Use of Personal Care Products and Semen Quality: A Cross-Sectional Study in Young Danish Men
Abstract
1. Introduction
2. Materials and Methods
2.1. The FEPOS Cohort
2.2. PCP Exposure
2.3. Outcome Measures
2.4. Statistical Analyses
2.5. Ethics
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jacobs, C.; Kerna, N.A.; Tulp, O.L. An overview of the causes and consequences of male fertility decline. Percept. Reprod. Med. 2019, 3, 192–194. [Google Scholar]
- Jorgensen, N.; Andersen, A.G.; Eustache, F.; Irvine, D.S.; Suominen, J.; Petersen, J.H.; Andersen, A.N.; Auger, J.; Cawood, E.H.H.; Horte, A.; et al. Regional differences in semen quality. Eur. Hum. Reprod. 2001, 16, 1012–1019. [Google Scholar] [CrossRef] [PubMed]
- Skakkebaek, N.E.; Rajpert, D.; Meyts, E.; Buck Louis, G.M.; Toppari, J.; Andersson, A.M.; Eisenberg, M.L.; Jensen, T.K.; Jørgensen, N.; Swan, S.H.; et al. Male Reproductive Disorders and Fertility Trends: Influences of Environment and Genetic Susceptibility. Physiol. Rev. 2016, 96, 55–97. [Google Scholar] [CrossRef] [PubMed]
- Priskorn, L.; Nordkap, L.; Bang, A.K.; Krause, M.; Holmboe, S.A.; Egeberg Palme, D.L.; Winge, S.; Mørup, N.; Carlsen, E.; Joensen, U.N.; et al. Average sperm count remains unchanged despite reduction in maternal smoking: Results from a large cross-sectional study with annual investigations over 21 years. Hum. Reprod. 2018, 33, 998–1008. [Google Scholar] [CrossRef] [PubMed]
- Witorsch, R.J.; Thomas, J.A. Personal care products and endocrine disruption: A critical review of the literature. Crit. Rev. Toxicol. 2010, 40 (Suppl. 3), 1–30. [Google Scholar] [CrossRef]
- Elsner, P. Overview and trends in male grooming. Br. J. Dermatol. 2012, 166 (Suppl. 1), 2–5. [Google Scholar] [CrossRef]
- Malinauskiene, L.; Blaziene, A.; Chomiciene, A.; Isaksson, M. Formaldehyde may be found in cosmetic products even when unlabelled. Open Med. 2015, 10, 323–328. [Google Scholar] [CrossRef]
- Borowska, S.; Brzoska, M.M. Metals in cosmetics: implications for human health. J. Appl. Toxicol. 2015, 35, 551–572. [Google Scholar] [CrossRef]
- Alaee, S.; Talaiekhozani, A.; Rezaei, S.; Alaee, K.; Yousefian, E. Cadmium and male infertility. J. Infertil. Reprod. Biol. 2014, 2, 62–69. [Google Scholar]
- Benoff, S.; Jacob, A.; Hurley, I.R. Male infertility and environmental exposure to lead and cadmium. Hum. Reprod. Update 2000, 6, 107–121. [Google Scholar] [CrossRef]
- EU. Commission Regulation (EU) No 344/2013 of 4 April 2013 amending Annexes II, III, V and VI to Regulation (EC) No 1223/2009 of the European Parliament and of the Council on cosmetic products. Off. J. Eur. Union 2013, 1–59. [Google Scholar]
- Ficheux, A.S.; Gomez-Berrada, M.P.; Roudot, A.C.; Ferret, P.J. Consumption and exposure to finished cosmetic products: A systematic review. Food Chem. Toxicol. 2019, 124, 280–299. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Hidalgo, E.; von Goetz, N.; Siegrist, M.; Hungerbuhler, K. Use-patterns of personal care and household cleaning products in Switzerland. Food Chem. Toxicol. 2017, 99, 24–39. [Google Scholar] [CrossRef] [PubMed]
- Biesterbos, J.W.; Dudzina, T.; Delmaar, C.J.; Bakker, M.I.; Russel, F.G.; von Goetz, N.; Scheepers, P.T.J.; Roeleveld, N. Usage patterns of personal care products: important factors for exposure assessment. Food Chem. Toxicol. 2013, 55, 8–17. [Google Scholar] [CrossRef] [PubMed]
- FDA—U.S. Food and Drug Administration. Fragrances in Cosmetics, 2018. Available online: https://www.fda.gov/cosmetics/cosmetic-ingredients/fragrances-cosmetics (accessed on 1 November 2019).
