Released from Rubber Crumb; RIVM Report 609300002; National Institute for Public Health and the Environment (RIVM): Bilthoven, The Netherlands, 2008; pp. 1–34.
- KMFDS (Korean Ministry of Food and Drug Safety). Standards and Specifications for Utensils, Containers and Packages; KMFDS: Cheongju, Korea, 2019.
- EC (European Commission). Commission Directive 93/11/EEC of 15 March 1993 concerning the release, of the N-nitrosamines and N-nitrosatable substances from elastomer or rubber teats and soothers. Off. J. Eur. Commun. 1993, 93, 37–38. [Google Scholar]
- U.S. FDA (U.S. Food and Drug Administration). COMPLIANCE POLICY GUIDE (CPG) CPG Sec 500.450 Volatile N-Nitrosamines in Rubber Baby Bottle Nipples; Center for Food Safety and Applied Nutrition Office of Regulatory Affairs: College Park, MD, USA, 2005.
- Government of Canada. Infant Feeding Bottle Nipples Regulations; SOR/2016-180; Department of Justice Canada: Ottawa, ON, Canada, 2021.
- NHFPC (The National Health and Family Planning Commission of the People’s Republic of China). National Food Safety Standard—Nipple; National Standard of The People’s Republic of China; GB 4806.2-2015; NHFPC: Beijing, China, 2015.
- MOH (Ministry of Health of the People’s Republic of China). National Food Safety Standards of Food Additives—Pullulan; Ministry of Health Bulletin; GB 28402-2012; Ministry of Health of the People’s Republic of China: Beijing, China, 2012.
- Incavo, J.A.; Schafer, M.A. Simplified method for the determination of N-nitrosamines in rubber vulcanizates. Anal. Chim. Acta 2006, 557, 256–261. [Google Scholar] [CrossRef]
- Hong, Y.; Kim, K.H.; Sang, B.I.; Kim, H. Simple quantification method for N-nitrosamines in atmospheric particulates based on facile pretreatment and GC-MS/MS. Environ. Pollut. 2017, 226, 324–334. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.C.; Park, J.H.; Lee, W. Determination of N-nitrosamines in Water by Gas Chromatography Coupled with Electron Impact Ionization Tandem Mass Spectrometry. J. Korean Soc. Environ. Eng. 2014, 36, 764–770. [Google Scholar] [CrossRef]
- Liu, J.; Xie, B.; Mai, B.; Cai, Q.; He, R.; Guo, D.; Zhang, Z.; Fan, J.; Zhang, W. Development of a sensitive and stable GC-MS/MS method for simultaneous determination of four N-nitrosamine genotoxic impurities in sartan substances. J. Anal. Sci. Technol. 2021, 12, 1–8. [Google Scholar] [CrossRef]
- Munch, J.W.; Bassett, M.V. Method 521: Determination of Nitrosamines in Drinking Water by Solid Phase Extraction and Capillary Column Gas Chromatography with Large Volume Injection and Chemical Ionization Tandem Mass Spectrometry (MS/MS); National Exposure Research Laboratory Office of Research and Development, US Environmental Protection Agency: Cincinnati, OH, USA, 2004. [Google Scholar]
- Yoon, W.H.; Lee, J.H.; Lee, H.J.; Lee, S.W.; Ahn, J.C.; Kim, B.S. Investigation of N-nitrosamines using GC-MS/MS in Han-river Water Supply Systems. J. Korean Soc. Water Environ. 2016, 32, 410–418. [Google Scholar] [CrossRef][Green Version]
- Chang, S.H.; Chang, C.C.; Wang, L.J.; Chen, W.C.; Fan, S.Y.; Zang, C.Z.; Hsu, Y.H.; Lin, M.C.; Tseng, S.H.; Wang, D.Y. A multi-analyte LC-MS/MS method for screening and quantification of nitrosamines in sartans. J. Food Drug Anal. 2020, 28. [Google Scholar] [CrossRef]
- Mutsuga, M.; Yamaguchi, M.; Kawamura, Y. Analysis of N-nitrosamine migration from rubber teats and soothers. Am. J. Analyt. Chem. 2013, 4, 277–285. [Google Scholar] [CrossRef]
- Park, N.Y.; Jung, W.; Kho, Y. Analysis method of N-nitrosamines in human urine by LC-MS/MS system. J. Korean Chem. Soc. 2017, 61, 51–56. [Google Scholar] [CrossRef][Green Version]
- Park, N.Y.; Kim, S.; Jung, W.; Kho, Y. Analysis of Nitrosamines Concentration in Condom by using LC-MS/MS. J. Korean Chem. Soc. 2018, 62, 181–186. [Google Scholar] [CrossRef]
- Park, S.J.; Jeong, M.J.; Park, S.R.; Choi, J.C.; Choi, H.; Kim, M. Release of N-nitrosamines and N-nitrosatable substances from baby bottle teats and rubber kitchen tools in Korea. Food Sci. Biotechnol. 2018, 27, 1519–1524. [Google Scholar] [CrossRef] [PubMed]
- U.S. FDA (U.S. Food and Drug Administration). Liquid Chromatography-High Resolution Mass Spectrometry (LC-HRMS) Method for the Determination of Six Nitrosamine Impurities in ARB Drugs; U.S. Food and Drug Administration: Silver Spring, MD, USA, 2019.
