ChemSkin Reference Chemical Database for the Development of an In Vitro Skin Irritation Test
Abstract
:1. Introduction
2. Materials and Methods
2.1. Classification Criteria for the Skin Irritancy of Chemicals
2.1.1. Classification Based on Primary Irritation Index (PII)
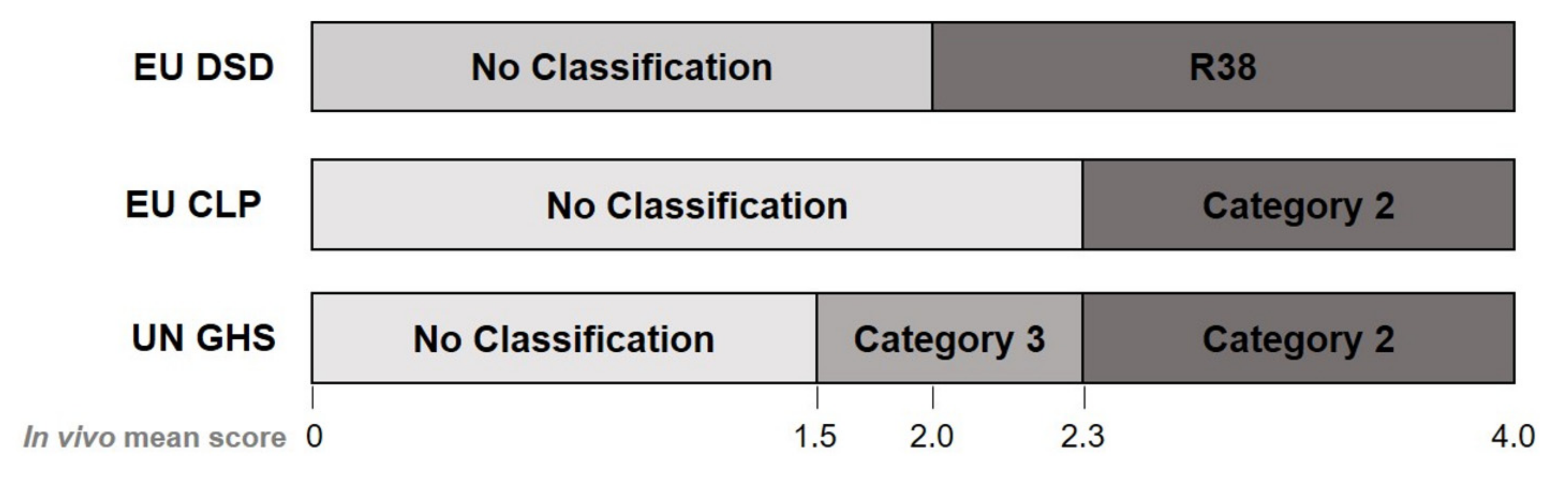
2.1.2. Classification Based on EU DSD/DPD and EU CLP
2.1.3. Classification Based on the Current UN GHS
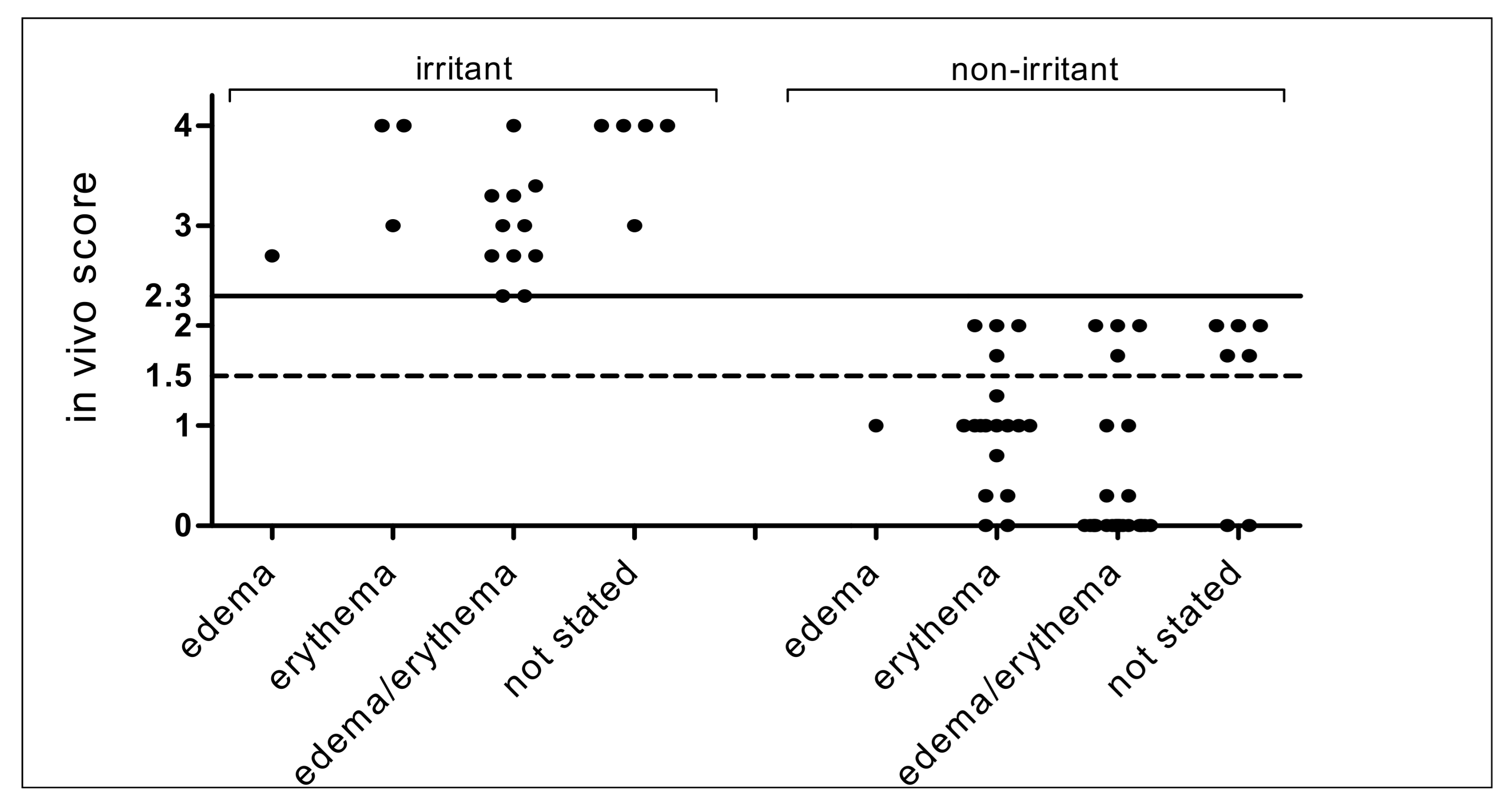
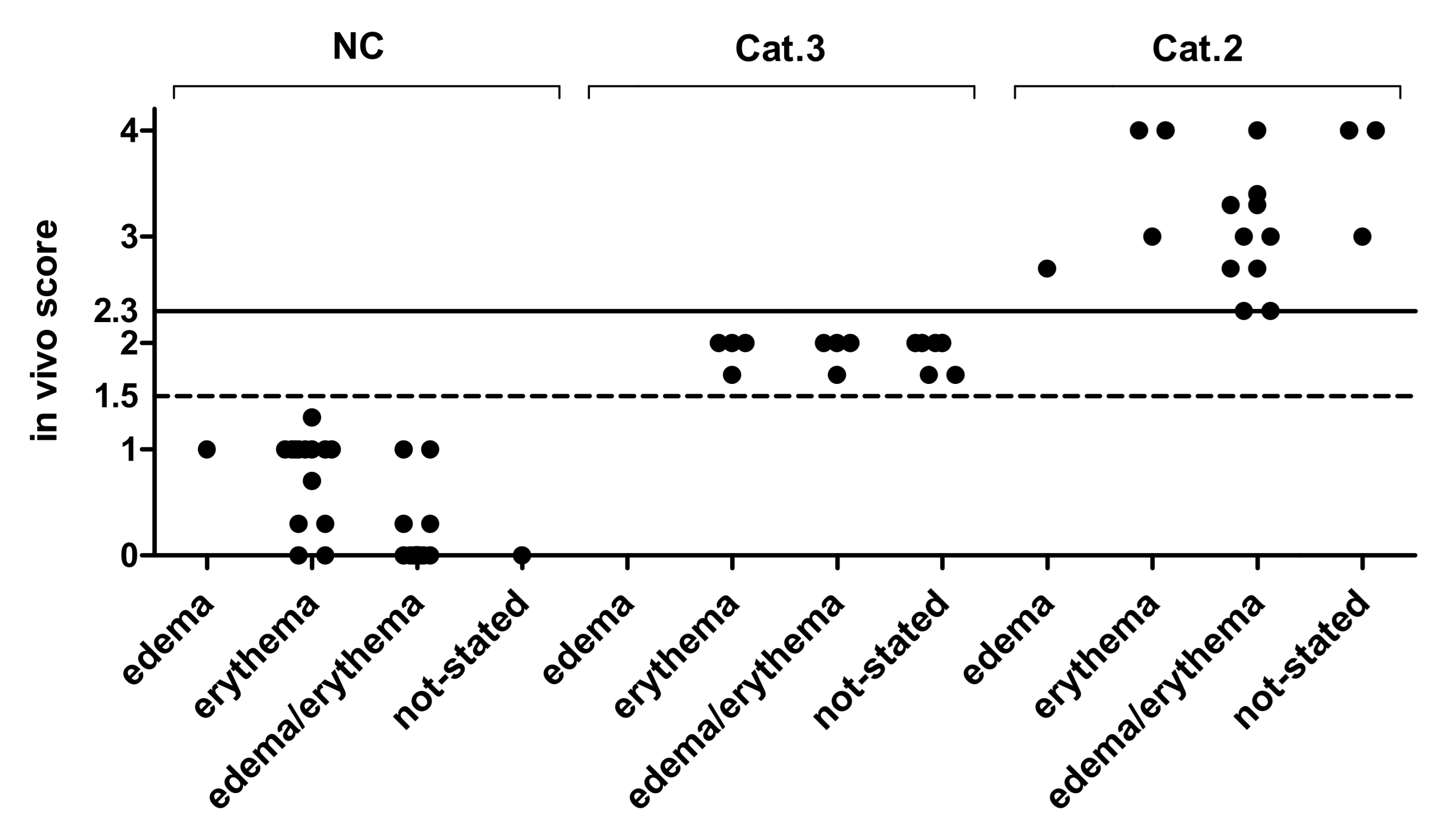
2.2. Classification of Edema/Erythema of 100 Reference Chemicals Based on the In Vivo Draize Test
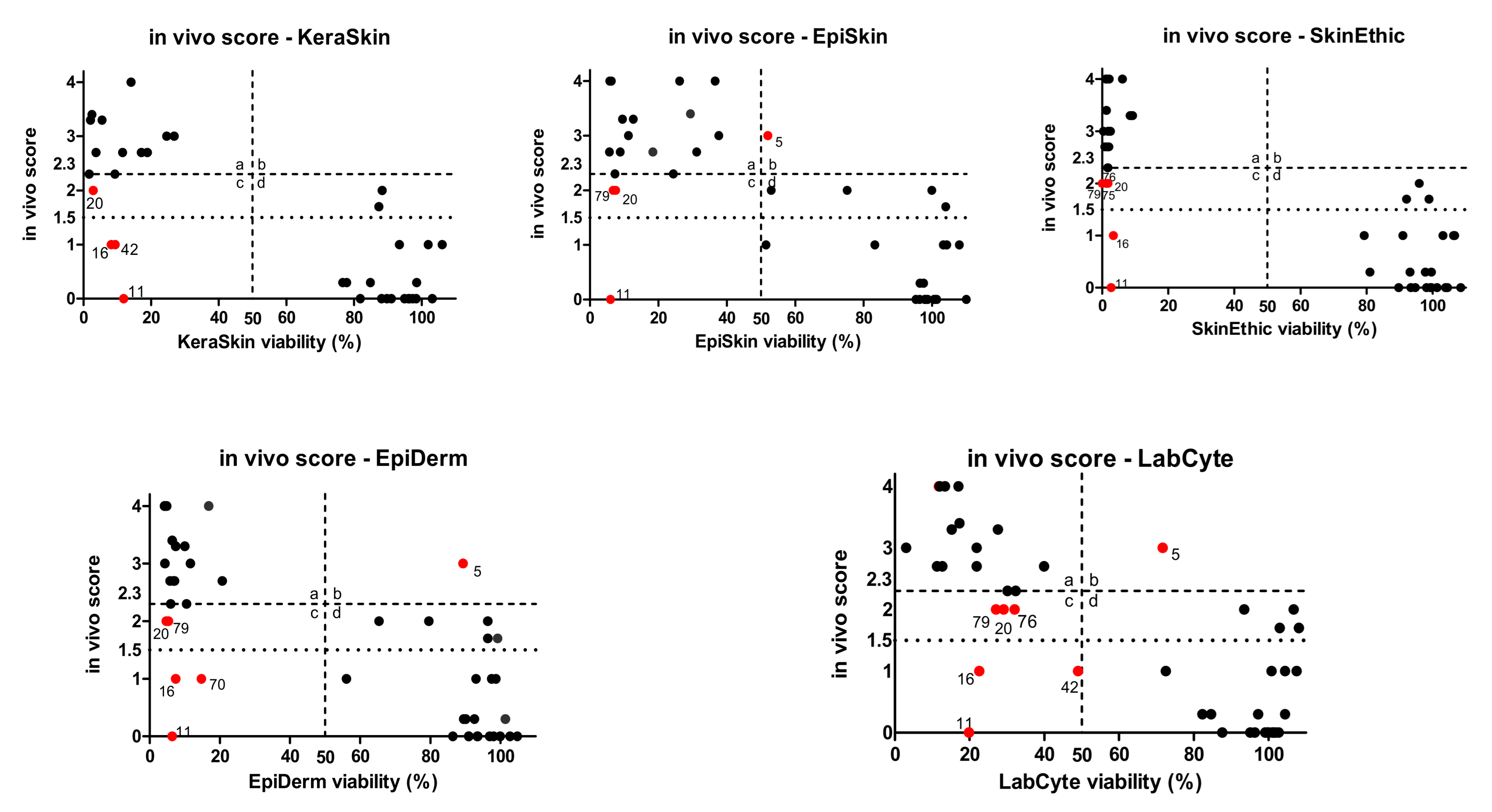
2.3. Comparison of In Vivo and In Vitro Data of ChemSkin DB Reference Chemicals
3. Results and Discussion
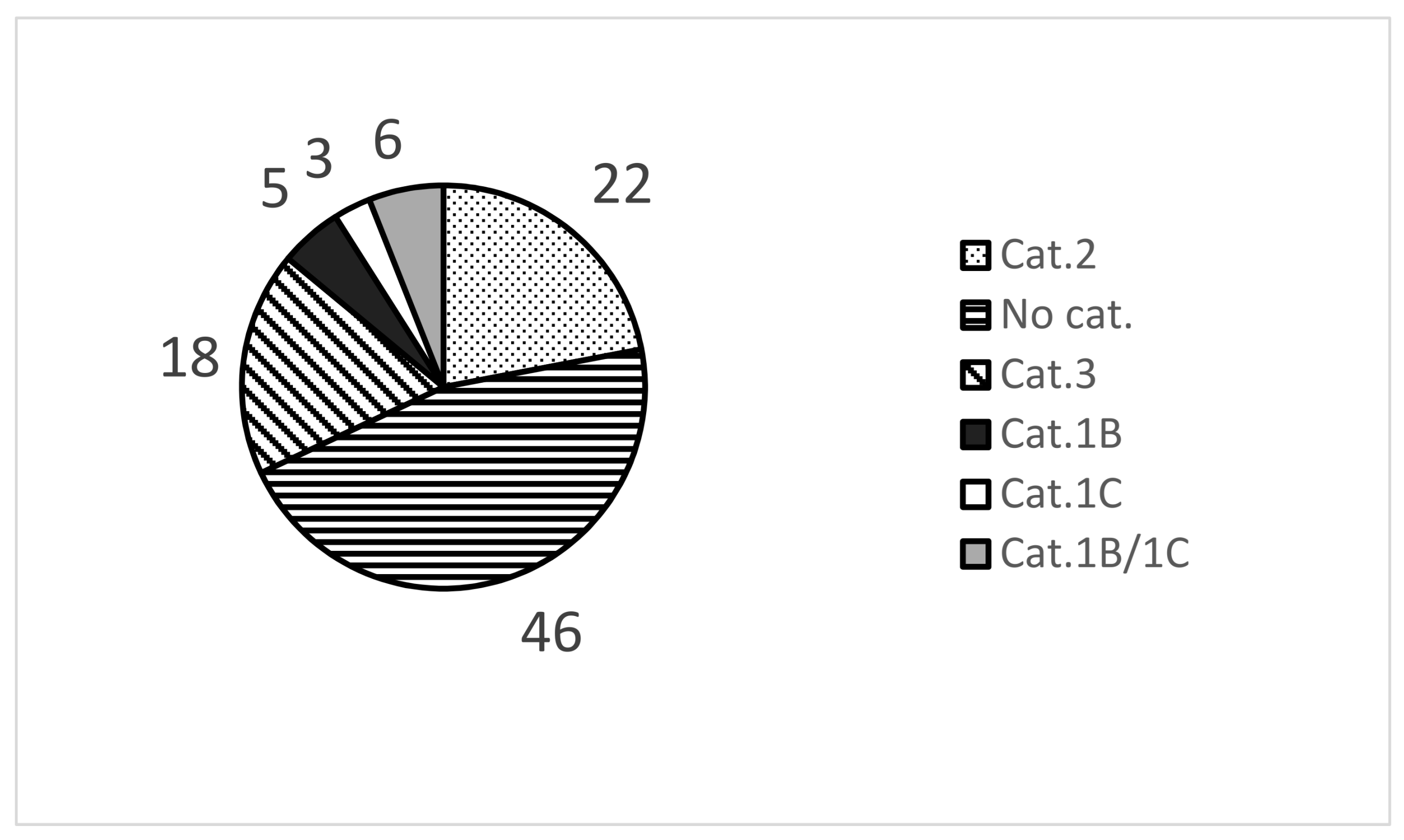
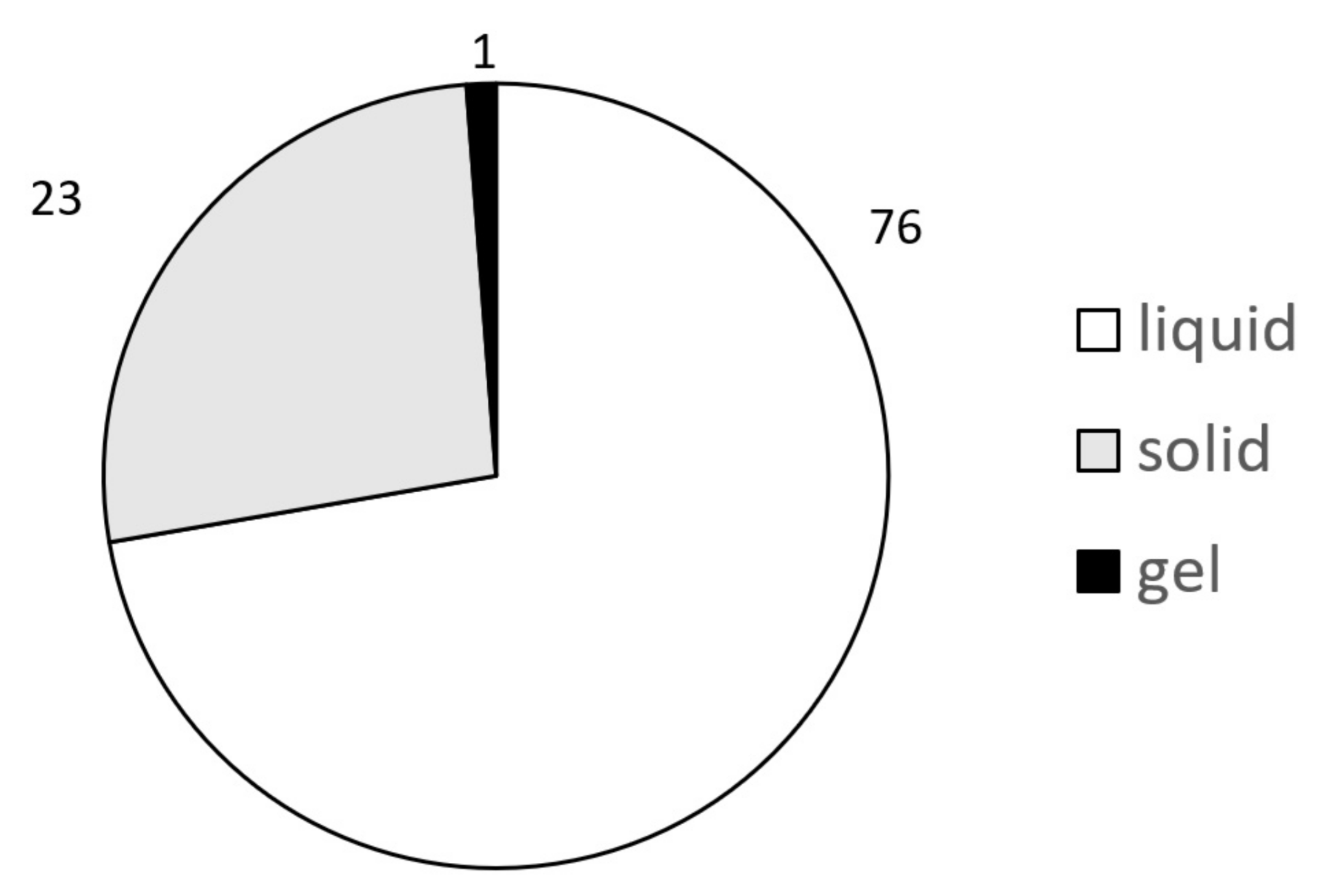
3.1. Chemical Selection for the Establishment of ChemSkin DB
3.2. In Vivo Draize Scores of 86 Chemicals in ChemSkin DB
3.3. Comparison of Prediction Results Produced by OECD TG 439 Test Methods for ChemSkin DB Substances
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Whitehead, H.D.; Venier, M.; Wu, Y.; Eastman, E.; Urbanik, S.; Diamond, M.L.; Shalin, A.; Schwartz-Narbonne, H.; Bruton, T.A.; Blum, A.; et al. Fluorinated Compounds in North American Cosmetics. Environ. Sci. Technol. Lett. 2021, 8, 538–544. [Google Scholar] [CrossRef]
- Adler, S.; Basketter, D.; Creton, S.; Pelkonen, O.; Van Benthem, J.; Zuang, V.; Andersen, K.E.; Angers-Loustau, A.; Aptula, A.; Bal-Price, A. Alternative (non-animal) methods for cosmetics testing: Current status and future prospects—2010. Arch. Toxicol. 2011, 85, 367–485. [Google Scholar] [CrossRef]
- Akbarsha, M.A.; Mascarenhas, B. Cosmetic Regulation and Alternatives to Animal Experimentation in India. In Alternatives to Animal Testing-Proceedings of Asian Congress 2016; Kojima, H., Seidle, T., Spielmann, H., Eds.; Springer: Singapore, 2019; pp. 57–62. [Google Scholar]
- Avonto, C.; Wang, M.; Chittiboyina, A.G.; Vukmanovic, S.; Khan, I.A. Chemical stability and in chemico reactivity of 24 fragrance ingredients of concern for skin sensitization risk assessment. Toxicol. In Vitro 2018, 46, 237–245. [Google Scholar] [CrossRef]
- Kim, J.Y.; Kim, M.K.; Kim, K.B.; Kim, H.S.; Lee, B.M. Quantitative structure-activity and quantitative structure-property relationship approaches as alternative skin sensitization risk assessment methods. J. Toxicol. Environ. Health Part A 2019, 82, 447–472. [Google Scholar] [CrossRef]
- Kim, M.K.; Kim, K.B.; Kim, H.S.; Lee, B.M. Alternative skin sensitization prediction and risk assessment using proinflammatory biomarkers, interleukin-1 beta (IL-1beta) and inducible nitric oxide synthase (iNOS). J. Toxicol. Environ. Health Part A 2019, 82, 361–378. [Google Scholar] [CrossRef] [PubMed]
- Organisation for Economic Co-Operation and Development. Series on Testing and Assessment No. 34: Guidance Document on the Validation and International Acceptance of New or Updated Test Methods for Hazard Assessment; Organisation for Economic Co-Operation and Development Environment: Paris, France, 2005. [Google Scholar]
