Short-Term Exposure to Wood Smoke Increases the Expression of Pro-Inflammatory Cytokines, Gelatinases, and TIMPs in Guinea Pigs
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Short-Term Model of Exposure to WS
2.3. Carboxyhemoglobin Analysis and WS Composition
2.4. Histology
2.5. Cell Profile Analysis in BAL Fluid
2.6. Total Collagen Content Measurement in BAL
2.7. Gene Expression by qRT-PCR in Lung Tissue
2.8. Quantification of Cytokines in BAL and Serum
2.9. Gelatinolytic Zymography Assay
2.10. Statistical Analysis
3. Results
3.1. WS Composition and Carboxyhemoglobin (COHb) Analysis
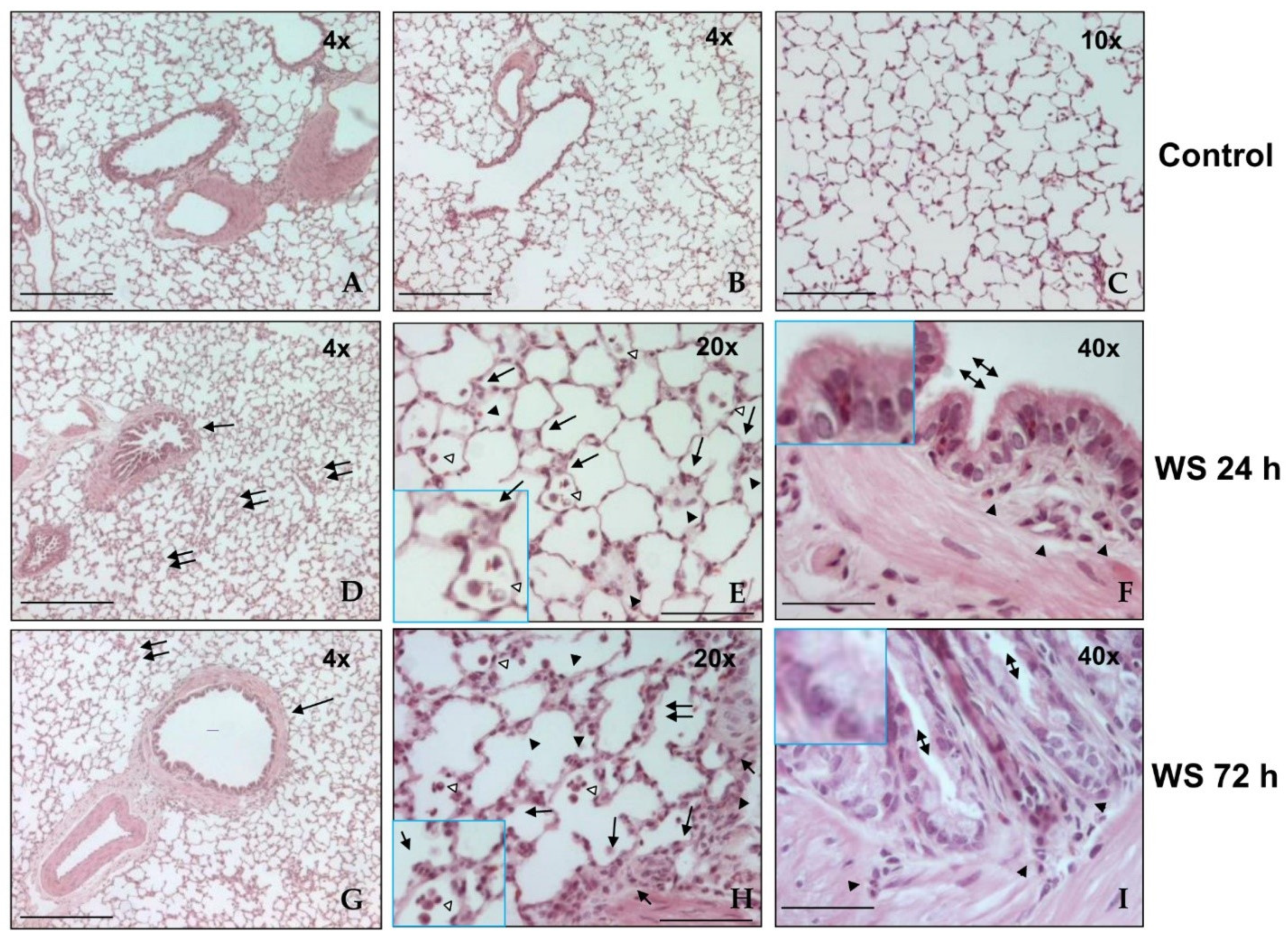
3.2. Histological Analysis
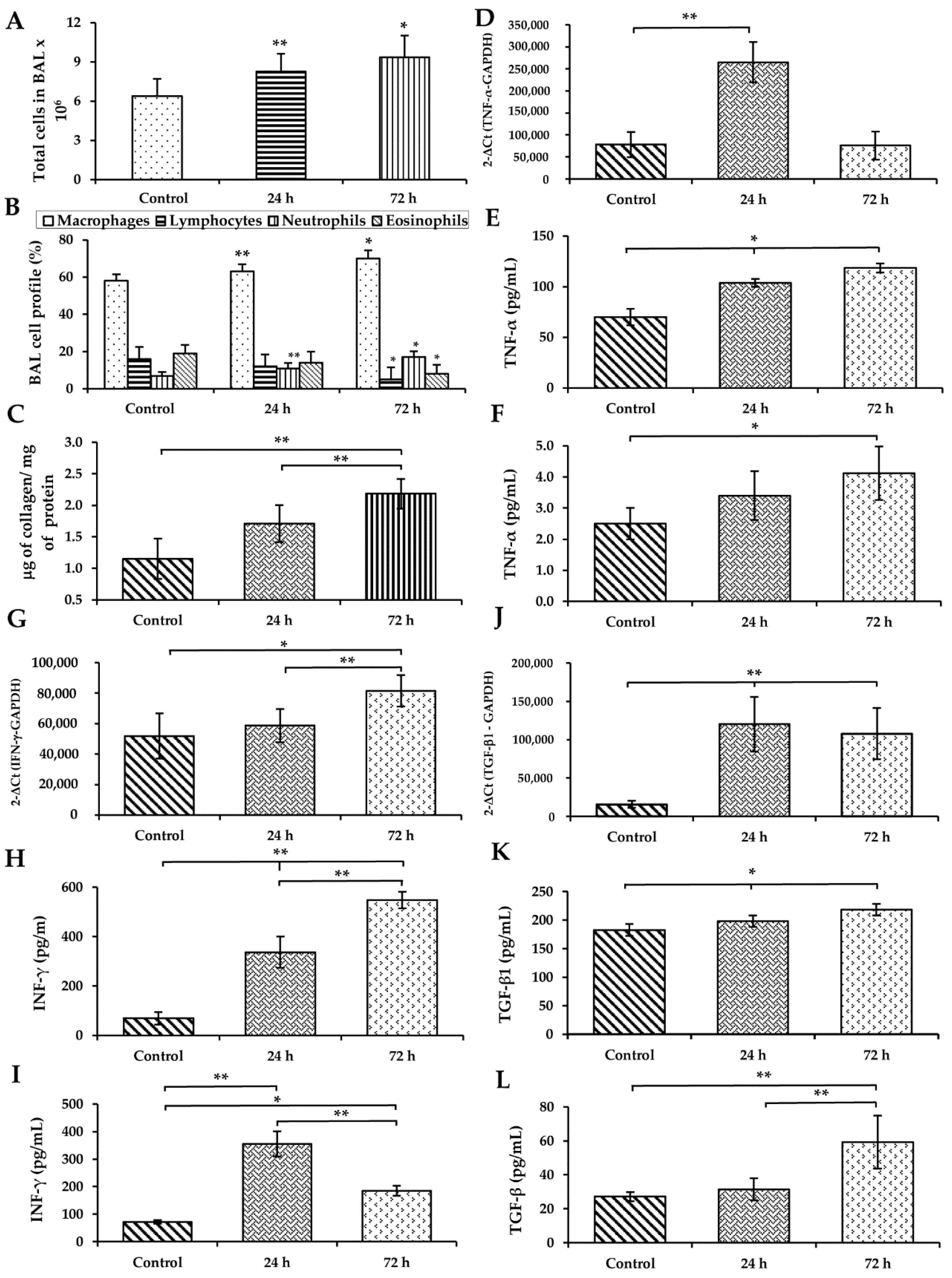