- U.S. FDA (U.S. Food and Drug Administration). Liquid Chromatography-Electrospray Ionization-High Resolution Mass Spectrometry (LC-ESI-HRMS); Method for the Determination of Nitrosamine Impurities in Metformin Drug Substance and Drug Product; U.S. Food and Drug Administration: Silver Spring, MD, USA, 2020.
- Lim, H.H.; Oh, Y.S.; Shin, H.S. Determination of N-nitrosodimethylamine and N-nitrosomethylethylamine in drug substances and products of sartans, metformin and ranitidine by precipitation and solid phase extraction and gas chromatography–tandem mass spectrometry. J. Pharm. Biomed. Anal. 2020, 189, 113460. [Google Scholar] [CrossRef] [PubMed]
- Perez, E.R.; Knapp, J.A.; Horn, C.K.; Stillman, S.L.; Evans, J.E.; Arfsten, D.P. Comparison of LC–MS-MS and GC–MS analysis of benzodiazepine compounds included in the drug demand reduction urinalysis program. J. Anal. Toxicol. 2016, 40, 201–207. [Google Scholar] [CrossRef]
- Yahaya, A.; Babatunde, D.; Olaniyan, L.W.; Agboola, O. Application of chromatographic techniques in the analysis of total nitrosamines in water. Heliyon 2020, 6, e03447. [Google Scholar] [CrossRef]
- Anna, V.; Rimma, S.; Lev, O.; Jenny, G. GC determination of N-nitrosamines by supersonic molecular beam MS equipped with triple quadrupole analyzer, GC/SMB/QQQ/MS. Anal. Chim. Acta 2011, 685, 162–169. [Google Scholar] [CrossRef]
- Bouma, K.; Nab, F.M.; Schothorst, R.C. Migration of N-nitrosamines, N-nitrosatable substances and 2-mercaptobenzthiazol from baby bottle teats and soothers: A Dutch retail survey. Food Addit. Contam. 2003, 20, 853–858. [Google Scholar] [CrossRef]
- KMFDS (Korean Ministry of Food and Drug Safety). Study on Establishment of Specification and Analytical Method for Nitrosamines in Rubber Food Contact Materials; Korean Ministry of Food and Drug Safety: Cheongju, Korea, 2009.
- Kühne, F.; Kappenstein, O.; Straβgütl, S.; Weese, F.; Weyer, J.; Pfaff, K.; Luch, A. N-nitrosamines migrating from food contact materials into food simulants: Analysis and quantification by means of hplc-apci-ms/ms. Food Addit. Contam. Part A 2018, 35, 793–806. [Google Scholar] [CrossRef]
- Suh, B.; Kwon, H. Factors Attributing to the Formation of N-Nitrosamines in Instant Food. J. Food Hyg. Saf. 2017, 32, 114–122. [Google Scholar] [CrossRef]
- Wang, W.; Yu, J.; An, W.; Yang, M. Occurrence and profiling of multiple nitrosamines in source water and drinking water of China. Sci. Total Environ. 2016, 551, 489–495. [Google Scholar] [CrossRef]
- Zhao, Y.Y.; Boyd, J.; Hrudey, S.E.; Li, X.F. Characterization of new nitrosamines in drinking water using liquid chromatography tandem mass spectrometry. Environ. Sci. Technol. 2006, 40, 7636–7641. [Google Scholar] [CrossRef] [PubMed]
- Bratinova, S.; Raffael, B.; Simoneau, C. Guidelines for performance criteria and validation procedures of analytical methods used in controls of food contact materials. JRC Sci. Techn. Rep. EUR 2009, 24105, 1–74. [Google Scholar]
- U.S. FDA (U.S. Food and Drug Administration) Guidance for Industry: Preparation of Premarket Submissions for Food Contact Substances: Chemistry Recommendations. Available online: http://www.fda.gov/Food/GuidanceRegulation/GuidanceDocumentsRegulatoryInformation/ucm081818. (accessed on 9 August 2021).