- Duty, S.M.; Ackerman, R.M.; Calafat, A.M.; Hauser, R. Personal care product use predicts urinary concentrations of some phthalate monoesters. Environ. Health Perspect. 2005, 113, 1530–1535. [Google Scholar] [CrossRef]
- Dodson, R.E.; Nishioka, M.; Standley, L.J.; Perovich, L.J.; Brody, J.G.; Rudel, R.A. Endocrine disruptors and asthma-associated chemicals in consumer products. Environ. Health Perspect. 2012, 120, 935–943. [Google Scholar] [CrossRef]
- Keglberg Hærvig, K.; Bonde, J.P.; Ramlau-Hansen, C.H.; Toft, G.; Hougaard, K.S.; Specht, I.O.; Giwercman, A.; Nybo Andersen, A.M.; Olsen, J.; Lindh, C.; et al. Fetal Programming of Semen Quality (FEPOS) Cohort—A DNBC Male Offspring Cohort. Clin. Epidemiol. 2020, 12, 757–770. [Google Scholar] [CrossRef]
- Olsen, J.; Melbye, M.; Olsen, S.F.; Sorensen, T.I.; Aaby, P.; Andersen, A.M.; Taxbøl, D.; Hansen, K.D.; Juhl, M.; Schow, T.B.; et al. The Danish National Birth Cohort—Its background, structure and aim. Scand. J. Public Health 2001, 29, 300–307. [Google Scholar] [CrossRef]
- Jacobsen, T.N.; Nohr, E.A.; Frydenberg, M. Selection by socioeconomic factors into the Danish National Birth Cohort. Eur. J. Epidemiol. 2010, 25, 349–355. [Google Scholar] [CrossRef]
- Bliddal, M.; Liew, Z.; Pottegard, A.; Kirkegaard, H.; Olsen, J.; Nohr, E.A. Examining Nonparticipation in the Maternal Follow-up within the Danish National Birth Cohort. Am. J. Epidemiol. 2018, 187, 1511–1519. [Google Scholar] [CrossRef]
- Pedersen, C.B. The Danish Civil Registration System. Scand. J. Public Health 2011, 39 (Suppl. 7), 22–25. [Google Scholar] [CrossRef]
- Schmidt, M.; Schmidt, S.A.; Sandegaard, J.L.; Ehrenstein, V.; Pedersen, L.; Sorensen, H.T. The Danish National Patient Registry: A review of content, data quality, and research potential. Clin. Epidemiol. 2015, 7, 449–490. [Google Scholar] [CrossRef]
- Park, J.Y.; Lee, K.; Hwang, Y.; Kim, J.H. Determining the exposure factors of personal and home care products for exposure assessment. Food Chem. Toxicol. 2015, 77, 105–110. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO Laboratory Manual for the Examination and Processing of Human Semen; WHO: Geneva, Switzerland, 2010. [Google Scholar]
- Etminan, M.; Collins, G.S.; Mansournia, M.A. Using Causal Diagrams to Improve the Design and Interpretation of Medical Research. Chest 2020, 158, S21–S28. [Google Scholar] [CrossRef] [PubMed]
- Danmarks Statistik. DISCO-88—Danmarks Statistiks Fagklassifikation; Danmarks Statistiks Trykkeri: Copenhagen, Denmark, 1996. [Google Scholar]
- Cocuzza, M.; Alvarenga, C.; Pagani, R. The epidemiology and etiology of azoospermia. Clinics (Sao Paulo) 2013, 68 (Suppl. 1), 15–26. [Google Scholar] [CrossRef]
- World Medical Association. WMA Declaration of Helsinki—Ethical Principles for Medical Research Involving Human Subjects. 2018. Available online: https://www.wma.net/policies-post/wma-declaration-of-helsinki-ethical-principles-for-medical-research-involving-human-subjects/ (accessed on 2 October 2019).