3.3. WS Induces an Increase in Macrophages and Neutrophils and Diminished Lymphocytes and Eosinophils in BAL
3.4. WS Induces an Increase in the Total Collagen Content in BAL
3.5. WS Induces the Upregulation of Cytokines in the Lung and Increases Their Levels in BAL and Serum
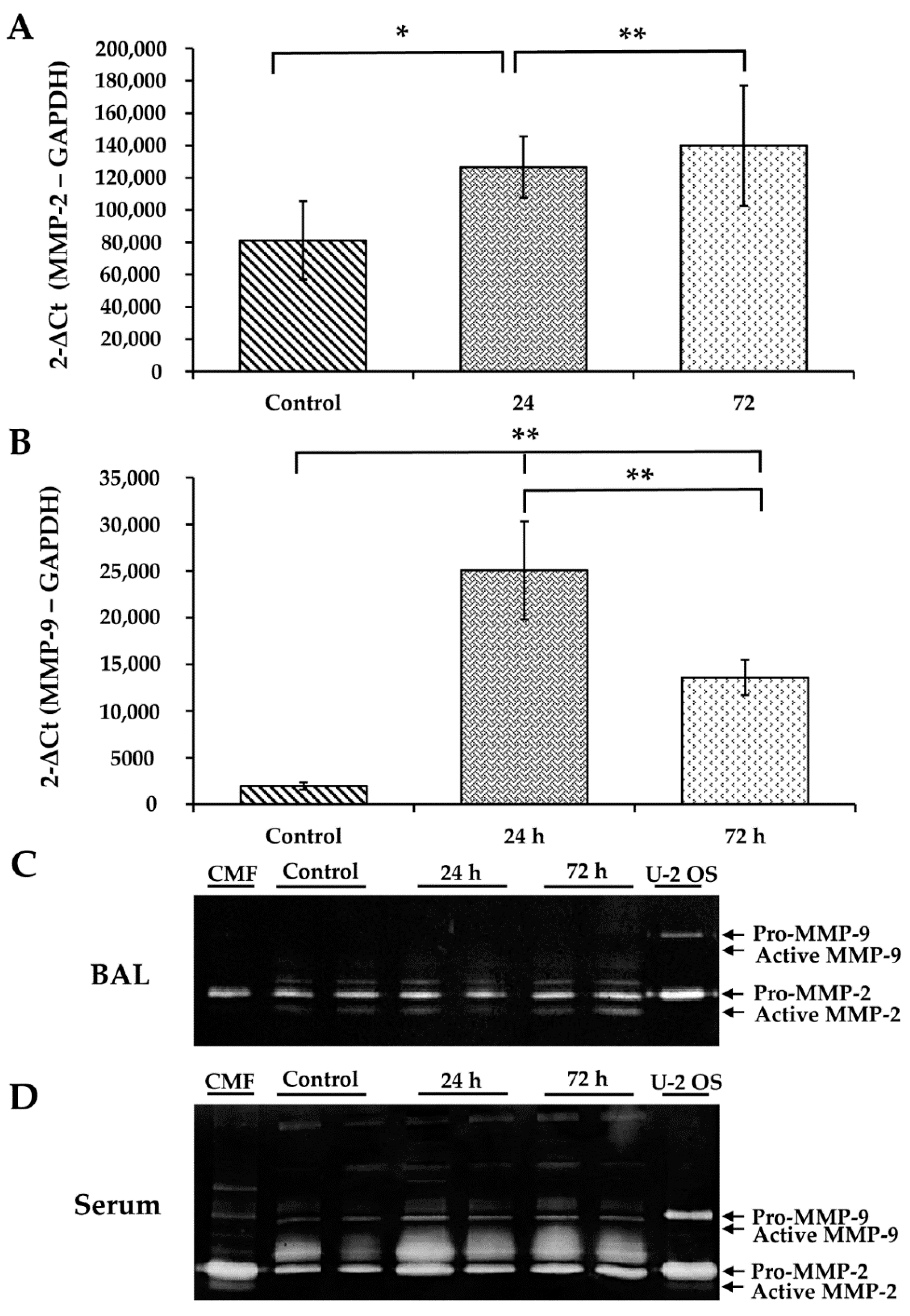
3.6. WS Upregulates MMP-2 and MMP-9 and Increases the Activity of MMP-2