- KMFDS (Korean Ministry of Food and Drug Safety). Safety Evaluation Model for Migrant from Utensils and Food Packaging Materials; Research and Development Report; Korean Ministry of Food and Drug Safety: Cheongju, Korea, 2017.
- Phillps, D.H.; Committee on the Carcinogenicity of Chemicals in Food, Consumer Products and the Environment (COC). Annual Report 2007. 2007. Available online: https://cot.food.gov.uk/sites/default/files/cot/cocsection05.pdf (accessed on 19 September 2021).
| Nitrosamines | R.T (min), Average ± SD (%RSD) | Precursor Ion | Product Ion | CE (V) | |
|---|---|---|---|---|---|
| Intra-Day (n = 3) | Inter-Day (n = 3) | ||||
| EI-GC-MS/MS | |||||
| NDMA | 7.78 ± 0.03 (0.34) | 7.76 ± 0.03 (0.32) | 74.1 | 44.0 | 5 |
| NDEA | 8.60 ± 0.03 (0.37) | 8.57 ± 0.04 (0.51) | 102.1 | 44.1 | 10 |
| NDPA | 10.10 ± 0.04 (0.37) | 10.05 ± 0.03 (0.32) | 130.0 | 43.0- | 10 |
| NDBA | 12.06 ± 0.05 (0.41) | 11.99 ± 0.03 (0.22) | 116.1 | 99.1 | 15 |
| NPIP | 12.34 ± 0.05 (0.41) | 12.29 ± 0.03 (0.26) | 114.0 | 84.1 | 5 |
| NPYR | 12.68 ± 0.04 (0.32) | 12.62 ± 0.03 (0.23) | 100.1 | 55.1 | 5 |
| NMOR | 13.21 ± 0.06 (0.42) | 13.16 ± 0.03 (0.24) | 86.1 | 56.1 | 15 |
| NDPhA | 18.61 ± 0.07 (0.39) | 18.54 ± 0.03 (0.14) | 169.1 | 168.1 | 10 |
| NMEA | 8.29 ± 0.02 (0.28) | 8.27 ± 0.02 (0.24) | 88.1 | 42.1 | 10 |
| NDPA-d14 | 10.03 ± 0.04 (0.36) | 9.99 ± 0.01 (0.06) | 78.1 | 46.1 | 15 |
| APCI-LC-MS/MS | |||||
| NDMA | 2.75 ± 0.04 (1.28) | 2.81 ± 0.07 (2.57) | 75.2 | 43.0 | 23 |
| NDEA | 4.12 ± 0.01 (0.14) | 4.12 ± 0.01 (0.14) | 103.2 | 75.0 | 17 |
| NDPA | 5.35 ± 0.00 (0.00) | 5.35 ± 0.01 (0.19) | 131.2 | 89.0 | 15 |
| NDBA | 6.37 ± 0.01 (0.18) | 6.36 ± 0.01 (0.09) | 159.3 | 57.0 | 23 |
| NPIP | 4.26 ± 0.01 (0.14) | 4.27 ± 0.01 (0.27) | 115.2 | 64.2 | 23 |
| NPYR | 3.34 ± 0.01 (0.17) | 3.34 ± 0.01 (0.17) | 101.2 | 55.0 | 25 |
| NMOR | 3.10 ± 0.04 (1.22) | 3.10 ± 0.03 (1.04) | 117.2 | 87.0 | 19 |
| NDPhA | - | - | 199.2 | 169.2 | 25 |
| NMEA | 3.41 ± 0.03 (0.74) | 3.43 ± 0.05 (1.32) | 89.2 | 61.0 | 17 |
| NDPA-d14 | 5.25 ± 0.01 (0.11) | 5.24 ± 0.01 (0.11) | 145.1 | 50.1 | 17 |
| ESI-LC-MS/MS | |||||
| NDMA | 2.15 ± 0.04 (2.07) | 2.15 ± 0.04 (1.68) | 75.1 | 43.0 | 18 |
| NDEA | 3.80 ± 0.03 (0.70) | 3.81 ± 0.03 (0.66) | 103.2 | 75.0 | 8 |
| NDPA | 5.61 ± 0.01 (0.20) | 5.56 ± 0.05 (0.91) | 131.2 | 43.0 | 12 |
| NDBA | 7.06 ± 0.05 (0.75) | 7.02 ± 0.01 (0.14) | 159.3 | 56.9 | 12 |
| NPIP | 4.07 ± 0.06 (1.35) | 4.07 ± 0.03 (0.85) | 115.2 | 41.0 | 22 |