- Manová, E.; von Goetz, N.; Keller, C.; Siegrist, M.; Hungerbühler, K. Use patterns of leave-on personal care products among Swiss-German children, adolescents, and adults. Int. J. Environ. Res. Public Health 2013, 10, 2778–2798. [Google Scholar] [CrossRef] [PubMed]
- Weismann, K.; Petersen, C.S.; Menné, T.; Grønhøj Larsen, F.; Skovgaard, G.L. Dermatologi og Venerologi—Lærebogen, 4th ed.; FADL’s Forlag A/S: Brøndby, Denmark, 2005. [Google Scholar]
- Moungkhem, C.; Surakiatpinyo, J. A Study of Factors Affecting on Men’s Skin Care Products Purchasing, Particularly in Karlstad; Karlstad University: Karlstad, Sweden, 2010. [Google Scholar]
- Zamkowska, D.; Karwacka, A.; Jurewicz, J.; Radwan, M. Environmental exposure to non-persistent endocrine disrupting chemicals and semen quality: An overview of the current epidemiological evidence. Int. J. Occup. Med. Environ. Health 2018, 31, 377–414. [Google Scholar] [CrossRef] [PubMed]
- Adoamnei, E.; Mendiola, J.; Monino-Garcia, M.; Vela-Soria, F.; Iribarne-Duran, L.M.; Fernandez, M.F.; Olea, N.; Jørgensen, N.; Swan, S.H.; Torres-Cantero, A.M. Urinary concentrations of parabens and reproductive parameters in young men. Sci. Total Environ. 2018, 621, 201–209. [Google Scholar] [CrossRef]
- Adoamnei, E.; Mendiola, J.; Monino-Garcia, M.; Vela-Soria, F.; Iribarne-Duran, L.M.; Fernandez, M.F.; Olea, N.; Jørgensen, N.; Swan, S.H.; Torres-Cantero, A.M. Urinary concentrations of benzophenone-type ultra violet light filters and reproductive parameters in young men. Int. J. Hyg. Environ. Health 2018, 221, 531–540. [Google Scholar] [CrossRef]
- Adoamnei, E.; Mendiola, J.; Vela-Soria, F.; Fernandez, M.F.; Olea, N.; Jørgensen, N.; Swan, S.H.; Torres-Cantero, A.M. Urinary bisphenol A concentrations are associated with reproductive parameters in young men. Environ. Res. 2018, 161, 122–128. [Google Scholar] [CrossRef]
- Al-Saleh, I.; Coskun, S.; Al-Doush, I.; Al-Rajudi, T.; Abduljabbar, M.; Al-Rougi, R.; Palawan, H.; Al-Hassan, S. The relationships between urinary phthalate metabolites, reproductive hormones and semen parameters in men attending in vitro fertilization clinic. Sci. Total Environ. 2019, 25, 982–995. [Google Scholar] [CrossRef]
- Jurewicz, J.; Radwan, M.; Wielgomas, B.; Dziewirska, E.; Karwacka, A.; Klimowska, A.; Kaluzny, P.; Radwan, P.; Bochenek, M.; Hanke, W. Human Semen Quality, Sperm DNA Damage, and the Level of Reproductive Hormones in Relation to Urinary Concentrations of Parabens. J. Occup. Environ. Med. 2017, 59, 1034–1040. [Google Scholar] [CrossRef] [PubMed]