- Engelke, M.; Zorn-Kruppa, M.; Gabel, D.; Reisinger, K.; Rusche, B.; Mewes, K. A human hemi-cornea model for eye irritation testing: Quality control of production, reliability and predictive capacity. Toxicol. In Vitro 2013, 27, 458–468. [Google Scholar] [CrossRef] [PubMed]
- Jírová, D.; Basketter, D.; Liebsch, M.; Bendová, H.; Kejlová, K.; Marriott, M.; Kandárová, H. Comparison of human skin irritation patch test data with In vitro skin irritation assays and animal data. Contact Dermat. 2010, 62, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Gerberick, F.G.; Ryan, C.A.; Kern, P.S.; Schlatter, H.; Dearman, R.J.; Kimber, I.; Patlewicz, G.Y.; Basketter, D.A. Compilation of historical local lymph node data for evaluation of skin sensitization alternative methods. Dermatitis 2005, 16, 157–202. [Google Scholar]
- Barroso, J.; Pfannenbecker, U.; Adriaens, E.; Alépée, N.; Cluzel, M.; De Smedt, A.; Hibatallah, J.; Klaric, M.; Mewes, K.R.; Millet, M. Cosmetics Europe compilation of historical serious eye damage/eye irritation in vivo data analysed by drivers of classification to support the selection of chemicals for development and evaluation of alternative methods/strategies: The Draize eye test Reference Database (DRD). Arch. Clin. Toxicol. 2017, 91, 521–547. [Google Scholar]
- Fentem, J.H.; Botham, P.A. ECVAM’s activities in validating alternative tests for skin corrosion and irritation. Altern. Lab. Anim. 2002, 30, 61–67. [Google Scholar] [CrossRef]
- Organisation for Economic Co-Operation and Development. Test Guideline No. 404: Acute Dermal Irritation/Corrosion. OECD Guidelines for the Testing of Chemicals; Organisation for Economic Cooperation and Development: Paris, France, 2015. [Google Scholar]
- Draize, J.H.; Woodard, G.; Calvery, H.O. Methods for the study of irritation and toxicity of substances applied topically to the skin and mucous membranes. Pharmacol. Exp. Ther. 1944, 82, 377–390. [Google Scholar]
- European Commission. Commission Directive 2001/59/EC of 6 August 2001 adapting to technical progress for the 28th time Council Directive 67/548/EEC on the approximation of laws, regulations and administrative provisions relating to the classification, packaging and labelling of dangerous substances. Off. J. Eur. Communities L 2001, 225, 1–333. [Google Scholar]
- European Commission. Regulation (EC) No. 1272/2008 of the European Parliament and of the Council of 16 December 2008 on Classification, Labelling and Packaging of substances and mixtures, amending and repealing Directives 67/548/EEC and 1999/45/EC, and amending Regulation (EC) No. 1907/2006. Off. J. Eur. Union L 2008, 50, 353. [Google Scholar]
- United Nations. Globally Harmonised System of Classification and Labelling of Chemicals (GHS); United Nations: New York, NY, USA; Geneva, Swizterland, 2019. [Google Scholar]
- Organisation for Economic Co-Operation and Development. Test Guideline No. 439: In Vitro Skin Irritation (Reconstructed Human Epidermis Test Method); Organisation for Economic Cooperation and Development: Paris, France, 2015. [Google Scholar]