| NPYR | 2.78 ± 0.03 (0.91) | 2.80 ± 0.03 (1.09) | 101.1 | 54.9 | 12 |
| NMOR | 2.54 ± 0.04 (1.42) | 2.52 ± 0.03 (1.05) | 117.1 | 87.0 | 8 |
| NDPhA | 7.05 ± 0.04 (0.59) | 7.11 ± 0.01 (0.08) | 199.2 | 65.9 | 15 |
| NMEA | 3.05 ± 0.05 (1.64) | 3.07 ± 0.04 (1.36) | 89.2 | 61.0 | 8 |
| NDPA-d14 | 5.52 ± 0.03 (0.48) | 5.50 ± 0.02 (0.36) | 145.1 | 50.1 | 8 |
| NitrosamInes | EI-GC-MS/MS | APCI-LC-MS/MS | ESI-LC-MS/MS | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Correlation Coefficients of the Slopes (R2) | ||||||||||||||||||
| Intra-Day (n = 3) | Inter-Day (n = 3) | Intra-Day (n = 3) | Inter-Day (n = 3) | Intra-Day (n = 3) | Inter-Day (n = 3) | |||||||||||||
| 1 | 2 | 3 | 1 | 2 | 3 | 1 | 2 | 3 | 1 | 2 | 3 | 1 | 2 | 3 | 1 | 2 | 3 | |
| NDMA | 0.9998 | 1.0000 | 0.9999 | 0.9999 | 0.9998 | 0.9995 | 0.9988 | 0.9975 | 0.9991 | 0.9992 | 0.9990 | 0.9995 | 0.9965 | 0.9988 | 0.9979 | 0.9982 | 0.9970 | 0.9992 |
| NDEA | 0.9997 | 0.9998 | 0.9998 | 0.9998 | 0.9999 | 0.9996 | 0.9999 | 0.9995 | 0.9997 | 0.9999 | 1.0000 | 0.9994 | 0.9984 | 0.9952 | 0.9973 | 0.9968 | 0.9970 | 0.9980 |
| NDPA | 0.9994 | 0.9998 | 0.9996 | 0.9996 | 0.9995 | 0.9995 | 0.9995 | 0.9992 | 0.9989 | 0.9987 | 0.9981 | 0.9999 | 1.0000 | 0.9999 | 0.9998 | 0.9997 | 1.0000 | 0.9996 |
| NDBA | 0.9997 | 0.9995 | 0.9997 | 0.9997 | 0.9996 | 0.9999 | 0.9991 | 0.9999 | 0.9995 | 0.9998 | 0.9992 | 0.9995 | 0.9999 | 0.9999 | 0.9998 | 0.9991 | 0.9990 | 0.9999 |
| NPIP | 0.9999 | 0.9994 | 0.9998 | 0.9995 | 0.9999 | 0.9998 | 0.9998 | 0.9979 | 0.9997 | 0.9982 | 0.9979 | 0.9988 | 0.9998 | 0.9997 | 0.9995 | 0.9999 | 1.0000 | 0.9992 |
| NPYR | 0.9994 | 0.9997 | 0.9995 | 0.9994 | 0.9997 | 0.9999 | 0.9986 | 0.9999 | 0.9992 | 0.9991 | 0.9993 | 0.9999 | 0.9995 | 0.9992 | 0.9999 | 0.9999 | 0.9991 | 0.9992 |
| NMOR | 0.9995 | 0.9999 | 0.9996 | 0.9999 | 0.9994 | 0.9999 | 1.0000 | 0.9999 | 0.9998 | 0.9992 | 0.9993 | 0.9980 | 0.9992 | 0.9991 | 0.9990 | 0.9998 | 0.9995 | 0.9989 |
| NDPhA | 0.9967 | 0.9999 | 0.9998 | 0.9987 | 0.9996 | 0.9999 | - | - | - | - | - | - | 0.9988 | 0.9999 | 0.9993 | 0.9995 | 1.0000 | 0.9991 |
| NMEA | 0.9988 | 0.9999 | 0.9999 | 0.9997 | 0.9998 | 0.9997 | 0.9968 | 0.9992 | 0.9978 | 0.9991 | 0.9989 | 0.9982 | 0.9999 | 0.9999 | 0.9999 | 0.9999 | 0.9998 | 0.9998 |
| Nitrosamines | LOD (µg/L) | LOQ (µg/L) | ||||
|---|---|---|---|---|---|---|
| GC-MS/MS | APCI-LC-MS/MS | ESI-LC-MS/MS | GC-MS/MS | APCI-LC-MS/MS | ESI-LC-MS/MS | |
| NDMA | 0.12 | 62.5 | 62.5 | 0.24 | 125 | 125 |
| NDEA | 0.24 | 0.48 | 7.81 | 0.48 | 0.96 | 15.62 |