- Jurewicz, J.; Radwan, M.; Wielgomas, B.; Kaluzny, P.; Klimowska, A.; Radwan, P.; Hanke, W. Environmental levels of triclosan and male fertility. Environ. Sci. Pollut. Res. Int. 2018, 25, 5484–5490. [Google Scholar] [CrossRef] [PubMed]
- Nishihama, Y.; Toshima, H.; Yoshinaga, J.; Mizumoto, Y.; Yoneyama, M.; Nakajima, D.; Shiraishi, H.; Tokuoka, S. Paraben exposure and semen quality of Japanese male partners of subfertile couples. Environ. Health Prev. Med. 2017, 22, 5. [Google Scholar] [CrossRef] [PubMed]
- Smarr, M.M.; Honda, M.; Kannan, K.; Chen, Z.; Kim, S.; Louis, G.M.B. Male urinary biomarkers of antimicrobial exposure and bi-directional associations with semen quality parameters. Reprod. Toxicol. 2018, 77, 103–108. [Google Scholar] [CrossRef] [PubMed]
- Smarr, M.M.; Kannan, K.; Sun, L.; Honda, M.; Wang, W.; Karthikraj, R.; Chen, Z.; Weck, J.; Louis, J.M.B. Preconception seminal plasma concentrations of endocrine disrupting chemicals in relation to semen quality parameters among male partners planning for pregnancy. Environ. Res. 2018, 167, 78–86. [Google Scholar] [CrossRef] [PubMed]
- Hipwell, A.E.; Kahn, L.G.; Factor-Litvak, P.; Porucznik, C.A.; Siegel, E.L.; Fichorova, R.N.; Hamman, R.F.; Klein-Fedyshin, M.; Harley, K.G.; Program collaborators for Environmental influences on Child Health Outcomes. Exposure to non-persistent chemicals in consumer products and fecundability: A systematic review. Hum. Reprod. Update 2019, 25, 51–71. [Google Scholar] [CrossRef]
- Kay, V.R.; Bloom, M.S.; Foster, W.G. Reproductive and developmental effects of phthalate diesters in males. Crit. Rev. Toxicol. 2014, 44, 467–498. [Google Scholar] [CrossRef]
- Michalek, I.M.; Liu, B.; Benn, E.K.T.; Caetano Dos Santos, F.L. Skin lightening products’ violations in Europe: An analysis of the rapid alert system for dangerous non-food products 2005-Regul. Toxicol. Pharmacol. 2019, 106, 50–54. [Google Scholar]
- Joensen, U.N.; Jorgensen, N.; Thyssen, J.P.; Szecsi, P.B.; Stender, S.; Petersen, J.H.; Andersson, A.-M.; Frederiksen, H. Urinary excretion of phenols, parabens and benzophenones in young men: Associations to reproductive hormones and semen quality are modified by mutations in the Filaggrin gene. Environ. Int. 2018, 121 Pt 1, 365–374. [Google Scholar] [CrossRef]
- Wilcox, A.J. Fertility and Pregnancy; Oxford University Press: New York, NY, USA, 2010. [Google Scholar]
- Allied Market Research. Men’s Personal Care Market to Reach $166 Billion, Globally, by 2022. 2016. Available online: https://www.prnewswire.com/news-releases/mens-personal-care-market-to-reach-166-billion-globally-by-2022-allied-market-research-597595471.html (accessed on 22 November 2019).