- Organisation for Economic Co-Operation and Development. Test Guideline No. 439: In Vitro skin Irritation (Reconstructed Human Epidermis Test Method); Organisation for Economic Cooperation and Development: Paris, France, 2021. [Google Scholar]
- Pedrosa, T.D.N.; Catarino, C.M.; Pennacchi, P.C.; Assis, S.R.; Gimenes, F.; Consolaro, M.E.L.; Barros, S.B.M.; Maria-Engler, S.S. A new reconstructed human epidermis for In vitro skin irritation testing. Toxicol. In Vitro 2017, 42, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Spielmann, H.; Hoffmann, S.; Liebsch, M.; Botham, P.; Fentem, J.H.; Eskes, C.; Roguet, R.; Cotovio, J.; Cole, T.; Worth, A.; et al. The ECVAM international validation study on In vitro tests for acute skin irritation: Report on the validity of the EPISKIN and EpiDerm assays and on the Skin Integrity Function Test. Altern. Lab. Anim. 2007, 35, 559–601. [Google Scholar] [CrossRef] [Green Version]
- Katoh, M.; Hamajima, F.; Ogasawara, T.; Hata, K. Assessment of human epidermal model LabCyte EPI-MODEL for In vitro skin irritation testing according to European Centre for the Validation of Alternative Methods (ECVAM)-validated protocol. J. Toxicol. Sci. 2009, 34, 327–334. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Katoh, M.; Hata, K. Refinement of LabCyte EPI-MODEL24 Skin Irritation Test Method for Adaptation to the Requirements of OECD Test Guideline 439. Altern. Anim. Test. Exp. 2011, 16, 111–122. [Google Scholar]
- Eskes, C.; Cole, T.; Hoffmann, S.; Worth, A.; Cockshott, A.; Gerner, I.; Zuang, V. The ECVAM international validation study on In vitro tests for acute skin irritation: Selection of test chemicals. Altern. Lab. Ani. 2007, 35, 603–619. [Google Scholar] [CrossRef]
- Shimazaki, T.; Nagai, T.; Kawamoto, Y.; Ozawa, M.; Suzuki, M. SAGAN COAT Photocatalyst Coating Solution TPX for Primary Skin Irritation in Rabbits; Japan Food Research Laboratories: Tokyo, Japan, 2007. [Google Scholar]
- Lee Rodgers, J.; Nicewander, W.A.J.T.A.S. Thirteen ways to look at the correlation coefficient. Am. Stat. 1988, 42, 59–66. [Google Scholar] [CrossRef]
- Zhou, H.; Deng, Z.; Xia, Y.; Fu, M. A new sampling method in particle filter based on Pearson correlation coefficient. Neurocomputing 2016, 216, 208–215. [Google Scholar] [CrossRef]
- Taylor, R. Interpretation of the correlation coefficient: A basic review. J. Diagn. Med. Sonogr. 1990, 6, 35–39. [Google Scholar] [CrossRef]
- Basketter, D.; Jírova, D.; Kandárová, H. Review of skin irritation/corrosion Hazards on the basis of human data: A regulatory perspective. Interdiscip. Toxicol. 2012, 5, 98–104. [Google Scholar] [CrossRef] [PubMed]
- Kandárová, H.; Hayden, P.; Klausner, M.; Kubilus, J.; Kearney, P.; Sheasgreen, J. In vitro skin irritation testing: Improving the sensitivity of the EpiDerm skin irritation test protocol. Altern. Lab. Anim. 2009, 37, 671–689. [Google Scholar] [CrossRef]
- Organisation for Economic Co-Operation and Development. Series on Testing and Assessment No. 137: Explanatory Background Document to the OECD Test Guideline on In Vitro Skin Irritation Testing; Organisation for Economic Co-Operation and Development Environment: Paris, France, 2010. [Google Scholar]