| NDPA | 0.24 | 0.98 | 7.81 | 0.48 | 1.96 | 15.62 |
| NDBA | 0.12 | 0.49 | 0.24 | 0.24 | 0.98 | 0.48 |
| NPIP | 0.12 | 0.24 | 0.48 | 0.24 | 0.48 | 0.96 |
| NPYR | 0.12 | 0.48 | 0.24 | 0.24 | 0.96 | 0.48 |
| NMOR | 0.24 | 3.12 | 0.24 | 0.48 | 6.25 | 0.48 |
| NDPhA | 0.24 | N.D. | 0.24 | 0.24 | - | 0.48 |
| NMEA | 0.48 | 3.12 | 62.5 | 0.98 | 6.25 | 125 |
| Nitrosamines | EI-GC-MS/MS | APCI-LC-MS/MS | ESI-LC-MS/MS | |||
|---|---|---|---|---|---|---|
| Spike Conc. (µg/L) | Avr. Recovery (%) | Spike Conc. (µg/L) | Avr. Recovery (%) | Spike Conc. (µg/L) | Avr. Recovery (%) | |
| NDMA | 1 | 105.46 | 10 | -1) | 10 | - |
| 5 | 88.53 | 100 | 90.12 | 100 | 79.26 | |
| NDEA | 1 | 115.33 | 10 | 110.83 | 10 | 73.50 |
| 5 | 105.82 | 100 | 110.98 | 100 | 75.36 | |
| NDPA | 1 | 109.40 | 10 | 108.93 | 10 | 90.52 |
| 5 | 102.86 | 100 | 101.54 | 100 | 114.75 | |
| NDBA | 1 | 117.39 | 10 | 111.45 | 10 | 96.47 |
| 5 | 111.46 | 100 | 115.76 | 100 | 100.35 | |
| NPIP | 1 | 113.20 | 10 | 106.76 | 10 | 83.60 |
| 5 | 106.33 | 100 | 106.80 | 100 | 108.49 | |
| NPYR | 1 | 110.07 | 10 | 103.87 | 10 | 93.51 |
| 5 | 99.97 | 100 | 97.42 | 100 | 102.16 | |
| NMOR | 1 | 105.11 | 10 | 101.16 | 10 | 87.42 |
| 5 | 99.01 | 100 | 93.77 | 100 | 114.31 | |
| NDPhA | 1 | 127.57 | 10 | N.D. | 10 | 80.60 |
| 5 | 126.23 | 100 | N.D. | 100 | 123.47 | |
| NMEA | 1 | 107.16 | 10 | 106.55 | 10 | 83.66 |
| 5 | 104.98 | 100 | 131.76 | 100 | 83.28 | |
| Nitrosamines | EI-GC-MS/MS | APCI-LC-MS/MS | ESI-LC-MS/MS | |||
|---|---|---|---|---|---|---|
| Spike Conc. (µg/L) | %RSD (%) | Spike Conc. (µg/L) | %RSD (%) | Spike Conc. (µg/L) | %RSD (%) | |
| NDMA | 1 | 3.90 | 10 | -1) | 10 | - |
| 5 | 1.25 | 100 | 5.20 | 100 | 12.55 | |
| NDEA | 1 | 2.34 | 10 | 11.08 | 10 | 10.42 |
| 5 | 1.96 | 100 | 0.92 | 100 | 11.94 | |
| NDPA | 1 | 0.82 | 10 | 1.96 | 10 | 10.28 |
| 5 | 1.78 | 100 | 4.97 | 100 | 2.28 | |
| NDBA | 1 | 0.88 | 10 | 9.50 | 10 | 4.60 |
| 5 | 5.49 | 100 | 2.54 | 100 | 8.70 | |
| NPIP | 1 | 2.10 | 10 | 9.98 | 10 | 8.60 |
| 5 | 4.05 | 100 | 2.07 | 100 | 8.38 | |
| NPYR | 1 | 1.64 | 10 | 5.66 | 10 | 12.84 |
| 5 | 4.49 | 100 | 7.48 | 100 | 8.42 | |
| NMOR | 1 | 3.48 | 10 | 4.63 | 10 | 13.19 |
| 5 | 4.12 | 100 | 6.98 | 100 | 7.48 | |
| NDPhA | 1 | 3.07 | 10 | N.D. | 10 | 9.70 |
| 5 | 6.26 | 100 | N.D. | 100 | 7.51 | |
| NMEA | 1 | 3.03 | 10 | 9.38 | 10 | 11.80 |
| 5 | 4.40 | 100 | 7.63 | 100 | 6.51 | |
| Resins | Nitrosamines | Previous Studies | Analytical Method | Sample | Detection Frequency | Monitoring Results in Previous Studies (µg/kg) | Monitoring Results in our Study (µg/kg) (Detection Frequency) |