- Shaw Nunez, N. The International Cosmetic Regulatory Framework. Master’s Thesis, University of Barcelona, Barcelona, Spain, 2015. [Google Scholar]
- Heerfordt, I.M. Sunscreen use at Danish beaches and how to improve coverage. Dan. Med. J. 2018, 65, B5476. [Google Scholar] [PubMed]
- Ingle, M.E.; Minguez-Alarcon, L.; Carignan, C.C.; Butt, C.M.; Stapleton, H.M.; Williams, P.L.; Hauser, R.; Meeker, J.D.; EARTH Study Team. The association of urinary phosphorous-containing flame retardant metabolites and self-reported personal care and household product use among couples seeking fertility treatment. J. Expo. Sci. Environ. Epidemiol. 2020, 30, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Carlsen, E.; Andersson, A.M.; Petersen, J.H.; Skakkebaek, N.E. History of febrile illness and variation in semen quality. Hum. Reprod. 2003, 18, 2089–2092. [Google Scholar] [CrossRef] [PubMed]
- National Research Council. When I’m 64; The National Academies Press: Washington, DC, USA, 2006. [Google Scholar]
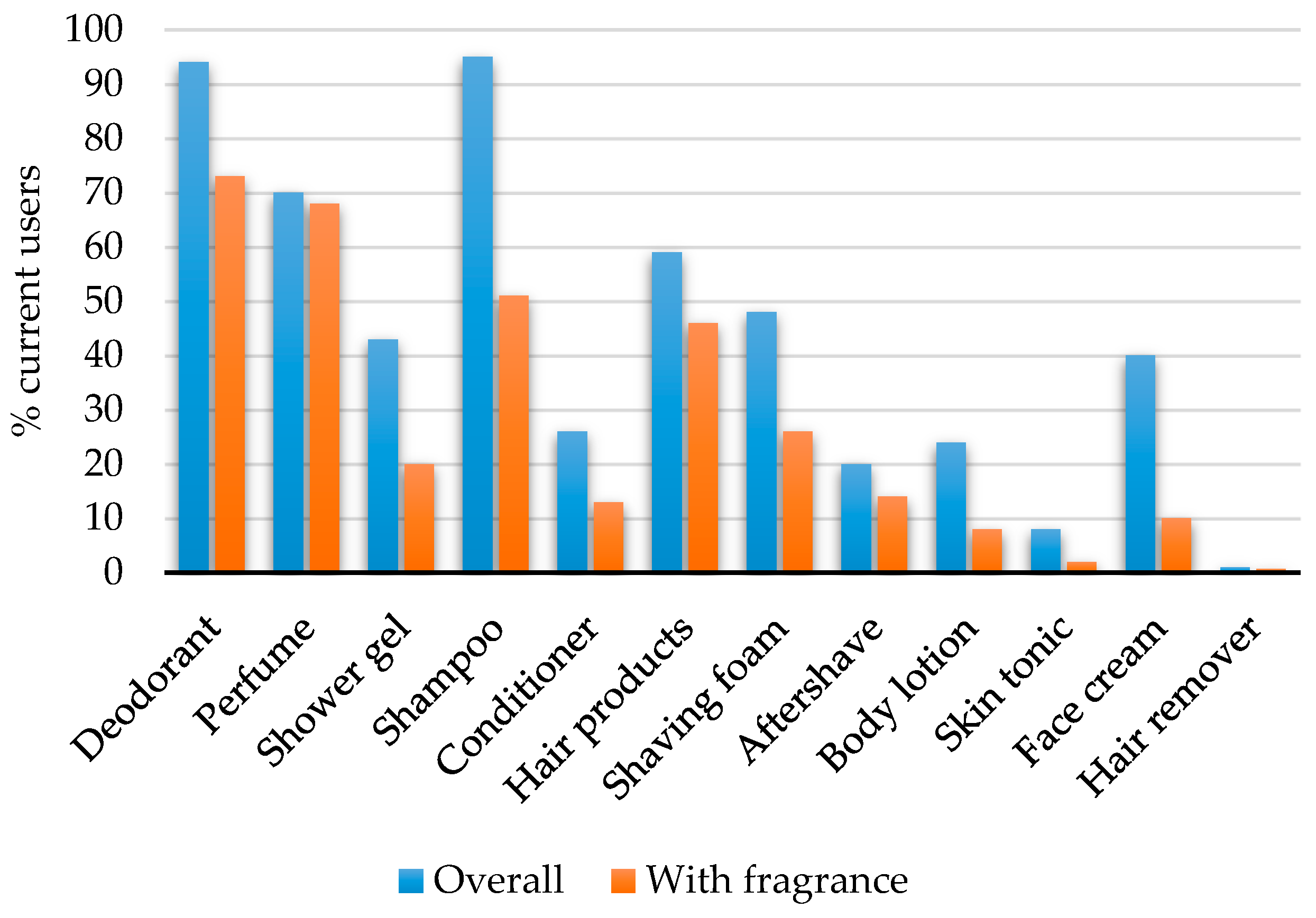
| Do You Use the Following Products Every Day or Several Times a Week? |
|---|
| Answer Options: No/Yes, Without Fragrance (i.e., Nordic Swan Ecolabel)/Yes, with Fragrance |
| Deodorant |
| Perfume |
| Shower gel |
| Shampoo |
| Conditioner |
| Hair products (gel, spray, etc.) |
| Shaving foam |
| Aftershave |
| Body lotion |
| Skin tonic |
| Face cream |
| Hair remover |
| Characteristics | Overall PCP Usage | ||
|---|---|---|---|
| Low | Medium | High | |
| (≤3 PCPs) | (4–6 PCPs) | (≥7 PCPs) | |
| Men | |||
| Total men, n | 203 | 578 | 265 |
| BMI, mean (SD) | 22.1 (3.1) | 22.6 (3.6) | 22.5 (3.0) |
| Alcohol ≥ once a week, n (%) | 97 (48) | 316 (55) | 151 (57) |
| Current smoking, n (%) | 64 (32) | 220 (38) | 129 (49) |
| Current vaping, n (%) | 21 (10) | 37 (6) | 17 (6) |
| Regular exercise, n (%) | 152 (75) | 479 (83) | 233 (88) |
| Sparse facial hair, n (%) 1 | 157(77) | 464 (80) | 230 (87) |
| Acne, n (%) | 66 (33) | 221 (38) | 103 (39) |
| Urogenital disorders, n (%) 2 | 36 (18) | 121 (21) | 55 (21) |
| Mothers | |||
| Age at birth of son, mean (SD) | 30.1 (4.0) | 30.6 (4.1) | 30.5 (4.4) |
| Smoking during pregnancy, n (%) | 45 (22) | 132 (23) | 66 (25) |
| Pre-pregnancy BMI, mean (SD) | 22.6 (3.8) | 22.9 (3.6) | 22.7 (3.5) |
| High family occupational status, n (%) 3 | 69 (34) | 192 (33) | 93 (35) |
| Semen sample parameters | |||