- Organisation for Economic Co-Operation and Development. Series on Testing and Assessment No. 220: Performance Standards for the Assessment of Proposed Similar or Modified In Vitro Reconstructed Human Epidermis (RhE) Test Methods for Skin Irritation Testing as Described in TG 439 (Intended for the Developers of New or Modifed Similar Test Methods); Organisation for Economic Co-Operation and Development Environment: Paris, France, 2015. [Google Scholar]
- Alépée, N.; Grandidier, M.-H.; Tornier, C.; Cotovio, J. An integrated testing strategy for In vitro skin corrosion and irritation assessment using SkinEthic™ Reconstructed Human Epidermis. Toxicol. In Vitro 2015, 29, 1779–1792. [Google Scholar] [CrossRef]
- Organisation for Economic Co-Operation and Development. Series on Testing and Assessment No.155: Peer Review Report of the Validation of the Skin Irritation Test Using Labcyte EPI-Model 24; Organisation for Economic Co-Operation and Development Environment: Paris, France, 2011. [Google Scholar]
- Tornier, C.; Amsellem, C.; de Fraissinette, A.D.B.; Alépée, N. Assessment of the optimized SkinEthic™ Reconstructed Human Epidermis (RHE) 42 bis skin irritation protocol over 39 test substances. Toxicol. In Vitro 2010, 24, 245–256. [Google Scholar] [CrossRef]
- Alépée, N.; Tornier, C.; Robert, C.; Amsellem, C.; Roux, M.-H.; Doucet, O.; Pachot, J.; Méloni, M.; de Fraissinette, A.D.B. A catch-up validation study on reconstructed human epidermis (SkinEthic™ RHE) for full replacement of the Draize skin irritation test. Toxicol. In Vitro 2010, 24, 257–266. [Google Scholar] [CrossRef]
- Li, N.; Liu, Y.; Qiu, J.; Zhong, L.; Alépée, N.; Cotovio, J.; Cai, Z. In vitro skin irritation assessment becomes a reality in China using a reconstructed human epidermis test method. Toxicol. In Vitro 2017, 41, 159–167. [Google Scholar] [CrossRef]
- Alépée, N.; Grandidier, M.-H.; Cotovio, J. Usefulness of the EpiSkin™ reconstructed human epidermis model within Integrated Approaches on Testing and Assessment (IATA) for skin corrosion and irritation. Toxicol. In Vitro 2019, 54, 147–167. [Google Scholar] [CrossRef] [PubMed]
- Tornier, C.; Rosdy, M.; Maibach, H.I. In vitro skin irritation testing on reconstituted human epidermis: Reproducibility for 50 chemicals tested with two protocols. Toxicol. In Vitro 2006, 20, 401–416. [Google Scholar] [CrossRef]
- Kandárová, H.; Liebsch, M.; Schmidt, E.; Genschow, E.; Traue, D.; Spielmann, H.; Meyer, K.; Steinhoff, C.; Tornier, C.; De Wever, B. Assessment of the skin irritation potential of chemicals by using the SkinEthic reconstructed human epidermal model and the common skin irritation protocol evaluated in the ECVAM skin irritation validation study. Altern. Lab. Anim. 2006, 34, 393–406. [Google Scholar] [CrossRef]
- European Chemicals Agency. Disseminated Registration Dossier for Heptanoic Acid (CAS No. 111-14-8): Irritation/Corrosion_Endpoint Summary. Available online: https://echa.europa.eu/registration-dossier/-/registered-dossier/15829/7/4/2/?documentUUID=6f6c78bb-666f-43b2-a71e-4048b573130d (accessed on 14 November 2021).