|---|---|---|---|---|---|---|---|
| Rubber (n = 49) | NDMA | KMFDS., 2009 | LC-MS/MS | Nipples | 0/349 | ND | 1.01~1.71 (2/49) |
| Bouma, K., et al., 2003 | GC-TEA | Nipples | 15/19 | 0.20~1.60 | |||
| Anna, V., et al., 2011 | GC-MS/MS | Nipples | 2/2 | 0.30~1.90 | |||
| Mutsuga, M., et al., 2013 | GC-MS | Nipples | 0/3 | ND | |||
| Suh et al., 2017 | PCI-GC-MS/MS | Nipples | 0/93 | ND | |||
| Kühne, F., et al., 2018 | APCI-LC-MS/MS | Elastomer | 18/96 | 0.72~5.22 | |||
| Park, S.J., et al., 2018 | LC-MS/MS | Nipples and baby products, kitchenware | 17/75 | 1.02~3.67 | |||
| NDEA | KMFDS., 2009 | LC-MS/MS | Nipples | 0/349 | ND | 0.33~0.56 (3/49) | |
| Anna, V., et al., 2011 | GC-MS/MS | Nipples | 0/2 | ND | |||
| Mutsuga, M., et al., 2013 | GC-MS | Nipples | 0/3 | ND | |||
| Suh et al., 2017 | PCI-GC-MS/MS | Food packaging | 0/93 | ND | |||
| Park, S.J., et al., 2018 | LC-MS/MS | Nipples and baby products, kitchenware | 0/75 | ND | |||
| NDPA | KMFDS., 2009 | LC-MS/MS | Nipples | 0/349 | ND | ND (0/49) | |
| Anna, V., et al., 2011 | GC-MS/MS | Nipples | 0/2 | ND | |||
| Mutsuga, M., et al., 2013 | GC-MS | Nipples | 0/3 | ND | |||
| Park, S.J. et al., 2018 | LC-MS/MS | Nipples and baby products, kitchenware | 0/75 | ND | |||
| NDBA | KMFDS., 2009 | LC-MS/MS | Nipples | 0/349 | ND | 0.12~0.64 (10/49) | |
| Anna, V., et al., 2011 | GC-MS/MS | Nipples | 0/2 | ND | |||
| Mutsuga, M., et al., 2013 | GC-MS | Nipples | 0/3 | ND | |||
| Kühne, F., et al., 2018 | APCI-LC-MS/MS | Rubber elastomer | 18/96 | 0.54~2.04 | |||
| Park, S.J., et al., 2018 | LC-MS/MS | Nipples and baby products, kitchenware | 0/75 | ND | |||
| Suh et al., 2017 | PCI-GC-MS/MS | Food packaging | 0/93 | ND | |||
| NPIP | KMFDS., 2009 | LC-MS/MS | Nipples | 0/349 | ND | 0.13~0.67 (12/49) | |
| Anna, V., et al., 2011 | GC-MS/MS | Nipples | 0/2 | ND | |||
| Mutsuga. M., et al., 2013 | GC-MS | Nipples | 0/3 | ND | |||
| Park, S.J., et al., 2018 | LC-MS/MS | Nipples and baby products, kitchenware | 3/75 | 0.38~0.55 | |||
| NPYR | KMFDS., 2009 | LC-MS/MS | Nipples | 0/349 | ND | 0.12~0.15 (2/49) | |
| Anna, V., et al., 2011 | GC-MS/MS | Nipples | 1/2 | 0.6 | |||
| Mutsuga, M., et al., 2013 | GC-MS | Nipples | 0/3 | ND | |||
| Park, S.J., et al., 2018 | LC-MS/MS | Nipples and baby products, kitchenware | 0/75 | ND | |||
| NMOR | KMFDS., 2009 | LC-MS/MS | Nipples | 0/349 | ND | 0.29~2.77 (29/49) | |
| Anna, V., et al., 2011 | GC-MS/MS | Nipples | 1/2 | 0.2 | |||