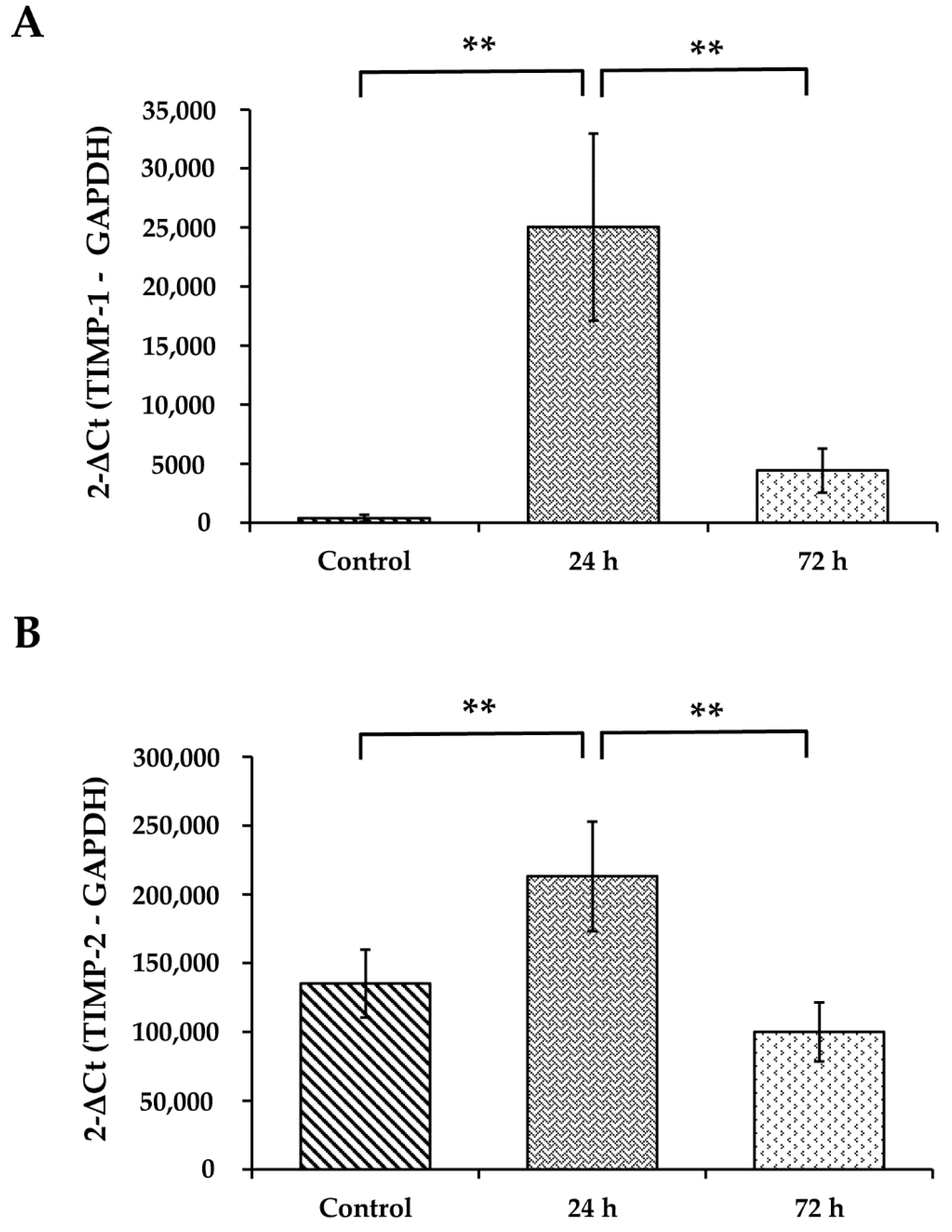
3.7. WS Upregulates the mRNA Expression Levels of TIMP-1 and TIMP-2
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Global Initiative for Chronic Obstructive Lung Disease. GOLD-REPORT-2021-v1.1-25Nov20_WMV.pdf (goldcopd.org). Available online: https://goldcopd.org/2021-gold-reports/ (accessed on 26 April 2021).