- European Centre for Ecotoxicology and Toxicology of Chemicals. Skin Irritation and Corrosion: Reference Chemicals Data Bank, Technical Report No 66; European Centre for Ecotoxicology and Toxicology of Chemicals: Brussels, Belguim, 1995; pp. 1–255. [Google Scholar]
- Jacobs, J.J.; Lehé, C.; Cammans, K.D.; Das, P.K.; Elliott, G.R. Methyl green-pyronine staining of porcine organotypic skin explant cultures: An alternative model for screening for skin irritants. Altern. Lab. Anim. 2000, 28, 279–292. [Google Scholar] [CrossRef]
- Sugiyama, M.; Akita, M.; Alépée, N.; Fujishiro, M.; Hagino, S.; Handa, Y.; Ikeda, H.; Imai, N.; Jitsukawa, S.; Katoh, M. Comparative assessment of 24-h primary skin irritation test and human patch test data with In vitro skin irritation tests according to OECD Test Guideline 439 (for quasi-drugs in Japan). Toxicol. Sci. 2018, 43, 751–768. [Google Scholar] [CrossRef]
- Hoffmann, S.; Saliner, A.G.; Patlewicz, G.; Eskes, C.; Zuang, V.; Worth, A.P. A feasibility study developing an integrated testing strategy assessing skin irritation potential of chemicals. Toxicol. Lett. 2008, 180, 9–20. [Google Scholar] [CrossRef] [PubMed]
- Alépée, N.; Barroso, J.; De Smedt, A.; De Wever, B.; Hibatallah, J.; Klaric, M.; Mewes, K.; Millet, M.; Pfannenbecker, U.; Tailhardat, M. Use of HPLC/UPLC-spectrophotometry for detection of formazan in In vitro reconstructed human tissue (RhT)-based test methods employing the MTT-reduction assay to expand their applicability to strongly coloured test chemicals. Toxicol. In Vitro 2015, 29, 741–761. [Google Scholar] [CrossRef]
- European Chemicals Agency. European Chemicals Agency. Disseminated Registration Dossier for Triclocarban (CAS No. 101-20-2): Irritation/Corrosion_Endpoint Summary. Available online: https://echa.europa.eu/registration-dossier/-/registered-dossier/12075/7/4/1 (accessed on 14 November 2021).
- European Chemicals Agency. Disseminated Registration Dossier for Propane-1,2-diol (CAS No. 57-55-6): Irritation/Corrosion_Endpoint Summary. Available online: https://echa.europa.eu/registration-dossier/-/registered-dossier/16001/7/4/1 (accessed on 14 November 2021).
- Golla, S.; Madihally, S.; Robinson, R.L., Jr.; Gasem, K.A. Quantitative structure–Property relationships modeling of skin irritation. Toxicol. In Vitro 2009, 23, 176–184. [Google Scholar] [CrossRef]
- Molinari, J.; Eskes, C.; Andres, E.; Remoué, N.; Sá-Rocha, V.; Hurtado, S.; Barrichello, C. Improved procedures for In vitro skin irritation testing of sticky and greasy natural botanicals. Toxicol. In Vitro 2013, 27, 441–450. [Google Scholar] [CrossRef]
- Scientific Committee on Consumer Products. Reports of the Scientific Committee on Cosmetology (Ninth Series); European Commission: Luxembourg, 2000. [Google Scholar]
- Alépée, N.; Hibatallah, J.; Klaric, M.; Mewes, K.; Pfannenbecker, U.; McNamee, P. Assessment of cosmetic ingredients in the In vitro reconstructed human epidermis test method EpiSkin™ using HPLC/UPLC-spectrophotometry in the MTT-reduction assay. Toxicol. In Vitro 2016, 33, 105–117. [Google Scholar] [CrossRef]
- Scientific Committee on Consumer Safety. Opinion on Tetrabromophenol, 4,4′-(4,5,6,7-Tetrabromo-1,1-Dioxido-3H-2,1-Benzoxathiol-3-Yliden)bis-2,6-Dibromophenol (C183)-Submission IV; European Union, Scientific Committee on Consumer Safety: Brussels, Belgium, 2019. [Google Scholar]
- Scientific Committee on Consumer Safety. Memorandum (Addendum) on the In Vitro Test EPISKIN™ for Skin Irritation Testing; European Union, Scientific Committee on Consumer Safety: Brussels, Belgium, 2010. [Google Scholar]
- Scientific Committee on Consumer Safety. Opinion on Alkyl (C16, C18, C22) Trimethylammonium Chloride: For Other Uses than as a Preservative COLIPA n°P72; European Union, Scientific Committee on Consumer Safety: Brussels, Belgium, 2009. [Google Scholar]
- European Chemicals Agency. Disseminated Registration Dossier for Oxybenzone (CAS No. 131-57-7): Irritation/Corrosion_Endpoint Summary. Available online: https://echa.europa.eu/registration-dossier/-/registered-dossier/5515/7/4/2 (accessed on 14 November 2021).
- Fiume, M.M.; Heldreth, B.; Bergfeld, W.F.; Belsito, D.V.; Hill, R.A.; Klaassen, C.D.; Liebler, D.C.; Marks, J.G., Jr.; Shank, R.C.; Slaga, T. Safety assessment of microbial polysaccharide gums as used in cosmetics. Int. J. Toxicol. 2016, 35, 5S–49S. [Google Scholar] [CrossRef] [PubMed]
- Joint FAO/WHO Expert Committee on Food Additives. Toxicological Evaluation of Certain Food Additives and Contaminants: WHO Food Additives Series 21_Xanthan Gum; Cambridge University Press: Cambridge, UK, 1987. [Google Scholar]
- Kandárová, H.; Liebsch, M.; Genschow, E.; Gerner, I.; Traue, D.; Slawik, B.; Spielmann, H. Optimisation of the EpiDerm test protocol for the upcoming ECVAM validation study on In vitro skin irritation tests. Altern. Anim. Exp. 2004, 21, 107–114. [Google Scholar]
- Liu, Y.; Li, N.; Chen, L.; Alépée, N.; Cai, Z. A ready-to-use integrated In vitro skin corrosion and irritation testing strategy using EpiSkin™ model in China. Toxicol. In Vitro 2020, 65, 104778. [Google Scholar] [CrossRef]
- Fentem, J.; Briggs, D.; Chesné, C.; Elliott, G.; Harbell, J.; Heylings, J.; Portes, P.; Roguet, R.; Van de Sandt, J.; Botham, P. A prevalidation study on In vitro tests for acute skin irritation: Results and evaluation by the Management Team. Toxicol. In Vitro 2001, 15, 57–93. [Google Scholar] [CrossRef]
- Alépée, N.; Grandidier, M.; Cotovio, J. Sub-categorisation of skin corrosive chemicals by the EpiSkin™ reconstructed human epidermis skin corrosion test method according to UN GHS: Revision of OECD Test Guideline 431. Toxicol. In Vitro 2014, 28, 131–145. [Google Scholar] [CrossRef] [PubMed]
- Organisation for Economic Co-Operation and Development. Series on Testing and Assessment No. 219: Performance Standards for the Assessment of Proposed Similar or Modified In Vitro Reconstructed Human Epidermis (RHE) Test Methods for Skin Corrosion Testing as Described in TG 431 (Intended for the Developers of New or Modified Similar Test Methods); Organisation for Economic Co-Operation and Development Environment: Paris, France, 2015. [Google Scholar]
- Ishii, S.; Ishii, K.; Nakadate, M.; Yamasaki, K. Correlation study in skin and eye irritation between rabbits and humans based on published literatures. Food Chem. Toxicol. 2013, 55, 596–601. [Google Scholar] [CrossRef] [PubMed]
- European Chemicals Agency. Disseminated Registration Dossier for Benzyldimethylamine (CAS No. 103-83-3): Irritation/corrosion_Endpoint summary. Available online: https://echa.europa.eu/registration-dossier/-/registered-dossier/13869 (accessed on 14 November 2021).