| Mutsuga, M., et al., 2013 | GC-MS | Nipples | 0/3 | ND | |||
| Kühne, F., et al., 2018 | APCI-LC-MS/MS | Rubber elastomer | 18/96 | 0.30~1.50 | |||
| Park, S.J., et al., 2018 | LC-MS/MS | Nipples and baby products, kitchenware | 4/75 | 0.89~1.96 | |||
| NDPhA | Anna, V., et al., 2011 | GC-MS/MS | Nipples | 2/2 | 0.1 | 0.27~1.88 (9/49) | |
| Kühne, F., et al., 2018 | APCI-LC-MS/MS | Rubber elastomer | 3/96 | 0.42~1.50 | |||
| Zhao, Y.Y., et al., 2006 | ESI-LC-MS/MS | River water | 3/4 | 0.0006~0.0010 | |||
| NMEA | Anna, V., et al., 2011 | GC-MS/MS | Nipples | 0/2 | ND | ND (0/49) | |
| Zhao, Y.Y., et al., 2006 | ESI-LC-MS/MS | River water | 0/4 | ND | |||
| Wang, X., et al., 2016 | ESI-LC-MS/MS | River water | 1/17 | 1.00 | |||
| TPE, TPU, PU (n = 8) | NDMA | -1) | - | - | - | - | ND (0/8) |
| NDEA | ND (0/8) | ||||||
| NDPA | ND (0/8) | ||||||
| NDBA | ND (0/8) | ||||||
| NPIP | ND (0/8) | ||||||
| NPYR | ND (0/8) | ||||||
| NMOR | 0.92 (1/8) | ||||||
| NDPhA | ND (0/8) | ||||||
| NMEA | ND (0/8) |
| Nitrosamines | Synthetic Resin | EDI (mg/kg bw/day) | BMDL10 (mg/kg bw/day) | MOE 1) |
|---|---|---|---|---|
| NDMA | Rubber | 8.10 × 10−08 | 0.027 | 333,165 |
| TPE | 5.70 × 10−08 | 473,684 | ||
| TPU | 6.00 × 10−09 | 4,500,000 | ||
| PU | 6.00 × 10−09 | 4,500,000 | ||
| NDEA | Rubber | 1.19 × 10−07 | 0.018 | 150,963 |
| TPE | 1.14 × 10−07 | 157,895 | ||
| TPU | 1.20 × 10−08 | 1,500,000 | ||
| PU | 1.20 × 10−08 | 1,500,000 | ||
| NPYR | Rubber | 5.73 × 10−08 | 0.16 | 2,792,769 |
| TPE | 5.70 × 10−08 | 2,807,018 | ||
| TPU | 6.00 × 10−09 | 26,666,667 | ||
| PU | 6.00 × 10−09 | 26,666,667 | ||
| NMOR | Rubber | 2.99 × 10−07 | 0.7 | 2,342,976 |
| TPE | 1.14 × 10−07 | 6,140,351 | ||
| TPU | 1.20 × 10−08 | 58,333,333 | ||
| PU | 4.60 × 10−08 | 15,217,391 |
| Nitrosamines | Synthetic Resin | EDI (mg/kg bw/day) | Cancer Slope Factor (mg/kg bw/day) | Carcinogenic Risk |
|---|---|---|---|---|
| NDMA | Rubber | 8.10 × 10−08 | 5.10 × 10+01 | 4.13 × 10−06 |
| TPE | 5.70 × 10−08 | 2.91 × 10−06 | ||
| TPU | 6.00 × 10−09 | 3.06 × 10−07 | ||
| PU | 6.00 × 10−09 | 3.06 × 10−07 | ||
| NDEA | Rubber | 1.19 × 10−07 | 1.50 × 10+02 | 1.79 × 10−05 |
| TPE | 1.14 × 10−07 | 1.71 × 10−05 | ||
| TPU | 1.20 × 10−08 | 1.80 × 10−06 | ||
| PU | 1.20 × 10−08 | 1.80 × 10−06 | ||
| NDPA | Rubber | 1.14 × 10−07 | 7.00 × 10+00 | 7.98 × 10−07 |
| TPE | 1.14 × 10−07 | 7.98 × 10−07 | ||
| TPU | 1.20 × 10−08 | 8.40 × 10−08 | ||
| PU | 1.20 × 10−08 | 8.40 × 10−08 | ||