| Days of abstinence, mean (SD) | 2.5 (2.0) | 2.3 (1.4) | 2.2 (1.3) |
| Spillage, yes n (%) | 33 (16) | 96 (17) | 50 (19) |
| Minutes from ejaculation to analysis, mean (SD) | 50 (19.9) | 49 (19.3) | 52 (19.1) |
| Sampling site, clinic n (%) | 176 (87) | 504 (87) | 224 (85) |
| Place of analysis, Copenhagen n (%) | 150 (74) | 442 (76) | 230 (87) |
| Item | General Hygiene | Hair Care | ||||
| Deodorant | Perfume | Shower Gel | Shampoo | Conditioner | Hair Products | |
| General hygiene | ||||||
| Deodorant | ||||||
| Perfume | 0.09 | |||||
| Shower gel | 0.12 | 0.09 | ||||
| Hair Care | ||||||
| Shampoo | 0 | 0.03 | −0.07 | |||
| Conditioner | 0 | 0.15 | 0.1 | 0.09 | ||
| Hair products | 0.08 | 0.29 | 0.05 | 0.09 | 0.05 | |
| Shaving products | ||||||
| Shaving foam | 0.03 | 0.17 | 0.05 | 0.05 | 0.04 | 0.11 |
| Aftershave | 0.05 | 0.14 | 0.14 | −0.03 | 0.11 | 0.02 |
| Skin care | ||||||
| Body lotion | 0.01 | 0.13 | 0.1 | −0.01 | 0.14 | 0.12 |
| Skin tonic | 0.03 | 0.06 | 0.1 | −0.00 | 0.06 | 0.06 |
| Face cream | 0.06 | 0.2 | 0.09 | −0.09 | 0.12 | 0.18 |
| Other | ||||||
| Hair remover | 0.03 | 0.03 | 0.04 | −0.01 | 0.08 | −0.01 |
| Item | Shaving Product | Skin Care | ||||
| Shaving Foam | Aftershave | Body Lotion | Skin Tonic | Face Cream | ||
| Aftershave | 0.27 | |||||
| Skin care | ||||||
| Body lotion | 0.05 | 0.08 | ||||
| Skin tonic | 0.08 | 0.14 | 0.08 | |||
| Face cream | 0.14 | 0.17 | 0.26 | 0.21 | ||
| Other | ||||||
| Hair remover | 0.03 | 0.06 | 0.07 | 0.12 | 0.02 | |
| Parameter | N 1 | Overall PCP Usage | |||||
|---|---|---|---|---|---|---|---|
| Low | Medium | High | |||||
| (≤3 PCPs) | (4–6 PCPs) | (≥7 PCPs) | |||||
| Md | P25%, P75% | Md | P25%, P75% | Md | P25%, P75% | ||
| Sperm concentration (106/mL) | 1046 | 38 | 18, 73 | 40 | 19, 76 | 38 | 20, 65 |
| Total sperm count (106) | 867 | 105 | 41, 204 | 99 | 45, 202 | 106 | 52, 196 |
| Semen volume (mL) | 867 | 2.9 | 2.0, 3.8 | 2.6 | 1.8, 3.6 | 2.7 | 2.0, 3.7 |
| Progressive motility (%) | 1031 | 64 | 52, 72 | 63 | 52, 74 | 63 | 53, 74 |
| Morphology, normal (%) | 1026 | 7 | 4, 10 | 6 | 3, 10 | 6 | 2, 10 |
| Characteristics | Model 1 | N | Overall PCP Usage | ||
| Low | Medium | High | |||
| (≤3 PCPs) | (4–6 PCPs) | (≥7 PCPs) | |||
| Reference | % Difference (95% CI) | % Difference (95% CI) | |||
| Sperm concentration (106/mL) | Crude | 1046 | 0 | 2 (−11, 18) | −8 (−22, 8) |
| Adjusted | 1037 | 0 | 9 (−5, 26) | −2 (−17, 15) | |
| Total sperm count (106) | Crude | 867 | 0 | −3 (−18, 15) | −4 (−21, 17) |
| Adjusted | 858 | 0 | −4 (−18, 13) | −3 (−20, 16) | |