- Lee, D.H.; Kim, S.-H.; Lee, J.H.; Yang, J.-Y.; Seok, J.-H.; Jung, K.; Lee, J.K. Flow cytometric evaluation of the potential of metal oxide nanoparticles for skin sensitization using 5-Bromo-2-deoxyuridine. Toxicol. Res. 2021, 37, 369–377. [Google Scholar] [CrossRef]
| Classification | Primary Irritation Index (PII) |
|---|---|
| Negligible | 0–0.4 |
| Slight Irritation | 0.5–1.9 |
| Moderate irritation | 2–4.9 |
| Severe irritation | 5–8 |
| c | Chemical | CAS No. | Physical State | In Vivo Category | In Vivo Score | Human In Vivo Result | In vivo Class | In Vivo Score Data Source References | KeraSkinTM SIT | Vendor | VRMs | VRM References | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| EpiSkin | SkinEthic | EpiDerm | LabCyte | ||||||||||||
| 1 | 1-Decanol #, ## | 112-30-1 | Liquid | Cat 2 | 2.3 | NC | I | [29,30,31,32] | 9.3 | Sigma | 7.3 | 1.4–2.4 | 6.0 | 32.3 | [23,30,33,34] |
| 2 | Cyclamen aldehyde # | 103-95-7 | Liquid | Cat 2 | 2.3 | - | I | [30,31,32] | 1.7 | TCI | 24.4 | 1.6–1.8 | 10.5 | 30.1 | [23,30,33,34,35,36,37] |
| 3 | 1-Bromohexane #, ### | 111-25-1 | Liquid | Cat 2 | 2.7 | I | I | [29,30,31,32] | 18.9 | Sigma | 18.4 | 1.1–1.7 | 20.7 | 39.9 | [23,30,33,34] |
| 4 | 2-Chloromethyl-3,5-dimethyl-4-methoxypyridine HCl # | 86604-75-3 | Solid | Cat 2 | 2.7 | - | I | [30,31,32] | 3.7 | Sigma | 5.7 | 0.7–0.8 | 6.7 | 12.7 | [23,30,33,34] |
| 5 | Di-n-propyl disulphide #, ## | 629-19-6 | Liquid | Cat 2 | 3.0 | NC | I | [9,29,30,31,32] | 26.8 | Sigma | 52.0 | 1.2–2.4 | 89.3 | 71.7 | [23,30,33,34] |
| 6 | Potassium hydroxide (5% aq.) # | 1310-58-3 | Liquid | Cat 2 | 3.0 | - | I | [23,33,34] | −0.5 | Sigma | 37.7 | 0.1–0.6 | 4.3 | 3.0 | [23,30,33,34] |
| 7 | Benzenethiol, 5-(1,1-dimethylethyl)-2-methyl # | 7340-90-1 | Liquid | Cat 2 | 3.3 | - | I | [23,33,34] | 2.1 | ACROS | 12.7 | 8.5 | 10.0 | 27.6 | [23,30,34,35] |
| 8 | 1-Methyl-3-phenyl-1-piperazine #, ### | 5271-27-2 | Solid | Cat 2 | 3.3 | - | ci | [29,30,32] | 5.5 | AK Scientific | 9.5 | 9.2 | 7.4 | 15.2 | [23,30,34,35] |
| 9 | Heptanal # | 111-71-7 | Liquid | Cat 2 | 3.4 | I | I | [29,30,31,32] | 2.5 | Sigma | 29.4 | 1.1–1.5 | 6.4 | 17.3 | [23,30,34,35] |
| 10 | Tetrachloroethylene # | 127-18-4 | Liquid | Cat 2 | 4.0 | - | I | [30,31,32] | 14.1 | Sigma | 26.2 | 0.6–1.5 | 4.8 | 17.0 | [23,30,33,34] |
| 11 | 1-Bromo-4-chlorobutane # | 6940-78-9 | Liquid | NC | 0 | NC | NI | [29,30,31,32] | 11.9 | Sigma | 6.0 | 1.6–4.9 | 6.4 | 19.8 | [23,30,34,35] |
| 12 | Diethyl phthalate # | 84-66-2 | Liquid | NC | 0 | - | NI | [30,31,32] | 88.1 | Sigma | 95.3 | 86.8–93.1 | 86.4 | 87.6 | [23,30,33,34] |
| 13 | Naphthalene acetic acid # | 86-87-3 | Solid | NC | 0 | NC | NI | [30,31,32] | 81.8 | Sigma | 96.4 | 95.5–99.9 | 104.8 | 99.7 | [23,30,33,34] |
| 14 | Allyl phenoxyacetate # | 7493-74-5 | Liquid | NC | 0.3 | - | NI | [30,31,32] | 77.8 | Sigma | 96.5 | 67.9–92.0 | 92.6 | 82.3 | [23,30,33,34] |
| 15 | Isopropanol # | 67-63-0 | Liquid | NC | 0.3 | NC | NI | [29,30,31,32] | 76.6 | Sigma | 97.5 | 91.3–104.1 | 90.1 | 84.6 | [23,30,33,34] |
| 16 | 4-Methylthio-benzaldehyde # | 3446-89-7 | Liquid | NC | 1.0 | NC | NI | [9,29,31,32] | 8.2 | Sigma | 51.5 | 2.8–4.2 | 7.4 | 22.6 | [23,30,33,34] |
| 17 | Methyl stearate # | 112-61-8 | Solid | NC | 1.0 | - | NI | [30,31,32] | 93.4 | Sigma | 103.3 | 99.4–108.2 | 98.7 | 104.4 | [23,30,33,34] |
| 18 | Heptyl butyrate # | 5870-93-9 | Liquid | Cat 3 | 1.7 | NC | NI | [29,30,31,32] | 87.3 | Sigma | 104.0 | 91.5–102.3 | 96.4 | 108.1 | [23,30,34,35,36] |
| 19 | Hexyl salicylate # | 6259-76-3 | Liquid | Cat 3 | 2.0 | NC | NI | [29,30,31,32] | 88.3 | Sigma | 99.9 | 93.7–99.2 | 96.4 | 106.7 | [23,30,34,35,36] |
| 20 | Cinnamaldehyde # | 104-55-2 | Liquid | Cat 3 | 2.0 | - | NI | [30,31,32] | 2.9 | Sigma | 7.5 | 1.2–1.7 | 4.7 | 29.1 | [23,30,33,34] |
| 21 | Nonanoic acid | 112-05-0 | Liquid | Cat 2 | 4.0 | I | I | [9,29,37] | 3.0 | Sigma | 4.6 * | 1.1 | – | – | [35,37] |
| 22 | Butyl methacrylate | 97-88-1 | Liquid | Cat 2 | 3.0 | NC | I | [9,29,30,31,33] | 24.6 | Sigma | 11.3 (5.7) * | 1.1–3.7 | 11.6 | 24.5–33.6 | [30,33,34,35,36] |
| 23 | Butyric acid | 107-92-6 | Liquid | Cat 1B | - | - | I | [33,38] | 2.2 | Sigma | 2.1–4.5 | 0.4–1.1 | – | – | [33,38] |
| 24 | Decanoic acid (capric acid) | 334-48-5 | Liquid | Cat 2 | 4.0 | I | I | [9,29,30] | 43.6 | Sigma | 3.1 | <10 | 5.5 | 6.1–17.6 | [30,34,35,39] |
| 25 | 1-Bromopentane | 110-53-2 | Liquid | Cat 2 | 2.7 | - | I | [30,31] | 11.6 | Sigma | 31.2 (9.9–45.1) * | <10 (2.0) ※ | 5.8 | 17.7–24.3 | [30,31,34,37,38,39,40] |
| 26 | alpha-Terpineol | 98-55-5 | Liquid | Cat 2 | 2.7 | NC | I | [9,29,30,31] | 17.1 | Sigma | 8.9 (2.8–15.2) ** | <10 (1.3–3.0) ※※ | 7.1 (17.9–70.9) ** | 7.3–14.5 | [30,31,34,36,39] |
| 27 | Heptanoic acid | 111-14-8 | Liquid | Cat 1B | (PII 5.6) | I | I | [29,39,41] | 2.2 | Sigma | – | <10 | – | – | [39] |
| 28 | Octanoic acid (caprylic acid) | 124-07-2 | Liquid | Cat 1B/1C | (PII 4.4) | I | I | [29,31,38,42,43] | 2.0 | Sigma | 4.5–6.1 | 0.5–1.1 | – | – | [33,38,39] |
| 29 | N,N-Dimethylisopropylamine | 996-35-0 | Liquid | Cat 1B/1C | (PII 5.6) | - | I | [33,38,42] | – | Sigma | 6.3–8.4 | 0.4–1.3 | – | – | [33,38] |
| 30 | Polyethylene glycol 400 (PEG-400) | 25322-68-3 | Liquid | NC | 0 | NC | NI | [29,30,34,39] | 96.1 | TCI | 101.4 *** | >80 (93.4) *** | 99.9 | 98.2–106.6 (98.2) *** | [30,34,39,44] |
| 31 | 3-(Chloropropyl) trimethoxysilane (Silane A-1430) | 2530-87-2 | Liquid | NC | 0 | - | NI | [31,35,45] | 3.3 | Sigma | 36.0–94.6 (10.8) * | 80.7 | 61.7–98.6 | – | [33,35,37,38] |
| 32 | 3,3′-Dithiodipropionic acid | 1119-62-6 | Solid | NC | 0 | - | NI | [30,31,45] | 96.3 | Sigma | 96.7–107.5 | 102.4–117.1 | 98.0 | 89.9–100 | [30,33,34,38] |
| 33 | 2-Phenylethanol (phenylethyl alcohol) | 60-12-8 | Liquid | NC | 1.0 | - | NI | [31,35] | 0.3 | Sigma | 63.1–104.4 (91.7) * | 5.6 | 45.7–92.9 | – | [30,35,37,38] |
| 34 | Benzyl salicylate | 118-58-1 | Liquid | NC | 0.3 | NC | NI | [29,30,45] | 98.5 | Sigma | 121.37 (96.4) * | 86.5–113.3 | 89.5 | 93.6–99.9 | [31,33,34,37] |