| NDBA | Rubber | 8.32 × 10−08 | 5.40 × 10+00 | 4.49 × 10−07 |
| TPE | 5.70 × 10−08 | 3.08 × 10−07 | ||
| TPU | 6.00 × 10−09 | 3.24 × 10−08 | ||
| PU | 6.00 × 10−09 | 3.24 × 10−08 | ||
| NPIP | Rubber | 7.29 × 10−08 | 9.40 × 10+00 | 6.85 × 10−07 |
| TPE | 5.70 × 10−08 | 5.36 × 10−07 | ||
| TPU | 6.00 × 10−09 | 5.64 × 10−08 | ||
| PU | 6.00 × 10−09 | 5.64 × 10−08 | ||
| NPYR | Rubber | 5.73 × 10−08 | 2.10 × 10+00 | 1.20 × 10−07 |
| TPE | 5.70 × 10−08 | 1.20 × 10−07 | ||
| TPU | 6.00 × 10−09 | 1.26 × 10−08 | ||
| PU | 6.00 × 10−09 | 1.26 × 10−08 | ||
| NMOR | Rubber | 2.99 × 10−07 | 6.70 × 10+00 | 2.00 × 10−06 |
| TPE | 1.14 × 10−07 | 7.64 × 10−07 | ||
| TPU | 1.20 × 10−08 | 8.04 × 10−08 | ||
| PU | 4.60 × 10−08 | 3.08 × 10−07 | ||
| NDPhA | Rubber | 9.01 × 10−08 | 4.90 × 10−03 | 4.41 × 10−10 |
| TPE | 5.70 × 10−08 | 2.79 × 10−10 | ||
| TPU | 6.00 × 10−09 | 2.94 × 10−11 | ||
| PU | 6.00 × 10−09 | 2.94 × 10−11 | ||
| NMEA | Rubber | 2.28 × 10−07 | 2.20 × 10+01 | 5.02 × 10−06 |
| TPE | 2.28 × 10−07 | 5.02 × 10−06 | ||
| TPU | 2.40 × 10−08 | 5.28 × 10−07 | ||
| PU | 2.40 × 10−08 | 5.28 × 10−07 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, H.; Sung, D.; Yu, H.; Jang, D.; Koo, Y.; Lee, S.; Lim, K.; Choi, D. Comparison of EI-GC-MS/MS, APCI-LC-MS/MS, and ESI-LC-MS/MS for the Simultaneous Analysis of Nine Nitrosamines Eluted from Synthetic Resins into Artificial Saliva and Health Risk Assessment. Toxics 2021, 9, 230. https://doi.org/10.3390/toxics9100230
Kim H, Sung D, Yu H, Jang D, Koo Y, Lee S, Lim K, Choi D. Comparison of EI-GC-MS/MS, APCI-LC-MS/MS, and ESI-LC-MS/MS for the Simultaneous Analysis of Nine Nitrosamines Eluted from Synthetic Resins into Artificial Saliva and Health Risk Assessment. Toxics. 2021; 9(10):230. https://doi.org/10.3390/toxics9100230
Chicago/Turabian StyleKim, Hyungsoo, Daekwan Sung, Honghyeon Yu, Daeyong Jang, Yeji Koo, Seungha Lee, Kyungmin Lim, and Dalwoong Choi. 2021. "Comparison of EI-GC-MS/MS, APCI-LC-MS/MS, and ESI-LC-MS/MS for the Simultaneous Analysis of Nine Nitrosamines Eluted from Synthetic Resins into Artificial Saliva and Health Risk Assessment" Toxics 9, no. 10: 230. https://doi.org/10.3390/toxics9100230
APA StyleKim, H., Sung, D., Yu, H., Jang, D., Koo, Y., Lee, S., Lim, K., & Choi, D. (2021). Comparison of EI-GC-MS/MS, APCI-LC-MS/MS, and ESI-LC-MS/MS for the Simultaneous Analysis of Nine Nitrosamines Eluted from Synthetic Resins into Artificial Saliva and Health Risk Assessment. Toxics, 9(10), 230. https://doi.org/10.3390/toxics9100230