- Gupta, K.; Mehrotra, M.; Kumar, P.; Gogia, A.R.; Prasad, A.; Fisher, J.A. Inhalation injury: Etiopathogenesis, diagnosis, and management. Indian J. Crit. Care Med. 2018, 22, 180–188. [Google Scholar] [CrossRef]
- Pathak, U.; Gupta, N.C.; Suri, J.C. Risk of COPD due to indoor air pollution from biomass cooking fuel: A systematic review and meta-analysis. Int. J. Environ. Health Res. 2020, 30, 75–88. [Google Scholar] [CrossRef] [PubMed]
- Walker, P.F.; Buehner, M.F.; Wood, L.A.; Boyer, N.L.; Driscoll, I.R.; Lundy, J.B.; Cancio, L.C.; Chung, K.K. Diagnosis and management of inhalation injury: An updated review. Crit. Care 2015, 19, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Swiston, J.R.; Davidson, W.; Attridge, S.; Brauer, L.M.; van Eeden, S.F. Wood smoke exposure induces a pulmonary and systemic inflammatory response in firefighters. Eur. Respir. J. 2008, 32, 129–138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hejl, A.; Adetona, O.; Diaz-Sanchez, D.; Carter, J.D.; Commodore, A.A.; Rathbun, S.L.; Naeher, L.P. Inflammatory effects of woodsmoke exposure among wildland firefighters working at prescribed burns at the Savannah River Site, SC. J. Occup. Environ. Hyg. 2013, 10, 173–180. [Google Scholar] [CrossRef]
- Toledo, R.T. Wood Smoke Components and Functional Properties. In International Smoked Seafood Conference Proceedings, Alaska Sea Grant College Program, Fairbanks; Kramer, D.E., Brown, L., Eds.; Alaska Sea Grant Program, University of Alaska Fairbanks: Fairbanks, AK, USA, 2008; pp. 55–61. [Google Scholar]
- Schwartz, C.; Bølling, A.K.; Carlsten, C. Controlled human exposures to wood smoke: A synthesis of the evidence. Part. Fibre. Toxicol. 2020, 17, 49. [Google Scholar] [CrossRef]
- Fricker, M.; Deane, A.; Hansbro, P.M. Animal models of chronic obstructive pulmonary disease. Expert. Opin. Drug. Discov. 2014, 9, 629–645. [Google Scholar] [CrossRef]
- David, P.; Dunsford, D.; Lu, J.; Moochhala, S. Animal models of smoke inhalation induced injuries. Front. Biosci. 2009, 14, 4618–4630. [Google Scholar]
- Regalado, J.; Pérez-Padilla, R.; Sansores, R.; Páramo Ramirez, J.I.; Brauer, M.; Paré, P.; Vedal, S. The effect of biomass burning on respiratory symptoms and lung function in rural Mexican women. Am. J. Respir. Crit. Care Med. 2006, 174, 901–905. [Google Scholar] [CrossRef]
- Ramos, C.; Pedraza-Chaverri, J.; Becerril, C.; Cisneros, J.; González-Ávila, G.; Rivera-Rosales, G.; Sommer, B.; Medina-Campos, O.N.; Montaño, M. Oxidative stress and lung injury induced by short-term exposure to wood smoke in guinea pigs. Toxicol. Mech. Methods 2013, 23, 711–722. [Google Scholar] [CrossRef]
- Granados-Castro, L.F.; Rodríguez-Rangel, D.S.; Montaño, M.; Ramos, C.; Pedraza-Chaverri, J. Wood smoke exposure induces a decrease in respiration parameters and in the activity of respiratory complexes I and IV in lung mitochondria from guinea pigs. Environ. Toxicol. 2015, 30, 461–471. [Google Scholar] [CrossRef] [PubMed]