| 35 | 1-(4-Chlorophenyl)-3-(3,4-dichlorophenyl) urea | 101-20-2 | Solid | NC | 0 | - | NI | [46,47] | 102.3 | Sigma | 111.2 | – | – | – | [46] |
| 36 | 3,3-Dimethylpentane | 562-49-2 | Liquid | NC | 0 | - | NI | [30,34] | 88.3 | ACROS | – | – | 102.4 | 72.3–90.8 | [30,34] |
| 37 | 4,4′-Methylenebis(2,6-di-tert-butylphenol) | 118-82-1 | Solid | NCu | 0 | - | NI | [30,31] | 97.3 | Sigma | 85.8–111.2 | 86.5–113.3 | 96.9 (94.4) ** | 100.0–101.3 | [30,31,33,34,38] |
| 38 | Dodecanoic acid (lauric acid) | 143-07-7 | Solid | NC | 0.3 | NC | NI | [29,30,31] | 84.8 | Sigma | 102.5–127.6 | 78.0–102.1 | 20.2 (101.4) ** | 94.0–110.0 | [30,31,33,34] |
| 39 | 1-Chloro-3-nitrobenzene (3-chloronitrobenzene) | 121-73-3 | Solid | NC | 0 | - | NI | [30,31] | 87.7 | Sigma | 90.0 (102.5) * | – | 96.9 | 95.2–104.3 | [30,31,34,38] |
| 40 | Benzyl benzoate | 120-51-4 | Liquid | NC | 0 | - | NI | [30,31] | 98.3 | Sigma | 110.0 | 84.3–104.1 | 93.4 (100.1) ** | 99.6–105.7 | [30,31,33,34] |
| 41 | 2-(Formylamino)-3-thiophenecarboxylic acid | 43028-69-9 | Solid | NC | 0 | - | NI | [31,45] | 100.9 | Sigma | 89.0–95.8 | 108.0 | 97.8–105.7 | – | [31,35] |
| 42 | Sodium bisulphite | 7631-90-5 | Solid | NC | 1.0 | - | NI | [30,34,45] | 9.4 | Sigma | 108.0 | 79.1–99.7 | 56.1 | 11.1–74.7 | [30,33,34] |
| 43 | 4-Acetoxy-2,5-dimethyl-3(2H)-furanone (2,5-dimethyl-4-oxo-4,5-dihydrofuran-3-yl acetate) | 4166-20-5 | Liquid | NC | 0 | - | NI | [31,35,45] | 4.6 | TCI | 98.2–114.3 | 102.3 | 80.3–91.2 | – | [31,35] |
| 44 | 4-Amino-4H-1,2,4-triazole (4-amino-1,2,4-triazole) | 584-13-4 | Solid | NC | 0 | - | NI | [30,31,45] | 89.7 | Sigma | 98.3 | 102.2–106.0 | 91.0 (92.1) ** | 97.4–101.0 | [30,31,34] |
| 45 | Dipropylene glycol | 25265-71-8 | Liquid | NC | 0 | NC | NI | [9,30,31,45] | 94.9 | Sigma | 81.5–111.5 | 95.9–103.5 | 93.5 | 92.9–109.9 | [30,33,34,38] |
| 46 | Dipropylene glycol butyl ether, mixture of isomers (dipropylene glycol monobutyl ether; DPnB) | 29911-28-2 | Liquid | NC | 0 | - | NI | [31,45] | 13.9 | Sigma | 85.0–113.9 | 97.4 | 101.2 | – | [33,35,38] |
| 47 | Erucamide | 112-84-5 | Solid | NC | 0 | - | NI | [30,31,45] | 103.1 | Sigma | 97.8–106.2 | 95.1–107.7 | 102.7 | 90.0–102.5 | [30,33,35,38] |
| 48 | Propylene glycol | 1-6 | Liquid | NC | 0 | NC | NI | [29,39,48,49] | 84.5 | Sigma | – | >80 | – | – | [39] |
| 49 | Triethylene glycol | 112-27-6 | Liquid | NC | 0 | - | NI | [31,35,45] | 76.6 | Sigma | 90.7–116.9 (101.9) * | 101.4 | 95.0–95.3 | – | [31,33,35,37,38] |
| 50 | Sodium bicarbonate | 144-55-8 | Solid | NC | 0 | - | NI | [30,31,45] | 91.0 | Sigma | 92.0–104.2 | 94.7–113.3 | 90.9 | 99.6–100.3 | [30,33,38] |
| 51 | Isopropyl palmitate | 142-91-6 | Liquid | NC | 1.0 | NC | NI | [29,30,31,45] | 106.0 | Sigma | 93.9–104.1 | 100.7–115.1 | 93.0 | 102.5–115.9 | [30,33,34,38] |
| 52 | Isopropyl myristate | 110-27-0 | Liquid | NC | 1.0 | NC | NI | [29,30,31,45] | 101.9 | Sigma | 102.9–118.6 | 98.6–110.5 | 97.5 | 97.2–107.9 | [30,33,34,38] |
| 53 | 2-Ethylhexyl 4-methoxycinnamate (octinoxate) | 5466-77-3 | Liquid | NC | - | - | NI | [50,51] | 106.5 | Sigma | – | 83.3–109.7 | – | – | [50] |
| 54 | Tetrabromophenol blue | 4430-25-5 | Solid | NC | - | - | NI | [46,52,53] | 89.9 | Sigma | 109.8 (110.8) **** | – | – | – | [46,52] |
| 55 | Piroctone olamine | 68890-66-4 | Solid | Cat 2 | - | - | I | [52,54] | 1.7 | TCI | 6.0 (7–11) ***** | – | – | – | [52,54] |
| 56 | 25% Cetyltrimethylammonium chloride solution (in D.W.) (cetrimonium chloride) | 112-02-7 | Liquid | Cat 2 | - | - | I | [52,54,55] | 1.1 | Sigma | 10.5 | – | – | – | [52] |
| 57 | 2-Hydroxy-4-methoxybenzophenone, (Benzophenone-3) | 131-57-7 | Solid | NC | - | - | NI | [52,54,56] | 99.3 | Sigma | 104.1 (64–119) ***** | – | – | – | [52,54] |
| 58 | Cyclohexadecanone | 2550-52-9 | Solid | NC | 0 | - | NI | [31,45] | 103.9 | Symrise | 112.4–121.9 | – | 7.9–113.6 | – | [31] |
| 59 | Methyl laurate | 111-82-0 | Liquid | Cat 3 | 2.0 | NC | NI | [29,31,45] | 91.2 | Sigma | 83.9 * | >80 | 103.3 | – | [31,37,39] |
| 60 | Capryl-isostearate | 209802-43-7 | Liquid | NC | 1.0 | - | NI | [31,45] | 94.1 | Nikko Chemical | 96.0–102.3 | – | 99.5–108.0 | – | [31] |
| 61 | 2-Ethylhexyl-4-aminobenzoate | 26218-04-2 | Solid | NC | 0.7 | - | NI | [31,45] | 103.1 | Santacruz | 90.9–111.4 | – | 91.9–107.3 | – | [31] |
| 62 | 2-Phenylhexanenitrile | 3508-98-3 | Liquid | Cat 3 | 1.7 | - | NI | [31,45] | 76.0 | IFF | 86.7–116.2 | – | 73.9–82.1 | – | [31] |
| 63 | Barium sulfate | 7727-43-7 | Solid | NC | - | - | NI | 94.8 | ACROS | 95.3 | 99.4 | 99.0 | 92.9 | [44] | |
| 64 | Diisopropyl sebacate | 7491-02-3 | Liquid | NC | - | - | NI | 93.8 | Nippon Fine Chemicals | 91.1 | 91.0 | – | 104.6 | [44] | |
| 65 | 10% Xanthan gum (in D.W.) | 11138-66-2 | Gel | NC | - | - | NI | [44,57,58] | 99.6 | Sigma | 101.3 | 98.4 | – | 94.0 | [44] |
| 66 | 3-Chloro-4-fluoronitrobenzene | 350-30-1 | Solid | NC | 1.0 | - | NI | [31,45] | 80.7 | Sigma | 11.0–54.9 (5.0) * | 101.6 | 53.2–101.7 | – | [31,35,37] |
| 67 | 1,5-Hexadiene | 592-42-7 | Liquid | NC | 0 | - | NI | [30,34] | – | Sigma | – | – | 100.4 | 85.6–95.6 | [30,34] |
| 68 | Glycerol | 56-81-5 | Liquid | NC | 0 | - | NI | [30,34] | – | Sigma | – | – | 99.7 | 98.1–125.7 | [30,34] |
| 69 | Benzyl acetate | 140-11-4 | Liquid | NC | 1.0 | - | NI | [30,31,45] | – | Sigma | 21.7 | – | 74.4 (102.8) ** | 11.3–37.4 | [30,31,34] |
| 70 | Hydroxycitronellal | 107-75-5 | Liquid | NC | 1.0 | NC | NI | [9,30,45] | – | Sigma | 83.3 | 79.3 | 14.7 | 19.8–32.3 | [30,33,34,35,38] |
| 71 | n-Butyl propionate | 590-01-2 | Liquid | NC | 1.0 | - | NI | [30,31,45] | – | Sigma | 92.1–104.0 (31.5) ※※※ | – | 20.8 ※※※ (108.7) ** | 23.2–45.9 | [30,31,34,38] |
| 72 | Benzyl alcohol | 100-51-6 | Liquid | NC | 1.3 | NC | NI | [29,30,31] | – | Sigma | 72.5 ※※※ | <10 | 5.5 ※※※ (89.2) ** | 5.6–17.8 | [30,31,34,39] |
| 73 | Allyl heptanoate | 142-19-8 | Liquid | Cat 3 | 1.7 | - | NI | [31,33,38] | – | Sigma | 88.6–112.5 (101.1) ※※※ | 74.4–101.7 | 97.0–102.1 (99.2) ※※※ | 95.6–108.9 | [30,31,33,34,38] |