| Semen volume (mL) | Crude | 866 | 0 | −8 (−17, 2) | −3 (−14, 9) |
| Adjusted | 857 | 0 | −4 (−14, 7) | 0 (−12, 13) | |
| Motility, 100-progressive (%) | Crude | 1031 | 0 | 0 (−4, 7) | 0 (−7, 8) |
| Adjusted | 1016 | 0 | 2 (−5, 8) | 3 (−4, 11) | |
| Morphology, normal (%) | Crude | 1026 | 0 | −8 (−19, 4) | −8 (−20, 6) |
| Adjusted | 1017 | 0 | −7 (−18, 5) | −8 (−20, 7) | |
| Characteristics Model 1 N | Fragranced PCP Usage | ||||
| Low | Medium | High | |||
| (≤2 PCPs) | (3–4 PCPs) | (≥5 PCPs) | |||
| Reference | % Difference (95% CI) | % Difference (95% CI) | |||
| Sperm concentration (106/mL) | Crude | 1046 | 0 | −5 (−16, 8) | −6 (−18, 9) |
| Adjusted | 1037 | 0 | 0 (−12, 14) | 6 (−9, 24) | |
| Total sperm count (106) | Crude | 867 | 0 | 1 (−13, 17) | −6 (−20, 11) |
| Adjusted | 858 | 0 | 5 (−10, 22) | 7 (−11, 28) | |
| Semen volume (mL) | Crude | 866 | 0 | 1 (−8, 10) | −4 (−14, 6) |
| Adjusted | 857 | 0 | 5 (−5, 15) | 1 (−10, 14) | |
| Motility, 100-progressive (%) | Crude | 1031 | 0 | 0 (−6, 5) | −4 (−10, 2) |
| Adjusted | 1016 | 0 | 0 (−6, 6) | −4 (−11, 3) | |
| Morphology, normal (%) | Crude | 1026 | 0 | −4 (−14, 7) | −3 (−14, 9) |
| Adjusted | 1017 | 0 | −1 (−12, 11) | 3 (−11, 18) | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ugelvig Petersen, K.; Balkiss, A.M.; Hærvig, K.K.; Ellekilde Bonde, J.P.; Hougaard, K.S.; Toft, G.; Ramlau-Hansen, C.H.; Tøttenborg, S.S. Use of Personal Care Products and Semen Quality: A Cross-Sectional Study in Young Danish Men. Toxics 2020, 8, 62. https://doi.org/10.3390/toxics8030062
Ugelvig Petersen K, Balkiss AM, Hærvig KK, Ellekilde Bonde JP, Hougaard KS, Toft G, Ramlau-Hansen CH, Tøttenborg SS. Use of Personal Care Products and Semen Quality: A Cross-Sectional Study in Young Danish Men. Toxics. 2020; 8(3):62. https://doi.org/10.3390/toxics8030062
Chicago/Turabian StyleUgelvig Petersen, Kajsa, Ahmad Mahmoud Balkiss, Katia Keglberg Hærvig, Jens Peter Ellekilde Bonde, Karin Sørig Hougaard, Gunnar Toft, Cecilia Høst Ramlau-Hansen, and Sandra Søgaard Tøttenborg. 2020. "Use of Personal Care Products and Semen Quality: A Cross-Sectional Study in Young Danish Men" Toxics 8, no. 3: 62. https://doi.org/10.3390/toxics8030062
APA StyleUgelvig Petersen, K., Balkiss, A. M., Hærvig, K. K., Ellekilde Bonde, J. P., Hougaard, K. S., Toft, G., Ramlau-Hansen, C. H., & Tøttenborg, S. S. (2020). Use of Personal Care Products and Semen Quality: A Cross-Sectional Study in Young Danish Men. Toxics, 8(3), 62. https://doi.org/10.3390/toxics8030062