- Vang, A.; Clements, R.T.; Chichger, H.; Kue, N.; Allawzi, A.; O’Connell, K.; Jeong, E.M.; Dudley, S.C., Jr.; Sakhatskyy, P.; Lu, Q.; et al. Effect of α7 nicotinic acetylcholine receptor activation on cardiac fibroblasts: A mechanism underlying RV fibrosis associated with cigarette smoke exposure. Am. J. Physiol. Lung Cell Mol. Physiol. 2017, 312, L748–L759. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative C T method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef] [PubMed]
- Chomczynski, P.; Sacchi, N. Single-step method of RNA isolation by acid guanidinium thiocyanatephenol-chloroform extraction. Anal. Biochem. 1987, 162, 156–159. [Google Scholar] [CrossRef]
- Esquivel, A.L.; Pérez-Ramos, J.; Cisneros, J.; Herrera, I.; Rivera-Rosales, R.; Montaño, M.; Ramos, C. The effect of obesity and tobacco smoke exposure on inflammatory mediators and matrix metalloproteinases in rat model. Toxicol. Mech. Methods 2014, 24, 633–643. [Google Scholar] [CrossRef]
- Naeher, L.P.; Brauer, M.; Lipsett, M.; Zelikoff, J.T.; Simpson, C.D.; Koenig, J.Q.; Smith, K.R. Woodsmoke health effects: A review. Inhal. Toxicol. 2007, 19, 67–106. [Google Scholar] [CrossRef]
- Demling, R.H. Smoke inhalation lung injury: An update. Eplasty 2008, 8, e27. [Google Scholar]
- Migliaccio, C.; Mauderly, J. Biomass smoke exposures: Toxicology and animal study design. Inhal. Toxicol. 2010, 22, 104–107. [Google Scholar] [CrossRef]
- Mehra, D.; Geraghty, P.; Hardigan, A.; Foronjy, R.A. A comparison of the inflammatory and proteolytic effects of dung biomass and cigarette smoke exposure in the lung. PLoS ONE 2012, 7, e52889. [Google Scholar]
- Wong, J.; Magun, B.; Wood, L. Lung inflammation caused by inhaled toxicants: A review. Int. J. Chron. Obstruct. Pulmon. Dis. 2016, 11, 1391–1401. [Google Scholar] [CrossRef] [Green Version]
- Sussan, T.E.; Ingole, V.; Kim, J.H.; McCormick, S.; Negherbon, J.; Fallica, J.; Akulian, J.; Yarmus, L.; Feller-Kopman, D.; Wills-Karp, M.; et al. Source of biomass cooking fuel determines pulmonary response to household air pollution. Am. J. Respir. Cell Mol. Biol. 2014, 50, 538–548. [Google Scholar] [CrossRef]
- Ghio, A.J.; Soukup, J.M.; Case, M.; Dailey, L.A.; Richards, J.; Berntsen, J.; Devlin, R.B.; Stone, S.; Rappold, A. Exposure to WS particles produces inflammation in healthy volunteers. Occup. Environ. Med. 2012, 69, 170–175. [Google Scholar] [CrossRef]
- Hiraiwa, K.; van Eeden, S.F. Contribution of lung macrophages to the inflammatory responses induced by exposure to air pollutants. Mediat. Inflamm. 2013, 2013, 619523. [Google Scholar] [CrossRef] [Green Version]
- Butterfield, T.A.; Best, T.M.; Merrick, M.A. The dual roles of neutrophils and macrophages in inflammation: A critical balance between tissue damage and repair. J. Athl. Train. 2006, 1, 57–465. [Google Scholar]
- Valledor, A.F.; Comalada, M.; Santamaría-Babi, L.F.; Lloberas, J.; Celada, A. Macrophage Proinflammatory Activation and Deactivation: A Question of Balance. Macrophage pro-inflammatory activation and deactivation: A question of balance. Adv. Immunol. 2010, 108, 1–20. [Google Scholar]