| 74 | 2-Ethoxy ethyl methacrylate | 2370-63-0 | Liquid | Cat 3 | 1.7 | - | NI | [31,38,45] | – | Sigma | 65.3–116.3 (6.7) ※※※※ | – | 6.9 ※※※ (99.0) ** | 29.3–74.4 | [30,31,33,34,38,46] |
| 75 | Linalyl acetate | 115-95-7 | Liquid | Cat 3 | 2.0 | NC | NI | [29,30,31] | – | Sigma | 50.0–103.8 (43.2) ※※※ | 1.25 | 79.6 | 86.8–101.2 | [30,34,35,38] |
| 76 | Terpinyl acetate | 80-26-2 | Liquid | Cat 3 | 2.0 | NC | NI | [29,30,31] | – | Sigma | 4.9–75.4 (53.0) ※※※ | 1.7–3.2 | 65.4 | 26.2–36.3 | [30,33,34,38] |
| 77 | Linalool | 78-70-6 | Liquid | Cat 3 | 2.0 | - | NI | [30,31,45] | – | Sigma | 14.7 | – | 4.7 | 7.9–25.4 | [30,34] |
| 78 | D-Limonene | 5989-27-5 | Liquid | Cat 3 | 2.0 | - | NI | [31,45] | – | Sigma | – | 1.1 | 10.4–23.6 (16.3) ** | – | [31,40,59] |
| 79 | Eugenol | 97-53-0 | Liquid | Cat 3 | 2.0 | NC | NI | [29,30,31,38] | – | Sigma | 5.1–8.3 | 0.0–0.1 | 5.26 | 18.5–32.2 | [30,33,34,38] |
| 80 | Methyl palmitate #### | 112-39-0 | Liquid | Cat 2 | 3.0 | NC | I | [29,31,33,45] | – | Sigma | 100.5–104.9 | 70.9–110.6 | 95.7 | – | [31,33,38] |
| 81 | 1,1,1-Trichloroethane | 71-55-6 | Liquid | Cat 2 | 4.0 | - | I | [30,31,45] | – | Sigma | 36.6 | <20 (6.1) ※ | 16.8 | 10.1–13.4 | [30,34,39,40] |
| 82 | SLS (50% aq.) | 151-21-3 | Liquid | Cat 2 | 4.0 | - | I | [31,33] | – | Sigma | 13.1–34.5 (2.3) * | 0.7–1.1 (1.6) ※ | 11.9 | – | [31,33,37,38,40] |
| 83 | SLS (20% aq.) | 151-21-3 | Liquid | Cat 2 | 4.0 | I | I | [29,30,31,33] | – | Sigma | 5.2–8.3 (61.3) ※※※ | 1.0–1.7 | 4.2 (13.2) ** | 9.4–13.4 | [23,30,31,33,34,38] |
| 84 | SLS (5% aq.) | 151-21-3 | Liquid | Cat 2 | 4.0 | - | I | [30,34] | – | Sigma | 5.8 *** | 2.1 *** | 4.3 | 11.4–14.5 | [30,34,44] |
| 85 | Tri-isobutyl phosphate | 126-71-6 | Liquid | Cat 3 | 2.0 | - | NI | [24,30,36,38,45] | – | Santa Cruz Biotechnology | 4.4–8.3 (5.9–7.1) ** | 1.3–1.7 | 6.4–10.6 (24.3–44.6) ** | – | [30,31,36,38] |
| 86 | 10-Undecenoic acid | 112-38-9 | Solid (Liquid at room temp.) | Cat 3 | 2.0 | NC | NI | [29,31,45,60] | – | Sigma | 6.0–15.3 (6.2) * | 2.7–14.1 | 10.0–17.5 (13.2) ** | – | [31,38,40,59,60] |
| 87 | dl-Citronellol | 106-22-9 | Liquid | Cat 3 | 2.0 | NC | NI | [29,31,45] | – | Sigma | 5.9–11.4 | 0.3–1.0 | 9.7–12.3 (11.1) ** | – | [31,40,59,61] |
| 88 | 33% Sodium undecylenate (in aqueous solution) | 3398-33-2 | Liquid | Cat 3 | (PII 1.7) | - | NI | [33,42] | – | Sigma | 9.4–28.0 | 0.8–1.7 | – | – | [33,38] |
| 89 | 2-Methoxyphenol (guaiacol) | 90-05-1 | Liquid | Cat 3 | (PII 2.4) | - | NI | [33,40,60] | – | Sigma | 0.7 | 0.5–0.6 | – | – | [33,60] |
| 90 | 1,9-Decadiene | 1647-16-1 | Liquid | Cat 3 | (PII 3.0) | - | NI | [33,42,60] | - | Sigma | 17.2–20.0 (10.6) * | 1.3–2.3 | – | – | [33,38,60] |
| 91 | 2-tert-Butylphenol | 88-18-6 | Liquid | Cat 1B/1C | (PII 5.6) | - | I | [38,42,62,63] | - | Sigma | 3.1–5.3 | 1.4–9.5 | – | – | [33,38] |
| 92 | Carvacrol | 499-75-2 | Liquid | Cat 1B/1C | (PII > 4) | - | I | [38,42,62] | - | Sigma | 4.5–5.6 | 0–27.1 | – | – | [33,38] |
| 93 | Cyclohexylamine | 108-91-8 | Liquid | Cat 1B/1C | - | I | I | [33,38,64] | - | Sigma | 4.3–6.6 (3.6) * | 0.5–1.9 | – | – | [33,38,60] |
| 94 | Lactic acid | 50-21-5 (598-82-3) | Liquid | Cat 1B/1C | - | I | I | [29,33,60,63] | - | Sigma | 8.4 | 0.5–1.5 | – | – | [33,60] |
| 95 | Ethanolamine | 141-43-5 | Liquid | Cat 1B | - | - | I | [33,60] | - | Sigma | 3.4 | 0.2–21.2 | – | – | [33,60] |
| 96 | Boron trifluoride acetic acid complex | 373-61-5 | Liquid | Cat 1B | - | - | I | [33,38] | - | Sigma | 3.4–4.5 | 0.4–1.0 | – | – | [33,38] |
| 97 | Propionic acid | 79-09-4 | Liquid | Cat 1B | - | - | I | [33,38,60] | - | Sigma | 3.0–5.5 (2.9) * | 0.6–0.7 | – | – | [33,38,60] |
| 98 | N,N-Dimethylbenzylamine | 103-83-3 | Liquid | Cat 1C | >4 | - | I | [33,38,60,65] | - | Sigma | 4.7–6.8 (0.3) * | 0.5–0.9 | – | – | [33,38,60] |
| 99 | Maleic anhydride | 108-31-6 | Solid | Cat 1C | - | I | I | [38,60,64] | - | Sigma | 4.7–7.5 (6.1) * | 0.4–0.5 | – | – | [33,38,60] |
| 100 | 48% Fluoroboric acid (in D.W.) (hydrogen tetrafluoroborate) | 16872-11-0 | Liquid | Cat 1C | - | - | I | [33,60] | - | Sigma | 2.4 | 0.6–1.1 | [33,60] | ||
| KeraSkinTM | EpiSkinTM | SkinEthicTM | EpiDermTM | LabCyte EPI-MODEL24 | PS | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| I | NI | I | NI | I | NI | I | NI | I | NI | ||
| I | 20 | 0 | 34 | 1 | 33 | 1 | 17 | 2 | 16 | 1 | |
| NI | 8 | 37 | 12 | 46 | 15 | 34 | 10 | 44 | 12 | 28 | |
| Total (n) | 65 | 93 | 83 | 73 | 57 | ||||||
| Sensitivity | 100% | 97.1% | 97.1% | 89.5% | 94.1% | 80% | |||||
| Specificity | 82.2% | 79.3% | 69.4% | 81.5% | 70.0% | 70% | |||||
| Accuracy | 87.5% | 86.0% | 80.7% | 83.6% | 77.2% | 75% | |||||
| In Vivo Score | KeraSkinTM | EpiSkinTM | SkinEthicTM RHE | EpiDermTM | LabCyte EPI-MODEL24 | |
|---|---|---|---|---|---|---|
| In vivo score | 1.000 | |||||
| KeraSkinTM | −0.773 * | 1.000 | ||||
| EpiSkinTM | −0.760 * | 0.899 * | 1.000 | |||
| SkinEthicTM RHE | −0.803 * | 0.938 * | 0.957 * | 1.000 | ||
| EpiDermTM | −0.749 * | 0.940 * | 0.933 * | 0.933 * | 1.000 | |
| LabCyte EPI-MODEL24 | −0.752 * | 0.960 * | 0.919 * | 0.938 * | 0.978 * | 1.000 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, J.; Lee, G.-Y.; Bae, G.; Kang, M.-J.; Lim, K.-M. ChemSkin Reference Chemical Database for the Development of an In Vitro Skin Irritation Test. Toxics 2021, 9, 314. https://doi.org/10.3390/toxics9110314
Han J, Lee G-Y, Bae G, Kang M-J, Lim K-M. ChemSkin Reference Chemical Database for the Development of an In Vitro Skin Irritation Test. Toxics. 2021; 9(11):314. https://doi.org/10.3390/toxics9110314
Chicago/Turabian StyleHan, Juhee, Ga-Young Lee, Green Bae, Mi-Jeong Kang, and Kyung-Min Lim. 2021. "ChemSkin Reference Chemical Database for the Development of an In Vitro Skin Irritation Test" Toxics 9, no. 11: 314. https://doi.org/10.3390/toxics9110314
APA StyleHan, J., Lee, G.-Y., Bae, G., Kang, M.-J., & Lim, K.-M. (2021). ChemSkin Reference Chemical Database for the Development of an In Vitro Skin Irritation Test. Toxics, 9(11), 314. https://doi.org/10.3390/toxics9110314