- Gonzalez-Avila, G.; Sommer, B.; Mendoza-Posada, D.A.; Ramos, C.; Garcia-Hernandez, A.A.; Falfan-Valencia, R. Matrix metalloproteinases participation in the metastatic process and their diagnostic and therapeutic applications in cancer. Crit. Rev. Oncol. Hematol. 2019, 137, 57–83. [Google Scholar] [CrossRef]
- Gonzalez-Avila, G.; Sommer, B.; García-Hernández, A.A.; Ramos, C. Matrix Metalloproteinases’ Role in Tumor Microenvironment. Adv. Exp. Med. Biol. 2020, 1245, 97–131. [Google Scholar] [PubMed]
- Jensen, A.; Karottki, D.G.; Christensen, J.M.; Bønløkke, J.H.; Sigsgaard, T.; Glasius, M.; Loft, S.; Møller, P. Biomarkers of oxidative stress and inflammation after wood smoke exposure in a reconstructed Viking Age house. Environ. Mol. Mutagen. 2014, 55, 652–661. [Google Scholar] [CrossRef] [PubMed]
- Rylance, J.; Fullerton, D.G.; Scriven, J.; Aljurayyan, A.N.; Mzinza, D.; Barrett, S.; Wright, A.K.A.; Wootton, D.G.; Glennie, S.J.; Baple, K.; et al. Household air pollution causes dose-dependent inflammation and altered phagocytosis in human macrophages. Am. J. Respir. Cell Mol. Biol. 2015, 52, 584–593. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olopade, C.O.; Frank, E.; Bartlett, E.; Alexander, D.; Dutta, A.; Ibigbami, T.; Adu, D.; Olamijulo, J.; Arinola, G.; Karrison, T.; et al. Effect of a clean stove intervention on inflammatory biomarkers in pregnant women in Ibadan, Nigeria: A randomized controlled study. Environ. Int. 2017, 98, 181–190. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, R.; Hebert, J.D.; Lee, T.A.; Xing, H.; Boussommier-Calleja, A.; Hynes, R.O.; Lauffenburger, D.A.; Kamm, R.D. Macrophage-Secreted TNFα and TGFβ1 Influence Migration Speed and Persistence of Cancer Cells in 3D Tissue Culture via Independent Pathways. Cancer Res. 2017, 77, 279–290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leonard, S.S.; Wang, S.; Shi, X.; Jordan, B.S.; Castranova, V.; Dubick, M.A. Wood smoke particles generate free radicals and cause lipid peroxidation, DNA damage, NFκB activation and TNF-α release in macrophages. Toxicology 2000, 150, 147–157. [Google Scholar] [CrossRef]
- Barnes, P.J. Targeting cytokines to treat asthma and chronic obstructive pulmonary disease. Nat. Rev. Immunol. 2018, 18, 454–466. [Google Scholar] [CrossRef]
- Costa, L.D.A.; Ottoni, M.H.F.; Dos Santos, M.G.; Meireles, A.B.; De Almeida, V.G.; Pereira, W.D.F.; De Avelar-Freitas, B.A.; Brito-Melo, G.E.A. Dimethyl sulfoxide (DMSO) decreases cell proliferation and TNF-α, IFN-γ, and IL-2 cytokines production in cultures of peripheral blood lymphocytes. Molecules 2017, 22, 1789. [Google Scholar] [CrossRef] [Green Version]
- Davis, G.; Pfeiffer, L.; Hemenway, D. Interferon-γ production by specific lung lymphocyte phenotypes in silicosis in mice. Am. J. Respir. Cell Mol. Biol. 2000, 22, 491–501. [Google Scholar] [CrossRef]
- Hinz, B. The extracellular matrix and transforming growth factor-β1: Tale of a strained relationship. Matrix Biol. 2015, 47, 54–65. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, T.; O’Reilly, P.; Antony, V.B.; Gaggar, A.; Thannickal, V.J. Matrix remodeling in pulmonary fibrosis and emphysema. Am. J. Respir. Cell Mol. Biol. 2016, 54, 751–760. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marchetti, S.; Longhin, E.; Bengalli, R.; Avino, P.; Stabile, L.; Buonanno, G.; Colombo, A.; Camatini, M.; Mantecca, P. In vitro lung toxicity of indoor PM10 from a stove fueled with different biomasses. Sci. Total Environ. 2019, 649, 1422–1433. [Google Scholar] [CrossRef]

| Gene | Primer Sequence (5′–3′) | Product Size (bp) |
|---|---|---|
| TNF-α | Forward AACTCCAGCCGGTGCCTAT Reverse GTTCAGCAGGCAGAAGAGGATT | 81 |
| INF-γ | Forward GGCCATCCAGAGGAGCATAG Reverse CCATGCTGCTGTTGAAGAAGTTAG | 68 |
| TGF-β1 | Forward GCGGCAGCTGTACATCGA Reverse GGCTCGTGAATCCACTTCCA | 57 |
| IL-1β | Forward CTTGAGGACTGGACCTTTTGC Reverse TCGTCACTGTGGTAAGCTGT | 80 |
| IL-6 | Forward GTTCAGCACGACTTCACAGC Reverse TGTGAAGCAGAGGTTGTTGGT | 212 |
| IL-8 | Forward TAGGGTGGCAGATTTAACTCA Reverse TCAGGAATTGGCTTGCTAC | 112 |
| IL-12 | Forward AAAACCAGCACCGTGAAAGC Reverse AAAACCAGCACCGTGAAAGC | 296 |
| MMP-2 | Forward CTACCCTTGTACCACCATCGA Reverse TTAGCTGACCGTCACCAATC | 462 |
| MMP-9 | Forward GTGACACCGCTCACCTTCAC Reverse GCGTGTGCCAGTAGACCATC | 122 |
| TIMP-1 | Forward GATCGGATGCCTTGGGACAT Reverse TTCTGGGACGGGTGGAAGTA | 326 |
| TIMP-2 | Forward GACCTGTCTGACGCCCTACCTCTT Reverse ATGGGAACCCCATCAAGCGA | 358 |
| GAPDH | Forward TCAGAGGGCTCCCTCAAAG Reverse CGCTGTTGAAGTCACAGGAC | 70 |
| Molecule | Concentration in Chamber |
|---|---|
| CO2 | 0.31 ± 0.13% |
| O2 | 19.8 ± 0.15% |
| PM10 | 479 ± 45 mg/m3 |
| PM2.5 | 361 ± 32 mg/m3 |
| Plasma COHb | (%) |
| Control | 3.36 ± 2.12 |
| WS 24 h | 12.96 ± 4.91 ** |
| WS 72 h | 14.36 ± 5.89 ** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramos, C.; Cañedo-Mondragón, R.; Becerril, C.; González-Ávila, G.; Esquivel, A.L.; Torres-Machorro, A.L.; Montaño, M. Short-Term Exposure to Wood Smoke Increases the Expression of Pro-Inflammatory Cytokines, Gelatinases, and TIMPs in Guinea Pigs. Toxics 2021, 9, 227. https://doi.org/10.3390/toxics9090227
Ramos C, Cañedo-Mondragón R, Becerril C, González-Ávila G, Esquivel AL, Torres-Machorro AL, Montaño M. Short-Term Exposure to Wood Smoke Increases the Expression of Pro-Inflammatory Cytokines, Gelatinases, and TIMPs in Guinea Pigs. Toxics. 2021; 9(9):227. https://doi.org/10.3390/toxics9090227
Chicago/Turabian StyleRamos, Carlos, Rebeca Cañedo-Mondragón, Carina Becerril, Georgina González-Ávila, Ana Laura Esquivel, Ana Lilia Torres-Machorro, and Martha Montaño. 2021. "Short-Term Exposure to Wood Smoke Increases the Expression of Pro-Inflammatory Cytokines, Gelatinases, and TIMPs in Guinea Pigs" Toxics 9, no. 9: 227. https://doi.org/10.3390/toxics9090227
APA StyleRamos, C., Cañedo-Mondragón, R., Becerril, C., González-Ávila, G., Esquivel, A. L., Torres-Machorro, A. L., & Montaño, M. (2021). Short-Term Exposure to Wood Smoke Increases the Expression of Pro-Inflammatory Cytokines, Gelatinases, and TIMPs in Guinea Pigs. Toxics, 9(9), 227. https://doi.org/10.3390/toxics9090227







